Abstract
The essential JIL-1 histone H3S10 kinase is a key regulator of chromatin structure that functions to maintain euchromatic domains while counteracting heterochromatization and gene silencing. In the absence of the JIL-1 kinase, two of the major heterochromatin markers H3K9me2 and HP1a spread in tandem to ectopic locations on the chromosome arms. Here we address the role of the third major heterochromatin component, the zinc-finger protein Su(var)3-7. We show that the lethality but not the chromosome morphology defects associated with the null JIL-1 phenotype to a large degree can be rescued by reducing the dose of the Su(var)3-7 gene and that Su(var)3-7 and JIL-1 loss-of-function mutations have an antagonistic and counterbalancing effect on position-effect variegation (PEV). Furthermore, we show that in the absence of JIL-1 kinase activity, Su(var)3-7 gets redistributed and upregulated on the chromosome arms. Reducing the dose of the Su(var)3-7 gene dramatically decreases this redistribution; however, the spreading of H3K9me2 to the chromosome arms was unaffected, strongly indicating that ectopic Su(var)3-9 activity is not a direct cause of lethality. These observations suggest a model where Su(var)3-7 functions as an effector downstream of Su(var)3-9 and H3K9 dimethylation in heterochromatic spreading and gene silencing that is normally counteracted by JIL-1 kinase activity.
SU(VAR)3-9, a histone methyltransferase, Su(var)2-5, HP1a, and Su(var)3-7, a 1250-residue zinc-finger protein are all inherent components of pericentric heterochromatin (Rea et al. 2000; Eissenberg and Elgin 2000; Schotta et al. 2002; Delattre et al. 2004; Ebert et al. 2004) and are important factors for silencing of reporter genes by heterochromatic spreading in Drosophila (for review see Weiler and Wakimoto 1995; Girton and Johansen 2008). Su(var)3-9 has been shown to catalyze most of the dimethylation of the histone H3K9 residue which in turn can promote HP1a and Su(var)3-7 recruitment (Schotta et al. 2002; Jaquet et al. 2006). In addition, Su(var)3-9, HP1a, and Su(var)3-7 can directly interact with each other, suggesting a model where interdependent interactions between Su(var)3-9, HP1a, and Su(var)3-7 lead to heterochromatin assembly at pericentric sites (Lachner et al. 2001; Schotta et al. 2002; Elgin and Grewal 2003; Jaquet et al. 2006). Heterochromatin formation in Drosophila is initiated early in development through active removal of H3K4 methylation by the LSD1 demethylase homolog Su(var)3-3 (Rudolph et al. 2007). Subsequently, a developmentally regulated balance between Su(var)3-3 H3K4 demethylase, Su(var)3-9 H3K9 methyltransferase, and RPD3 H3K9 deacetylase activity contribute to conserve the distinction between euchromatic and heterochromatic domains (Rudolph et al. 2007). Thus, highly complex interactions between multiple heterochromatic and euchromatic factors are likely to contribute to the regulation of a dynamic balance between the distinct chromatin environments promoting gene activity and gene silencing.
It has recently been demonstrated that activity of the essential JIL-1 histone H3S10 kinase (Jin et al. 1999; Wang et al. 2001) is a major regulator of chromatin structure (Deng et al. 2005; 2008) and that it functions to maintain euchromatic domains while counteracting heterochromatization and gene silencing (Ebert et al. 2004; Zhang et al. 2006; Lerach et al. 2006; Bao et al. 2007). In the absence of the JIL-1 kinase, the major heterochromatin markers H3K9me2 and HP1a spread in tandem to ectopic locations on the chromosome arms with the most pronounced increase on the X chromosomes (Zhang et al. 2006; Deng et al. 2007). However, overall levels of the H3K9me2 mark and HP1a were unchanged, suggesting that the spreading was accompanied by a redistribution that reduces the levels in pericentromeric heterochromatin. Genetic interaction assays demonstrated that the lethality as well as some of the chromosome morphology defects associated with the null JIL-1 phenotype to a large degree can be rescued by reducing the dose of the Su(var)3-9 gene (Zhang et al. 2006; Deng et al. 2007). This is in contrast to similar experiments performed with alleles of the Su(var)2-5 gene where no genetic interactions were detectable between JIL-1 and Su(var)2-5 (Deng et al. 2007) Thus, these findings indicate that while Su(var)3-9 histone methyltransferase activity may be a factor in the lethality and chromatin structure perturbations associated with loss of the JIL-1 histone H3S10 kinase, these effects are likely to be uncoupled from HP1a. However, the potential role of the third major heterochromatin component, Su(var)3-7, was not addressed in these studies. Here we show that Su(var)3-7, like Su(var)3-9, genetically interacts with JIL-1, that reducing the dose of Su(var)3-7 significantly reduces the lethality of JIL-1 null mutants, and that Su(var)3-7 and JIL-1 loss-of-function mutations have an antagonistic and counterbalacing effect on position-effect variegation (PEV).
MATERIALS AND METHODS
Drosophila melanogaster stocks and PEV assays:
Fly stocks were maintained according to standard protocols (Roberts 1998). Canton S was used for wild-type preparations. The JIL-1z28, JIL-1z60, and JIL-1z2 alleles are described in Wang et al. (2001) and in Zhang et al. (2003). The Su(var)3-77.1A, Su(var)3-714, and Su(var)3-7R2a8 alleles are described in Seum et al. (2002) and in Spierer et al. (2005). The Su(var)3-901 and Su(var)3-902 stocks were obtained from the Umeå Stock Center. The hsp83 promoter-driven JIL-1-GFP transgene GF29.1 is described in Jin et al. (1999) and in Wang et al. (2001) and the hsp70 promoter-driven JIL-1-V5 transgene JIL-1-FL is described in Bao et al. (2008). The hsp83 and hsp70 promoters are leaky and promote expression at or above wild-type levels under non-heat-shock conditions (Wang et al. 2001; Bao et al. 2008). Recombinant JIL-1z2 Su(var)3-77.1A, JIL-1z2 Su(var)3-714, JIL-1z2 Su(var)3-7R2a8, JIL-1z60 Su(var)3-7R2a8, and JIL-1z2 Hsp70-Gal4 chromosomes were generated as described in Ji et al. (2005) except that the Su(var)3-7 alleles were identified by a yellow reporter gene and the presence of JIL-1z2 or JIL-1z60 was confirmed by PCR as in Zhang et al. (2003). The In(1)wm4 and DX1 alleles were obtained from the Bloomington Stock Center and the P-element insertion line 118E-15 was the generous gift of L. Wallrath. Balancer chromosomes and markers are described in Lindsley and Zimm (1992).
PEV assays were performed as previously described in Lerach et al. (2006) and in Bao et al. (2007). In short, various combinations of JIL-1, Su(var)3-7, or JIL-1 Su(var)3-7 recombinant alleles were introduced into each of the three PEV arrangements by standard crossing. To quantify the variegated phenotype, newly eclosed adults were collected, aged for 5 days at 25°, and were then sorted into different classes on the basis of the percentage of the eye that was red. Eyes from representative individuals from these crosses were photographed using an Olympus stereo microscope and a Spot digital camera (Diagnostic Instruments).
Immunohistochemistry:
Polytene chromosome squash preparations were performed as in Kelley et al. (1999) using the 1-min or 5-min fixation protocol and labeled with antibody as described in Johansen et al. (2009). The preparations were labeled with H3K9me2 pAb (Upstate Biotechnology) or with Su(var)3-7 pAb (Cleard et al. 1997) and DNA was visualized by staining with Hoechst 33258 (Molecular Probes) in PBS. The appropriate Texas Red-, TRITC-, or FITC-conjugated secondary antibodies (Cappel/ICN, Southern Biotech) were used (1:200 dilution) to visualize primary antibody labeling. The final preparations were mounted in 90% glycerol containing 0.5% n-propyl gallate. The preparations were examined using epifluorescence optics on a Zeiss Axioskop microscope and images were captured and digitized using a high-resolution Spot CCD camera. Images were imported into Photoshop where they were pseudocolored, image processed, and merged. In some images nonlinear adjustments were made to the channel with Hoechst labeling for optimal visualization of chromosomes.
RESULTS
Viability and chromosome morphology in JIL-1 and Su(var)3-7 double mutants:
The seven-zinc-finger protein Su(var)3-7 is a major heterochromatic factor that interacts and cooperates with both Su(var)3-9 and HP1a at pericentric heterochromatic regions (jaquet et al. 2006). To determine whether Su(var)3-7, like Su(var)3-9, genetically interacts with JIL-1 in the same pathway in vivo, we explored interactions between mutant alleles of Su(var)3-7 and JIL-1 by generating double mutant individuals. Since Su(var)3-7 and JIL-1 both are located on the third chromosome, we first recombined the Su(var)3-714, Su(var)3-77.1A, and Su(var)3-7R2a8 alleles onto the JIL-1z2 chromosome. JIL-1z2 is a null allele generated by P-element mobilization (Wang et al. 2001; Zhang et al. 2003), whereas the Su(var)3-714, Su(var)3-77.1A, and Su(var)3-7R2a8 alleles were isolated by homologous recombination (Seum et al. 2002; Spierer et al. 2005). The Su(var)3-714 and Su(var)3-7R2a8 alleles behave genetically as null mutations, whereas the Su(var)3-77.1A allele is a strong hypomorph (Seum et al. 2002; Spierer et al. 2005). Due to maternal effects, the homozygous Su(var)3-7 mutant flies from heterozygous parents are viable and fertile. However, in the second generation all the homozygous progeny of homozygous females die during second instar larval stages (Seum et al. 2002; H. Deng, unpublished observations). To determine whether a reduction of Su(var)3-7 levels can rescue the lethality normally associated with a null JIL-1z2/JIL-1z2 mutant background, we crossed JIL-1z2 Su(var)3-77.1A/TM6 Sb Tb males, JIL-1z2 Su(var)3-714/TM6 Sb Tb males, or JIL-1z2 Su(var)3-7R2a8/TM6 Sb Tb males with JIL-1z2/TM6 Sb Tb virgin females generating JIL-1z2 Su(var)3-77.1A/JIL-1z2, JIL-1z2 Su(var)3-714/JIL-1z2, or JIL-1z2 Su(var)3-7R2a8/JIL-1z2 animals identified as non-Sb (Table 1). In control experiments in which JIL-1z2/TM6 Sb Tb males were crossed with JIL-1z2/TM6 Sb Tb virgin females generating JIL-1z2/JIL-1z2 progeny, no flies of the JIL-1z2/JIL-1z2 genotype were observed out of a total of 596 eclosed flies, indicating complete lethality (Table 1). However, introduction of one copy of either of the Su(var)3-7 mutant alleles dramatically increased the number of surviving flies with the JIL-1z2/JIL-1z2 genotype. In these crosses one-third of the eclosed flies would be expected to be of the JIL-1z2/JIL-1z2 Su(var)3-7 genotype assuming full rescue. Therefore, the reduction of Su(var)3-7 levels in these animals resulted in a >60% viability rate compared to a rate of 0% for JIL-1z2/JIL-1z2 flies without the reduction in Su(var)3-7 levels (Table 1). Similar results were obtained with crosses using the JIL-1z60 and Su(var)3-7R2a8 alleles (supporting information, Table S1). Both males and females were rescued but the rate of rescue was higher for females than for males (Table S2). Interestingly, while at least some of the rescued males were fertile, all of the rescued females tested were sterile. In crosses generating double homozygous JIL-1z2 Su(var)3-77.1A/JIL-1z2 Su(var)3-77.1A and JIL-1z2 Su(var)3-714/JIL-1z2 Su(var)3-714, rescue of viability was still observed but at a greatly reduced rate of only ∼16% (Table S3). To ensure that the JIL-1z2 chromosome did not have a second site lethal, we performed rescue experiments with the hsp83 promoter-driven full-length JIL-1-GFP transgene, GF29.1 at 25° (Jin et al. 1999; Wang et al. 2001). In crosses generating double homozygous GF29.1/GF29.1; JIL-1z2/JIL-1z2 flies, viability was restored to 65% (Table S4), strongly indicating that the complete lethality of JIL-1z2/JIL-1z2 flies (Table 1) is not due to a second site lethal. These results were confirmed in crosses with the hsp70 promoter-driven full-length JIL-1-V5 transgene, JIL-1-FL (Bao et al. 2008). In these crosses, viability was restored to 54.8% at 25° (Table S5) and to 94.2% at 21° (Table S6). Thus, these results suggest that the lethality in null JIL-1 mutant backgrounds to a substantial degree is dependent on the dose of Su(var)3-7. Furthermore, since this effect was observed with three different alleles of Su(var)3-7 it is likely to be specific to Su(var)3-7 and not to second site modifiers.
TABLE 1.
Genetic interaction between JIL-1 and Su(var)3-7 alleles
| Cross | Genotypes (no. of adult flies) | % of expected ratioa | |
|---|---|---|---|
| JIL-1z2/TM6 × JIL-1z2/TM6 | JIL-1z2/TM6596 | JIL-1z2/JIL-1z20 | 0.0 |
| JIL-1z2/TM6 × JIL-1z2 Su(var)3-77.1A/TM6 | JIL-1z2/TM6 or JIL-1z2 Su(var)3-7.1A/TM6531 | JIL-1z2/JIL-1z2 Su(var)3-77.1A201 | 82.5 |
| JIL-1z2/TM6 × JIL-1z2 Su(var)3-714/TM6 | JIL-1z2/TM6 or JIL-1z2 Su(var)3-714/TM478 | JIL-1z2/JIL-1z2 Su(var)3-714185 | 83.7 |
| JIL-1z2/TM6 × JIL-1z2 Su(var)3-7R2a8/TM6 | JIL-1z2/TM6 or JIL-1z2 Su(var)3-7R2a8/TM6359 | JIL-1z2/JIL-1z2 Su(var)3-7R2a897 | 63.8 |
In these crosses, the TM6 chromosome was identified by the Stubble marker. Consequently, the experimental genotypes could be distinguished from balanced heterozygotic flies by the absence of the Stubble marker. The expected Mendelian ratio of non-Stubble to Stubble flies was 1:2 since TM6/TM6 is embryonic lethal. The percentage of expected genotypic ratios was calculated as: observed non-Stubble flies × 300/total observed flies.
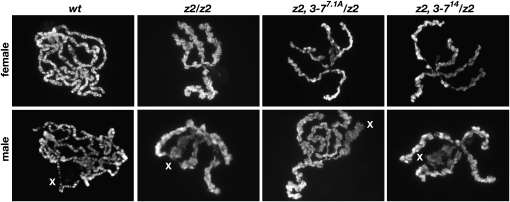
It has previously been demonstrated that a reduction in the levels of the heterochromatin factor Su(var)3-9 to a large degree can rescue the severely perturbed polytene chromosome morphology observed in null JIL-1z2 homozygous larvae (Zhang et al. 2006; Deng et al. 2007). We therefore investigated whether a reduction in the dose of Su(var)3-7 would have a similar effect. For this analysis we prepared squashes of polytene chromosomes labeled with Hoechst from JIL-1z2 homozygous null and wild-type third instar larvae and compared them with squashes from double mutant homozygous JIL-1z2 larvae with either the Su(var)3-714 or the Su(var)3-77.1A allele. As illustrated in Figure 1, loss of JIL-1 histone H3S10 kinase activity leads to misalignment of the interband chromatin fibrils, coiling of the chromosomes, and an increase of ectopic contacts between nonhomologous regions. This results in a shortening and folding of the chromosomes with a nonorderly intermixing of euchromatin and the compacted chromatin characteristic of banded regions (Deng et al. 2005). The extreme of this phenotype is exhibited by the male X polytene chromosome where no remnants of coherent banded regions can be observed (Figure 1). However, we found that in homozygous JIL-1z2 double mutant combinations with a reduced dosage of Su(var)3-7 there was little or no improvement in polytene chromosome morphology including that of the male X chromosome (Figure 1).
Figure 1.—
Morphology of polytene chromosomes in JIL-1 and Su(var)3-7 double mutant backgrounds. Polytene chromosome preparations from third instar male and female larvae were labeled with Hoechst to visualize the chromatin. Note the misalignment and intermixing of interband and banded regions and the extensive coiling and folding of the chromosome arms in JIL-1z2/JIL-1z2 (z2/z2) mutant chromosomes as compared to wild type (wt). The male X chromosome (X) was particularly affected and no remnants of banded regions were discernible. In JIL-1 and Su(var)3-7 double mutant backgrounds from male and female JIL-1z2 Su(var)3-77.1A/JIL-1z2 (z2, 3-77.1A/z2) and JIL-1z2 Su(var)3-714/JIL-1z2 (z2, 3-714/z2) larvae, the polytene chromosome morphology was indistiguishable from that of JIL-1z2/JIL-1z2 homozygous null mutants.
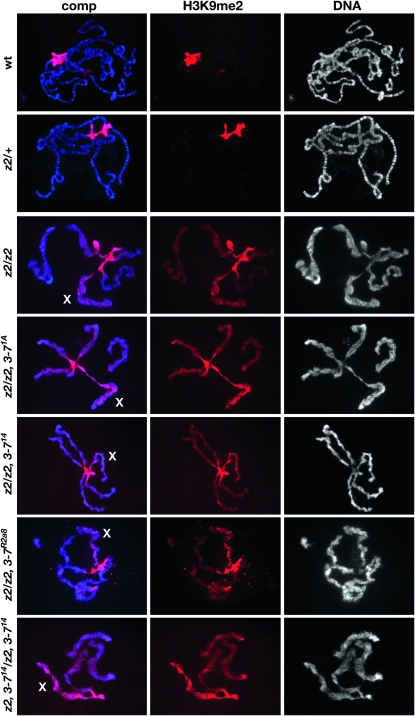
Since Zhang et al. (2006) and Deng et al. (2007) have shown that ectopic Su(var)3-9 histone methyltransferase activity may be a major factor in causing the lethality and chromatin structure perturbations associated with the loss of JIL-1 H3S10 kinase activity, we explored whether a reduction in the dose of Su(var)3-7 affected the distribution of the H3K9me2 mark in JIL-1 null mutants. Polytene squashes from third instar larval salivary glands from JIL-1 and Su(var)3-7 double mutant combinations were double labeled with Hoechst and an antibody to histone H3K9me2 and compared to wild-type and JIL-1z2/+ heterozygous preparations (Figure 2). In JIL-1 null animals histone H3K9 dimethylation is dramatically upregulated on all the chromosome arms; however, the upregulation is most pronounced on the X chromosome (Zhang et al. 2006; Deng et al. 2007) (Figure 2). In JIL-1z2/+ heterozygous preparations both chromosome morphology and H3K9me2 distribution is indistinguishable from wild-type preparations (Figure 2). As further illustrated in Figure 2, a reduction in the dose of Su(var)3-7 affected neither chromosome morphology nor the ectopic spreading of H3K9 dimethylation in JIL-1z2 Su(var)3-77.1A/JIL-1z2, JIL-1z2 Su(var)3-714/JIL-1z2, JIL-1z2 Su(var)3-7R2a8/JIL-1z2, or JIL-1z2 Su(var)3-714/JIL-1z2 Su(var)3-714 mutant larvae. Taken together these results suggest that JIL-1 interacts with Su(var)3-7 in a genetic pathway and that Su(var)3-7 contributes to the lethality but not the disruption of chromosome morphology observed in JIL-1 loss-of-function mutants.
Figure 2.—
Localization of H3K9me2 in polytene chromosomes from JIL-1 and Su(var)3-7 mutant female third instar larvae. The polytene squash preparations were labeled with antibody to H3K9me2 (in red) and with Hoechst (DNA, in blue/gray). The X chromosome is indicated by an X. Preparations from wild-type (wt), heterozygous JIL-1z2/+ (z2/+), homozygous JIL-1z2/JIL-1z2 (z2/z2), JIL-1z2 Su(var)3-77.1A/JIL-1z2 (z2, 3-77.1A/z2), JIL-1z2 Su(var)3-714/JIL-1z2 (z2, 3-714/z2), JIL-1z2 Su(var)3-7R2a8/JIL-1z2 (z2, 3-7R2a8/z2), and JIL-1z2 Su(var)3-714/JIL-1z2 Su(var)3-714 (z2, 3-714/z2, 3-714) larvae are shown. In wild-type and JIL-1z2/+ preparations, H3K9me2 labeling was mainly localized to and abundant at the chromocenter; however, in the absence of the JIL-1 kinase, the H3K9me2 labeling spread to the autosomes and particularly to the X chromosome (see also Zhang et al. 2006; Deng et al. 2007). In JIL-1z2 Su(var)3-77.1A/JIL-1z2, JIL-1z2 Su(var)3-714/JIL-1z2, and JIL-1z2 Su(var)3-714/JIL-1z2 Su(var)3-714 double mutant larvae, the H3K9me2 labeling was indistiguishable from that of JIL-1z2/JIL-1z2 homozygous null mutants.
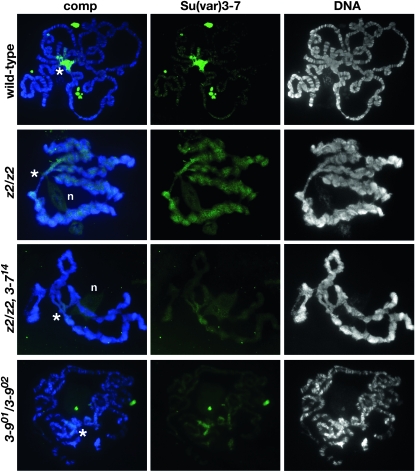
To determine whether the distribution of Su(var)3-7 was affected in JIL-1 null mutants, polytene chromosomes from JIL-1 and Su(var)3-7 double mutant combinations were double labeled with Hoechst and an antibody to Su(var)3-7 (Cleard et al. 1997) and compared to wild-type preparations (Figure 3). In wild-type polytene chromosomes, Su(var)3-7 is predominantly located to the chromocenter and the fourth chromosome (Cleard et al. 1997); however, in the absence of JIL-1 the labeling of the chromosome arms is dramatically upregulated in conjunction with a reduced presence at the chromocenter (Figure 3). In contrast to the redistribution of HP1a and H3K9me2 where the upregulation is most pronounced on the X chromosome (Zhang et al. 2006; Deng et al. 2007), we did not observe a difference in Su(var)3-7 levels between the X chromosome and the autosomes in the JIL-1 null background (Figure 3). In JIL-1z2 Su(var)3-714/JIL-1z2 mutant larvae Su(var)3-7 labeling was substantially reduced at the chromocenter with very little Su(var)3-7 detectable on the chromosome arms (Figure 3). For comparison we also labeled polytene chromosomes heterozygous for the Su(var)3-9 null alleles Su(var)3-901 and Su(var)3-902 (Reuter et al. 1986; Tschiersch et al. 1994; Ebert et al. 2004) with Su(var)3-7 antibody. Figure 3 shows that the binding of Su(var)3-7 is greatly reduced at the chromocenter without any spreading to the chromosome arms (Figure 3). This reduced binding pattern in the Su(var)3-9 null background is similar to that previous reported for the other major heterochromatin component HP1a (Schotta et al. 2002). Taken together these results suggest that the distribution pattern of Su(var)3-7 is dependent on both Su(var)3-9 and JIL-1 levels and/or activity.
Figure 3.—
Localization of Su(var)3-7 in polytene chromosomes from JIL-1, Su(var)3-7, and Su(var)3-9 mutant female third instar larvae. The polytene squashes were labeled with antibody to Su(var)3-7 (in green) and with Hoechst (DNA, in blue/gray). The chromocenter is indicated with an asterisk and n indicates weak background labeling of the nucleolus in some of the preparations. Preparations from wild-type, JIL-1z2 homozygous (z2/z2), JIL-1z2 Su(var)3-714/JIL-1z2 (z2, 3-714/z2), and Su(var)3-901/Su(var)3-902 (3-901/3-902) larvae are shown. In wild-type preparations, Su(var)3-7 labeling was mainly localized to and abundant at the chromocenter; however, in the absence of the JIL-1 kinase, the Su(var)3-7 labeling spread to the chromosome arms with a concomitant decrease at the chromocenter. In JIL-1z2 Su(var)3-714/JIL-1z2 and Su(var)3-901/Su(var)3-902 mutant larvae Su(var)3-7 labeling was greatly reduced and mainly confined to the chromocenter.
JIL-1 and Su(var)3-7 counteract each other's effect on PEV:
PEV in Drosophila occurs when euchromatic genes are transcriptionally silenced as a result of their placement in or near heterochromatin (reviewed in Girton and Johansen 2008). Repression typically occurs in only a subset of cells and can be heritable, leading to mosaic patterns of gene expression. It has been demonstrated that loss-of-function JIL-1 alleles can act as enhancers of PEV, resulting in increased silencing of gene expression (Bao et al. 2007), whereas loci for structural components of heterochromation such as Su(var)3-9, Su(var)2-5, and Su(var)3-7 act as strong haplosuppressors (Eissenberg et al. 1990; Reuter et al. 1990; Tschiersch et al. 1994). This together with the finding that JIL-1 and Su(var)3-7 interact genetically suggest that JIL-1 and Su(var)3-7 may potentially have a counterbalancing effect on the regulation of PEV. To test this hypothesis we explored the effect of various combinations of loss-of-function alleles of JIL-1 and Su(var)3-7 on PEV caused by both P-element insertions of reporter genes (118E-15 and DX1) as well as of a chromosome rearrangement (wm4).
118E-15:
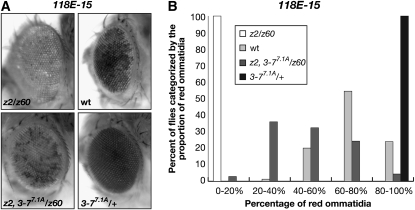
Insertion of the P element (P[hsp26-pt, hsp70-w]) into euchromatic sites results in a uniform red eye phenotype, whereas insertion into a known heterochromatin region of the fourth chromosome (line 118E-15) results in a variegating eye phenotype (Figure 4 and Table S7) (Wallrath and Elgin 1995; Wallrath et al. 1996; Cryderman et al. 1998). In the experiments, the transgenic reporter line was crossed into JIL-1z60/JIL-1z2 and Su(var)3-77.1A/+ mutant backgrounds as well as into the JIL-1z2 Su(var)3-77.1A/JIL-1z60 double mutant background. The JIL-1z60 allele is a strong hypomorph producing only 0.3% of wild-type JIL-1 protein levels (Wang et al. 2001; Zhang et al. 2003). The JIL-1z2/JIL-1z60 heteroallelic combination is semilethal and only a limited number of eclosed animals from large-scale crosses could be analyzed (Zhang et al. 2003). Flies from each of the different genotypes were scored for the percentage of the eye that had red ommatidia and compared to flies containing wild-type levels of the JIL-1 and Su(var)3-7 proteins (Figure 4 and Table S3). Although both male and female flies were scored, due to sex differences, only results from male flies are shown. However, the trend observed in female flies was identical to that in male flies. As illustrated in Figure 4 the hypomorphic allelic combination of the JIL-1 alleles JIL-1z60 and JIL-1z2 leads to a strong enhancement of PEV as indicated by the nearly completely white eye phenotype, whereas in contrast, the heterozygous Su(var)3-77.1A/+ allele leads to strong suppression of PEV as indicated by the nearly completely red eyes. However, in the JIL-1z2 Su(var)3-77.1A/JIL-1z60 double mutant background variegation of the proportion of red ommatitidia was substantially restored and closer to the distribution when wild-type levels of the JIL-1 and Su(var)3-7 proteins were present (Figure 4 and Table S7).
Figure 4.—
Counterbalancing effect of JIL-1 and Su(var)3-7 loss-of-function alleles on PEV of the P-element insertion line 118E-15. (A) Examples of the degree of PEV in the eyes of wild-type JIL-1 and Su(var)3-7 (wt), JIL-1z60/JIL-1z2 (z2/z60), Su(var)3-77.1A/+, and JIL-1z2 Su(var)3-77.1A/JIL-1z60 (z2, 3-77.1A/z60) flies in a 118E-15 background. All images are from male flies. (B) Histograms of the distribution of the percentage of red ommatidia in wt, JIL-1z60/JIL-1z2 (z2/z60), Su(var)3-77.1A/+, and JIL-1z2 Su(var)3-77.1A/JIL-1z60 (z2, 3-77.1A/z60) male flies homozygous for 118E-15.
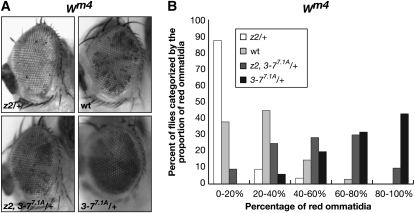
wm4:
The In(1)wm4 X chromosome contains an inversion that juxtaposes the euchromatic white gene and heterochromatic sequences adjacent to the centromere (Muller 1930; Schultz 1936). The resulting somatic variegation of wm4 expression occurs in clonal patches in the eye, reflecting heterochromatic spreading from the inversion breakpoint that silences wm4 expression in the white patches and euchromatic packaging of the w gene in those patches that appear red (reviewed in Grewal and Elgin 2002). Studies of this effect suggest that the degree of spreading may depend on the amount of heterochromatic factors at the breakpoint (reviewed in Weiler and Wakimoto 1995; Girton and Johansen 2008). Interestingly, strong hypomorphic combinations of JIL-1 alleles, in which heterochromatic factors spread to ectopic locations (Zhang et al. 2006; Deng et al. 2007), act as suppressors not enhancers of PEV of the wm4 allele (Lerach et al. 2006). On the basis of these findings, Lerach et al. (2006) proposed a model where the suppression of PEV of wm4 in strong JIL-1 hypomorphic backgrounds is due to a reduction in the level of heterochromatic factors at the pericentromeric heterochromatin near the inversion breakpoint site that reduces its potential for heterochromatic spreading and silencing. However, as illustrated in Figure 2, in heterozygous preparations of the null JIL-1z2 allele both chromosome morphology and H3K9me2 distribution are indistinguishable from wild-type preparations. Therefore, a prediction of the model of Lerach et al. (2006) is that JIL-1 should have no effect or act as a haploenhancer of PEV at the wm4 allele. To test this hypothesis, the In(1)wm4 chromosome was crossed into different heterozygous JIL-1 mutant backgrounds of hypomorphic and null JIL-1 alleles (JIL-1z28, JIL-1z60, and JIL-1z2). The JIL-1z28 allele is a weak hypomorph producing 45% the normal level of wild-type JIL-1 protein (Zhang et al. 2003). The strong hypomorphic JIL-1z2/JIL-1z60 heteroallelic combination was included for comparison. Male flies with the different genotypes were scored for the percentage of the eye that was red and variegated wm4; +/+ flies containing wild-type levels of JIL-1 protein were used as controls (Table 2). As shown in Table 2, all three heterozygous JIL-1 alleles reduced the proportion of red ommatidia as compared to +/+ flies, whereas the strong hypomorphic JIL-1z2/JIL-1z60 heteroallelic combination resulted in completely red eyes. Thus, these results strongly indicate that JIL-1 acts as a haploenhancer of PEV of wm4 in male flies.
TABLE 2.
JIL-1 alleles act as haploenhancers of PEV of wm4
| Percentage of flies categorized by the proportion of red ommatidia |
||||||
|---|---|---|---|---|---|---|
| Genotypea | n | 0% red | 0–25% red | 25–75% red | 75–99% red | 100% red |
| +/+ | 274 | 0.0 | 23.0 | 59.0 | 19.0 | 0.0 |
| zz28/+ | 182 | 8.8 | 64.3 | 26.9 | 0.0 | 0.0 |
| z60/+ | 126 | 2.4 | 83.3 | 14.3 | 0.0 | 0.0 |
| z2/+ | 162 | 21.0 | 65.4 | 13.6 | 0.0 | 0.0 |
| z60/z2 | 19 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
Genotype of the third chromosome. Only male flies hemizygous for wm4 on the X chromosome were tabulated.
To test whether a heterozygous JIL-1 allele could counterbalance the suppression of a Su(var)3-7 hypomorphic allele of PEV of wm4, we performed experiments similar to those described above for 118E-15. In the experiments, the In(1)wm4 chromosome was crossed into JIL-1z2/+ and Su(var)3-77.1A/+ mutant backgrounds as well as into the JIL-1z2 Su(var)3-77.1A/+ double mutant background. As illustrated in Figure 5, heterozygous JIL-1z2/+ led to enhancement of PEV as indicated by the increased proportion of white ommatidia, whereas in contrast, the heterozygous Su(var)3-77.1A/+ allele led to suppression of PEV as indicated by an increase of the proportion of red ommatidia. However, in the JIL-1z2 Su(var)3-77.1A/+ double mutant background, variegation of the proportion of red ommatitidia was intermediate and closer to the distribution when wild-type levels of the JIL-1 and Su(var)3-7 proteins were present (Figure 5 and Table S7). These results suggest that the haploenhancer effect of JIL-1 can counterbalance the haplosupressor effect of Su(var)3-7 on PEV of wm4.
Figure 5.—
Counterbalancing effect of JIL-1 and Su(var)3-7 loss-of-function alleles on PEV in the eyes of wm4 flies. (A) Examples of the degree of PEV in the eyes of wild-type JIL-1 and Su(var)3-7 (wt), JIL-1z2/+ (z2/+), Su(var)3-77.1A/+, and JIL-1z2 Su(var)3-77.1A/+ (z2, 3-77.1A/+) flies in a wm4 background. All images are from male flies. (B) Histograms of the distribution of the percentage of red ommatidia in wt, JIL-1z2/+ (z2/+), Su(var)3-77.1A/+, and JIL-1z2 Su(var)3-77.1A/+ (z2, 3-77.1A/+) male flies in a wm4 background.
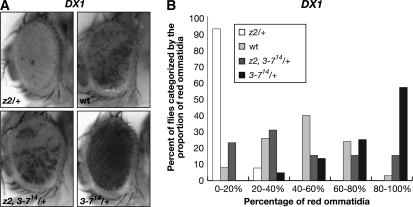
DX1:
We also tested the counterbalancing effect of JIL-1 and Su(var)3-7 alleles on PEV of the DX1 transgenic line. In the DX1 line, seven tandem copies of the p[lacW] transgene, which contains a mini-white and a lacZ gene, were inserted into the 50C euchromatic region of the second chromosome (Dorer and Henikoff 1994). The tandem sequence repetition induces heterochromatin formation resulting in partial silencing of the mini-white reporter and a variegated eye phenotype (Dorer and Henikoff 1994). In the experiments, the DX1 reporter line was crossed into JIL-1z2/+ and Su(var)3-714/+ mutant backgrounds as well as into the JIL-1z2 Su(var)3-714/+ double mutant background. As illustrated in Figure 6, heterozygous JIL-1z2/+ led to strong enhancement of PEV as indicated by the increased proportion of white ommatidia, whereas in contrast, the heterozygous Su(var)3-714/+ allele led to suppression of PEV as indicated by an increase of the proportion of red ommatidia. However, in the JIL-1z2 Su(var)3-714/+ double mutant background, variegation of the proportion of red ommatitidia was intermediate and closer to the distribution when wild-type levels of the JIL-1 and Su(var)3-7 proteins were present (Figure 6 and Table S7). These results suggest that the haploenhancer effect of JIL-1 also can counterbalance the haplosupressor effect of Su(var)3-7 on PEV of the DX1 transgenic insertion line.
Figure 6.—
Counterbalancing effect of JIL-1 and Su(var)3-7 loss-of-function alleles on PEV in the eyes of DX1 flies. (A) Examples of the degree of PEV in the eyes of wild-type JIL-1 and Su(var)3-7 (wt), JIL-1z2/+ (z2/+), Su(var)3-714/+, and JIL-1z2 Su(var)3-714/+ (z2, 3-714/+) flies in a DX1 background. All images are from male flies. (B) Histograms of the distribution of the percentage of red ommatidia in wt, JIL-1z2/+ (z2/+), Su(var)3-714/+, and JIL-1z2 Su(var)3-714/+ (z2, 3-714/+) male flies in a DX1 background.
DISCUSSION
While Su(var)3-9, Su(var)3-7, and HP1a reciprocal interactions are well documented at pericentric regions (Schotta et al. 2002; Greil et al. 2003; Danzer and Wallrath 2004) they are not universal. For example, HP1 binding on the fourth chromosome has been shown to be independent of Su(var)3-9 (Schotta et al. 2002), and Danzer and Wallrath (2004) using a tethering system to recruit HP1a to euchromatic sites have shown that HP1a-mediated silencing can operate in a Su(var)3-9-independent manner. Moreover, Deng et al. (2007) have provided evidence that at least two different molecular mechanisms regulate Su(var)3-9 localization, one dependent on HP1 and one dependent on the JIL-1 kinase. These findings indicate that although Su(var)3-9, Su(var)3-7, and HP1a cooperate in heterochromatin formation and gene silencing at pericentric chromosome sites, they may function independently at other regions such as the chromosome arms. In this study we show that the lethality but not the chromosome morphology defects associated with the null JIL-1 phenotype to a large degree can be rescued by reducing the dose of the Su(var)3-7 gene. This effect was observed with three different alleles of Su(var)3-7, strongly suggesting it is likely to be specific to Su(var)3-7 and not to second site modifiers. Furthermore, we provide evidence that JIL-1 levels and/or activity regulate the chromosome localization of Su(var)3-7 and that Su(var)3-7 levels are dramatically redistributed to the chromosome arms in conjunction with a reduced presence at the chromocenter in the absence of JIL-1.
Previously, it has been demonstrated that JIL-1 genetically interacts with Su(var)3-9 but not with Su(var)2-5, suggesting that the lethality and disruption of chromosome morphology observed when JIL-1 levels are decreased are associated with ectopic Su(var)3-9 activity on the chromosomal arms and unrelated to HP1a recruitment (Deng et al. 2007). In this scenario, the spreading of the H3K9me2 mark to ectopic locations on the chromosomes is likely to lead to heterochromatization and repression of gene expression at these sites, leading to increased lethality (Zhang et al. 2006; Deng et al. 2007). This hypothesis has been supported by genetic interaction assays that demonstrated that the lethality of JIL-1 null mutants could be almost completely rescued by a reduction in Su(var)3-9 dosage that prevented ectopic dimethylation of histone H3K9 (Deng et al. 2007). However, in this study we show that while reducing the dose of Su(var)3-7 also rescues viability of JIL-1 null mutant larvae, H3K9me2 in polytene squashes still spreads to the chromosome arms, strongly indicating that ectopic Su(var)3-9 activity is not a direct cause of lethality, but rather that Su(var)3-9-mediated recruitment of Su(var)3-7 is a necessary factor. Futhermore, since viability was rescued despite no obvious improvement in chromosome morphology, the lethality caused by loss of JIL-1 function is not likely to be a consequence of perturbed chromosome morphology. Taken together these observations give rise to a model where Su(var)3-7 functions as an effector downstream of Su(var)3-9 and H3K9 dimethylation in heterochromatic spreading and gene silencing that is normally counteracted by JIL-1 kinase activity. How Su(var)3-7 may mediate these effects is unknown and will require additional studies.
The inherent components of heterochromatin Su(var)3-9, HP1a, and Su(var)3-7 display a haplosuppressor/triploenhancer dosage-dependent effect on PEV (Schotta et al. 2002). Additional copies of all three genes cause strong enhancement of white variegation in wm4, and in genetic interaction tests, the suppressor effect of Su(var)3-9 null mutations dominates the triplo-dependent enhancer effect of Su(var)2-5) and Su(var)3-7 (Schotta et al. 2002). Furthermore, it has been recently demonstrated that the gain-of-function JIL-1Su(var)3-1 allele is one of the strongest suppressors of PEV so far described at all the PEV arrangements that have been tested (Ebert et al. 2004; Lerach et al. 2006; Bao et al. 2007). This allele even counteracts gene repression that is caused by overexpression of the major determinants of heterochromatin formation, e.g., Su(var)3-9, Su(var)2-5, and Su(var)3-7 (Ebert et al. 2004). The JIL-1Su(var)3-1 allele generates truncated proteins with COOH-terminal deletions that mislocalize to ectopic chromatin sites, leading to expanded histone H3S10 phosphorylation (Ebert et al. 2004; Zhang et al. 2006; Bao et al. 2008). On the basis of these findings, Ebert et al. (2004) proposed a model for a dynamic balance between euchromatin and heterochromatin, where as can be monitored in PEV arrangements, the boundary between these two states is determined by antagonistic functions of euchromatic regulators (JIL-1) and the determinants of heterochromatin assembly. In this study we have further tested this hypothesis using JIL-1 loss-of-function alleles which we show can act as haploenhancers of PEV. This included PEV of the wm4 allele where, interestingly, combinations of strong hypomorphic JIL-1 alleles act as suppressors, not enhancers. Lerach et al. (2006) have proposed that this is due to a reduction in the levels of heterochromatic factors near the inversion breakpoint that reduces its potential for heterochromatic spreading and silencing (reviewed in Girton and Johansen 2008). As predicted by this hypothesis we show that in chromosome squash preparations from JILz2/+ larvae there was no discernible redistribution of the H3K9me2 heterochromatic mark. We further demonstrate that JIL-1 and Su(var)3-7 alleles can counteract each other's effect on PEV. In all three PEV arrangements tested, Su(var)3-7 loss-of-function alleles acted as strong haplosuppressors as indicated by a high proportion of nearly completely red eyes, whereas JIL-1 loss-of-function alleles acted as strong haploenhancers as indicated by a high proportion of flies with nearly completely white ommatidia. However, in double mutant backgrounds, variegation of the proportion of red ommatidia was substantively restored and closer to the distribution when wild-type levels of JIL-1 and Su(var)3-7 proteins were present. These results strongly support a genetic interaction between JIL-1 and Su(var)3-7 and provide evidence that a finely tuned balance between the levels of JIL-1 and Su(var)3-7 contributes to the regulation of PEV.
While several potential mechanisms for heterochromatin spreading and gene silencing have been identified (reviewed in Girton and Johansen 2008), the concept of a dynamic balance between euchromatin and heterochromatin implies that euchromatic factors may have similar spreading potential. However, the mechanisms that actively may lead to the expansion of euchromatic domains have received comparatively less attention. In Drosophila, the studies of the JIL-1 kinase have demonstrated that histone H3S10 phosphorylation is an important epigenetic modification potentially regulating both the establishment and maintenance of euchromatin (reviewed in Johansen and Johansen 2006). For example, Deng et al. (2008) have shown using a LacI tethering system, that JIL-1-mediated ectopic H3S10 phosphorylation can cause a change in higher-order chromatin structure from a condensed heterochromatin-like state to a more open euchromatic state. Thus, spreading of JIL-1 activity has the potential to expand euchromatic domains and counteract gene silencing in heterochromatic regions. However, while it has been shown that the COOH-terminal region of JIL-1 can directly interact with the histone H3 tail region (Bao et al. 2008), it remains to be established how JIL-1 targeting to specific chromatin regions is regulated and how dynamic this regulation is.
Acknowledgments
We thank members of the laboratory for discussion, advice, and critical reading of the manuscript. We also acknowledge V. Lephart for maintenance of fly stocks and Laurence Woodruff for technical assistance. We especially thank P. Spierer and A. Csink for providing fly stocks, P. Spierer for the Su(var)3-7 antibody, and L. Wallrath for the P-element insertion line 118E-15. Work in the laboratory of K.M.J. and J.J. was supported by National Institutes of Health grant GM62916. M.D. was supported by the Swiss National Science Foundation and the state of Geneva.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.117150/DC1.
References
- Bao, X., H. Deng, J. Johansen, J. Girton and K. M. Johansen, 2007. Loss-of-function alleles of the JIL-1 histone H3S10 kinase enhance position-effect-variegation at pericentric sites in Drosophila heterochromatin. Genetics 176 1355–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, X., W. Cai, H. Deng, W. Zhang, R. Krencik et al., 2008. The COOH-terminal domain of the JIL-1 histone H3S10 kinase interacts with histone H3 and is required for correct targeting to chromatin. J. Biol. Chem. 283 32741–32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleard, F., M. Delattre and P. Spierer, 1997. Su(var)3–7, a Drosophila heterochromatin-associated protein and companion of HP1 in the genomic silencing of position-effect variegation. EMBO J. 16 5280–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryderman, D. E., M. H. Cuaycong, S. C. R. Elgin and L. L. Wallrath, 1998. Characterization of sequences associated with position-effect-variegation at pericentric sites in Drosophila heterochromatin. Chromosoma 107 277–285. [DOI] [PubMed] [Google Scholar]
- Danzer, J. R., and L. L. Wallrath, 2004. Mechanisms of HP1-mediated gene silencing in Drosophila. Development 131 3571–3580. [DOI] [PubMed] [Google Scholar]
- Delattre, M., A. Spierer, Y. Jaquet and P. Spierer, 2004. Increased expression of Drosophila Su(var)3–7 triggers Su(var)3–9-dependent heterochromatin formation. J. Cell. Sci. 117 6239–6247. [DOI] [PubMed] [Google Scholar]
- Deng, H., W. Zhang, X. Bao, J. N. Martin, J. Girton et al., 2005. The JIL-1 kinase regulates the structure of Drosophila polytene chromosomes. Chromosoma 114 173–182. [DOI] [PubMed] [Google Scholar]
- Deng, H., X. Bao, W. Zhang, J. Girton, J. Johansen et al., 2007. Reduced levels of Su(var)3–9 but not Su(var)2–5 (HP1) counteract the effects on chromatin structure and viability in loss-of-function mutants of the JIL-1 histone H3S10 kinase. Genetics 177 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H., X. Bao, W. Cai, M. J. Blacketer, A. S. Belmont et al., 2008. Ectopic histone H3S10 phosphorylation causes chromatin structure remodeling in Drosophila. Development 135 699–705. [DOI] [PubMed] [Google Scholar]
- Dorer, D. R., and S. Henikoff, 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77 993–1002. [DOI] [PubMed] [Google Scholar]
- Ebert, A., G. Schotta, S. Lein, S. Kubicek, V. Krauss et al., 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, J. C., and S. C. Elgin, 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10 204–210. [DOI] [PubMed] [Google Scholar]
- Eissenberg, J. C., T. James, D. Foster-Hartnett, D. Hartnett, V. Ngan et al., 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87 9923–9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin, S. C., and S. I. Grewal, 2003. Heterochromatin: silence is golden. Curr. Biol. 13 R895–R898. [DOI] [PubMed] [Google Scholar]
- Girton, J., and K. M. Johansen, 2008. Chromatin structure and regulation of gene expression: the lessons of PEV in Drosophila. Adv. Genet. 61 1–43. [DOI] [PubMed] [Google Scholar]
- Greil, F., I. Van Der Kraan, J. Delrow, J. F. Smothers, E. De Wit et al., 2003. Distinct HP1 and Su(var)3–9 complexes bind to sets of developmentally coexpressed genes depending on chromosomal location. Genes Dev. 17 2825–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal, S. I., and S. C. Elgin, 2002. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12 178–187. [DOI] [PubMed] [Google Scholar]
- Jaquet, Y., M. Delattre, J. Montoya-Burgos, A. Spirer and P. Spierer, 2006. Conserved domains control heterochromatin localization and silencing properties of Su(var)3–7. Chromosoma 115 139–150. [DOI] [PubMed] [Google Scholar]
- Ji, Y., U. Rath, J. Girton, K. M. Johansen and J. Johansen, 2005. D-Hillarin, a novel W180-domain protein, affects cytokinesis through interaction with the septin family member Pnut. J. Neurobiol. 64 157–169. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, D. L. Walker, H. Dong, C. Conley et al., 1999. JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4 129–135. [DOI] [PubMed] [Google Scholar]
- Johansen, K. M., and J. Johansen, 2006. Regulation of chromatin structure by histone H3S10 phosphorylation. Chromosome Res. 14 393–404. [DOI] [PubMed] [Google Scholar]
- Johansen, K. M., W. Cai, H. Deng, X. Bao, W. Zhang et al., 2009. Methods for studying transcription and epigenetic chromatin modification in Drosophila polytene chromosome squash preparations using antibodies. Methods 48 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. L., V. H. Meller, P. R. Gordadze, G. Roman, R. L. Davis et al., 1999. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98 513–522. [DOI] [PubMed] [Google Scholar]
- Lachner, M., D. O'Carroll, S. Rea, K. Mechtler and T. Jenuwein, 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 116–120. [DOI] [PubMed] [Google Scholar]
- Lerach, S., W. Zhang, X. Bao, H. Deng, J. Girton et al., 2006. Loss-of-function alleles of the JIL-1 kinase are strong suppressors of position effect variegation of the wm4 allele in Drosophila. Genetics 173 2403–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, New York.
- Muller, H. J., 1930. Types of visible variegations induced by X-rays in Drosophila. J. Genet. 22 299–335. [Google Scholar]
- Rea, S., F. Eisenhaber, D. O'Carroll, B. D. Strahl, Z. W. Sun et al., 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406 481–487. [DOI] [PubMed] [Google Scholar]
- Reuter, G., R. Dorn, G. Wustmann, B. Friede and G. Rauh, 1986. Third chromosome suppressor of position-effect variegation loci in Drosophila melanogaster. Mol. Gen. Genet. 202 481–487. [Google Scholar]
- Reuter, G., M. Giarre, J. Farah, J. Gausz, A. Spierer et al., 1990. Dependence of position-effect varigation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature 344 219–223. [DOI] [PubMed] [Google Scholar]
- Roberts, D. B., 1998. Drosophila: A Practical Approach. IRL Press, Oxford.
- Rudolph, T., M. Yonezawa, S. Lein, K. Heidrich, S. Kubicek et al., 2007. Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog Su(var)3–3. Mol. Cell 26 103–115. [DOI] [PubMed] [Google Scholar]
- Schotta, G., A. Ebert, V. Krauss, A. Fischer, J. Hoffmann et al., 2002. Central role of Drosophila SU(VAR)3–9 in histone H3–K9 methylation and heterochromatic gene silencing. EMBO J. 21 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, J., 1936. Variegation in Drosophila and the inert chromosome regions. Proc. Natl. Acad. Sci. USA 22 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seum, C., D. Pauli, M. Delattre, Y. Jaquet, A. Spierer et al., 2002. Isolation of Su(var)3–7 mutations by homologous recombination in Drosophila melanogaster. Genetics 161 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer, A., C. Seum, M. Delattre and P. Spierer, 2005. Loss of the modifiers of variegation Su(var)3–7 or HP1 impacts male X polytene chromosome morphology and dosage compensation. J. Cell Sci. 118 5047–5057. [DOI] [PubMed] [Google Scholar]
- Tschiersch, B., A. Hofmann, V. Krauss, R. Dorn, G. Korge et al., 1994. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3–9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 13 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrath, L. L., and S. C. R. Elgin, 1995. Position effect variegation in Drosophila is associated with altered chromatin structure. Genes Dev. 9 1263–1277. [DOI] [PubMed] [Google Scholar]
- Wallrath, L. L., V. P. Guntur, L. E. Rosman and S. C. R. Elgin, 1996. DNA representation of variegating heterochromatic P-element inserts in diploid and polytene tissues of Drosophila melanogaster. Chromosoma 104 519–527. [DOI] [PubMed] [Google Scholar]
- Wang, Y., W. Zhang, Y. Jin, J. Johansen and K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105 433–443. [DOI] [PubMed] [Google Scholar]
- Weiler, K. S., and B. T. Wakimoto, 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29 577–605. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Y. Jin, Y. Ji, J. Girton, J. Johansen et al., 2003. Genetic and phenotypic analysis of alleles of the Drosophila chromosomal JIL-1 kinase reveals a functional requirement at multiple developmental stages. Genetics 165 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., H. Deng, X. Bao, S. Lerach, J. Girton et al., 2006. The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133 229–235. [DOI] [PubMed] [Google Scholar]