Abstract
Sensory communication depends on the precise matching between the emission and the perception of sex- and species-specific signals; understanding both the coevolutionary process and the genes involved in both production and detection is a major challenge. desat1 determines both aspects of communication—a mutation in desat1 simultaneously alters both sex pheromone emission and perception in Drosophila melanogaster flies. We investigated whether the alteration of pheromonal perception is a consequence of the altered production of pheromones or if the two phenotypes are independently controlled by the same locus. Using several genetic tools, we were able to separately manipulate the two pheromonal phenotypes, implying that desat1 is the sole gene responsible, exerting a pleiotropic effect on both transmission and detection. The levels of the five desat1 trancripts, measured in the head and body of manipulated flies, were related to variation in pheromone production. This suggests that the pleiotropic action of desat1 on pheromonal communication depends on the fine regulation of its transcriptional activity.
PHEROMONES are frequently used for sexual communication by vertebrate and invertebrates alike (Bradbury and Vehrencamp 1998; Wyatt 2003). In particular, pheromones play an important role in attraction and discrimination of sexual partners (Johansson and Jones 2007) and can be subjected to sexual selection (Darwin 1883). In moths, pheromones can affect premating behavior (Roelofs and Rooney 2003; Smadja and Butlin 2009). Pheromones have also been studied in several groups of Drosophila species (Toolson and Kupersimbron 1989; Tompkins et al. 1993; Higgie et al. 2000; Ferveur 2005), including Drosophila melanogaster, a model species used to dissect the molecular, genetic, physiological, neural, and evolutionary basis of pheromonal communication (Coyne et al. 1994; Jallon and Wicker-Thomas 2003; Lacaille et al. 2007; Koganezawa et al. 2010).
In moths, sex pheromone communication has evolved to make use of complex blends of relatively simple long-chain fatty acid precursors and species specificity is derived from the introduction of double bonds into exact locations along the hydrocarbon backbone of fatty acids (Rooney 2009). In D. melanogaster, the predominant hydrocarbons on the cuticle of mature flies radically differ between the sexes in both their structure and effect. Males produce cuticular hydrocarbons (CHs) with one double bond (monoenes), which stimulate conspecific females and inhibit conspecific males (Jallon 1984; Ferveur and Sureau 1996; Grillet et al. 2006; Lacaille et al. 2007). Females produce dienes (with two double bonds), which slightly stimulate conspecific males but strongly inhibit males of the sibling D. simulans species (Savarit et al. 1999). The biosynthesis of these sexually dimorphic CHs requires several desaturase enzymes that show sex- and species-specific expression (Legendre et al. 2008). We previously characterized the mutational effects of a specific PGal4 transposable element inserted in the regulatory sequence of the desat1 gene (1573-1). This mutation affected not only the production of monoenes and dienes in male and female flies (Marcillac et al. 2005a) but also the male discrimination of sex pheromones (Marcillac et al. 2005b). The precise excision of the transposon completely rescued the two phenotypes, demonstrating that desat1 can simultaneously affect the emission and the reception of sex pheromones.
To explore the genetic relationship between these two pheromonal phenotypes, we used flies (i) homozygous for desat1 excision alleles (Marcillac et al. 2005a), (ii) combining PUAS deregulation elements (Ep) (Rorth 1996) inserted in the desat1 regulatory region, driven or not by a PGal4 desat1 enhancer trap element. Two PGal4 desat1 enhancer traps, containing either a complete or a deleted desat1 coding region (Marcillac et al. 2005a), were used to induce Ep deregulation in these two desat1 backgrounds. The effect of genetic deregulation was also measured on the relative levels of the five desat1 transcripts.
MATERIALS AND METHODS
D. melanogaster stocks and crosses:
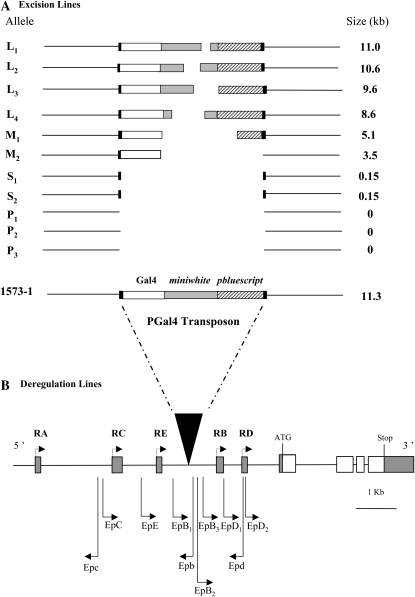
All D. melanogaster strains were raised on yeast/cornmeal/agar medium and kept at 24 ± 0.5° with 65 ± 5% humidity on a 12-hr light:12-hr dark cycle. Dijon2000 (DIJ) and Canton-S (Cs) are wild-type strains used as controls. Crosses were performed using standard techniques and genetic tools (Lindsley and Zimm 1992). We used 11 homozygous derivative excision lines of desat11573-1 (1573-1) (Marcillac et al. 2005b; Figure 1A). Among these excision alleles, 6 retained a substantial fragment (11–3.5 kb) of the original PGal4 transposon. The fragment was long in the L1–L4 alleles (A4, C1, F3, and H6, respectively) and of medium size in M1 and M2 alleles (L6 and H3). In these alleles, the mini-white sequence was partially or totally removed. In some alleles, the pbluescript sequence was also partly removed, whereas the Gal4 sequence was not or only slightly affected. In two other alleles, S1 and S2, the transposon had a small size (F7 and F4, respectively), since most of the transposon was removed at the exception of the “feet” (0.15 kb). The three other alleles, P1–P3, resulted from the precise excision of the transposon (O5, A′2, and N2, respectively).
Figure 1.—
Genetic tools used to dissect desat1 functional pleiotropy. (A) Excision lines were derived from the remobilization of the PGal4 transposon from the original 1573-1 allele. The transposon contains the Gal4, miniwhite, and pbluescript sequences (respectively shown as open, solid, and hatched horizontal bars). The two ends of the transposon are delimitated by two feet (shown as small dark bars). The remobilization (Marcillac et al. 2005a) induced either (i) precise excision of the transposon in the three P1, P2, and P3 alleles or (ii) incomplete excision of the transposon in the eight alleles (L1–S2) shown from top to bottom, with a decreased size of the remaining transposon fragment (indicated in kb on the right). Excision alleles have been designated according to the approximate size of the remaining insertion: long (L1–L4), medium (M1 and M2), or short (S1 and S2). (B) Schematic representation of the desat1 locus and Ep deregulation lines. The insertion position of the PGal4 transposon (solid triangle) and of the PUAS (Ep transposons) is shown together with the Ep orientation relative to desat1 transcription (arrow to the right, sense; arrow to the left, antisense). For Ep lines, the letter corresponds to the regulatory region of the exon in which the transgene is inserted, and its size to the orientation relative to the desat1 gene transcription (uppercase, sense; lowercase, antisense). The index indicates that multiple Ep (only sense orientation) are inserted in the same regulatory region. The five solid boxes represent the five specific alternative 5′-UTR exons for each transcript (RA, RC, RE, RB, and RD), and the open boxes represent the translated desat1 region. Note that the transposon and the desat1 DNA are not shown at the same scale.
We also used 10 desat1 Ep deregulation lines that contain a PUAS transgene inserted in the 5′ noncoding region of the desat1 gene and whose expression can be deregulated under the conditional activation of Gal4 (Rorth 1996). These transgenes were named according to both the position and orientation of the Ep element in the desat1 regulatory sequence. (The letter indicates the regulatory region of the corresponding transcript: capital letter = sens; lower case letter = antisens; Figure 1B). Most strains were purchased from Genexel with the exception of EpB2 (EpGSV6) (Aigaki et al. 2003), which was kindly provided by T. Aigaki (University of Tokyo). The Ep transgenes were tested in four combinations. (1) The dominant effect of the Ep transgenes was measured in heterozygous flies combining one copy of each Ep transgene with the desat1–P3 precisely excised allele (P3/Ep) (Marcillac et al. 2005b). (2 and 3) To potentially affect desat1 transcriptional activity and deregulate its expression, each Ep transgene was driven either by the hypomorph desat1 1573-1–Gal4 allele (1573-1>Ep) or with the null desat1 1573-C′1–Gal4 allele (C′1>Ep) (Marcillac et al. 2005b). This allowed us to compare the effect of desat1 deregulation in hetero- and hemizygous desat1 genetic backgrounds, respectively. (4) We also measured the recessive effect of these Ep transgenes in homozygous flies (Ep/Ep) and their complementation in double heterozygote flies.
Behavior:
All flies were isolated 0–4 hr after eclosion under CO2 anesthesia. Tester male flies (i.e., those whose sexual response to target flies was measured) were held individually in fresh glass food vials for 5 days before testing. Target flies were similarly treated but they were held in groups of five for the same period. All tests were performed in a room at 24 ± 0.5° with 65 ± 5% humidity. Tester males were individually aspirated (without anesthesia) under a watch glass used as a courtship observation chamber (1.6 cm3). After 5 min, necessary for the tester male to habituate to the chamber, the two control target flies (a male and a female) were introduced and the observation period started.
To characterize male discrimination of sex pheromones, we measured the proportion of time spent by tester males in actively courting (wing vibration, licking, and attempting copulation; total = courtship index, CI) each target. For each male, we obtained two values corresponding to the CI directed to the male (CIm) and to the female target (CIf). Tests were carried out under a dim red light (25W with a Kodak Safe-Light Filter n°1) to remove all visual stimuli (Boll and Noll 2002) and with decapitated target flies to remove most acoustic and behavioral signals (Ferveur et al. 1995).
Besides the parametric CI parameters providing a global average measure of behavioral activity, we also designed, for each genotype, a new parameter to obtain a picture of the distribution of sexual discrimination in individual males on the basis of their respective CIf and CIm. We named this parameter “distribution of courtship index discrimination” [DCID = (CIf − CIm)/(CIf + CIm)]. Three classes of discrimination behavior were delimitated according to the dominant sexual orientation of each tester male: (1) heterosexual (DCID ≥ +0.35), or (2) homosexual (DCID ≤ −0.35), or (3) bisexual (−0.35 < DCDI < +0.35). Since low CI values could yield a biased ratio, we considered only individual males with a total CI (CIm + CIf) > 10%.
We measured the locomotor activity of single 5-day-old males. Each fly was introduced in a courtship chamber placed over a pattern delimitating 12 equal areas. After a habituation period of 5 min, we counted the number of lines (separating the areas) crossed during five periods of 10 sec and the total count number (during 50 sec) was used as the individual locomotor activity index (LAI) (Balakireva et al. 2000). This measurement was simultaneously and sequentially performed on four flies of different genotypes during a total period of 5 min. All tests lasted 5 min and took place 1–4 hr after lights on. For each strain, tests were performed over several days.
Cuticular hydrocarbons:
Cuticular hydrocarbons (CHs) were extracted from 5-day-old intact individual flies by gas chromatography following a brief wash in hexane according to standard procedures (Ferveur 1991). Analyses were performed with a Varian CP3380 chromatograph, equipped with a Cp-sil 25-m capillary column with hydrogen as the carrier gas. All the D. melanogaster predominant CHs have already been identified and characterized (Antony and Jallon 1982; Pechine et al. 1985). Twenty-four CHs were systematically detected in female flies, and 14 in male flies, both with a chain length ranging from 23 to 29 carbons (Marcillac et al. 2005b). Each CH was characterized both by (i) its percentage relative to the sum of all CHs (∑CHs) and (ii) to the area of an internal standard (hexacosane) used to calculate its absolute amount (in nanograms). For the sake of clarity, we show only four principal CH parameters: (1) the total amount of CHs (in ng; ∑CHs), the total percentage of (2) desaturated CHs (∑% Desat), (3) linear saturated CHs (∑% Lin), and (4) ramified CHs (∑% Br). The percentages (parameters 2–4) were calculated relative to ∑CHs (Note that ∑% Desat + ∑% Lin + ∑% Br =100% and corresponds to ∑CHs).
Desat1 transcripts:
To find a relationship betwen the alteration of the two pheromonal phenotypes and the deregulation of desat1, we measured the relative amount of the five desat1transcripts (RA, RC, RE, RB, and RD) separately in heads and in headless bodies of 5-day-old males. The nine tested genotypes include four experimental genotypes (C′1>1573-1, >Epb, >Epd, and >EpB2; which induced the strongest phenotypic effects; see results) and five control genotypes combining the precisely excised P3 allele with the five alleles present in the experimental genotypes. Note that RE was never detected in the head. All measures were compared with those obtained with P3 control males.
RNAs were extracted by the Trizol method (GIBCO BRL) and treated with RNase-free DNase to avoid contamination by genomic DNA. Total RNA (2 μg) was reverse transcribed with the iScript cDNA synthesis kit (Biorad). Quantative PCR reaction were performed with the IQ SYBR Green supermix (Biorad) in a thermal cycler (MyIQ, Biorad) according to the procedure recommended by the manufacturer. The qPCR reaction was done in a volume of 20 μl, by 40 cycles (95° for 30 sec, melting temperature (Tm) for 30 sec, and 72° for 30 sec), preceded by a 3-min denaturation step at 98°, and followed by a 1-min elongation step at 72°. TM of the hybridization step depends on the primer pair used. Generally, we used a TM of 60° for RC (RC forward: GGACGTGTGCTTTCGCCACT; RC reverse: CTGCGATCAGTGAGTCTGAGAT), RE (RE forward: GATACAACATCCTAAACAAATCGGG; RE reverse: CTGCGATCAGTGAGTCTGAGAT), RB (RB forward: TAATGGCCCCATCCTGGT; RB reverse CTGCGATCAGTGAGTCTGAGAT), RD (RD forward: CGAAACGGCTTGTTAATTTCTAGC; RD reverse: CTGCGATCAGTGAGTCTGAGAT), and control actine 5C (Act60 forward: TAACAAATTCAAGGCGTGAAA; Act60 reverse: TTCAGTCGGTTTATTCCAGTCA), except for RA amplification where 62.5° was used (RA forward: GCCATCACTAAACCAGGAGAATA; RA reverse: CGGTGGTTTCCACATCGCACTCGAA) and control actine 5C (Act62.5 forward: CAGATCATGTTCGAGACCTTCAA; Act62.5 reverse: ATCTTCATCAGGTAGTCGGTCAA). Each reaction was performed in triplicate and the mean of the three independent biological replicates was calculated. All results were normalized to the Actine5C mRNA level.
Statistics:
We compared CH levels between genotypes with a Kruskal–Wallis test. Within each strain, the difference between CIm and CIf was measured with a Student's t-test. The CI toward each target was tested between genotypes with a ANOVA completed by a multiple pairwise comparison using Bonferroni post hoc tests. DCIDs were compared between genotypes with a Khi2 homogeneity test. Only P-values <0.05 were considered to be statistically significant. Statistical analyses were performed using XLSTAT software. Significant differences in transcript level ratios between control and sample strain (body and head) were detected with the Relative Expression Software Tool (REST, REST-MCS beta software version 2 (Pfaffl 2001), where the iteration number was fixed at 2000. This test is based on the probability of an effect as large as that observed under the null hypothesis (no effect of the treatment), using a randomization test (pairwise fixed reallocation randomization test; Pfaffl et al. 2002).
RESULTS
Our experimental strategy principally consisted of measuring the production of CHs and male sexual behavior in two series of genetically manipulated flies. First, we used flies homozygous for 11 excision alleles resulting from the un/precise remobilization of the PGal4 transposon (Figure 1A). Second, we measured the effect of 10 PUAS transgenes (Ep) inserted in the desat1 regulatory region (Figure 1B) in various genetic combinations (see materials and methods). Our goal consisted of determining whether and to what extent the two phenotypes can be separately affected.
Effect of desat1 excision alleles on cuticular hydrocarbon production:
For the sake of clarity, we show only the total absolute amount of CHs (∑CHs; in ng) and the total relative levels of desaturated CHs (∑% Desat), of linear CHs (∑% Lin), and of methyl branched CHs (∑% Br). The variation of individual CHs is not shown since it was similar to that of their principal group.
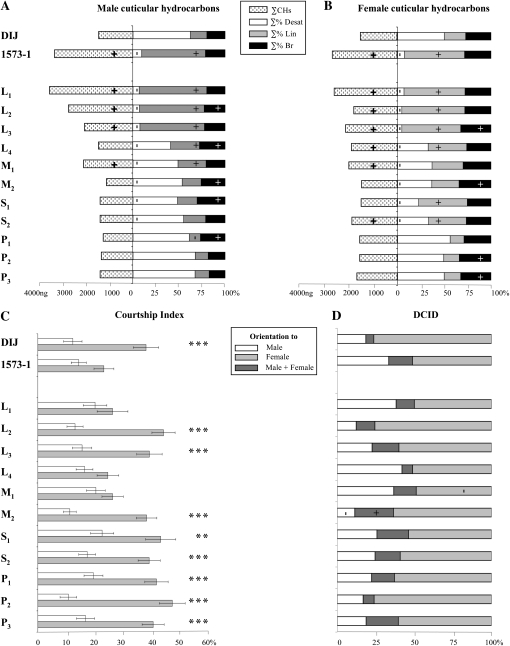
The production of male CHs was strongly affected in the three excision alleles containing the largest transposon fragment inserted (L1–L3; Figure 2A; see also supporting information, Table S1A). Their ∑% Desat was very reduced (7%), whereas their ∑% Lin was strongly increased (71%) compared to control males (62% and 18%, respectively). L1 and L2 males also showed strongly increased ∑CHs (3466 and 2716 ng, respectively). Overall, the CH levels of L1–L3 males were very similar to those of the original 1573-1 mutant (10% Desat, 69% Lin; ∑CHs = 3215 ng). Male CH levels were less affected in the five other imprecise excision alleles: their ∑% Desat was generally higher than ∑% Lin. P1–P3 males showed rescued CH profiles. Homozygous females of excision lines showed CH profiles very similar to respective excision males (Figure 2B; Table S1B). Note that S1 and S2 females showed a higher ∑% Lin than sibling males. Overall, these data reveal a relationship between the degree of alteration of male and female hydrocarbon profiles and the size of the transposon fragment inserted in the desat1 regulatory region.
Figure 2.—
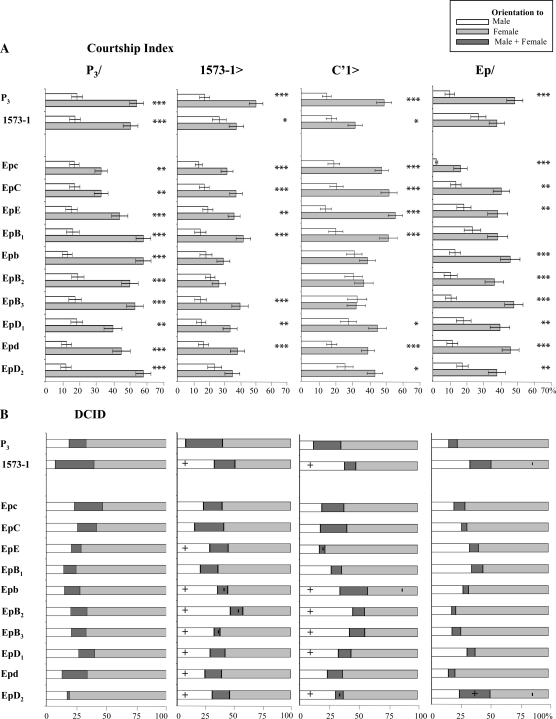
Pheromonal phenotypes in flies homozygous for various excision alleles. The genotype of excision strains is indicated on the left (for the molecular structure of excision alleles, see Figure 1). Two reference strains were also used: wild-type Dijon strain (DIJ) and the original desat11573-1 mutant (1573-1). (A and B) represent the production of cuticular hydrocarbons in mature male and female flies, respectively. For each sex, dotted bars on the left indicate the mean total absolute amounts of hydrocarbons (∑CHs, in ng), and bars on the right represent the total percentage of desaturated hydrocarbons (∑% Desat, open bars), of saturated linear hydrocarbon (∑% Lin, shaded bars), and of ramified hydrocarbons (∑% Br, solid bars). Note that ∑% Desat + ∑% Lin + ∑% Br = 100% = ∑CHs. The symbol within each bar indicates a significant variation (+, increase; −, decrease; P < 0.05; Kruskal–Wallis tests) with the DIJ control strain. N = 9–18. (C) Mean (±SEM) male courtship index to control target male (open bars) and control target female (lightly shaded bars). Bars are paired since both decapitated male and female target flies were simultaneously presented to a single tester male, under red light, during a 5-min period. The statistical significance for male ability to discriminate sex targets within each genotype is shown above each pair of bars: ***P < 0.001; **P < 0.01; *P < 0.05. N = 40–50. (D) The distribution for courtship index discrimination (DCID) indicates predominant sexual orientation in individual males: homosexual (open bars); bisexual (darkly shaded bars); and heterosexual (lightly shaded bars). Only males with a total courtship index (CIf + CIm) ≥10 were kept. The symbols within bars indicate a significant variation (+, increase; −, decrease; P < 0.05; χ2 test) of sexual orientation with the DIJ control strain. All flies were 5 days old. N = 40–50.
Effect of desat1 excision alleles on male courtship and sexual discrimination:
The second principal phenotype controlled by desat1 is the male discrimination of sex pheromones. To determine this, we measured the amount of courtship, or courtship index (CI) that single males directed toward a pair of immobilized control target female and male flies presented simultaneously. For each genotype, we compared their mean CI to the target female (CIf) and to the target male (CIm). Besides CIs, which represent the mean (±SEM) intensity of each tester male genotype toward each sex target, we designed a new parameter showing the distribution of individual male ability to discriminate (DCID; see materials and methods).
Control and homozygous P1–P3, L2, L3, M2, and S2 males showed a normally high discrimination: their CIf was significantly higher than the CIm (P < 0.001; Figure 2C). On the other hand, L1, L4, and M1 males showed no discrimination. In comparison to the DCID of control males, fewer M1 males showed heterosexual orientation, whereas M2 males were more often bisexual and less often heterosexual (Figure 2D). Therefore, no relationship was found between the gravity of the discrimination phenotype and the size of the transposon fragment.
desat1 deregulation and the production of male cuticular hydrocarbons:
We measured the potential dominant and recessive effect of each Ep transgene in Ep hetero- and homozygous flies, respectively. Some Ep transgenes were tested in double heterozygous flies to assess for complementation with regard to each phenotypes. To measure their effect on deregulation, each Ep transgene was also driven by either 1573-1 or C′1 enhancer trap. Both drivers contain a similar Gal4 element inserted at the same desat1 genomic site (e.g., able to reveal the activity of the same desat1 regulatory sequences), but C′1 is a null allele resulting from the total excision of the Desat1 coding region. This allowed us to compare the effect of the deregulation in a hypomorphic (1573-1) and in a null (C′1) desat1 context.
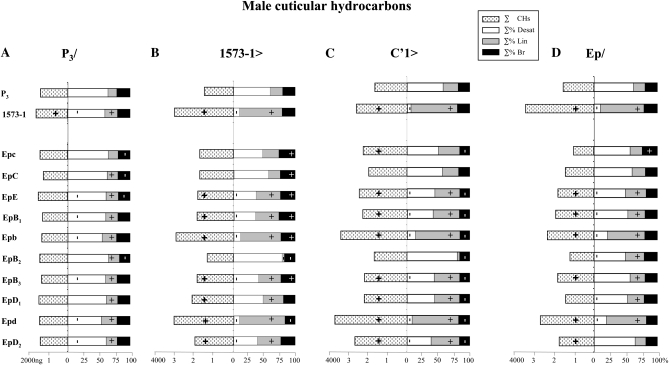
P3/Ep males showed no or very slight CH difference with P3 control males (Figure 3A; Table S1A). Their ∑% Desat (55–62%) and ∑% Lin (18–25%), respectively, corresponding to 700–900 ng (850 ng in P3 males) and 200–350 ng (200 ng in P3 males). Therefore, no Ep transgene had a dominant effect on male CH production.
Figure 3.—
Production of cuticular hydrocarbons in male flies of various strains combining Ep transgenes. The Ep transgenes used are indicated on the left (Epc–EpD2) below two reference desat1 alleles: the precisely excised P3 allele and the original 1573-1 mutant. Reference and Ep alleles were tested in four combinations (shown on the top, from left to right): (A) P3/ with P3; (B) 1573-1> driven by 1573-1; (C) C′1> driven by the null C′1 desat1 allele; and (D) EP/homozygous. Dotted bars on the left indicate the total absolute amounts of hydrocarbons (∑CHs, in ng). Bars on the right represent the total percentage of desaturated hydrocarbons (∑% Desat, open bars), saturated linear hydrocarbon (∑% Lin, shaded bars), and ramified hydrocarbons (∑% Br, solid bars). The symbol within each bar indicates a significant variation (+, increase; −, decrease; P < 0.05; Kruskal–Wallis tests) with the respective P3 control strain. For more information, see Figure 2 legend. N = 5–15.
The 1573-1>Ep males often showed a much higher ∑CHs than control 1573-1>P3 males (Figure 3B). In particular, 1573-1>Epb and >Epd males produced the double ∑CHs (respectively, 3000 and 3200 ng) of control males (1500 ng). The two former males also strongly decreased their ∑% Desat/Lin ratio (12/65% and 10/74%, respectively) compared to control males (59/20%). Reciprocally, 1573-1>EpB2 males showed a dramatically increased ∑% Desat/Lin ratio (80/3%). Concretely, 1573-1>Epd males produced 300 ng Desat CHs and 2300 ng Lin CHs against, respectively, 1400 and 60 ng in 1573-1>EpB2 males (900 and 300 ng in controls). In summary, 1573-1 combined with Epb and Epd tended to decrease the ∑% Desat/Lin ratio whereas EpB2 had a reciprocal effect on male CHs.
C′1>Epb and >Epd produced much higher ∑CHs (3400 and 3800 ng) than both control C′1> P3 males (1700 ng) and C′1>1573-1 males (2600 ng). If the two former males also strongly decreased their ∑% Desat/Lin ratio (14/71% and 7/66%, respectively) compared to control males (57/24%) and to C′1>EpB2 males (80/4%), they did not differ from C′1>1573-1 males (6/74%). Therefore, if C′1 induced a very similar effect to 1573-1 with regard to respective Ep transgene, C′1 tended to increase ∑CHs (+400–700 ng) compared to 1573-1 (when driving Epc, EpE, Epb, Epd, and EpD2).
Homozygous Epb and Epd males showed strongly increased ∑CHs (2300 and 2600 ng) and decreased ∑% Desat/Lin ratio (21/59% and 20/63%, respectively) compared to control males (1400 ng; 62/18%, respectively). However, these variations were less important than in 1573-1 mutant males (3200 ng; 10/69%). Overall, the amplitude of the CH variation was always lower in homozygous Ep males than in 1573-1>Ep and C′1>Ep deregulation males, with respect to Ep transgenes. Epb and Epd alleles did not complement each other since the general CH pattern (∑% Desat, ∑% Lin, and ∑CHs) of Epb/Epd males was similarly altered to that of either homozygous Epb or Epd males (Table S2A). EpE/EpB3 males (combining two mild-effect transgenes) showed no marked difference with either parental male. Epb/EpB3 and Epd/EpD2 males (combining a mild- and a strong-effect transgene) showed hydrocarbon phenotypes intermediate between those of both parents. In summary, some Ep transgenes induced semidominant effect (on male hydrocarbon production) depending on their insertion position in the desat1 regulatory region.
desat1 deregulation and the production of female cuticular hydrocarbons:
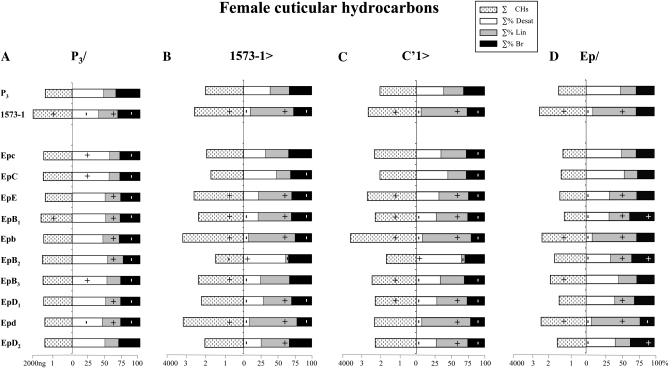
P3/Ep females showed no or slight CH difference with P3 control females (Figure 4A; Table S1B). Their average ∑% Desat/∑% Lin ratio was 51/19%. Therefore, no Ep transgene induced a dominant effect on female CHs.
Figure 4.—
Production of cuticular hydrocarbons in females flies of various strains combining Ep transgenes. The Ep transgenes (Epc–EpD2) and the two reference desat1 alleles used (P3, 1573-1) are indicated on the left. These alleles were tested in four combinations: (A) P3/with P3; (B) 1573-1> driven by 1573-1; (C) C′1> driven by the null C′1 desat1 allele; and (D) EP/homozygous. For more information, see legends for Figures 2 and 3. N = 8–15.
1573-1>Epb and >Epd females strongly increased their ∑CHs (3200 ng) compared to control females (2000 ng; Figure 4B). Reciprocally, 1573-1>EpB2 showed significantly reduced ∑CHs (1450 ng). In parallel to this variation, the ∑% Desat/Lin ratio was much lower in 1573-1>Epb and >Epd females (8/67% and 10/68%, respectively) than in control females (38/28%), whereas it strongly increased in 1573-1>EpB2 (63/4%). Concretely, 1573-1>Epb females produced 250 ng Desat CHs and 2150 ng Lin CHs against 900 and 60 ng, respectively, in 1571-1>EpB2 females (750 and 550 ng in control females). In summary, both Epb and Epd transgenes driven by 1573-1 tended to strongly decrease the ∑% Desat/Lin ratio, whereas EpB2 had a reciprocal effect on female CHs.
C′1>Ep females also showed important variations (Figure 4C), which were, however, not always parallel to those detected in 1573-1>Ep females. If C′1>Epb females strongly increased their ∑CHs (3650 ng) compared to control females (2000 ng) this did not vary either in C′1>Epd females (1900 ng) or in C′1>EpB2 (1670 ng). Nevertheless, C′1>Epb and >Epd decreased their ∑% Desat/Lin ratio (7/71%) similarly to C′1>1573-1 females (7/67%), compared to C′1>P3 control females (40/28%), whereas C′1>EpB2 females showed a strongly increased ratio (64/5%). Therefore, C′1 induced a similar effect on female CHs as 1573-1, except for Epd, which showed a less dramatic effect.
Homozygous Epb and Epd females produced much higher ∑CHs (2400 and 2250 ng, respectively), and much lower ∑% Desat/Lin ratio (7/67% and 7/71%, respectively) than control P3 females (1450 ng; 50/23%, respectively; Figure 4D). The CH levels found in Epb and Epd females were close to those of 1573-1 homozygous females (2600 ng; 8/63%). Epb/Epd females and EpE/EpB3 showed female hydrocarbon patterns resembling those of their respective parents (Table S2B). Epb/EpB3 and Epd/EpD2 females (combining a strong- and a mild-effect transgene) showed phenotypes intermediate between both parental females. Therefore, Epb and Epd transgenes showed a strong recessive effect and no complementation with regard to female CHs.
In conclusion, three Ep transgenes induced strong variation on the cuticular profiles of male and female flies. Both Epb and Epd—either homozygous, combined together, or with each PGal4 driver—strongly decreased the ∑% Desat/Lin ratio and increased the ∑CHs. On the other hand, EpB2 combined with either PGal4 driver strongly increased the ∑% Desat/Lin ratio.
Desat1 deregulation and male discrimination behavior:
P3/Ep males showed a high discrimination ability similar to that of control P3 males (P < 0.01–0.001; Figure 5A). The males with the lowest discrimination ability (P3/Epc, P3/EpC and P3/EpD1) showed significantly decreased CIf (t = 3.12–3.54; P = 0.0023–0.0013). No CIm variation was detected between genotypes. Therefore, no Ep transgene induced a strong dominant effect with regard to male discrimination ability.
Figure 5.—
Courtship behavior in males of various transgenic strains. The Ep transgenes (Epc–EpD2) and the two reference desat1 alleles (P3 and 1573-1) used are indicated on the left. Reference and Ep alleles were tested in four combinations (shown on the top) with P3 (P3/), with 1573-1 (1573-1>), with the null C′1 desat1 allele (C′1>), and homozygous (Ep/). (A) Mean (±SEM) male courtship index to control target male (open bars) and female (lightly shaded bars). A pair of decapitated control target flies (a male and a female) was simultaneously presented to a single tester male, under red light, during a 5-min period. The male ability to discriminate sex targets within each strain is shown above each pair of bars: ***P < 0.001; **P < 0.01; *P < 0.05. (B) The distribution for courtship index discrimination (DCID) is a newly designed parameter representing the distribution of predominant sexual orientation in individual males: homosexual (open bars); bisexual (darkly shaded bars); and heterosexual (lightly shaded bars). Only males with a total courtship index ≥10 were kept. The symbol within each bar/sexual orientation indicates a significant variation (+, increase; −, decrease; P < 0.05; χ2) with each respective P3 control strain. For more information, see legends for Figures 2 and 3. N = 38–59.
The 1573-1>Epb, >EpB2, and >EpD2 males did not discriminate the sex of their partners (P > 0.05; Figure 5A). The decreased CIf of 1573-1>EpB2 males (28 vs. 50; F10,468 = 2,177; P = 0.018) could explain their strongly increased frequency of homosexual males (DCID) compared to control males (47 vs. 7%; P < 0.01; Figure 5B). Several other genotypes also showed an increased frequency of homosexual males. Therefore, Ep deregulation driven by 1573-1 could strongly affect male discrimination, especially with EpB2 transgene.
C′1>Epb, >EpB2, and >EpB3 males showed no sexual discrimination and C′1>EpD1 and >EpD2 a slightly reduced discrimination ability (P < 0.05; Figure 5A). Their DCID revealed more frequent “homosexual” males. The frequency of “heterosexual” males only decreased in C′1>Epb. If 1573-1 and C′1 drivers induced similar effects on male discrimination when combined with Epb and EpB2, C′1 induced a stronger effect with EpB3 and a weaker effect with EpD2.
Only homozygous EpB1 males showed no discrimination ability similarly to homozygous 1573-1 mutant males (Figure 5A): Their CIm increased (23 and 27, respectively) compared to control males (10; F11,541 = 2,575; P = 0.0034). Strikingly, Epc males showed a high discrimination ability but dramatically reduced CIf and CIm. This low sexual activity was not related to a general behavioral defect since Epc males exhibited a locomotor activity index (LAI = 54), which was not significantly different from that of control P3 males (LAI = 60) and of 1573-1 mutant males (LAI = 48; K2df = 9.277, P = 0.01). Moreover, if 55% of Epc males did not court during the observation period (5–11% in the other genotypes), the 45% courting males generally showed a delayed courtship latency (data not shown). Fewer “heterosexual” and more “bisexual” males were found in the EpD2 genotype (Figure 5B). No complementation (no discrimination) was shown by Epd/EpD2 males, which were less frequently “heterosexual” compared to Epd males (but not to EpD2 males; Figure S1). On the other hand, EpE/EpB3 and Epb/EpB3 males showed no significant alteration of their discrimination phenotype. In summary, two Ep transgenes differently affected male sexual behavior: Epc altered general sexual activity, whereas EpB1 altered male discrimination. Moreover Epd and EpD2 transgenes did not complement each other with regard to male discrimination.
Desat1 deregulation and the transcript levels:
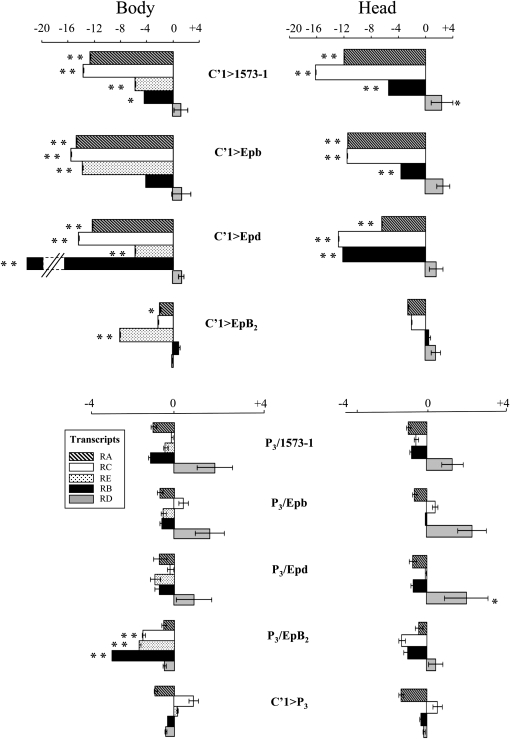
Our data reveal a very mild transcriptional variation in control genotypes but a strong variation in experimental genotypes (Figure 6). In experimental males, RA and RC strongly decreased in the head and body of C′1>1573-1, >Epb, and >Epd males. RB also decreased in the head of these males (P < 0.01) and in the body of C′1>1573-1 and >Epd males (P < 0.05 and 0.01, respectively). RE level also strongly decreased in the body of the four experimental genotypes (P < 0.01) but was never detected in the head of either experimental or reference P3 genotypes. Therefore, desat1 deregulation seems to strongly affect most transcripts in the head and body of males combining C′1 with Epb, Epd, or 1573-1 transgenes, whereas this effect is less important (and restricted to the body) in C′1>EpB2 males. Control genotypes only showed lower and sporadic decrease of transcript level. In summary, the quantitative variation of some transcripts could be related to the alteration of CH production but not with that of male discrimination.
Figure 6.—
—Relative levels of desat1 transcripts in various transgenic males. Transcripts were quantified with q-PCR separately in the body (left) and in the head (right) of nine transgenic males. The top four experimental genotypes combine the C′1 allele (C′1>) with either 1573-1, Epb, Epd, or EpB2. The five bottom males represent control genotypes combining the P3 rescued allele with the five transgenes present in the four experimental genotypes. Bars represent the mean (±SEM) for the relative levels of the five desat1 transcripts: RA (hatched), RC (open), RE (dotted; not detected in the head), RB (solid), and RD (shaded). Negative and positive values, respectively, indicate significantly decreased or increased levels compared to P3 control genotype (**P < 0.01; *P < 0.05). RB was not detected in the body of C′1>Epd males. Note that the Log2 scales differ between experimental and control genotypes. For each biological extraction, q-PCR was replicated three times. N = 3 for each tissue and genotype.
DISCUSSION
Desat1 induces pleiotropic effects on D. melanogaster pheromonal communication:
After showing that a single mutation in the desat1 gene affected both the production of cuticular pheromones and the male discrimination of these pheromones (Marcillac et al. 2005a,b), the present study reveals that these two phenotypes are independently regulated by desat1 (Table 1). First, the simultaneous rescue of both “production” and “discrimination” phenotypes in P1–P3 alleles confirms that the PGal4 transposon inserted in desat1 induced both defects. Second, several genetic manipulations allowed us to separately rescue each phenotype (Table 1; see below). The absence of a complete positive relationship between the two phenotypes indicates that they are independently controlled by desat1.
TABLE 1.
Synthesis of the principal results obtained in this study
| A. Excision lines |
1573-1 |
L1 |
L2 |
L3 |
L4 |
M1 |
M2 |
S1 |
S2 |
P1 |
P2 |
P3 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHs | Σ% Desat | − | − | − | − | − | − | − | − | − | |||
| ΣCHs | + | + | + | + | (+) | + | (+) | ||||||
| Discrimination | −−− | −−− | −−− | −−− | − | ||||||||
| B. Ep lines | 1573-1 | Epc | EpC1 | EpE | EpB1 | Epb | EpB2 | EpB3 | EpD1 | Epd | EpD2 | ||
| CHs | Σ% Desat | 1573-1> | − | (−)* | − | − | (+) | − | (−) | − | − | ||
| C′1> | − | (−)* | − | − | (+) | − | (−) | − | − | ||||
| Homozygous | − | − | − | − | − | (−)* | − | (−) | |||||
| ΣCHs | 1573-1> | + | + | + | + | (−) | + | (+)* | + | (+)* | |||
| C′1> | + | (+)* | + | + | + | + | + | (+)* | (+)* | ||||
| Homozygous | + | (+)* | (+)* | + | + | + | (+)* | ||||||
| Discrimination | 1573-1> | −− | − | −−− | −−− | − | −−− | ||||||
| C′1> | −− | −−− | −−− | −−− | −− | −− | |||||||
| Homozygous | −−− | − | − | −−− | − | − | |||||||
(A) Excision alleles and (B) Ep transgenes (driven by 1573-1 = 1573-1>; driven by 1573-C′1 = C′1>; homozygous). For the production of cuticular hydrocarbons (CHs), we only show the total CHs (∑CHs), and the total relative amounts of desaturated CHs (∑% Desat). The “+” and “−” signs indicate an increase and a decrease of the CH level. These variations were parallel in both sexes except when indicated as: ( ) = only in female; ( )* = only in male. For male discrimination, the number of minus (−) signs indicate the degree of alteration for the ability to discriminate: “−” = slight reduction; “−−” = important reduction; “−−−” = no discrimination. For both phenotypes, the absence of sign indicates no significant variation relatively to control genotypes.
Qualitative and quantitative effects on the production of cuticular hydrocarbons:
The comparison of male and female CH profiles induced by desat1 excision alleles revealed a positive relationship between the gravity of this phenotype and the size of the transposon fragment inserted in desat1 regulatory region: Alleles with the larger fragment showed a very low Desat/Lin ratio whereas shorter-fragment alleles showed a Desat/Lin ratio ≥1. Such a similar “molecular–phenotypic” relationship has been described with a series of Voila-prospero alleles (resulting from the remobilization of a PGal4 transposon) (Grosjean 2002; Grosjean et al. 2003).
Three Ep transgenes strongly affected pheromone production: Epb and Epd (i) combined with each PGal4 driver or (ii) homozygous or (iii) combined together showed strongly decreased Desat/Lin ratio. The EpB2 transgene driven by either PGal4 highly increased the Desat/Lin ratio. Since flies with strongly decreased Desat/Lin ratio often showed highly increased ∑CHs, this suggests that Desat CH biosynthesis requires more fatty acid precursors than Lin CH. This agrees with the finding that desat1 mutation also affects the total levels and fatty acid composition of several phospholipid species in Drosophila (Kohler et al. 2009). The few exceptions noted in the “low ratio-high ∑CHs” rule in our study (Table 1; L4 males and C′1>Epd females) suggest that desat1 separately controlled quantitative and qualitative aspects of pheromonal production. Moreover, some sex-specific effects were noted (Table 1). They could be related to the influence of sex determination genes such as doublesex, which target desat genes and other genes involved in sex-specific characters (Waterbury et al. 1999; Shirangi et al. 2009).
Moreover, Epb and Epd—but not EpB2—transgenes show an antisense orientation (relative to the direction of desat1 transcription), and their deregulation affected some transcript(s) differently. If RE decreased in the body of all experimental genotypes, RA and RC levels strongly decreased in the head and body (as well as RB in the head) of C′1>Epb, >Epd, but not of C′1>EpB2. Interestingly, C′1>1573-1 and homozygous 1573-1 males, both genotypes with a strongly decreased Desat/Lin ratio, also showed a drastic general reduction of RA, RC, and RE (Marcillac et al. 2005a). Therefore, if the alteration of the pheromonal production is linked to the differential variation of RC, RA, and/or RE in the fly body, RE decrease could induce a general alteration of the CH profile, whereas RC and/or RA decrease could induce the decreased Desat/Lin ratio. Our current data suggest that RC is preferentially expressed in the fat body, while RA is likely expressed in the central nervous system (F. Bousquet and J.-F. Ferveur, unpublished data). Given that (i) the fat body contains the fatty acid precursors necessary for hydrocarbon biosynthesis (Savarit and Ferveur 2002; Zinke et al. 2002; Vihervaara and Puig 2008) and (ii) the negative relationship between the RC level and the ∑CHs variation in C′1>Epb and >Epd—but not in C′1>EpB2—flies, the RC transcript may act to repress the overproduction of CHs. On the other hand, a brain hormonal factor (Wicker and Jallon 1995) linked with the strong decrease of RA in the head of C′1>1573-1, >Epb, and >Epd—but not in C′1>EpB2—flies could change the Desat/Lin ratio. Note that RC, RE, and RB also decreased in the body of P3/EpB2 control males, which, however, showed no significant CH variation. This can be explained by the fact that (i) RA did not vary in the head of these control males and/or (ii) their RC decrease was much weaker than in C′1>Ep males.
Effects on male discrimination of sex pheromones and courtship:
No clear relationship was found between the discrimination phenotype and the variation of (i) the transposon fragment size or (ii) the transcripts level. Males with severely affected discrimination often showed decreased CIf (≤40) and increased CIm (≥25). They may have lost the ability to perceive the pheromones produced by male and/or female flies (Table 1). Similar cases of reduced sex pheromones discrimination occurred after genetic misexpression in Drosophila chemosensory peripheral nervous system (PNS) (Svetec and Ferveur 2005; Xu et al. 2005; Park et al. 2006; Koganezawa et al. 2010) or central nervous system (CNS) (Ferveur et al. 1995). Given that desat1 is expressed in the chemosensory PNS (sensilla on the proboscis, tarsae, antennae) necessary for pheromonal perception (Marcillac et al. 2005a), the fact that we did not detect any relationship between the variation of transcripts and of male discrimination could be explained if two or more transcripts are simultaneously expressed at a very low level in a small number of neural cells. Alternatively, the RNA variation potentially occurring in these cells may have been diluted in the larger global amount of tissue used for q-PCR analysis.
Surprisingly, Epc males showed high sexual discrimination but very low general sexual activity, likely resulting from their delayed courtship initiation (Figure 5A). This indicates that they still perceive sex pheromones but not other non-sex-specific pheromones, which normally elicit male courtship initiation (Savarit et al. 1999). Further experiments are needed to verify this hypothesis.
Evolution of desat1 regulation:
Our data clearly show that distinct desat1 regulatory regions and/or transcripts separately control the two major aspects—emission and perception—of pheromonal communication in D. melanogaster. Many Drosophila genes affecting behavior have pleiotropic effects. For example, prospero affects several aspects of the nervous system development from early embryogenesis to adult life as well as larval and adult behaviors (Knoblich et al. 1995; Grosjean et al. 2001; Behan et al. 2005; Guenin et al. 2007). The evolution of cis-regulatory elements of two pigmentation genes (yellow and Bric-à-brac) was shown to change not only the pigmentation pattern of the fly but also sexually dimorphic traits including courtship behavior (Prud'homme et al. 2006; Williams et al. 2008).
Several genetic mechanisms are associated with the evolution of chemical communication and courtship behavior in Drosophila such as (i) recruitment of new chimeric genes combining genetic sequences with unrelated functions (Dai et al. 2008) or (ii) coordinated transcription of functionally related genes organized in a operon-like manner (Ben-Shahar et al. 2007). A third evolutionary manner would consist of increasing the complexity of regulatory sequences controlling the expression of a unique product, as in desat1. This was the first gene described to control several aspects of sensory communication (Marcillac et al. 2005b). The rarity of this mechanism may be explained by the dominant theory that proposed that genes coding for the emission and for the reception of sensory signals should be different (Boake 1991; Andersson and Simmons 2006). Two more loci affecting several aspects of sensory communication have recently been described: The color interfere locus changes visual recognition between sex partners in the teleost fish Medaka (Fukamachi et al. 2009), and a single QTL linkage group influences acoustic communication between two Hawaiian cricket species (Shaw and Lesnick 2009).
The sequence of desat1 is highly conserved in insects (Knipple et al. 2002) and desaturase genes may have played a key role in the formation of new species by creating new pheromonal molecules after the introduction of double bonds in new positions (Roelofs et al. 2002; Rooney 2009). In Drosophila, some very fast evolving desat genes (desat2 and desatF) originating from the ancestral desat1 gene may have been crucial in creating pheromonal diversity (Fang et al. 2009; Shirangi et al. 2009). If the primary function of desaturated cuticular hydrocarbons resides in the protection against water loss (Gibbs 2002), their pheromonal role was acquired more recently. Since the D. melanogaster subgroup is thought to have evolved 3–4 millions years ago (Lachaise et al. 1988), the evolution of desat1 regulatory elements involved in pheromonal perception may have occurred during that period.
In conclusion, our genetic dissection of desat1 reveals that both complex pheromonal phenotypes (production and perception) depend upon separate genetic controls. Given that some traits of the two phenotypes (the Desat/Lin ratio and ∑CHs for the hydrocarbon production; discrimination and initiation for male behavior) can also be genetically dissociated, this suggests that they depend on the precise control of one or several desat1 transcripts in specific tissues. Our next goal will consist of analyzing at a fine-grain resolution the expression of desat1 transcript in these tissues.
Acknowledgments
We thank Sylvie Chaudy, Laurence Dartevelle, Serge Loquin, and José Solonot for their help in maintaining the stocks and two anonymous reviewers for their comments on the manuscript. This work was partially funded by the Centre National de la Recherche Scientifique, by the Burgundy Regional Council and by the Agence Nationale pour la Recherche (INSAVEL).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.117226/DC1.
References
- Aigaki, T., T. Kaneuchi, T. Matsuo, K. H. Seong and T. Togawa, 2003. Genetic bases of oxidative stress resistance and life span in Drosophila. J. Clin. Biochem. Nutr. 34 77–83. [Google Scholar]
- Andersson, M., and L. W. Simmons, 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21 296–302. [DOI] [PubMed] [Google Scholar]
- Antony, C., and J. M. Jallon, 1982. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 28 873–880. [Google Scholar]
- Balakireva, M., N. Gendre, R. F. Stocker and J. F. Ferveur, 2000. The genetic variant Voila(1) causes gustatory defects during Drosophila development. J. Neurosci. 20 3425–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan, K. J., J. Fair, S. Singh, M. Bogwitz, T. Perry et al., 2005. Alternative splicing removes an Ets interaction domain from Lozenge during Drosophila eye development. Dev. Genes Evol. 215 423–435. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar, Y., K. Nannapaneni, T. L. Casavant, T. E. Scheetz and M. J. Welsh, 2007. Eukaryotic operon-like transcription of functionally related genes in Drosophila. Proc. Natl. Acad. Sci. USA 104 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boake, C. R. B., 1991. Coevolution of senders and receivers of sexual signals - genetic coupling and genetic correlations. Trends Ecol. Evol. 6 225–227. [DOI] [PubMed] [Google Scholar]
- Boll, W., and M. Noll, 2002. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129 5667–5681. [DOI] [PubMed] [Google Scholar]
- Bradbury, J. W., and S. L. Vehrencamp, 1998. Principles of Animal Communication. Sinauer Associates, Sunderland, MA.
- Coyne, J. A., A. P. Crittenden and K. Mah, 1994. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265 1461–1464. [DOI] [PubMed] [Google Scholar]
- Dai, H. Z., Y. Chen, S. D. Chen, Q. Y. Mao, D. Kennedy et al., 2008. The evolution of courtship behaviors through the origination of a new gene in Drosophila. Proc. Natl. Acad. Sci. USA 105 7478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C., 1883. The Descent of Man and Selection Relation to Sex, Ed 3. Murray, London.
- Fang, S., C. T. Ting, C. R. Lee, K. H. Chu, C. C. Wang et al., 2009. Molecular evolution and functional diversification of fatty acid desaturases after recurrent gene duplication in Drosophila. Mol. Biol. Evol. 26 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur, J. F., 1991. Genetic control of pheromones in Drosophila simulans. 1. Ngbo, a locus on the 2nd chromosome. Genetics 128: 293–301. [DOI] [PMC free article] [PubMed]
- Ferveur, J. F., 2005. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35 279–295. [DOI] [PubMed] [Google Scholar]
- Ferveur, J. F., and G. Sureau, 1996. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 263 967–973. [DOI] [PubMed] [Google Scholar]
- Ferveur, J. F., K. F. Stortkuhl, R. F. Stocker and R. J. Greenspan, 1995. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 267 902–905. [DOI] [PubMed] [Google Scholar]
- Fukamachi, S., M. Kinoshita, K. Aizawa, S. Oda, A. Meyer et al., 2009. Dual control by a single gene of secondary sexual characters and mating preferences in medaka. BMC Biol. 9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, A. G., 2002. Lipid melting and cuticular permeability: new insights into an old problem. J. Insect Physiol. 48 391–400. [DOI] [PubMed] [Google Scholar]
- Grillet, M., L. Dartevelle and J. F. Ferveur, 2006. A Drosophila male pheromone affects female sexual receptivity. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 273 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, Y., 2002. Caractérisation génétique, moléculaire, histologique et comportementale du mutant Voila dans le gène prospero, chez Drosophila melanogaster. Ph.D. Thesis, University of Burgundy, Dijon, France.
- Grosjean, Y., M. Balakireva, L. Dartevelle and J. F. Ferveur, 2001. PGal4 excision reveals the pleiotropic effects of Voila, a Drosophila locus that affects development and courtship behaviour. Genet. Res. 77 239–250. [DOI] [PubMed] [Google Scholar]
- Grosjean, Y., F. Lacaille, A. Acebes, J. Clemencet and J. F. Ferveur, 2003. Taste, movement, and death: varying effects of new prospero mutants during Drosophila development. J. Neurobiol. 55 1–13. [DOI] [PubMed] [Google Scholar]
- Guenin, L., Y. Grosjean, S. Fraichard, A. Acebes, F. Baba-Aissa et al., 2007. Spatio-temporal expression of Prospero is finely tuned to allow the correct development and function of the nervous system in Drosophila melanogaster. Dev. Biol. 304 62–74. [DOI] [PubMed] [Google Scholar]
- Higgie, M., S. Chenoweth and M. W. Blows, 2000. Natural selection and the reinforcement of mate recognition. Science 290 519–521. [DOI] [PubMed] [Google Scholar]
- Jallon, J. M., 1984. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14 441–478. [DOI] [PubMed] [Google Scholar]
- Jallon, J. M., and C. Wicker-Thomas, 2003. Genetic studies on pheromone production in Drosophila, pp. 253–280 in Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detecion of Pheromones and Plant Volatiles, edited by G. J. Blomquist and R. G. Vogt. Elsevier Academic Press, Amsterdam, The Netherlands.
- Johansson, B. G., and T. M. Jones, 2007. The role of chemical communication in mate choice. Biol. Rev. 82 265–289. [DOI] [PubMed] [Google Scholar]
- Knipple, D. C., C. L. Rosenfield, R. Nielsen, K. M. You and S. E. Jeong, 2002. Evolution of the integral membrane desaturase gene family in moths and flies. Genetics 162 1737–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich, J. A., L. Y. Jan and Y. N. Jan, 1995. Asymmetric segregation of Numb and Prospero during cell division. Nat. 377 624–627. [DOI] [PubMed] [Google Scholar]
- Koganezawa, M., D. Haba, T. Matsuo and D. Yamamoto, 2010. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 20 1–8. [DOI] [PubMed] [Google Scholar]
- Kohler, K., E. Brunner, X. L. Guan, K. Boucke, U. F. Greber et al., 2009. A combined proteomic and genetic analysis identifies a role for the lipid desaturase Desat1 in starvation-induced autophagy in Drosophila. Autophagy 5 980–990. [DOI] [PubMed] [Google Scholar]
- Lacaille, F., M. Hiroi, R. Twele, T. Inoshita, D. Umemoto et al., 2007. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2(e661): 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise, D., M. L. Cariou, J. R. David, F. Lemeunier, L. Tsacas et al., 1988. Historical biogeography of the Drosophila melanogaster species subgroup. Evol. Biol. 22 159–225. [Google Scholar]
- Legendre, A., X. X. Miao, J. L. Da Lage and C. Wicker-Thomas, 2008. Evolution of a desaturase involved in female pheromonal cuticular hydrocarbon biosynthesis and courtship behavior in Drosophila. Insect Biochem. Mol. Biol. 38 244–255. [DOI] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, London.
- Marcillac, F., F. Bousquet, J. Alabouvette, F. Savarit and J. F. Ferveur, 2005. a A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics 171 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac, F., Y. Grosjean and J. F. Ferveur, 2005. b A single mutation alters production and discrimination of Drosophila sex pheromones. Proc. R. Soc. Ser. B-Biol. Sci. 272 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. K., K. J. Mann, H. Lin, E. Starostina, A. Kolski-Andreaco et al., 2006. A Drosophila protein specific to pheromone-sensing gustatory hairs delays males' copulation attempts. Curr. Biol. 16 1154–1159. [DOI] [PubMed] [Google Scholar]
- Pechine, J. M., F. Perez, C. Antony and J. M. Jallon, 1985. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal. Biochem. 145 177–182. [DOI] [PubMed] [Google Scholar]
- Pfaffl, M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W., G. W. Horgan and L. Dempfle, 2002. Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme, B., N. Gompel, A. Rokas, V. A. Kassner, T. M. Williams et al., 2006. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440 1050–1053. [DOI] [PubMed] [Google Scholar]
- Roelofs, W. L., and A. P. Rooney, 2003. Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proc. Natl. Acad. Sci. USA 100 9179–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs, W. L., W. T. Liu, G. X. Hao, H. M. Jiao, A. P. Rooney et al., 2002. Evolution of moth sex pheromones via ancestral genes. Proc. Natl. Acad. Sci. USA 99 13621–13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, A. P., 2009. Evolution of moth sex pheromone desaturases. Ann. N. Y. Acad. Sci. 1170 506–510. [DOI] [PubMed] [Google Scholar]
- Rorth, P., 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA 93 12418–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarit, F., and J. F. Ferveur, 2002. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. 79 23–40. [DOI] [PubMed] [Google Scholar]
- Savarit, F., G. Sureau, M. Cobb and J. F. Ferveur, 1999. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl. Acad. Sci. USA 96 9015–9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, K. L., and S. C. Lesnick, 2009. Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. Proc. Natl. Acad.Sci. USA 106 9737–9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi, T. R., H. D. Dufour, T. M. Williams and S. B. Carroll, 2009. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 7 e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja, C., and R. K. Butlin, 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102 77–97. [DOI] [PubMed] [Google Scholar]
- Svetec, N., and J. F. Ferveur, 2005. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J. Exp. Biol. 208 891–898. [DOI] [PubMed] [Google Scholar]
- Tompkins, L., S. P. McRobert and K. Y. Kaneshiro, 1993. Chemical communication in Hawaiian Drosophila. Evolution 47 1407–1419. [DOI] [PubMed] [Google Scholar]
- Toolson, E. C., and R. Kupersimbron, 1989. Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura: effects on sexual dimorphism and thermal-acclimation ability. Evolution 43 468–473. [DOI] [PubMed] [Google Scholar]
- Vihervaara, T., and O. Puig, 2008. dFOXO regulates transcription of a Drosophila acid lipase. J. Mol. Biol. 376 1215–1223. [DOI] [PubMed] [Google Scholar]
- Waterbury, J. A., L. L. Jackson and P. Schedl, 1999. Analysis of the doublesex female protein in Drosophila melanogaster: role in sexual differentiation and behavior and dependence on intersex. Genetics 152 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker, C., and J. M. Jallon, 1995. Hormonal-control of sex-pheromone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 41 65–70. [Google Scholar]
- Williams, T. M., J. E. Selegue, T. Werner, N. Gompel, A. Kopp et al., 2008. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, T. D., 2003. Pheromones and Animal Behaviour. Communication by Smell and Taste. Cambridge University Press, Cambridge.
- Xu, P. X., R. Atkinson, D. N. M. Jones and D. P. Smith, 2005. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45 193–200. [DOI] [PubMed] [Google Scholar]
- Zinke, I., C. S. Schutz, J. D. Katzenberger, M. Bauer and M. J. Pankratz, 2002. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 21 6162–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]