Abstract
Biomaterial scaffolds that serve as vehicles for gene delivery to promote expression of inductive factors have numerous regenerative medicine applications. In this report, we investigate plasmid delivery from biomaterial scaffolds using a surface immobilization strategy. Porous scaffolds were fabricated from poly(lactide-co-glycolide) (PLG), and plasmids were immobilized by drying. In vitro plasmid release indicated that the majority (>70%) of adsorbed plasmid was released within 24 hours and >98% within 3 days; however, in vivo implantation of the scaffolds at the subcutaneous site yielded transgene expression that persisted for at least 28 weeks and was localized to the site of implantation. Histological analysis of DNA-adsorbed scaffolds indicated that macrophages at the scaffold were transfected in the first two weeks following implantation, whereas muscle cells adjacent to the implant primarily expressed the transgene at 4 weeks. In addition to localized gene expression, a secreted protein (human factor IX) was retained at the implant site and not available systemically after 3 days, indicating minimal off-target effects. These findings demonstrate that surface immobilization of plasmid onto microporous PLG scaffolds can produce localized and long-term gene expression in vivo, which may be employed to enhance the bioactivity of scaffolds used for regenerative medicine.
Introduction
Gene delivery from biomaterials has been used to induce local expression of tissue inductive or therapeutic factors for numerous regenerative medicine applications1–4. The biomaterial serves to maintain an elevated vector concentration locally, which provides enhanced opportunities for cellular transfection. Various approaches have been employed for vector delivery, including incorporation into gels5–7, encapsulation into micro- and nanoparticles8– 10, and the use of 3-D scaffolds3,11,12. Porous 3-D scaffolds are particularly attractive for regenerative medicine applications since they function to create and maintain a space for tissue growth and their porosity initially supports the diffusion of nutrients throughout the scaffold, which promotes cell infiltration, and thus integration with the host. Scaffolds can be made from biodegradable polymers, such as the copolymers of lactide and glycolide (PLG), and can thus avoid the need for a second surgical procedure to remove the scaffold. Porous scaffolds are being employed in numerous biomedical applications, such as islet transplantation, nerve and bone regeneration, vaccines, and dental tissue engineering13–16. The delivery of vectors from scaffolds can target cells transplanted on the scaffold17,18 or infiltrating host cells19, both of which can act as local bioreactors for the protein of interest. The expression of tissue inductive factors can be employed to promote cellular processes that lead to tissue regeneration or encourage cell engraftment 20.
The approaches used for scaffold-based gene delivery can be broadly classified into two categories: polymeric encapsulation and surface immobilization. In polymeric encapsulation, the vector is encapsulated or otherwise physically incorporated into the scaffold with degradation or hydration of the polymer resulting in release of the vector. This strategy, however, requires that the vector be integrated into the scaffold during fabrication, and thus it must remain stable throughout the processing steps. An alternative approach that avoids vector exposure to the polymer processing steps is surface immobilization. In this approach, vectors are immobilized to the material after the scaffold has been fabricated. Scaffolds are modified to interact with the vector to limit release and maintain elevated concentrations locally, with complexed DNA being primarily employed to mediate interactions between the vector and biomaterial21. For delivery of plasmids by an immobilization strategy, the extent and duration of transgene expression in vivo and the distribution of cells that express the transgene have not been well-characterized.
In this report, we investigate gene transfer in vivo using surface immobilization of naked plasmid to porous PLG scaffolds. Porous PLG scaffolds have been widely employed for numerous applications in regenerative medicine, and delivery of gene therapy vectors has the potential to enhance their bioactivity. Plasmid adsorption efficiency and release were measured in vitro and the magnitude, duration and location of transgene expression was monitored in vivo using a noninvasive imaging system. Immunohistochemistry was used to characterize the type and distribution of transfected cells over time. We also investigated whether the protein encoded by the plasmid would be restricted to the implantation site, thereby minimizing the potential for off-target effects. The studies will identify the utility of surface immobilization of plasmid in promoting localized and sustained transgene expression, which may provide a simple approach to enhance the bioactivity of scaffolds for regenerative medicine.
Results
In vitro plasmid release
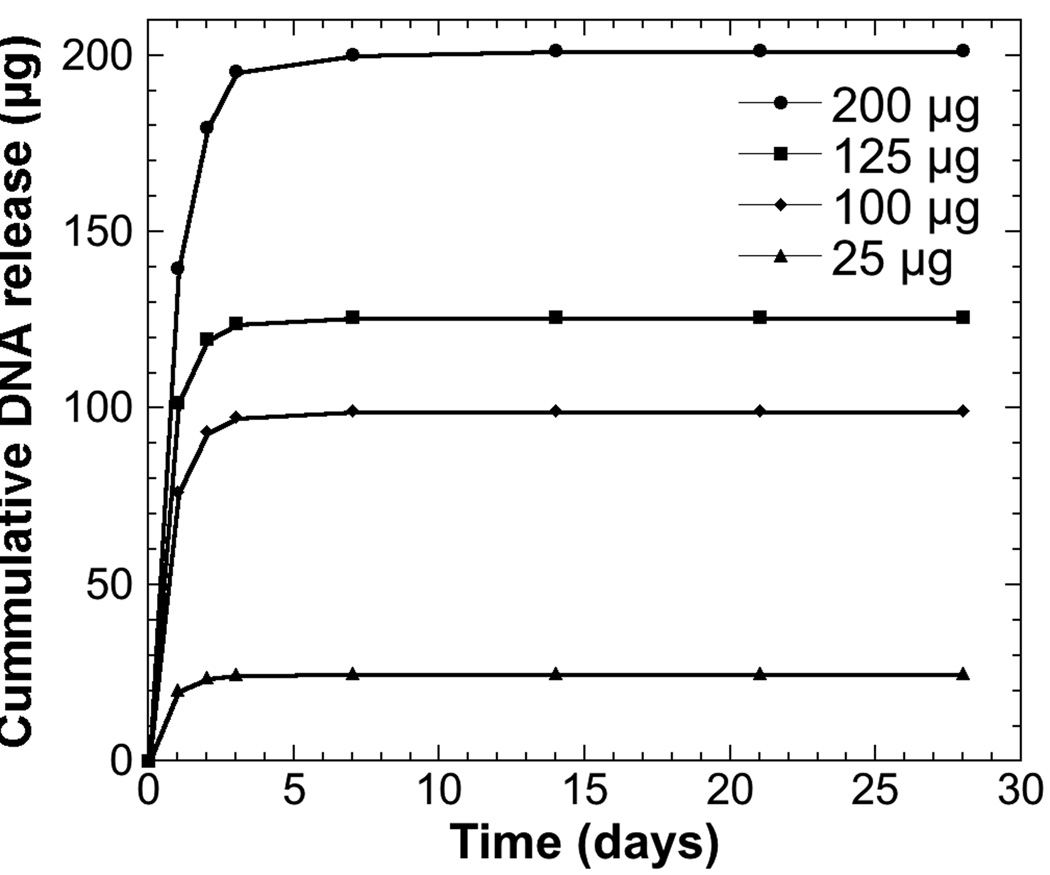
We initially investigated plasmid release as a function of the quantity of plasmid adsorbed to the scaffold. A large burst of plasmid was observed after 24 hours, with the amount of DNA released increasing with the amount adsorbed (Figure 1). Although the quantities released varied with the amount adsorbed, the percentage of plasmid released was similar for all conditions, ranging from 70.0 ± 3.9% to 81.4 ± 10.4%. After 3 days, greater than 98% of adsorbed plasmid had been released for all conditions. In all cases, the amount of plasmid released as a percent of total adsorbed was not significantly different at each time point among the four experimental conditions. At days 21 and 28, no plasmid was detected in the release media for any condition. Additionally, no plasmid was detected in the dissolved scaffolds after 28 days, indicating that all adsorbed plasmid had been released.
Figure 1.
Release kinetics of plasmid from microporous PLG scaffolds. Cumulative plasmid release from scaffolds adsorbed with 25 (●), 100 (■), 125 (◆) or 200 µg (▲) of plasmid (n = 3 per dose). Values are reported as mean ± SEM.
In vivo luciferase expression
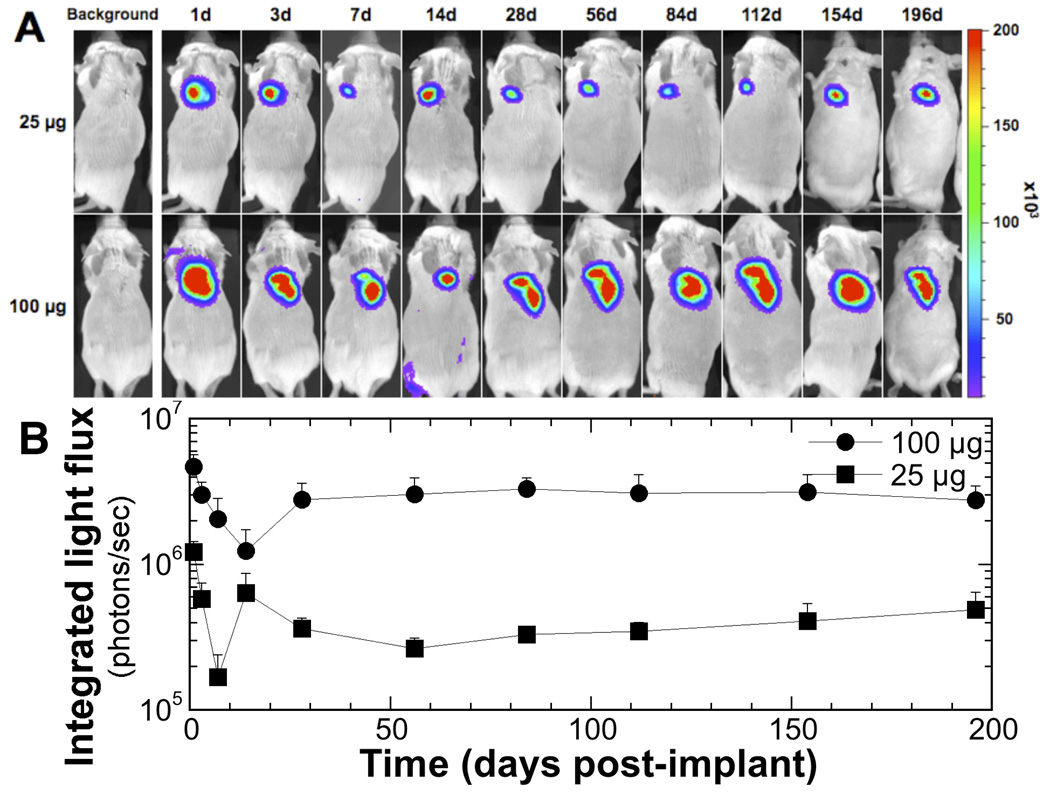
Scaffolds with adsorbed pLuc were implanted into the subcutaneous space, which induced luciferase transgene expression in vivo for at least 28 weeks (Figure 2A). Expression was observed in 100% of animals tested (n = 8) for each of the two doses of pLuc. The extent of expression was greater at the higher dose and light emission was localized to the implant site with no off-target foci of emission observed. For both plasmid doses, light emission decreased from the initial values at day 1, reaching the lowest values on days 7 and 14 (Figure 2B). In both cases, the photon flux subsequently increased. By day 28, the photon flux had returned to levels approximately 50% of those observed on day 1, and remained at this level for the remainder of the study. For 100 µg pLuc, the levels of light emission after day 28 was approximately an order of magnitude greater than the levels obtained with 25 µg pLuc. Mice implanted with scaffolds adsorbed with pbeta-gal or no DNA had measured light emission equivalent to background levels (<104 photons/sec; data not shown).
Figure 2.
In vivo transgene expression following implantation of plasmid-immobilized PLG scaffold. (A) Bioluminescence imaging and quantification of firefly luciferase expression in representative mice at 28 weeks following subcutaneous implantation of PLGA scaffolds immobilized with 25 (●) or 100 µg (■) of plasmid expressing luciferase (pLuc). (B) Integrated light flux (photons/s) as measured using constant-size ROIs over the implant site (n = 8 for both conditions). Values are reported as mean ± SEM. Control conditions not shown (n = 3; signal intensity at background levels; <104 photons/s).
Transfected cell distribution
The dynamic expression profile observed by bioluminescence imaging was subsequently investigated by characterizing the distribution and identity of transfected cells using antibodies to GFP (Figure 3). Transfection (i.e., positive staining for GFP) was observed at the scaffold (Figure 3A) and its interface with the host tissue (Figure 3B), and at a distance of approximately 100 µm from the scaffold (Figure 3C). To identify transfected macrophages, dual staining was performed with antibodies to GFP and F4/80 (Figures 3D-F).
Figure 3.
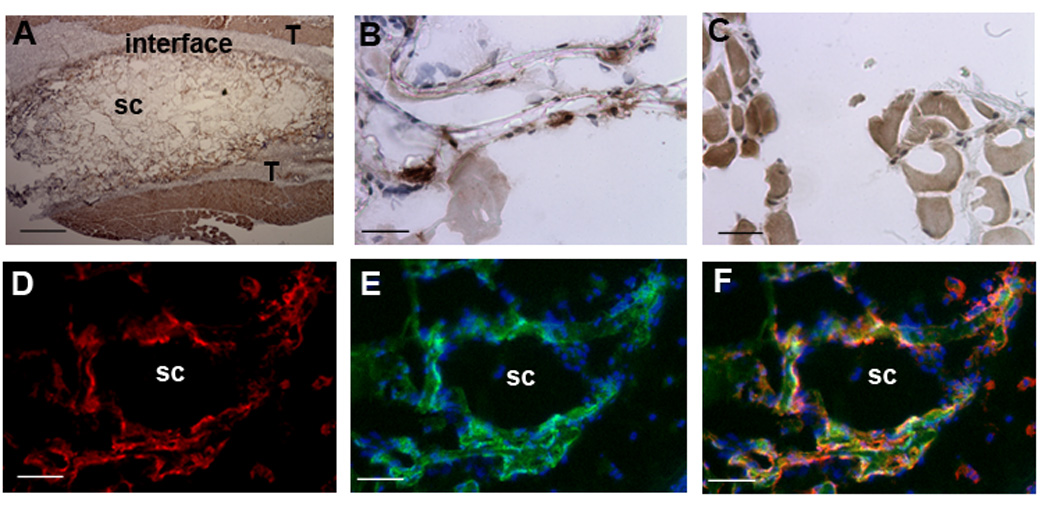
Transfection at the implant site. Images captured for sections stained with antibodies to GFP (A-C) using a biotinylated secondary antibody for HRP staining (brown), and (E-F) fluorescently labeled secondary antibody (green). (A) Image of the scaffold (sc) and surrounding tissue (T) at low magnification. Images captured within the scaffold (B) and at a distance of approximately 100 µm from the scaffold-host tissue interface (C). Sections were also stained with antibodies to F4/80 (D, red). An overlay of GFP (green) and Hoechst (blue, nuclei stain) (E), and (F) an overlay of panels D and E. Scale bars: A, 400 µm; B-C, 25 µm, D-F, 50 µm.
At the earliest time point, the greatest number of transfected cells were observed at the scaffold-tissue interface, with few transfected cells observed within the scaffold as the extent of cell infiltration at 3 days post-implantation was low (Figure 4A). Substantial staining for F4/80 was observed at the host-tissue interface (Figure 4B), and many of these cells were also GFP positive (Figure 4B). At 7 days post-implantation, numerous cells were observed within the scaffold, some of which were GFP-positive, and GFP-positive staining increased at the scaffoldtissue interface (Figure 4C). These GFP-positive cells within the scaffold and at the host tissue interface also stained positively for F4/80 (Figure 4D). By day 14, the extent of GFP-staining within the scaffold remained similar to levels observed at day 7, whereas the GFP-staining at the scaffold-host interface had decreased (Figure 4E). The number of cells that were stained with both GFP and F4/80 decreased from the numbers observed at day 7. On days 21 and 28, few GFP-positive cells were observed within the scaffold (Figures 4G-J), and the extent of GFP staining at the scaffold interface continued to decline, with few positive-staining cells remaining at 28 days. Samples from day 21 and day 28, however, were positive for F4/80 staining within or around the scaffold (Figures 3H and J). Staining of control scaffolds (no plasmid) with both primary and secondary antibodies for F4/80 and GFP did not indicate the presence of transfected macrophages, nor did staining of sections from experimental scaffolds in which the primary antibody was omitted (Figures 4 K and L).
Figure 4.
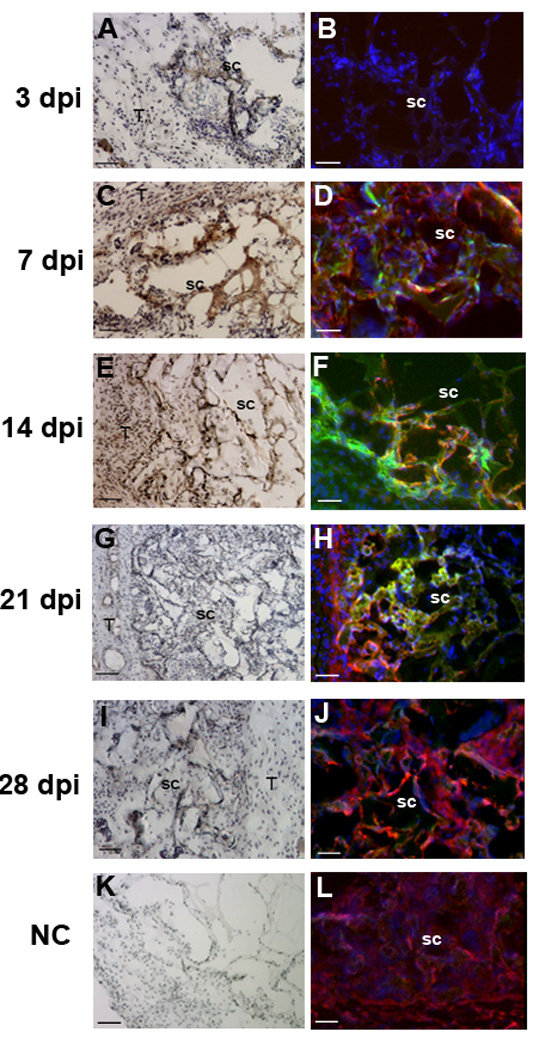
Transfection at the host-scaffold interface. Images captured within the scaffold (sc) and at the scaffold-host tissue interface (T). Samples were retrieved at 3 days (A,B); 7 days (C,D), 14 days (E,F), 21 days (G,H) and 28 days (I,J), which is indicated to the left of the panels. Sections stained with antibodies to GFP using a biotinylated secondary for HRP staining (A, C, E, G and I). An overlay of staining with immunofluorescence using antibodies to GFP (green) and F4/80 (red), along with Hoechst staining (blue) (B, D, F, H and J). (K,L) Negative controls with omission of primary antibody. For panels A, C, E, G, I and K, scale bars equal 100 µm. For panels B, D, F, H, J and L, scale bars equal 50 µm.
Transfected cells were also observed at a distance of approximately 100 µm from the scaffold, within tissue that morphologically appeared as muscle (Figure 5). At day 3, few isolated cells in this region stained positive for GFP (Figures 5A and B). By day 7, staining for GFP increased in this muscle layer (Figure 5C), which continued to increase through day 14 (Figure 5E). In samples from day 21 and day 28, strong GFP-positive staining within muscle fibers was evident (Figures 5G and I). Within the muscle layer, a small number of the GFP positive cells were also positive for F4/80, indicating that few of the transfected cells in the muscle layer were macrophages (Figures 5B, D, F, H and J).
Figure 5.
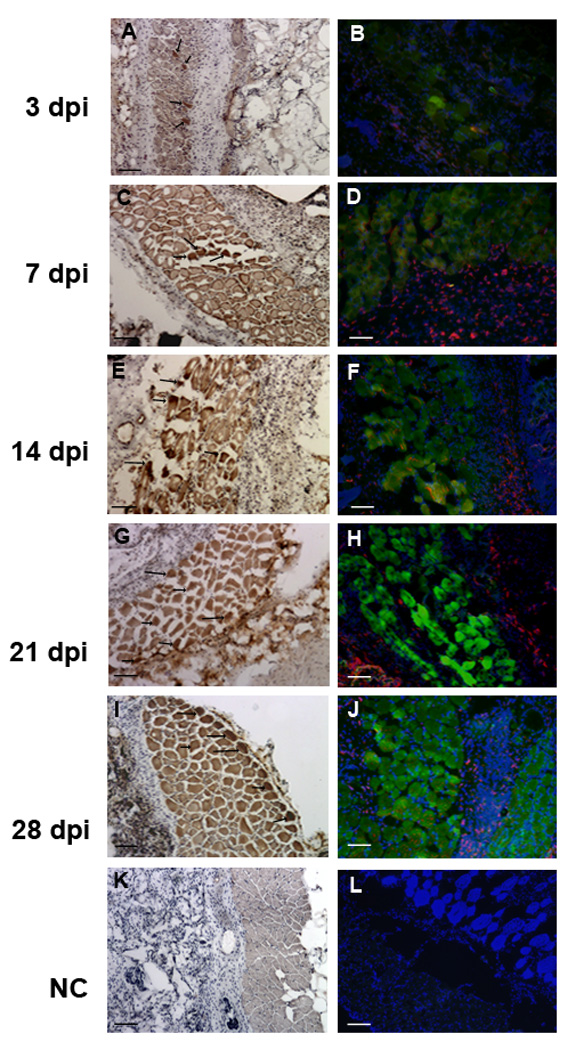
Transfection in the adjacent muscle tissue. (A-J) Images captured at muscle tissue adjacent to the scaffold. Samples were retrieved at 3 days (A,B); 7 days (C,D), 14 days (E,F), 21 days (G,H) and 28 days (I,J), which is indicated to the left of the panels. Sections stained with antibodies to GFP using a biotinylated secondary with HRP staining (A, C, E, G and I). An overlay of immunofluorescence images with antibodies to GFP (green) and F4/80 (red), along with Hoechst staining (blue) (B, D, F, H and J). (K,L) Negative control with omission of primary antibody. Arrows indicate several of the transfected cells. Scale bars equal 100 µm in all panels.
Localization of secreted protein
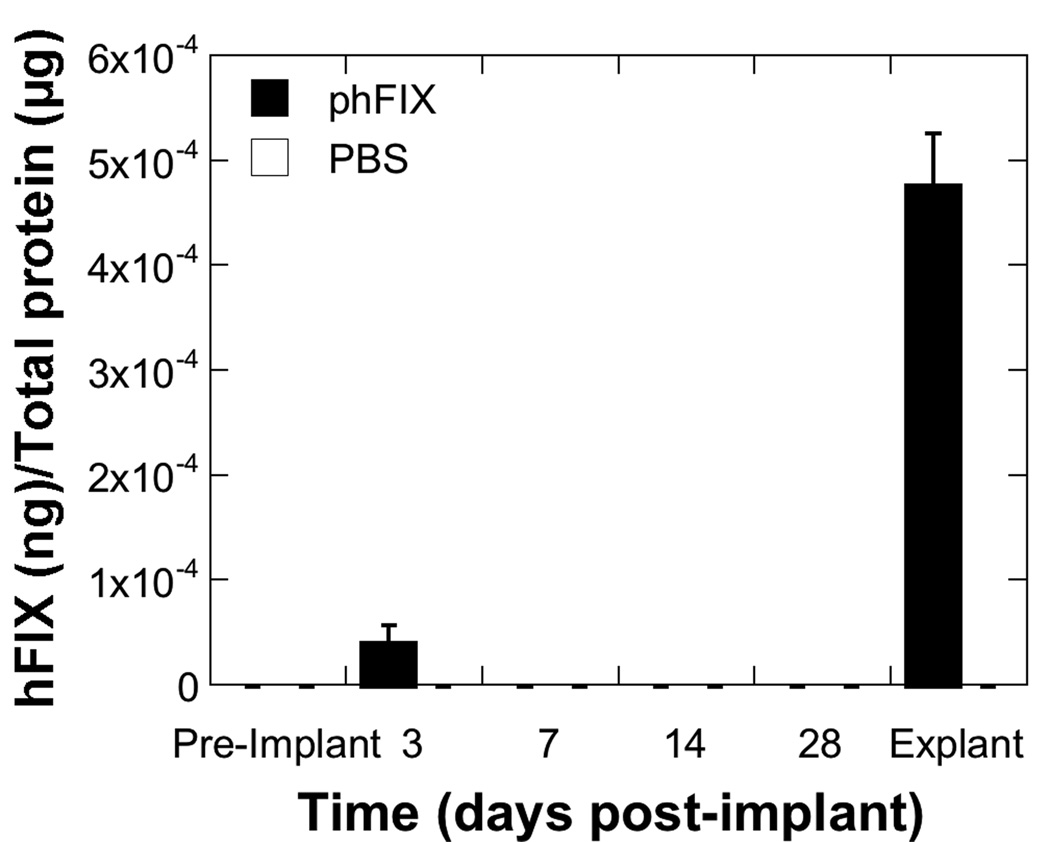
Although in vivo imaging data indicated that transgene expression remained localized to the implantation site, we investigated whether the protein produced locally would create substantial quantities only within the local microenvironment or achieve substantial quantities systemically. A plasmid encoding a humanized form of Factor IX, whose accumulation in the blood or in the tissue could be determined by ELISA, was immobilized to a scaffold and implanted subcutaneously. Scaffolds implanted with 200 µg of phFIX produced levels in blood equal to 4.1×10−5 ± 1.7×10−5 ng hFIX/µg total protein at day 3 (Figure 6), which was slightly above background levels. By day 7, the local levels of hFIX were below background levels that could be measured by the assay. hFIX was not detectable in the blood throughout the remainder of 28 day study. In tissue removed from the implant site on day 28 after implantation, the levels of Factor IX were 4.8×10−4 ± 4.9×10−5 ng hFIX/µg total protein. Taken together, these results indicate that the hFIX protein was maintained locally and did not substantially accumulate in the blood.
Figure 6.
Levels of hFIX in blood and tissue. Tail vein blood was collected from mice implanted with scaffolds adsorbed with 200 µg of phFIX or 100 µL of PBS prior to implantation and periodically thereafter (n = 5 for each condition). On day 28, scaffolds and a minimal amount of surrounding tissue were retrieved for hFIX measurement by ELISA. Values are reported as mean ± SEM.
Discussion
This report describes the delivery of plasmid, which was immobilized by a simple strategy, from a porous PLG scaffold at a subcutaneous implantation site. Regenerative medicine aims to create functional tissue replacements, typically by creating a controlled environment that promotes and directs the differentiation of stem or progenitor cells, either endogenous or transplanted. Scaffolds serve a central role in many strategies by providing the means to control the local environment, and gene delivery from the scaffold represents a versatile approach to manipulate the local microenvironment. Strategies to achieve controlled, localized gene expression within tissue engineering scaffolds have broad application in the regeneration of many tissues and have been applied to enhance vascularization, wound healing and promote bone and spinal cord regeneration11,22. Strategies for creating scaffolds capable of localized gene delivery usually require specialized equipment, yet we investigated a simple strategy that involves drying plasmid onto scaffolds following their fabrication, with localized release of the plasmid resulting in long-term and localized gene expression.
Long-term expression was observed following implantation of scaffolds with dried plasmid, which was unexpected given that previous systems have focused on encapsulating plasmid within the scaffold. Plasmid has been encapsulated within polymer microspheres23,24, and into microporous scaffolds11,12,21,22. Entrapment of plasmid resulted in in vitro release over approximately 7 days12, and promoted in vivo expression at both the subcutaneous19 and peritoneal fat sites22. For the dried plasmid, in vitro release studies indicate that most of the plasmid was released within 24 hours; however, transgene expression persisted in vivo for at least 28 weeks. Expression declined from the initial value by 5-fold during the initial 7 to 14 days, and then increased to levels that persisted through the remainder of the study. Interestingly, these scaffolds were not observed in histological sections after approximately 3-4 months in vivo 11, thus gene expression persists after the scaffolds have degraded. The scaffold degrades into lactic acid and glycolic acid, which are natural metabolites of the body. These materials are generally considered biocompatible25, as evidenced by the relatively mild foreign body response. Using scaffolds adsorbed with phFIX, hFIX was detected in tissue in and around the scaffold at 4 weeks post-implant, whereas it was undetectable in the blood after day 3. These results with non-viral delivery contrast with reports using delivery of viral vectors, which have been employed to obtain systemic levels of hFIX26. For regenerative medicine strategies, localized availability of protein is desirable to promote regeneration while avoiding off-target effects.
The transfection of macrophages at the implant site contributes to the expression dynamics during the first few weeks after implantation. Macrophage infiltration is one component of the foreign body response that is observed following implantation, and has been observed for these scaffolds previously 14,22. The fusion of macrophages to form foreign body giant cells is not readily observed with these scaffolds. Initially, after scaffold implantation, a large number of transfected macrophages were observed immediately adjacent to the scaffold, consistent with previous reports22. Macrophages are expected to be present based on their established role in wound healing and foreign body response. Regarding transfection, macrophages have cell-surface receptors that have been shown to recognize and promote the internalization of a variety of anionic macromolecules27,28. In this report, the number of transfected macrophages decreased with time, which corresponded to the 5-fold decrease in gene expression observed during the first two weeks post-implantation. The decline in transfected macrophages can potentially result from a number of mechanisms, such as silencing of the plasmid due to CpG motifs29, or turnover of the macrophages at this site30.
The initial decline in expression was reversed and stabilized by day 28, which corresponded with increased cellular transfection in the adjacent muscle tissue. Injection of naked plasmid directly into muscle has produced transgene expression that gradually declines over time scales of multiple months 23,31,32. In contrast, polymer microspheres with encapsulated plasmid injected into muscle produced transgene expression that increased with time23. In this report, an increased transfection of muscle was observed between days 3 and 21. Although transfected muscle tissue was observed at early time points, the extent of transfection was low and likely did not substantially contribute to the level of transgene expression. The increase in transfected muscle tissue is proposed as the mechanism by which expression increased after the minimum observed at day 7 or 14. Numerous reports have identified muscle for its ability to internalize plasmid and promote long-term transgene expression33–35; however, the increasing transfection of muscle in the present study was unexpected given the time scale over which the increase was observed (14 days) and the distance the released plasmid must diffuse from the scaffold (approximately 100 µm) to access muscle tissue. The increased transfection over time may result from the time required for plasmid to diffuse this distance. Interestingly, this prolonged duration of expression may be specific to the properties of PLG, as collagen or gelatin hydrogels that similarly deliver naked plasmid have been shown to result in substantially shorter expression36,37. These differences between the PLG and collagen scaffolds may result from processes such as cell infiltration or the foreign body response (e.g., macrophages), both of which impact the cell population and its activation state that would access the released plasmid. The ability to transfect muscle tissue by implantation of scaffolds in the adjacent tissue may be useful in selecting target sites for cell transplantation, in which inductive factors may be produced long-term to promote graft survival and function.
Microporous PLG scaffolds adsorbed with plasmid resulted in in vivo gene transfer at a subcutaneous site for up to 28 weeks. Increasing plasmid dose increased the magnitude and area of expression around the implant site. For both doses tested, transgene expression was highest immediately following implantation and decreased over the first 7 to 14 days but then rebounded and stabilized by day 28. Immunohistochemical analysis demonstrated a large number of transfected cells within and around the scaffold, which was well infiltrated by host cells by day 14. While muscle cells were transfected as early as day 3, and continued to express the transgene through at least day 28, transfected macrophages were initially abundant but decreased significantly over time and were no longer detected by 21 days post-implantation. Delivery of phFIX, a plasmid encoding a small, soluble protein factor normally found in blood, resulted in localized expression through day 28, but was not found systemically in the blood after day 3. These findings indicate that adsorption of naked plasmid to the scaffold surface can induces localized gene transfer and long-term expression in vivo with expression remaining localized to the site of the implant.
Materials and Methods
Plasmid production
Plasmids were purified from bacterial culture using Qiagen (Santa Clara, CA, USA) reagents and stored in Tris-EDTA (TE) buffer at −20°C until use. All plasmids used in this study contain a cytomegalovirus (CMV) promoter. The plasmids pLuc (5.7 kb) and pbeta-gal (7.5 kb) contain the genes encoding firefly luciferase and beta-galactosidase, respectively, within the pNGVL vector backbone (National Gene Vector Labs, University of Michigan). The pEGFP-C2 plasmid (4.7 kb; Clontech, Mountain View, CA, USA) encodes a red-shifted variant of wild-type GFP, which has been optimized for brighter fluorescence and higher expression in mammalian cells. The plasmid phFIX (6.2 kb) was kindly provided by Dr. Michele Calos (Stanford University, Stanford, CA, USA) and encodes the human form of Factor IX.
Scaffold fabrication and vector immobilization
PLG microspheres were made as previously described12 using a single emulsion/solvent evaporation process and used as building blocks for scaffold fabrication. Briefly, poly (D,Llactide- co-glycolide) (PLG) (75:25 mole ratio of D,L-lactide-co-glycolide; i.v. 0.6 – 0.8; MW 92 – 142 kD) (Lakeshore Biomaterials, Birmingham, AL, USA) was dissolved in methylene chloride (Sigma, St. Louis, MO, USA) to make a 6% (w/v) solution. This solution was emulsified in an aqueous 1% (w/v) poly(vinyl alcohol) (PVA) (Acros Organics, Fair Lawn, NJ, USA) solution by homogenization at 7,000 rpm for 45 seconds. This homogenized solution was diluted in deionized (DI) water and stirred for 3 hours at room temperature to evaporate the organic solvent. Microspheres were collected by centrifugation (4,000 rpm for 10 minutes), washed three times with DI water to remove residual PVA, lyophilized to form a powder and stored in a desiccator under vacuum until use.
Scaffolds were fabricated by mixing 7 mg of PLG microspheres with 190 mg of sodium chloride (NaCl) crystals (250 µm < diameter < 425 µm), and loaded into a cylindrical stainless steel KBr die with an internal diameter of 5 mm (International Crystal Labs, Garfield, NJ, USA). The mixture was compression molded at 1500 psi for 30 seconds using a Carver laboratory press (Carver, Muncie, IN, USA). The compressed pellets were then incubated with 95% humidity at 37°C for 24 h to fuse the salt crystals into an interconnected structure. After incubation, the pellet was dried under vacuum and equilibrated with CO2 (800 psi) for 16 h in a custom-made pressure vessel. Release of CO2 resulted in fusion of the polymer microspheres into a continuous matrix. To create a porous structure, the salt was removed by immersing scaffolds in four changes of sterile water over 2 h. Leached scaffolds were dried overnight and stored in a vacuum desiccator until use. Prior to DNA adsorption, scaffolds were rehydrated by soaking in 70% ethanol for 30 seconds, then in PBS for 2 minutes. Scaffolds were placed on sterile gauze in a tissue culture hood to remove excess fluid, transferred to a petri dish, and then plasmid was added drop-wise to the scaffold in a maximum of 25 µL increments. Between deposition of each 25 µL increment, scaffolds were incubated at room temperature for 15 minutes. Upon complete addition of plasmid, scaffolds were dried inside the hood for 4 hours prior to transfer into sterile dishes and placement in a dessicator under vacuum until needed.
Characterization of plasmid release in vitro
Release kinetics in vitro were determined for scaffolds adsorbed with multiple quantities (25 – 200 µg) of pLuc. DNA-adsorbed scaffolds were placed in 500 µL of PBS (pH 7.4) in microcentrifuge tubes and incubated at 37°C. At the indicated time points, scaffolds were transferred to new tubes containing fresh PBS into which DNA was released (tubes containing released DNA were stored at −20°C until study completion). At the end of the study, any nonreleased DNA was collected by dissolving scaffolds in 600 µl of chloroform and vortexing for 15 seconds, followed by addition of 400 µl of TE buffer and additional vortexing for 1 min. This two-phase mixture was then centrifuged at >16,000×g for 3 minutes followed by transfer of the aqueous layer to a new microcentrifuge tube. Additional TE buffer was added to the organic phase and the above process repeated two more times to fully extract the DNA. The concentration of released DNA for all samples was measured using a NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA). Scaffolds not adsorbed with DNA were used as negative controls.
In vivo transgene expression
Transgene expression was monitored in vivo using the IVIS imaging system (Caliper Life Sciences, Hopkinton, MA, USA). Scaffolds with adsorbed pLuc were implanted subcutaneously at the mid-thoracic region of male CD-1 mice (20 - 22 g) (Charles River Laboratories, Wilmington, MA, USA). Scaffolds adsorbed with 100 µg of pbeta-gal or no DNA were used as negative controls. For imaging, animals were injected intraperitoneally with D-luciferin potassium salt (150 mg/kg body weight; Caliper Life Sciences) dissolved in PBS. Following injection, animals were placed in a light-tight chamber and images were acquired every 2 minutes until the peak light emission was determined. Gray-scale photographs and bioluminescence images were superimposed using Living Image v2.6.1 software (Caliper Life Sciences). To quantify light output, constant size regions of interest (ROI) were drawn over the implantation site and the signal intensity reported as an integrated light flux (photons/sec) after background levels were subtracted (background measurements were obtained using the same procedures described above but prior to injection of D-luciferin), which was determined by IGOR Pro software (WaveMetrics, Portland, OR, USA). All animal studies followed procedures approved by the Northwestern University Animal Care and Use Committee and in accordance with all National Institutes of Health animal-handling guidelines.
Histological analysis and immunohistochemistry
Histological analysis was performed to determine the location and type of cells transfected following implantation of plasmid-immobilized scaffolds. PLG scaffolds were immobilized with 100 µg of pEGFP-C2. Tissue was collected by necropsy, snap-frozen in an isopentane bath cooled on dry ice, and stored at −80°C until needed. Prior to sectioning, samples were embedded in O.C.T. compound (Sakura Finetek, Torrance, CA, USA) and 14 µm sections were cut with a cryostat. The sections were mounted onto microscope slides and stored at −80°C.
Immunohistochemistry was performed to visualize the location of transfected cells. Multiple sections per sample were stained with polyclonal rabbit anti-GFP (1:800 dilution; Invitrogen, Carlsbad, CA, USA) in conjunction with an appropriate biotinylated secondary antibody. Briefly, sections were fixed in 4% PFA, after which endogenous peroxidases were blocked with 0.3% H202 in 100% methanol for 30 min. Following incubation with the primary antibody, the secondary antibody was applied and GFP-positive cells were visualized using avidin-biotin immunoperoxidase staining with a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) using 3,3’-diaminobenzidine (Vector) as the chromogen. Routine H&E staining was performed on selected sections to determine the extent of cellular infiltration and general tissue morphology.
The identity of transfected cells was investigated using double-immunofluorescence staining. Transfected cells were visualized by anti-GFP (1:500) antibody and an antibody directed against the macrophage surface marker, F4/80 (1:100; AdD Serotec, Raleigh, NC, USA)22. After blocking in a 10% solution of serum in which the relevant secondary antibody was made, the two primary antibodies, rat anti-F4/80 and rabbit anti-GFP, were applied simultaneously to the sections for 2 h at room temperature. Secondary antibodies (Alexa Fluor 546 conjugated to goat anti-rat (1:500 dilution; Invitrogen) and Alexa Fluor 488 conjugated to goat anti-rabbit (1:500 dilution; Invitrogen) were used to visualize the antigens. Lastly, sections were incubated with Hoechst 33258 (10 mg/mL; 1:2000 dilution; Invitrogen) for 5 min to identify cell nuclei. Negative controls included sections in which the primary antibody had been omitted and scaffolds not containing the GFP plasmid, for each time point. Digital images were acquired using a Spot camera via the accompanying image analysis software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) attached to a Nikon Eclipse 50i microscope (Nikon, Tokyo, Japan).
Characterization of protein distribution
The presence of the gene product locally and systemically was investigated using scaffolds immobilized with a plasmid encoding for a human-specific form of Factor IX (phFIX), a clotting factor normally found in blood. Scaffolds adsorbed with 200 µg of phFIX were subcutaneously implanted in the same manner described above. At the indicated times, blood was collected from the tail vein using EDTA-coated capillary tubes (Iris Sample Processing, Westwood, MA, USA). Following centrifugation, the plasma fraction was collected and analyzed for hFIX by ELISA38. To assess levels of hFIX at the implantation site, scaffolds and their surrounding tissue were removed at the last time point. Tissue samples were weighed and then homogenized in 10 mL/g of Tissue-PE LB Buffer (G-Biosciences, St. Louis, MO, USA) containing a protease inhibitor cocktail (G-Biosciences) and 5 mM EDTA. The tissue lysate was centrifuged at 20,000×g for 30 minutes at 4°C and the supernatant collected for determination of hFIX concentration in the same manner described above. Total protein concentration was determined for each sample using a BCA assay (Pierce, Rockford, IL, USA). Values are reported as a ratio of the amount of hFIX (ng) to total protein content (µg) in each sample. Scaffolds wetted with PBS prior to implantation were used as negative controls.
Statistical analysis
All values are reported as the mean ± SEM. Statistical calculations were performed using KaleidaGraph v4.03 (Synergy Software, Reading, PA, USA). Group comparisons were performed using a Student’s t-test and a P-value of less than 0.05 was considered statistically significant.
Acknowledgments
The authors are grateful to Prof. Michele Calos for helpful discussions and the Factor IX construct. Financial support for this research was provided by NIH RO1EB003806 and RO1EB005678.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Chandler LA, Gu DL, Ma C, Gonzalez AM, Doukas J, Nguyen T, et al. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000;8:473–479. doi: 10.1046/j.1524-475x.2000.00473.x. [DOI] [PubMed] [Google Scholar]
- 2.Endo M, Kuroda S, Kondo H, Maruoka Y, Ohya K, Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489–497. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 3.Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, et al. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2006;27:1095–1103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Pan H, Jiang H, Chen W. Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials. 2006;27:3209–3220. doi: 10.1016/j.biomaterials.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Kong HJ, Kim ES, Huang YC, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res. 2008;25:1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 6.Wieland JA, Houchin-Ray TL, Shea LD. Non-viral vector delivery from PEG-hyaluronic acid hydrogels. J Control Release. 2007;120:233–241. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005;11:546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 8.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129:66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman K, Sarkar R, Raut S, Leong KW. Gene transfer to hemophilia A mice via oral delivery of FVIII-chitosan nanoparticles. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern M, Ulrich K, Geddes DM, Alton EW. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 2003;10:1282–1288. doi: 10.1038/sj.gt.3301994. [DOI] [PubMed] [Google Scholar]
- 11.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86:157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 13.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr., et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85:1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009;17:318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang G, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan R, et al. Stem/progenitor Cell-Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Ayoubi R, Eliopoulos N, Diraddo R, Galipeau J, Yousefi AM. Design and fabrication of 3D porous scaffolds to facilitate cell-based gene therapy. Tissue Eng Part A. 2008;14:1037–1048. doi: 10.1089/ten.tea.2006.0418. [DOI] [PubMed] [Google Scholar]
- 18.Sun XD, Jeng L, Bolliet C, Olsen BR, Spector M. Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials. 2009;30:1222–1231. doi: 10.1016/j.biomaterials.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Jang JH, Rives CB, Shea LD. Plasmid Delivery in Vivo from Porous Tissue-Engineering Scaffolds: Transgene Expression and Cellular Transfection. Mol Ther. 2005 doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J Periodontal Res. 1999;34:413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 21.Jang JH, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50–58. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rives CB, Rieux AD, Zelivyanskaya M, Stock SR, Lowe WL, Jr., Shea LD. Layered PLG scaffolds for in vivo plasmid delivery. Biomaterials. 2008 doi: 10.1016/j.biomaterials.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang JH, Shea LD. Intramuscular delivery of DNA releasing microspheres: microsphere properties and transgene expression. J Control Release. 2006;112:120–128. doi: 10.1016/j.jconrel.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nof M, Shea LD. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res. 2002;59:349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- 25.Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190:1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 27.Takagi T, Hashiguchi M, Mahato RI, Tokuda H, Takakura Y, Hashida M. Involvement of specific mechanism in plasmid DNA uptake by mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;245:729–733. doi: 10.1006/bbrc.1998.8521. [DOI] [PubMed] [Google Scholar]
- 28.Takakura Y, Takagi T, Hashiguchi M, Nishikawa M, Yamashita F, Doi T, et al. Characterization of plasmid DNA binding and uptake by peritoneal macrophages from class A scavenger receptor knockout mice. Pharm Res. 1999;16:503–508. doi: 10.1023/a:1018842210588. [DOI] [PubMed] [Google Scholar]
- 29.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 30.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 31.Meuli M, Liu Y, Liggitt D, Kashani-Sabet M, Knauer S, Meuli-Simmen C, et al. Efficient gene expression in skin wound sites following local plasmid injection. J Invest Dermatol. 2001;116:131–135. doi: 10.1046/j.1523-1747.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar N, Blomberg P, Wardell E, Eskandarpour M, Sylven C, Drvota V, et al. Nonsurgical direct delivery of plasmid DNA into rat heart: time course, dose response, and the influence of different promoters on gene expression. J Cardiovasc Pharmacol. 2002;39:215–224. doi: 10.1097/00005344-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 34.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 35.van Deutekom JC, Hoffman EP, Huard J. Muscle maturation: implications for gene therapy. Mol Med Today. 1998;4:214–220. doi: 10.1016/s1357-4310(98)01231-3. [DOI] [PubMed] [Google Scholar]
- 36.Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J Gene Med. 2002;4:634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]
- 37.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivares EC, Hollis RP, Chalberg TW, Meuse L, Kay MA, Calos MP. Site-specific genomic integration produces therapeutic Factor IX levels in mice. Nat Biotechnol. 2002;20:1124–1128. doi: 10.1038/nbt753. [DOI] [PubMed] [Google Scholar]