Abstract
Background
The 92 capsular serotypes of Streptococcus pneumoniae differ greatly in nasopharyngeal carriage prevalence, invasiveness and disease incidence. There has been some debate, though, as to whether serotype independently affects the outcome of invasive pneumococcal disease (IPD). Published studies have shown variable results with regards to case-fatality ratios for specific serotypes and the role of host factors in affecting these relationships. We evaluated whether risk of death from IPD is a stable serotype-associated property across studies, and then compared the pooled effect estimates with epidemiologic and biological correlates.
Methods
We performed a systematic review and meta-analysis of serotype-specific disease outcome for pneumonia and meningitis cases. Study-specific estimates of risk of death (risk ratio, RR) were pooled from 9 studies that provided serotype-specific data on pneumonia and meningitis using a random-effects method with serotype 14 as the reference. Pooled RRs were compared to RRs from adult cases with low co-morbidity scores to evaluate potential confounding by host factors.
Results
There were significant differences in the RR estimates between serotypes among bacteremic pneumonia cases. Overall, types 1, 7F and 8 were associated with decreased RRs and types 3, 6A, 6B, 9N and 19F were associated with increased RRs. Outcomes among meningitis cases did not differ significantly between types. Serotypes with increased RRs tended to have a high carriage prevalence, low invasiveness, and were more heavily encapsulated in vitro. These results suggest that IPD outcome, like other epidemiologic measures, is a stable serotype-associated property.
Keywords: Serotype, pneumococcus, case-fatality ratio, mortality, capsule, meta-analysis
Streptococcus pneumoniae, or pneumococcus, is an important cause of pneumonia, meningitis, otitis media, and septicemia and is associated with significant morbidity and mortality worldwide. There are 92 known pneumococcal serotypes, and each produces a unique polysaccharide capsule that protects the bacterium against host immune effectors [1].
Serotype affects many aspects of pneumococcal epidemiology. The rank orders of serotypes found in nasopharyngeal carriage [2] and invasive disease [3, 4] are similar worldwide with a few exceptions. Likewise, the invasiveness of a serotype, or the frequency with which it causes invasive disease per carriage episode, is a stable property [5]. There is an inverse relationship between the carriage prevalence of a serotype and its invasiveness [5] and between disease severity and invasiveness [6].
The outcome of a case of invasive pneumococcal disease (IPD) can be affected both by bacterial factors, such as serotype, and by host characteristics, such as old age, very young age, low socioeconomic status, quality of care, alcoholism, immunodeficiency, and other underlying conditions[7–10]. Some studies have found that even after controlling for relevant host factors, certain serotypes are independently associated with more severe outcomes [11–13]. Likewise, experimental studies in mice have shown that serotypes differ in their ability to cause severe disease [14], and strains with larger capsules are more virulent in animals than strains of the same serotype with smaller capsules [15, 16]. It was long ago noted that differences in polysaccharide production between types 1, 2, and 3 correlated with the case-fatality ratios (CFR) for these serotypes in humans [7, 17].
While a number of studies have investigated the relationship between serotype and disease outcome, they differ in the kinds of clinical syndromes included, the age of the populations studied, and the covariates included when deriving effect estimates. As a result, published studies differ in the magnitude and direction of effect estimates for certain serotypes, and these studies have not previously been compared to determine whether stable patterns of virulence exist. We performed a systematic review and meta-analysis of IPD outcome by serotype to evaluate the stability of these estimates between studies. We found that clinical outcome in bacteremic pneumonia, like carriage prevalence and invasiveness, is a stable serotype-associated property, and we hypothesize about the biological reasons for these patterns.
METHODS
Literature review, inclusion criteria, and sources of data
We performed a Pubmed search with combinations of the terms “pneumococcus,” “pneumococcal,” “invasive pneumococcal disease,” “serotype,” “type,” “fatality,” “mortality,” and “severity” and reviewed abstracts for content. Additional studies were identified from reference lists and from published texts [7]. English language studies from 1928 to the present were considered that provided serotype-specific data on the number of invasive disease cases and number of deaths, though many of the older studies were excluded because they did not use contemporary diagnostic procedures and did not require the isolation of bacteria from sterile sites. A number of studies, including some with high quality data, were excluded, as described in Table S3. Reasons for exclusion included: results not stratified by syndrome, case definitions that did not require the isolation of bacteria from a normally sterile site, or if the study did not contain fatalities in the reference group, did not contain extractable data in the publication, or overlapped with other studies included in the analysis. Additionally, given our primary focus on bacteremic pneumonia, we did not seek or include studies that focused exclusively on meningitis. For studies that did not provide sufficient detail in the original text, we attempted to contact the corresponding authors of the studies for additional data.
Cases from the identified studies were included if they had pneumococcal isolates obtained from blood or cerebrospinal fluid (CSF) with a clinical diagnosis of pneumonia or meningitis. Non-bacteremic pneumonias were not included in this analysis. We used the diagnoses reported in the original studies, among which the clinical case definitions of these syndromes varied. Cases diagnosed with both pneumonia and meningitis were classified as meningitis cases.
Additional data on pneumonia outcome in adult cases with no known comorbidities (Charlson comorbidity score = 0) were derived from the dataset recently described by Harboe et al. [11]. Serotype-specific estimates of invasiveness and carriage frequency (number of times a serotype was detected in the population) were extracted from published data as described in the text.
As previously described, we measured the degree of encapsulation in vitro using capsule-switch variants that were created in the laboratory on the TIGR4 genetic background [18]. The strains were grown on TSAII plates, resuspended in PBS, and mixed with FITC-dextan, a large macromolecule that is excluded from the dense capsular region. We then measured the area of FITC-dextran exclusion for 100–250 bacteria with a Nikon Eclipse 80i. Degree of encapsulation measurements are presented as the mean area of the zone of FITC-dextran exclusion in pixels.
Selection of serotypes for comparison
To be included in the meta-analysis, we only considered serotypes that were found in at least three different studies with at least 10 isolates in each study for the pneumonia meta-analysis or at least two different studies for the meningitis meta-analysis. All available studies, regardless of their sample size, were used to calculate the pooled effect estimates and to examine heterogeneity.
Calculation of the risk ratio
We calculated the serotype-specific risk of death (risk ratio, RR) and 95% confidence intervals (95% CI) compared to serotype 14 [5]. Serotype 14 was chosen because it is a common cause of IPD and is the only serotype that contained non-zero numbers of fatalities in all studies. In situations where there were no fatalities, a value of 0.5 was added to each component of the risk ratio prior to calculation.
Statistics
Pooled RRs for each serotype were calculated by using a random effects model [19] using the “metan” package in Intercooled Stata v9.2. There could be true differences in the RRs between study locations attributable to different circulating bacterial strains or to differences in the host population. While a fixed effect approach calculates weights based only on the variance of the studies and assumes that all of the RRs are drawn from the same underlying population, the random effects model accounts for potential differences between studies and distributes the weights more equally. Separate analyses were performed for cases of pneumonia and meningitis, and we have stratified the meta-analysis by age group—pediatric or adult as defined in Table 1—of the population. While it would be preferable to use smaller age sub-groups, we did not have sufficient data for such an analysis. To further evaluate whether serotype is associated with outcome independent of age, we used data from the Dutch study [20] and the multicenter study [9] in logistic regressions to calculate the odds ratio (OR) of death for each serotype compared to type 14 and performed the regression either with or without a predictor for subject age (cubic spline). We found that adjusting for age did not greatly affect the ORs in the two studies we examined (Figure S1), suggesting that serotype independently affects disease outcome, and this is consistent with published findings [11].
Table 1.
Characteristics of studies included in the meta-analyses.
| Location | New York† [35] | Chicago [36] | Multicenter†* [9] | The Netherlands* [20] | Denmark* [11] | Kenya* (unpub) | Germany * [12] | Israel * (unpub) | [37] The Gambia*‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study years | 1952–1962 | 1967–1970 | 1998–2001 | 2004–2006 | 1997–2007 | 1994–2008 | 1997–2003 | 1999–2006 | 2000–2004 | |||||
| Age years | 52 | 69.4 | 1.75 | 1.5 | 1.16 | 0.98 | ||||||||
| median (range) | (> 12yrs) ¶ | (> 14 yrs)§ | (14–97) | (18–100) | (≥ 12) | (< 12) | (0–12) | (0–15) | (0–15.7) | (0.2–2.5) | ||||
| # Cases included | 321 | 204 | 582 | 841 | 5374 | 137 | 436 | 264 | 66 | 28 | ||||
| Pneumonia (dead/total) serotype | ||||||||||||||
| 1 | 6/78 | 6/21 | 10/75 | 5/60 | 53/853 | - | 7/82 | 0/15 | - | 0/1 | ||||
| 3 | 18/35 | 9/18 | 13/55 | 14/53 | 98/330 | - | 4/9 | 0/3 | - | - | ||||
| 4 | 8/43 | 7/33 | 4/68 | 14/88 | 63/587 | - | 2/11 | 0/3 | - | 0/1 | ||||
| 5 | 2/23 | 3/22 | 2/27 | 0/5 | 4/49 | - | 1/18 | - | - | 1/6 | ||||
| 6A | 3/10 | 1/5 | 3/18 | 2/22 | 31/134 | - | 3/24 | 0/2 | - | 0/1 | ||||
| 6B | - | - | 9/35 | 5/16 | 33/135 | - | 4/30 | 0/4 | - | 1/3 | ||||
| 7F | - | 4/20 | 0/14 | 10/115 | 41/482 | - | 0/1 | 1/7 | - | - | ||||
| 8 | 8/54 | 2/33 | 0/19 | 9/73 | 35/347 | - | 0/2 | - | - | - | ||||
| 9N | 2/6 | 4/14 | 2/11 | 4/17 | 50/210 | - | - | - | - | - | ||||
| 9V | - | - | 8/30 | 11/85 | 43/396 | - | 1/7 | 0/2 | - | 1/1 | ||||
| 12F | 7/32 | 3/21 | 1/22 | 0/8 | 31/214 | - | 2/6 | - | - | - | ||||
| 14 | 1/14 | 1/7 | 13/70 | 18/123 | 95/605 | - | 3/32 | 1/31 | - | 1/6 | ||||
| 19A | - | - | 3/20 | 3/28 | 27/111 | - | 2/18 | 0/4 | - | 0/4 | ||||
| 19F | 6/14 | 1/5 | 9/28 | 4/17 | 41/115 | - | 5/12 | 0/2 | - | 0/1 | ||||
| 22F | 1/6 | 0/5 | 2/12 | 2/13 | 21/158 | - | - | - | - | - | ||||
| 23F | 3/6 | - | 7/37 | 6/49 | 38/184 | - | 5/20 | 1/3 | - | 2/4 | ||||
| Meningitis (dead/total) serotypel | ||||||||||||||
| 1 | - | - | 5/6 | 6/34 | - | 27/71 | 0/5 | 1/8 | - | |||||
| 6A | - | - | 1/3 | 2/5 | 8/32 | 0/11 | 7/17 | 1/5 | 1/7 | - | ||||
| 6B | - | - | 0/3 | 0/4 | 7/21 | 3/45 | 8/16 | 2/24 | 0/9 | - | ||||
| 7F | - | - | 1/3 | 1/8 | 11/59 | 1/32 | 1/2 | 4/15 | 0/2 | - | ||||
| 8 | - | - | 1/1 | 3/8 | 9/38 | - | - | 0/1 | 0/2 | - | ||||
| 9N | - | - | 0/1 | 2/4 | 6/28 | - | - | 0/5 | - | - | ||||
| 9V | - | - | 1/4 | 2/5 | 15/39 | - | 1/2 | 1/10 | 0/2 | - | ||||
| 12F | - | - | 4/5 | 1/3 | 18/76 | - | 3/6 | - | 0/5 | - | ||||
| 14 | - | - | 1/5 | 0/6 | 12/44 | 1/35 | 7/20 | 7/72 | 1/9 | - | ||||
| 19A | - | - | - | 6/15 | - | 1/3 | 0/10 | 0/3 | - | |||||
| 19F | - | - | 1/3 | 3/7 | 5/33 | 0/14 | 4/11 | 1/21 | 0/5 | - | ||||
| 23F | - | - | 2/5 | 6/15 | 11/45 | - | 6/16 | 2/20 | 1/14 | - | ||||
Additional data provided by investigators.
Pneumonia without extrapulmonary focus.
Unvaccinated controls only.
9.6% of cases 12–29 years old, 38.8% were 30–49 years, 33.8% were 50–69 years, 17.8% were 70+ years.
11.5% of cases were 14–29 years old, 49.6% were 30–49 years old, 33.2% were 50–69 years old, 5.7% were > 70 years old. The data from New York, Chicago, Kenya, and the multicenter study come from hospital studies. Data from The Netherlands, Denmark and Germany come from surveillance systems in the respective countries. The data from The Gambia comes from the control arm of a vaccine trial.
In an effort to evaluate the influence of host comorbidities, we compared RRs from adult Danish cases with no known comorbidities with the overall pooled risk ratios, which were recalculated to exclude these cases.
Spearman’s rank correlations were used to compare the pooled RRs with carriage prevalence and invasiveness data and with in vitro measurements.
Heterogeneity between studies was evaluated using the I-squared approach, which determines the percent of variability between studies that can be attributed to true heterogeneity rather than random variation [21]. It has been suggested that an I2 value of less than 25% reflects low levels of heterogeneity [21].
RESULTS
Risk of death from pneumonia is a stable serotype-associated property
We analyzed 9 studies (summarized in Table 1) drawn from the United States, Europe, Africa and the Middle East covering years between 1952 and the present. Five of the datasets included pediatric cases.
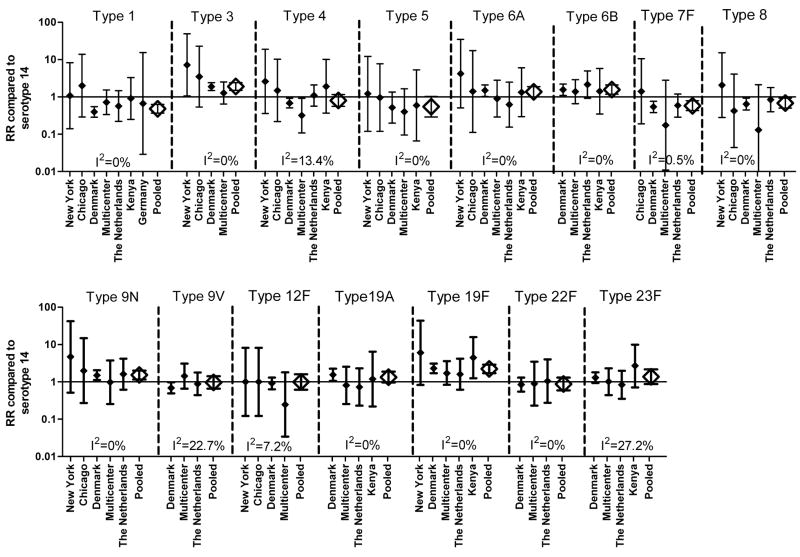
Among bacteremic pneumonia cases, we found significant differences in the pooled RRs between serotypes (Figure 1, Table S1). Overall, cases of pneumonia caused by types 3, 6A, 6B, 9N, and 19F were significantly more likely to be associated with death than those cases with type 14. Additionally, for types 19A and 23F, the RR was increased, albeit not statistically significantly. In contrast, cases infected with types 1, 7F, and 8 were significantly less likely to die than those infected with type 14, and the RRs for types 4 and 5 were also decreased but not significantly so. In most instances, we did not observe a significant difference between the RR estimates among adults and children (Table S1), though the estimates for children were less precise due to the relatively small number of pediatric deaths included in this meta-analysis. For some serotypes the RR appeared to be substantially higher among children than among adults. However, this could be attributed, in part, to the bias introduced in the small pediatric studies by the correction factor of adding 0.5 to the components of the risk ratio in instances when there were no fatalities. This correction leads to the appearance of an artificially large RR for serotypes with few isolates. All of the serotypes exhibited low levels of heterogeneity between studies, based on the I2 values, with the exception of types 9V and 23F, which exhibited moderate levels of heterogeneity (Figure 1, Table S1).
Figure 1.
Study specific and pooled risk ratios (RRs) for death from bacteremic pneumonia compared to serotype 14. Closed diamonds represent study-specific RR+/−95% CI. Open diamonds represent the pooled RR+/−95% CI. I2 denotes the amount of variation in the RR due to heterogeneity. Only studies with ≥ 10 isolates of the serotype are shown though all studies were used to calculate the RR and evaluate heterogeneity.
Among meningitis cases, we found that the differences in the RRs between serotypes were less pronounced than for pneumonia, and there was no serotype for which the RR was significantly different from 1 (Table S2).
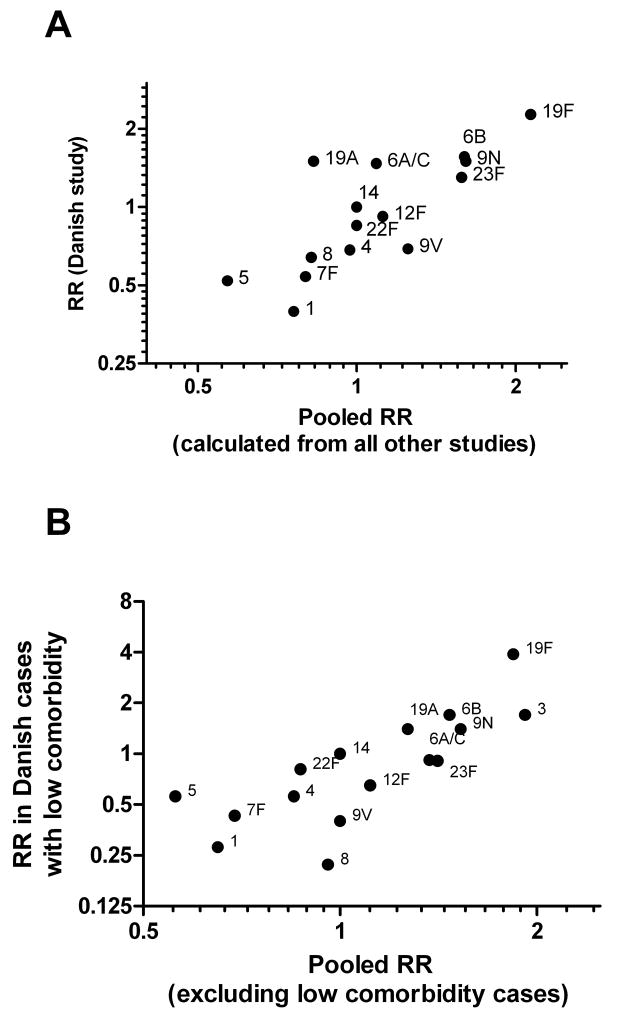
Given that nearly 70% of the cases used in the pneumonia meta-analysis were derived from the Danish study, we considered whether the pneumonia RRs could be dominated by the inclusion of this heavily weighted study. We recalculated the pooled effect estimates without the Danish data and found a strong correlation between the RRs calculated without the Danish data and the Danish estimates alone (Figure 2A, ρ = 0.82, p < 0.001), indicating that the estimates are not unduly influenced by the inclusion of this large study.
Figure 2.
(a) Comparison of RRs calculated among Danish adult bacteremic pneumonia cases compared to the pooled RR from all other studies. (b) Comparison of RRs calculated among adult Danish bacteremic pneumonia cases with no known comorbidities with the overall pooled RRs representing all studies except for the Danish low-comorbidity cases.
Pooled pneumonia estimates correlate with RRs from cases with no known comorbidities
Host comorbidities could affect the outcome of IPD and may make it appear that serotypes that are more frequently found in cases with pre-existing conditions are more virulent [6, 9]. As a result, we evaluated whether cases without known co-morbidities would have a similar pattern of disease severity between serotypes compared to the overall pooled RRs. Approximately 47% of the adult bacteremic pneumonia cases in the Danish study did not have a diagnosed co-morbidity. We calculated RRs from these cases and also recalculated the overall pooled RRs to exclude these cases with no known comorbidities. There was a strong correlation (Figure 2B, ρ = 0.83) between the RRs of the cases with no known comorbidites and the recalculated overall pooled RRs, indicating that the RRs are unlikely to be strongly biased by host comorbidities and could reflect true differences between serotypes.
Serotype-specific RR is associated with carriage prevalence, invasiveness and degree of encapsulation of the infecting serotypes
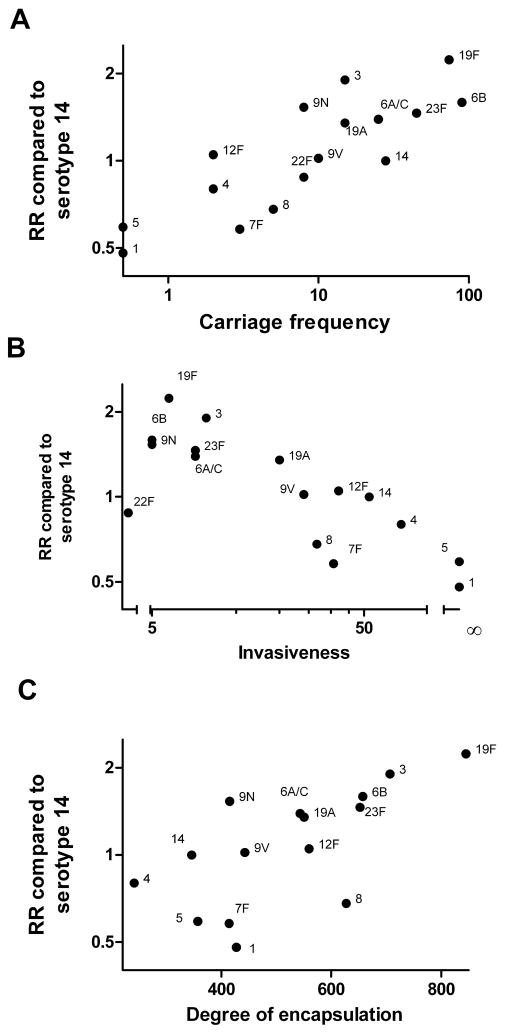
Next, we compared the pooled RRs from bacteremic pneumonia cases with serotype specific carriage prevalence. The pooled RRs also correlated with pre-PCV7 carriage prevalence data from studies in England (< 2 years, ρ = 0.78, p < 0.001) [22], The Gambia (9–15 month placebo controls, ρ = 0.67, p < 0.01) [23], The Netherlands (< 20 years old, ρ = 0.66, p < 0.01) [24], USA (< 7 years old, ρ = 0.67, p < 0.01) [25], and Canada (most < 5 years old, ρ = 0.71, p < 0.01) [26]. Among children from Kenya, this association was stronger in < 5 year olds (ρ = 0.64, p < 0.01) than in older children from the same population (ρ = 0.46, ns) [27].
Interestingly, serotypes with high carriage prevalence and high RRs tended to be less invasive [22]. Finally, we compared the pooled RRs for bacteremic pneumonia with in vitro measurements of capsule size and found that heavily encapsulated serotypes tended to be associated with increased RRs (ρ = 0.70, p < 0.01; Figure 3).
Figure 3.
Relationship between serotype-specific RRs and epidemiologic and microbiological measures. Serotype specific RR among pneumonia cases is related to (a) carriage prevalence in a pediatric study in England (number of nasopharyngeal isolates) [22], (b) invasiveness [22], and (c) degree of encapsulation (area in pixels) [18]. There were no carriage isolates for types 1 or 5.
DISCUSSION
In this study, we found that among bacteremic pneumonia cases, the risk of death varies by serotype, and, at least in adults, is stable between studies across time and in diverse geographic locations. Additionally, we found that the RR estimates were directly correlated with the carriage prevalence of the serotypes and inversely correlated with invasiveness. Finally, the serotypes that were more frequently associated with fatal outcomes tended to be more heavily encapsulated in vitro. While it was long ago suggested that there is a link between the degree of encapsulation and virulence [7], this is the first time, to our knowledge, that such a comparison has been formally made across a large number of serotypes.
The findings of this study suggest a potential mechanism for the epidemiologic relationships between serotypes. More heavily encapsulated types tend to be more prevalent among pediatric carriage isolates and are less likely to cause bacteremic invasive disease, but they tend to cause more severe disease when they do invade. The thick capsule could allow the bacterium to persist in the nasopharynx, lungs, and blood by protecting against host immune effectors. However, a large capsule could hinder the invasion process itself since invasion into tissues or the bloodstream could involve either direct transcytosis across epithelial cells [28] or the induction of an inflammatory response which disrupts the epithelial barrier [29]. In either case, a heavily encapsulated strain would interact with the host less efficiently and might be less likely to cross the epithelium.
We excluded several pre-antibiotic era studies that evaluated the relationship between serotype and disease outcome [29–31]. These studies found a pattern similar to what we report here, with type 3 having a higher CFR and types 5 and 14 having lower CFRs, and this is despite major changes in diagnostic procedures since that time. Our results are also consistent with some other individual studies that have addressed this topic but did not meet our inclusion criteria ([6, 32, 33], Table S3).
Bacterial factors in addition to capsule likely contribute to disease severity. Animal studies demonstrate different strains of the same serotype differ in virulence [34]. Consequently, regional differences or changes over time of the circulating clones could influence the virulence associated with a specific serotype.
The measurements of capsule size described here were performed on strains created in the laboratory and then isolated from the noses of mice and were confirmed using clinical carriage isolates (data not shown). It is possible, though, that among disease isolates, the capsule size measurements would follow a different pattern.
While we have focused on the importance of microbiological characteristics, host factors also have an important role. A number of studies have found that serotype independently affects disease outcome, while others have suggested that host factors were more important [6, 9–12]. While we did not have sufficient data to adjust for age and co-morbidity prior to pooling the risk-ratios, we found good agreement between our pooled RRs and estimates from pneumonia cases that have no known comorbidities.
The relatively low fatality rate in children makes estimating the pooled RRs difficult. However, in many instances where there was sufficient data, the directions of the RRs were the same in adults and children. In order to generalize our findings to neonates, where there is a high burden of disease, or to subsets of cases with specific syndromes, such as empyema, further study would be required among these groups.
Differences in socioeconomic status, access to healthcare, HIV prevalence, or inherent host differences could potentially affect the virulence of the serotypes and could contribute to the heterogeneity that we see between study populations. However, while the absolute case-fatality ratios differ between studies, the directions of the RRs are similar in populations from North America, Europe, and Africa suggesting that serotype-associated factors have an independent effect on disease outcome.
Aside from age and chronic co-morbidities, recent infections with respiratory viruses could influence the risk of death from pneumococcal pneumonia. If specific serotypes have an increased tendency to infect individuals with recent respiratory illnesses, then it might appear that these serotypes are more virulent when in fact they might just have different host preferences
Antimicrobial resistance tends to be associated with serotype, leading to the possibility that differences in resistance patterns could influence outcome differences between serotypes. However, Denmark has extremely low levels of antimicrobial resistance (2–5%) [11], and we see similar mortality patterns in the Danish study and in the other datasets. This suggests that resistance is unlikely to lead to the mortality patterns observed between serotypes.
We found a notable difference in the patterns of disease outcome among pneumonia and meningitis cases. This could reflect the fact that bacteremic pneumonias encompasses a broad range of disease severities while meningitis is, by its nature, a more serious condition with a distinct pathophysiology, so serotype might have a different and less important role in this context. Additionally, there tends to be a higher prevalence of comorbidities among adult meningitis cases, which could overshadow difference between serotypes.
Overall, we did not find substantial heterogeneity between studies. However, there were some differences in the magnitude and direction of the estimates. Some estimates are based on small numbers and are subject to considerable uncertainty. In particular, death from IPD was relatively rare in the pediatric studies included in this review, so the 95% CIs are especially wide around these estimates. Additionally, our decision to use a single serotype as the reference group could account for some differences between studies. In most instances, the CFR for serotype 14 was similar to the mean from the study, but for Austrian [8], type 14 had a substantially lower CFR than the mean CFR for all cases, potentially biasing some estimates.
The results of this study support an important and stable role for serotype in determining the outcome of pneumonia. Given the correlations between serotype-specific disease outcome, carriage prevalence, and invasiveness, there is likely a common microbial explanation for these stable patterns. Understanding these patterns of disease severity and transmission will help to determine the potential benefits of using vaccines with increased coverage that might target serotypes associated with higher mortality.
Acknowledgments
The authors would like to thank Richard Malley for his critical reading of the manuscript and expert clinical advice. Thanks to all of the investigators who contributed to the original studies including Drs Shaheen Alanee, Victor Yu, and the International Pneumococcal Study Group, and the German Pneumococcal Study Group. Thanks to Yin Bun Cheung for providing additional carriage data from The Gambia.
Funding: DMW is supported by NRSA training program T32 A1007535. The study was supported in part by NIH grants R01 AI048935 to ML and R01 AI066304.
Footnotes
Author Contributions: JAS, KLO, ML, and DMW conceived of and designed the analysis. ZBH, EAS, MN, KPK, SR, RD, RA, and FC contributed raw data for the meta-analysis. DMW and HJ performed the literature review. DMW performed all analyses and wrote the manuscript, and all authors contributed to revisions.
Conflicts of interest: SR has received travel grants from Wyeth; EAMS reports receiving unrestricted grants from Wyeth and Baxter for research, consulting fees for Wyeth and GlaxoSmithKline, lecturing fees from Wyeth and grant support from Wyeth and GlaxoSmithKline for vaccine studies. ML has received honoraria or consulting income from Pfizer, Novartis, and the Avian/Pandemic Flu Registry (Outcome Sciences), sponsored in part by Roche. KPK has received consulting and research support from Pfizer Vaccines, and consulting for Merck and Novartis. The rest of the authors declare no conflicts.
References
- 1.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Micro. 2008;6(4):288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, de Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff William P, Bryant J, Paradiso Peter R, Siber George R. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part I. Clin Infect Dis. 2000;30(1):100–21. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien KL. Pneumococcal Regional Serotype Distribution for Pneumococcal AMC TPP. 2008 [cited; Available from: http://www.vaccineamc.org/files/TPP_Codebook.pdf.
- 5.Brueggemann AB, Peto TEA, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and Geographic Stability of the Serogroup-Specific Invasive Disease Potential of Streptococcus pneumoniae in Children. J Infect Dis. 2004;190(7):1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom K, Spindler C, Ortqvist A, et al. Clonal and Capsular Types Decide Whether Pneumococci Will Act as a Primary or Opportunistic Pathogen. Clin Infect Dis. 2006;42(4):451–9. doi: 10.1086/499242. [DOI] [PubMed] [Google Scholar]
- 7.Heffron R. Pneumonia: with special reference to pneumococcus lobar pneumonia. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- 8.Austrian R, Gold J. Pneumococcal Bacteremia with Especial Reference to Bacteremic Pneumococcal Pneumonia. Annals of Internal Medicine. 1964 May;60(5):759. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 9.Alanee S, McGee L, Jackson D, et al. Association of Serotypes of Streptococcus pneumoniae with Disease Severity and Outcome in Adults: An International Study. Clinical Infectious Diseases. 2007;45(1):46–51. doi: 10.1086/518538. [DOI] [PubMed] [Google Scholar]
- 10.Berg S, Trollfors B, Persson E, et al. Serotypes of Streptococcus pneumoniae isolated from blood and cerebrospinal fluid related to vaccine serotypes and to clinical characteristics. Scandinavian Journal of Infectious Diseases. 2006;38(6):427–32. doi: 10.1080/00365540500532852. [DOI] [PubMed] [Google Scholar]
- 11.Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal Serotypes and Mortality following Invasive Pneumococcal Disease: A Population-Based Cohort Study. PLoS Med. 2009 May 26;6(5):e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rückinger S, von Kries R, Siedler A, van der Linden M. Association of Serotype of Streptococcus pneumoniae With Risk of Severe and Fatal Outcome. Pediatric Infectious Disease Journal. 2009;28(2):118–22. doi: 10.1097/INF.0b013e318187e215. [DOI] [PubMed] [Google Scholar]
- 13.Martens P, Worm S, Lundgren B, Konradsen H, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infectious Diseases. 2004;4(1):21. doi: 10.1186/1471-2334-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992 January 1;60(1):111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLeod CM, Krauss MR. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950 July 1;92(1):1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magee AD, Yother J. Requirement for Capsule in Colonization by Streptococcus pneumoniae. Infect Immun. 2001 June 1;69(6):3755–61. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruickshank R. PNEUMOCOCCAL INFECTIONS. The Lancet. 1933;221(5716):563–9. [Google Scholar]
- 18.Weinberger DM, Trzcinski K, Lu Y-J, et al. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog. 2009 Jun 12;5(6):e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Jansen A, Rodenburg G, van der Ende A, et al. Invasive Pneumococcal Disease among Adults: Associations among Serotypes, Disease Characteristics, and Outcome. Clinical Infectious Diseases. 2009;49(2):e23–e9. doi: 10.1086/600045. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 September 6;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sleeman KL, Griffiths D, Shackley F, et al. Capsular Serotype-Specific Attack Rates and Duration of Carriage of Streptococcus pneumoniae in a Population of Children. J Infect Dis. 2006;194(5):682–8. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 23.Cheung Y-BP, Zaman SMAP, Nsekpong EDB, et al. Nasopharyngeal Carriage of Streptococcus pneumoniae in Gambian Children who Participated in a 9-valent Pneumococcal Conjugate Vaccine Trial and in Their Younger Siblings. Pediatric Infectious Disease Journal. 2009;28(11):990–5. doi: 10.1097/INF.0b013e3181a78185. [DOI] [PubMed] [Google Scholar]
- 24.Bogaert D, Sluijter M, Toom N, et al. Dynamics of pneumococcal colonization in healthy Dutch children. Microbiology. 2006 February 1;152(2):377–85. doi: 10.1099/mic.0.28394-0. [DOI] [PubMed] [Google Scholar]
- 25.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 Changes in Colonizing Pneumococcal Serotypes in 16 Massachusetts Communities, 2001 and 2004. Pediatrics. 2005 September 1;116(3):e408–13. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 26.Kellner JD, Ford-Jones EL Members of the Toronto Child Care Centre Study G. Streptococcus pneumoniae Carriage in Children Attending 59 Canadian Child Care Centers. 1999;153:495–502. doi: 10.1001/archpedi.153.5.495. [DOI] [PubMed] [Google Scholar]
- 27.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27(1):59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ring A, Weiser J, Tuomanen E. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102(2):347–60. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullowa J. Management of pneumonias. New York: Oxford University Press; 1937. [Google Scholar]
- 30.Finland M. Significance of specific pneumococcus types in disease, including Types IV to XXXII (Cooper) Annals of Internals Medicine. 1937;10:1531. [Google Scholar]
- 31.Raia A, NP, Shultz S. New types of pneumococci in the pneumonias of children. Am J Dis Child. 1931;42(1):57–68. [Google Scholar]
- 32.Henriques B, Kalin M, Ortqvist A, et al. Molecular epidemiology of Streptococcus pneumoniae causing invasive disease in 5 countries. J Infect Dis. 2000;182:833–9. doi: 10.1086/315761. [DOI] [PubMed] [Google Scholar]
- 33.Mirzanejad Y, Roman S, Talbot J, Nicolle L. Pneumococcal bacteremia in two tertiary care hospitals in Winnipeg, Canada. Chest. 1996;109:173–8. doi: 10.1378/chest.109.1.173. [DOI] [PubMed] [Google Scholar]
- 34.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60(1):111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60(5):759–76. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 36.Mufson MA, Kruss DM, Wasil RE, Metzger WI. Capsula types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974;134:505–10. [PubMed] [Google Scholar]
- 37.Antonio M, Dada-Adegbola H, Biney E, et al. Molecular epidemiology of pneumococci obtained from Gambian children aged 2–29 months with invasive pneumococcal disease during a trial of a 9-valent pneumococcal conjugate vaccine. BMC Infectious Diseases. 2008;8(1):81. doi: 10.1186/1471-2334-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]