Abstract
Epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acid (AA) catalyzed by cytochrome P450 (CYP), have many essential biologic roles in the cardiovascular system including inhibition of apoptosis in cardiomyocytes. In the present study, we tested the potential of 8,9-EET and derivatives to protect pulmonary artery smooth muscle cells (PASMCs) from starvation induced apoptosis. We found 8,9-epoxy-eicos-11(Z)-enoic acid (8,9-EET analog(214)), but not 8,9-EET, increased cell viability, decreased activation of caspase-3 and caspase-9, and decreased TUNEL-positive cells or nuclear condensation induced by serum deprivation (SD) in PASMCs. These effects were reversed after blocking the Rho-kinase (ROCK) pathway with Y-27632 or HA-1077. Therefore, 8,9-EET analog(214) protects PASMC from serum deprivation-induced apoptosis, mediated at least in part via the ROCK pathway. Serum deprivation of PASMCs resulted in mitochondrial membrane depolarization, decreased expression of Bcl-2 and enhanced expression of Bax, all effects were reversed by 8,9-EET analog(214) in a ROCK dependent manner. Because 8,9-EET and not the 8,9-EET analog(214) protects pulmonary artery endothelial cells (PAECs), these observations suggest the potential to differentially promote apoptosis or survival with 8,9-EET or analogs in pulmonary arteries.
Keywords: 8,9-EET; PASMCs; Rho-kinase; apoptosis; 8,9-EET analog
Introduction
Similar to eicosanoid metabolites such as prostaglandin E2, prostacyclin and HETEs, epoxyeicosatrienoic acids (EETs) have been characterized as pro-angiogenic molecules in most cell types [1]. In the vasculature, the function of the EETs has been widely studied where they have been found to play important roles in modulation of vascular tone, proliferation and apoptosis [2–5]. 8,9-EET, one of four EET regioisomers, is a cytochrome P450 (CYP) metabolite synthesized from the essential fatty acid arachidonic acid [3]. EET formation is predominantly catalyzed by cytochrome P450 epoxygenases [6]. Products of these isoforms contribute to the pathophysiology of inflammation, cancer, and cardiovascular diseases. 8,9-EET has recently also been described as angiogenic in subcutaneous sponges implanted in mice [1,3].
Several signaling transduction pathways that activate 8,9-EET have also been elucidated. For example, protection of cardiomyocytes from apoptosis requires activation of the PI3K/Akt pathway [4]. However, the role of this or other downstream pathways in mediation of 8,9-EET induced protection of pulmonary artery smooth muscle cells has not been reported.
Rho-kinases (ROCKs) are the first and the best-characterized RhoA effectors [7]. Lipid messengers such as arachidonic acid (AA) are able to efficiently stimulate ROCK activity [8]. A lot of evidence has now been obtained regarding the role(s) of ROCK in vascular physiology, particularly in pulmonary arteries [7]. ROCK mediates hypoxia-induced decreases in endothelial NO synthase (eNOS) expression and phosphorylates phosphatase and tensin homologue (PTEN) to stimulate phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling in pulmonary endothelial cells [9–11]. Several studies indicate that activation of the RhoA/ROCK pathway contributes to both vasoconstriction and vascular remodeling in the lung [12]. The ROCK pathway may potentiate cardiovascular diseases through multiple mechanisms, including promotion of smooth muscle cells proliferation and inhibition of apoptosis. ROCK inhibitors, Y-27632 and fasudil, both attenuate pulmonary vessel vasoconstriction and reduce vascular remodeling in pulmonary arterial hypertension [13,14].
As EETs may decrease inflammation and protect against apoptosis, they are attractive compounds for the treatment of some cardiovascular disorders. The half life of EETs is rather short; therefore, 8,9-EET derivatives with longer half lives may improve the therapeutic efficacy of these compounds. We hypothesized that some EET derivatives would have the same anti-apoptotic effect as natural 8,9-EET and that protection would depend upon activation of ROCK signaling pathway in pulmonary artery smooth muscle cells (PASMCs). To demonstrate our hypothesis, we examined the effects of 8,9-EET and 5 analogs on apoptotic endpoints such as MTT, apoptotic proteins (procaspase-3, Bcl-2, Bax and caspase-9) expression, nuclear morphology determination, mitochondrial membrane potential, caspase-3 activity and DNA nick end labeling (TUNEL). To our surprise, we found that the natural 8,9-EET afforded no protection against starvation to PASMCs, but one analog, 8,9-EET analog(214), (missing double bonds between carbons 5/6 and 14/15), did protect these cells. We further tested the contribution of ROCK signaling pathway in the protection of PASMCs from apoptosis by the 8,9-EET analog(214). Our results show that 8,9-epoxy-eicos-11(Z)-enoic acid (8,9-EET analog(214)) protects PASMCs apoptosis through ROCK pathway and modulation of starvation induced effects on mitochondrial function.
Material and methods
Materials
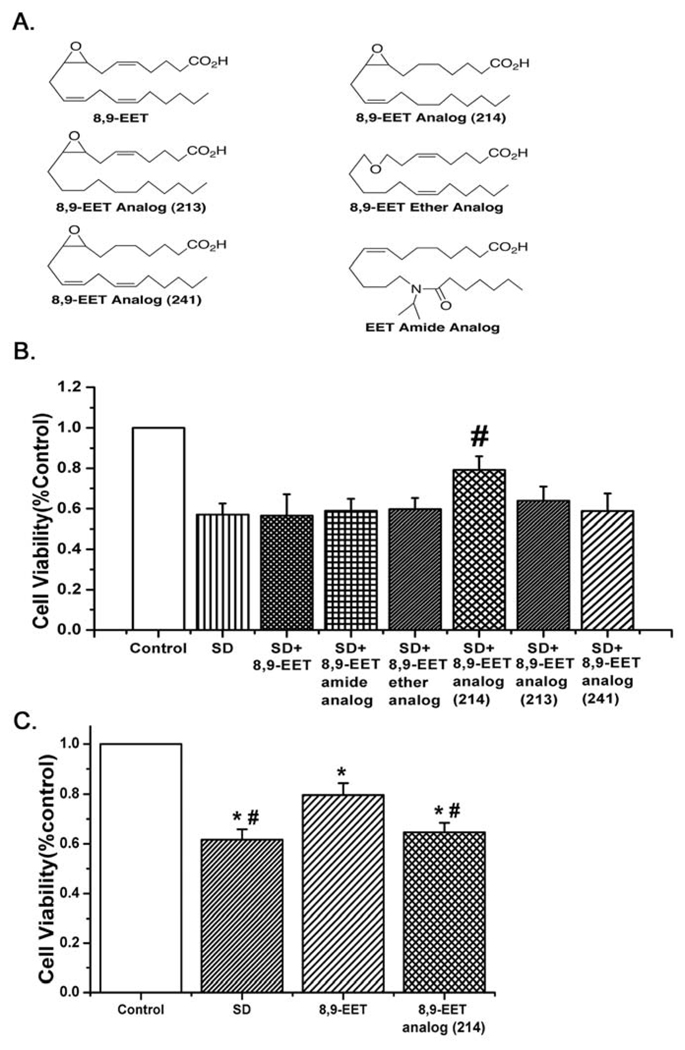
8,9-EET derivatives were synthesized in the laboratory of Dr. J. R. Falck. The structure of 8,9-EET and its derivatives are shown in Fig. 1A.
Fig. 1.
A: The structures of 8,9-EET and the analogs tested. B: Survival of control or serum deprived PASMCs treated with 8,9-EET and analogs. Only the 8,9-epoxy-eicos-11(Z)-enoic acid (8,9-EET analog(214)) improved survival (for each group n= 6; #p<0.05 compared with SD). C: Survival of starved pulmonary artery endothelial cells was increased by native 8,9-EET but not 8,9-EET analog(214) (n=12; *p<0.05 compared with Control; #p<0.05 compared with 8,9-EET).
Antibodies against Rac1, procaspase-3, Bcl-2, Bax, caspase-9 and β-actin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, Catalog # sc-95, Catalog # sc-7148, Catalog # sc-492, Catalog # sc-493, Catalog # 9507, Catalog # sc-1616-R, respectively). Rabbit anti-Cdc42, anti-MYPT1 and phosphor-specific antibodies against MYPT1 were from Cell Signaling Technology (Danvers, MA, Catalog # 2462, Catalog # 2634, Catalog # 4563). The polyclonal antibodies against ROCK I and ROCK II were obtained from Boster Biological Techology Co. Ltd (Wuhan, China, Catalog # BA1766, Catalog # BA1701). Caspase-3 activity kit (Catalog # C1116), JC-1 probe (Catalog # C2006), and the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) cell apoptosis detection kits (Catalog # C1086) were from Beyotime Institute of Biotechnology (Haimen, China). Enhanced chemiluminesence (ECL) reagents were obtained from Amersham International (Amersham, UK, Catalog # RPN2135). HA-1077 (Catalog No. 10010559) and Soluble Epoxide Hydrolase Cell-Based Assay kit (Catalog No. 600090) were from Cayman Company. Y-27632 was purchased from BIOMOL International (USA, Catalog # EI-299). All other reagents were from common commercial sources.
Animals
We used male Wistar rats (150–200 g) in the study. The rats were housed in the Animal Research Center of Harbin Medical University, which was fully accredited by the Institutional Animal Care and Use Committee (IACUC), at a controlled ambient temperature of 22 ± 2°C with 50 ± 10 % relative humidity and at a 12-h light–dark cycle (lights on at 8: 00 AM). Standard rat chow and water ad libitum were provided to all rats.
Cell Preparation and Culture
PASMCs were collected according to our previously published protocol. Cell viability as determined by Trypan Blue exclusion was consistently greater than 98%. The purity of PASMCs in the primary cultures was confirmed by the specific monoclonal antibody raised against smooth muscle α-actin (Boehringer Mannheim, Germany) and cells were cultured in 20% fetal bovine serum (FBS)-DMEM in a 37°C, 5% CO2 humidified incubator. Apoptosis in PASMCs was induced by serum deprivation, experimental cells were incubated in DMEM without serum for 24 or 48 hours. Passages 2–5 were used for further experimentation. Pulmonary artery endothelial cells were harvested and cultured per our previously published protocols [15].
MTT
PASMCs were plated in 96-well culture clusters (1 × 104 per well), after 24 hours growing, the cells were subjected to growth arrest for another 24 hours, and then the cells were placed in either complete medium (DMEM with 20% FBS) as control or switched to basal medium (DMEM) for the next 48 hours. Starved cells were treated with vehicle control, 8,9-EET analog(214) (1 µM) alone, Y-27632 (1 µM) alone, or Y-27632 (1 µM) plus 8,9-EET analog(214) (1 µM). The concentration of ethanol in the medium was less than 0.1% (v/v). Ethanol, Y-27632 and 8,9-EET analog(214) were added at the indicated concentration a second time 24 hours later. After 48 h of culture under test conditions (e.g. starvation with EET +/− inhibitors), cells were incubated with 0.5% of the yellow mitochondrial dye 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT), which was prepared in PBS at a concentration of 5 g/L and 20 µL per well, for 4 hours at 37°C. Then we added DMSO to the medium to terminate the MTT reaction followed by incubation for 10 min at 37°C. The absorbance was read at 540 nm in a spectrophotometer to assess for cell viability.
Western blot analysis
PASMCs in 6-well culture clusters were growth-arrested for 24 h, and then treated with vehicle, Y-27632(1 µM), 8,9-EET analog(214) (1 µM) in complete medium, and 8,9-EET analog(214) (1 µM), Y-27632 (1 µM) or 8,9-EET analog(214) (1 µM) plus Y-27632 (1 µM) in serum deprivation medium. Cells cultured in complete medium with vehicles served as controls. After this treatment for 24 h, the cells were lysed by lysis buffer (Tris 50 mM, pH 7.4, NaCl 150 mM, Triton X-100 1%, EDTA 1 mM, and PMSF 2 mM) and incubated for 30 min on ice. Then the lysates were sonicated and centrifuged at 12,000 r/min for 10 min, and the insoluble fractions were discarded. The protein concentrations in the supernatant were determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL) with bovine serum albumin (BSA) as a standard. Samples with 50 µg protein were subjected to 8% SDS-PAGE or 12% SDS-PAGE and then transferred to nitrocellulose membranes. After 1 hour incubation at 22–24°C in a blocking buffer (Tris 20 mM, pH 7.6, NaCl 150 mM, and Tween 20 0.1%) containing 5% nonfat dry milk powder, the membranes were reacted with appropriate antibody (ROCK I, ROCK II, procaspase-3, Bcl-2, Bax and Caspase-9) overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents.
RT-PCR
RNAs were extracted from PASMCs by using Trizol reagent and then determined by ultraviolet spectrophotometry (absorbance at 260nm/280nm). Total RNAs were reverse-transcribed using Superscript First-Strand Synthesis System for RT-PCR. Gene-specific primers were designed from coding regions and the nucleotide sequences of primers were as follows: ROCK2 (Genebank accession No. NM_013022.1): sense 5’-TTACTATGGACGAGAATGT-3’, antisense 5’-AGTTGCTGCTGTCTATGT-3’, 344bp; ROCK1 (Genebank accession No. NM_031098.1): sense 5’-AACCCAGGTAAAGGAACT-3’, antisense 5’-AGCCGACTAACGGTATGA-3’, 252bp; and β-actin (Genebank accession No. NM_031144.2): sense 5’- TTGTAACCAACTGGGACGATATGG -3’, antisense 5’- GATCTTGATCTTCATGGTGCTAGG -3’, 764bp. Our samples, cDNA, were amplified in a DNA thermal cycler (PxG20809, Thermo Electron Co.USA) by applying Taq polymerase. The PCR products were visualized by ethidium bromide-stained agarose gel electrophoresis. Images were recorded and band intensities were analyzed using gel imaging analysis system (Gene Genius, Syngene, England). β-actin was used as an internal control. The ratio of the optical density (OD) values of ROCK1, ROCK2 and β-actin were used for semi-quantitative assay.
Measurement of caspase-3 activity
Cleavage of chromogenic caspase substrate, Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide), a caspase-3 substrate, was examined for caspase-3 activity, and its absorbance was measured at 405 nm as directed by the kit. Samples were prepared as the methods for western blot analysis detailed above. Sample supernatants containing 50 µg of total protein were added to a reaction buffer containing Ac-DEVD-pNA (2 mM), incubated for 4 hours at 37°C, and then the absorbance of yellow pNA was measured by a spectrometer at 405 nm. The specific caspase-3 activity was normalized for total protein and then expressed as fold of the baseline caspase-3 activity of control cells cultured in DMEM with 10% FBS.
Mitochondrial depolarization assay
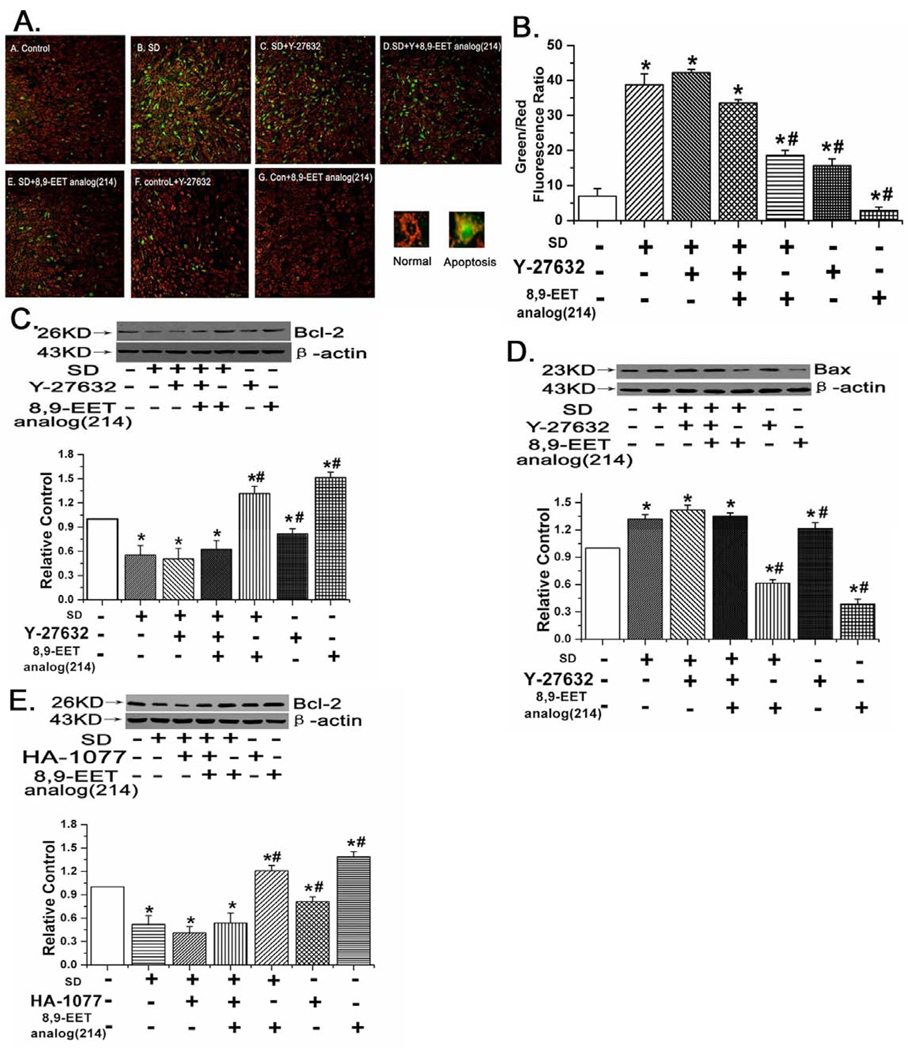
We indirectly assessed mitochondrial function by tracking relative mitochondrial transmembrane potential through detecting JC-1 fluorescence. Images obtained by a fluorescent microscope of PASMCs incubated under conditions detailed below and in the results section and reacted with 5,5′,6,6′ -tetrachloro-1,1′,3,3 ′ -tetraethylbenzimidazolcarbocyanine iodide (JC-1), were analyzed for green and red fluorescence. After growth-arrest for 24 h, cells in 6-well culture plates were treated with vehicle, 8,9-EET analog(214) (1 µM), Y-27632 (1 µM) or 8,9-EET analog(214) (1 µM) plus Y-27632 (1 µM) in serum deprivation condition for another 48 hours. Then we monitored the mitochondrial membrane potentials by determining the relative amounts or dual emissions from both mitochondrial JC-1 monomers (green) and aggregates (red) by using an Olympus fluorescent microscope under Argon-ion 488 nm laser excitation. Mitochondrial depolarization was expressed by an increase in the intensity ratio of green/red fluorescence.
Nuclear morphology determination
Quantitative nuclear chromatin morphology was assessed as another index of apoptosis. PASMCs were cultured in a six-well culture cluster to ~60% confluency. Seven groups of cells as described for the mitochondrial depolarization assay were studied. At the termination of the study, they were stained with 5µl of acridine orange (AO) (5mg/ml, Sigma) in 1 ml of basal medium and incubated for 10 min at room temperature (22–24°C). Then washed Stained cells three times with PBS and imaged under a fluorescent microscope by using 488 nm laser excitation and 405 nm emission. For each well, 15–25 high power fields were randomly imaged to determine the percentage of apoptotic cells based on the morphological characteristics of apoptosis. We described cells undergoing nuclear crenation, nuclear condensation and nuclear fractionation as apoptotic cells.
TUNEL
We performed TdT-UTP nick end labeling (TUNEL) method to label 3’-end of fragmented DNA of the apoptotic PASMCs. Five groups of cells cultured in 6-well plates were treated as described in the mitochondrial depolarization assay, then fixed with 4% paraform phosphate buffer saline, rinsed with PBS, and permeabilized by 0.1% TritonX-100 for 2 min on ice followed by TUNEL for 1 hour at 37°C. The FITC-labeled TUNEL-positive cells were imaged using fluorescent microscopy with 488-nm excitation and 530-nm emission wavelengths. The cells with green fluorescence were described as apoptotic cells.
Measurement of sEH activity
PASMCs and PAECs were seeded in a 96-well plate at a density of 5× 104 cells per well in 100 µl of culture medium and cultured them in a 37°C, 5% CO2 humidified incubator for 24 hours. Then Soluble Epoxide Hydrolase Cell-Based Assay kit (Catalog No. 600090, Cayman) was performed and the results were analyzed according to the experimental protocol provided by Cayman Chemical Company.
Statistics
The composite data are expressed as means ± S.E.M. Statistical analysis was performed with Student’s t-test or one-way ANOVA followed by Dunnett’s test where appropriate. Differences were considered to be significant at p≤0.05.
Results
Protection of PASMCs from apoptosis by 8,9-EET(214) analog but not 8,9-EET
First, we examined the potential of 8,9-EET and 5 analogs of this epoxyeicosatrienoic acid to protect against starvation-induced decreases in survival of PASMCs as estimated by the MTT assay. 8,9-EET was not effective in protection from starvation, and 4 additional analogs similarly had no efficacy in improving survival. However, the 8,9-EET analog(214) with the double bonds missing between carbons 5,6 and 14,15 did increase survival of PASMCs (see Fig. 1B). In contrast, native 8,9-EET protected pulmonary artery endothelial cells from starvation induced apoptosis, whereas the 8,9-EET analog(214) did not (Fig. 1C, n=12, p<0.05).
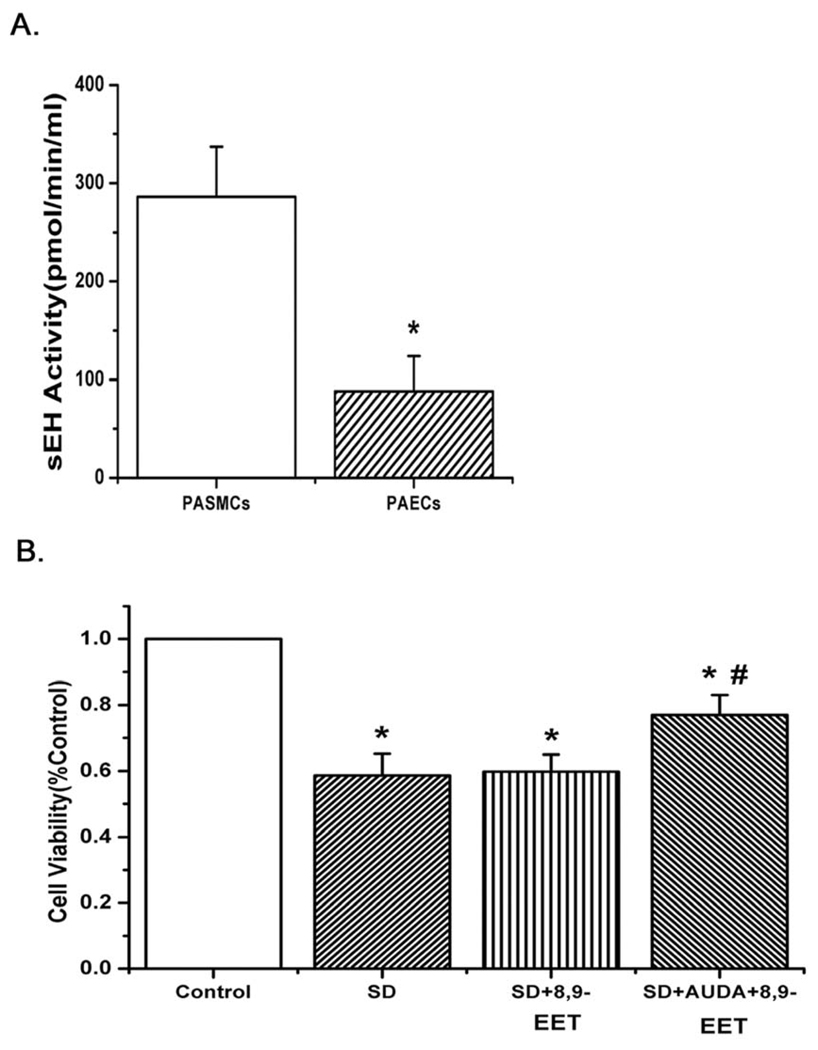
The activity of sEH was higher in PASMCs than that in PAECs, which significantly attenuated the protective effect of native 8,9-EET in PASMCs
To account for the lack of effect of native 8,9-EET in PASMCs, we examined whether there were differences in soluble epoxide hydrolase (sEH) activity between PASMCs and PAECs. The results showed that sEH activity of PASMCs was much higher than that of PAECs (Fig. 2A, n=6, p<0.05). Moreover, after blocking the soluble epoxide hydrolase in PASMCs with 1 µM 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA), an inhibitor of sEH, native 8,9-EET also had a protective effect against starvation-induced decreases in survival of PASMCs (Fig. 2B, n=6, p<0.05). The results show that PASMCs likely hydrolyze 8,9-EET rapidly and render it inactive.
Fig. 2.
A: The activity of soluble epoxide hydrolase (sEH) was examined in both PASMCs and PAECs. The activity was much higher in PASMCs (n= 6; *p<0.05). B: Native 8,9-EET protected against starvation-induced decrease in survival of PASMCs after inhibiting the activity of sEH with the sEH inhibitor (AUDA) (n= 6; *p<0.05 compared with Control; #p<0.05 compared with SD). All values are denoted as means ± S.E.M. from three or more independent batches of cells
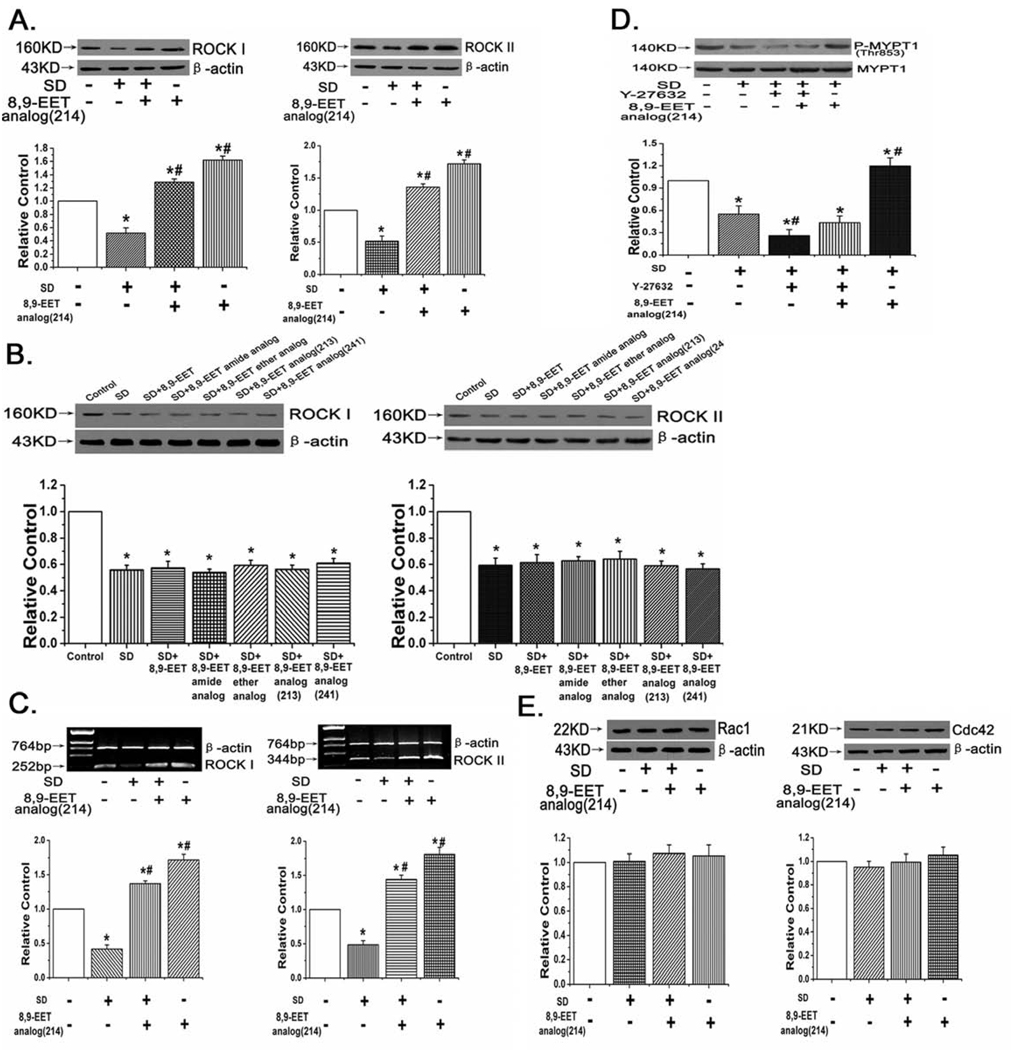
8,9-EET analog(214) up-regulated ROCK I and ROCK II expression in transcriptional level and increased the activity of ROCK in cultured PASMCs
To investigate the mechanisms 8,9-EET analog(214) protection against starvation-induced apoptosis in PASMCs, we first examined the role of ROCK in mediating pro-survival effects of the 8,9-EET analog(214). Western blot demonstrated that treatment with 8,9-EET analog(214) for 24 hours increased ROCK I and ROCK II protein expression (Fig. 3A, n=3, p<0.05), while other 8,9-EET analogs had no noticeable effects on the expression of ROCK I and ROCK II in starved PASMCs (Fig. 3B, n=3, p<0.05). Consistently, we found the mRNA expression of ROCK I and ROCK II was increased by 8,9-EET analog(214) (Fig. 3C, n=3, P<0.05), indicating that the effect of 8,9-EET analog(214) on ROCK protein was in transcriptional level. Moreover, we also found that after 24 hours stimulation 8,9-EET analog(214) increased the amount of phospho-Thr853 in total MYPT1, a downstream target of ROCK (Fig. 3D, n=3,P<0.05). In contrast, 8,9-EET analog(214) did not affect the expression of Rac1 and Cdc42 (Fig. 3E, n=3,P<0.05), suggesting Rac/Cdc42-dependent pathway was probably not required for the biological functions of 8,9-EET analog(214). These results indicate that 8,9-EET analog(214) blocks the apoptosis of PASMCs induced by serum deprivation via a ROCK-dependent pathway but not through Rac/Cdc42-dependent pathway.
Fig. 3.
Protein and mRNA expression of ROCK I and ROCK II, and the activity of ROCK after treatment with 8,9-EET analog(214). A: 8,9-EET analog(214) up-regulated the protein expression of ROCK I and ROCK II under serum deprived condition. B: Other 8,9-EET analogs did not affect the expression of ROCK I and ROCK II after serum deprivation in PASMCs. C: 8,9-EET analog(214) induced the mRNA expression of ROCK I and ROCK II. D: 8,9-EET analog(214) increased the activity of ROCK in PASMCs. E: The Rac1 and Cdc42 expression were not affected by 8,9-EET analog(214). “SD” means serum deprivation. All values are denoted as means ± S.E.M. from three or more independent batches of cells (*p<0.05 compared with Control; #p<0.05 compared with SD).
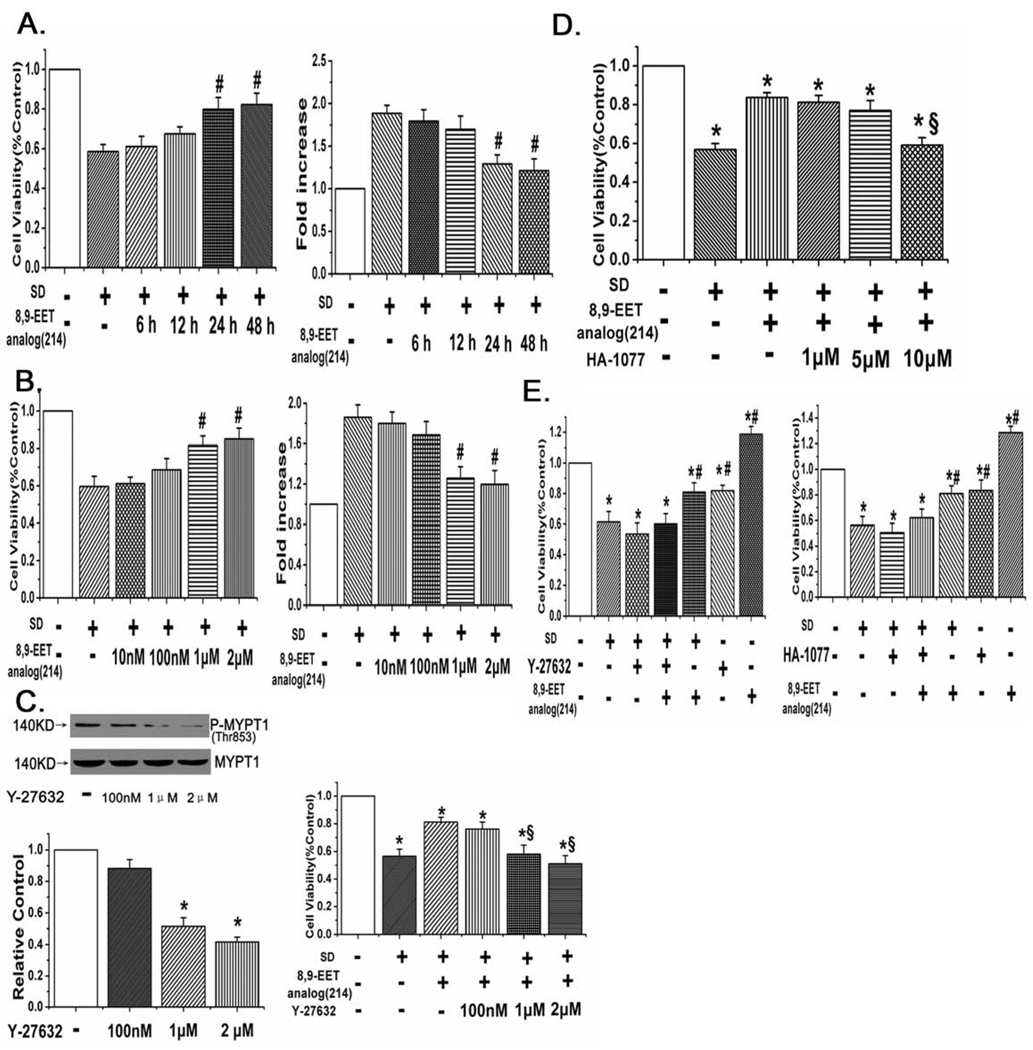
The protective effect of 8,9-EET analog(214) on PASMCs survival after serum deprivation was via ROCK pathway in PASMCs with time and concentration dependent manners
MTT assay and measurement of caspase-3 activity were applied to determine the protective effect of 8,9-EET analog(214) in PASMCs after serum deprivation at different time points and concentrations. We found 8,9-EET analog(214) improved the cell viability and decreased the caspase-3 activity in time- and concentration-dependent manners (Fig. 4A,B, n=6, p<0.05). We further studied the inhibitory effects of Y-27632 and HA-1077 (fasudil) on 8,9-EET analog(214) protection from PASMCs apoptosis, the data showed that Y-27632 weakened the protective effects of 8,9-EET analog(214) and decreased the amount of phospho-Thr853 in total MYPT1 in PASMCs with a dose-dependent way (Fig. 4C, n=6, p<0.05). A similar result was acquired when applying HA-1077 in PASMCs (Fig. 4D, n=6, p<0.05).
Fig. 4.
The inhibitory effects of 8,9-EET analog(214) on PASMCs apoptosis induced by serum deprivation are time- and concentration-dependent. A: 8,9-EET analog(214) improved the cell viability and decreased the activity of caspase-3 in a time-dependent manner. B: 8,9-EET analog(214) promoted survival and inhibited caspase-3 activation in a concentration-dependent way. C: The inhibitory effects of Y-27632 on ROCK activity and 8,9-EET analog(214) protection were dose-dependent. D: HA-1077 weakened the protective effect of 8,9-EET analog(214) in a dose-dependent manner. E: The viability decreased by serum deprivation was partially rescued by the 8,9-EET analog(214), Y-27632 and HA-1077 (ROCK inhibitors) inhibited the protective effect of 8,9-EET analog(214) on cell viability in PASMCs. “SD” means serum deprivation. All values are denoted as means ± S.E.M. from three or more independent batches of cells (*p<0.05 compared with Control; #p<0.05 compared with SD; §p<0.05 compared with SD+8,9-EET analog(214)).
In a separate study, cell viability was again determined by measuring colorimetric conversion of MTT to formazan. We found that enhanced survival afforded by 8,9-EET analog(214) in cells stressed with serum deprivation was inhibited by 1 µM Y-27632 or 10 µM HA-1077 (Fig. 4E, n=6, p<0.05), further proved that 8,9-EET analog(214) improved cell viability via ROCK pathway in PASMCs.
8,9-EET analog(214) prevented mitochondrial depolarization, induced Bcl-2 expression and suppressed Bax expression in a ROCK dependent manner
To ascertain whether the mitochondrial-dependent apoptosis pathway was involved in 8,9-EET analog(214) protection from apoptosis in PASMCs, we measured mitochondrial membrane potentials and expression of mitochondrial membrane proteins (Bcl-2 and Bax) in serum starved PASMCs treated with 8,9-EET analog(214) or vehicle control. Control PASMCs stained with JC-1 emitted predominantly mitochondrial orange-red fluorescence with minimal green fluorescence, while JC-1 was converted to the monomeric form (green fluorescence) in apoptotic cells. Quantitative analysis of JC-1–stained cells revealed an increase in the green (low ΔΨm) to the red (high ΔΨm) ratio in SD-treated cells when compared with control cells, which were cultured in the presence of 20% FBS (p<0.05, n=10). Treatment of SD cells with 8,9-EET analog(214) significantly increased the red fluorescence, but SD cells treated with 8,9-EET analog(214) and Y-27632 (ROCK inhibitor) were not protected, exhibiting changes in ΔΨm indistinguishable from that of SD cells treated with vehicle (Fig. 5A, n=10, P<0.05). These results indicate that 8,9-EET analog(214) protects against SD-induced loss of ΔΨm and maintains mitochondrial integrity through ROCK pathway.
Fig. 5.
8,9-EET analog(214) protects against SD evoked loss of mitochondrial membrane potentials in a ROCK dependent manner. A: The cells were stained with JC-1 probe and imaged by the fluorescent microscope. Representative images of cells from each of the 7 groups are shown as well as average green/red ratios for each group. Increases in green (and hence the green to red ratio) correlate with mitochondrial depolarization. B: Quantitative analysis of the shift of mitochondrial green fluorescence to red fluorescence from ten randomly selected fields obtained from each group. All values are denoted as means ± S.E.M. from ten independent photographs shot in each group. C: The expression of Bcl-2 in rat PASMCs. Representative images and averaged data of Bcl-2 and beta actin (to serve as a loading control) in rat PASMCs are shown. The relative ratio of Bcl-2 to actin in control cells was set at 1.0 and data from other cell groups normalized to the same. Bcl-2 expression was decreased by SD, but this decrease was partially mitigated by 8,9-EET analog(214). Protection against starvation associated loss of expression of Bcl-2 by exogenous 8,9-EET analog(214) was partly blocked by Y-27632 (ROCK inhibitor). D: The expression of Bax in rat PASMCs. Representative images and averaged data of Bax and beta actin (to serve as a loading control) in rat PASMCs are shown. The raio of Bax to beta actin in control cells was set at 1.0 and data from other groups normalized. Starvation increased expression of Bax, and this increase was largely blocked by treatment with the 8,9-EET analog(214). Expression of Bax in cells treated with both 8,9-EET analog(214) and Y-27632 (ROCK inhibitor) or Y-27632 alone was indistinguishable from that of serum starved cells alone. E: The expression of Bcl-2 in rat PASMCs. The relative ratio of Bcl-2 to actin in control cells was set at 1.0 and data from other cell groups normalized to the same. The inhibitory effect of 8,9-EET analog(214) on starvation associated loss of expression of Bcl-2 was partly blocked by HA-1077 (ROCK inhibitor). All values are denoted as means ± S.E.M. from three or more independent batches of cells (*p<0.05 compared with Control; #p<0.05 compared with SD).
Bcl-2 and Bax, which are localized on the mitochondrial membrane, were examined by western blot. Starvation decreased expression of Bcl-2 and increased expression of Bax, both pro-apoptotic shifts. 8,9-EET analog(214) induced Bcl-2 expression and inhibited Bax expression, but these effects were largely eliminated after blocking the ROCK pathway (Fig. 5C–E, n=3, P<0.05). The results further support a protective effect of 8,9-EET analog(214) that depends upon mitochondrial membrane effects.
The effect of 8,9-EET analog(214) on inhibition of caspase-9 was blocked by ROCK inhibitor (Y-27632)
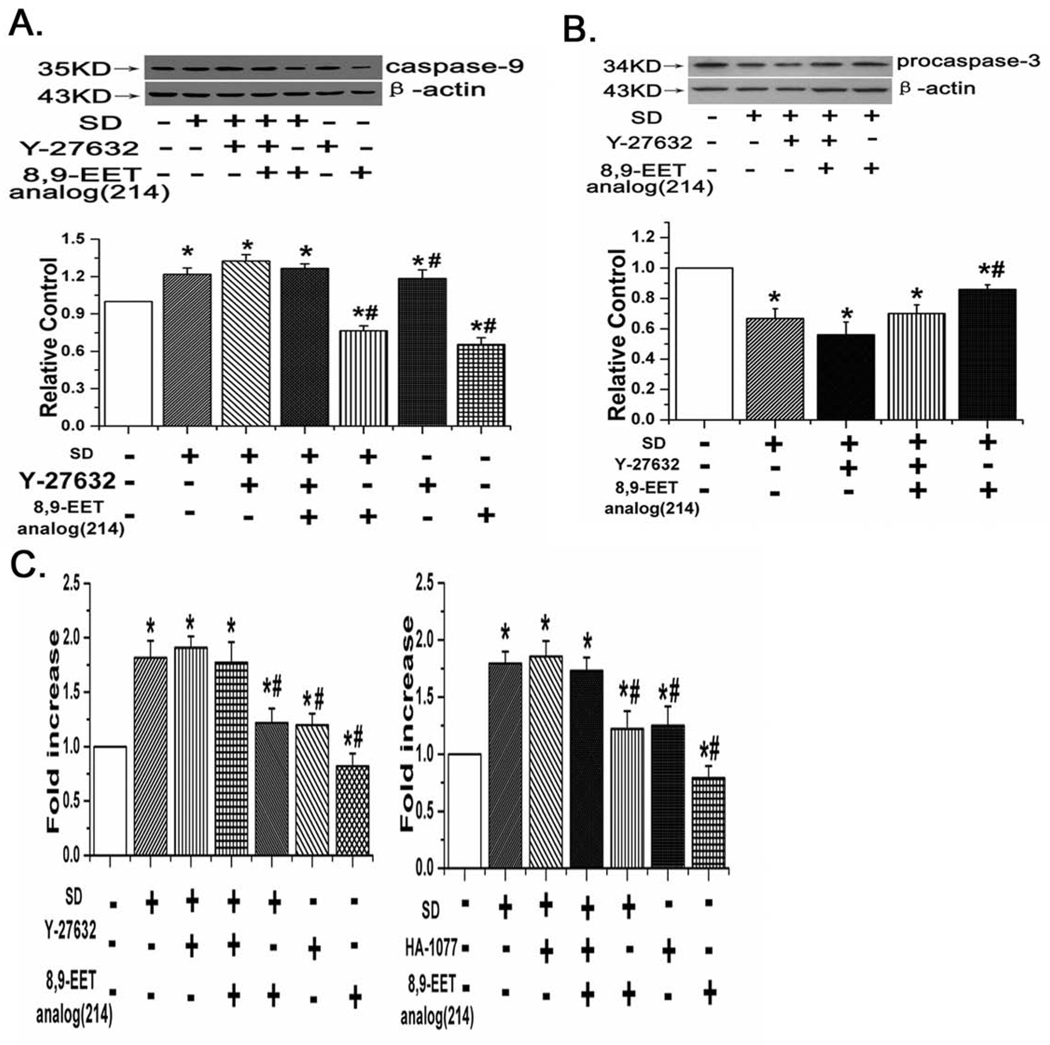
Western blots to detect caspase-9 expression were performed in the 7 groups of cells defined above. We found that SD activated/increased expression of caspase-9, while adding 8,9-EET analog(214) immediately prior to and during SD decreased expression of caspase-9. Treatment with Y-27632 reversed the effect of 8,9-EET analog(214) on SD enhanced caspase-9 expression (Fig. 6A, n=3, P<0.05).
Fig. 6.
8,9-EET analog(214) inhibits the caspase-3 activation, the expression of caspase-9 and cleavage of procaspase-3 through ROCK pathway. A: The expression of caspase-9 in rat PASMCs. B: The expression of procaspase-3 in rat PASMCs. C: The activity of Caspase-3 in rat PASMCs. 8,9-EET analog(214) decreased the activity of caspase-3, suppressed the cleavage of procaspase-3 and reduced the expression of caspase-9 in PASMCs under serum deprivation. However, its inhibitory effects were abolished after blocking the ROCK pathway with Y-27632 or HA-1077. “SD” means serum deprivation. All values are denoted as means ± S.E.M. from three or more independent batches of cells (*p<0.05 compared with Control; #p<0.05 compared with SD).
8,9-EET analog(214) inhibited the cleavage of procaspase-3 and decreased caspase-3 activity in a ROCK dependent manner
Caspase-3 is synthesized as a precursor protein procaspase-3 that undergoes cleavage in response to apoptotic stimuli and becomes activated. So we assayed for expression of procaspase-3 and caspase-3 activity. We found that SD promoted the cleavage of procaspase-3, and 8,9-EET analog(214) inhibited this effect. Treatment with Y-27632 partly reversed the effect of 8,9-EET analog(214) on the expression of procaspase-3 (Fig. 6B, n=3, p<0.05). Similar results were observed when we measured the activity of caspase-3. Serum deprivation activated caspase-3 activity in a manner that could be partly blocked by 8,9-EET analog(214). Blocking the ROCK pathway with inhibitors Y-27632 and HA-1077 eliminated the protective effect of 8,9-EET analog(214) with respect to activation of caspase-3 (Fig. 6C, n=3, P<0.05).
The effect of ROCK pathway on 8,9-EET analog(214)-inhibited nuclear shrinkage of PASMCs
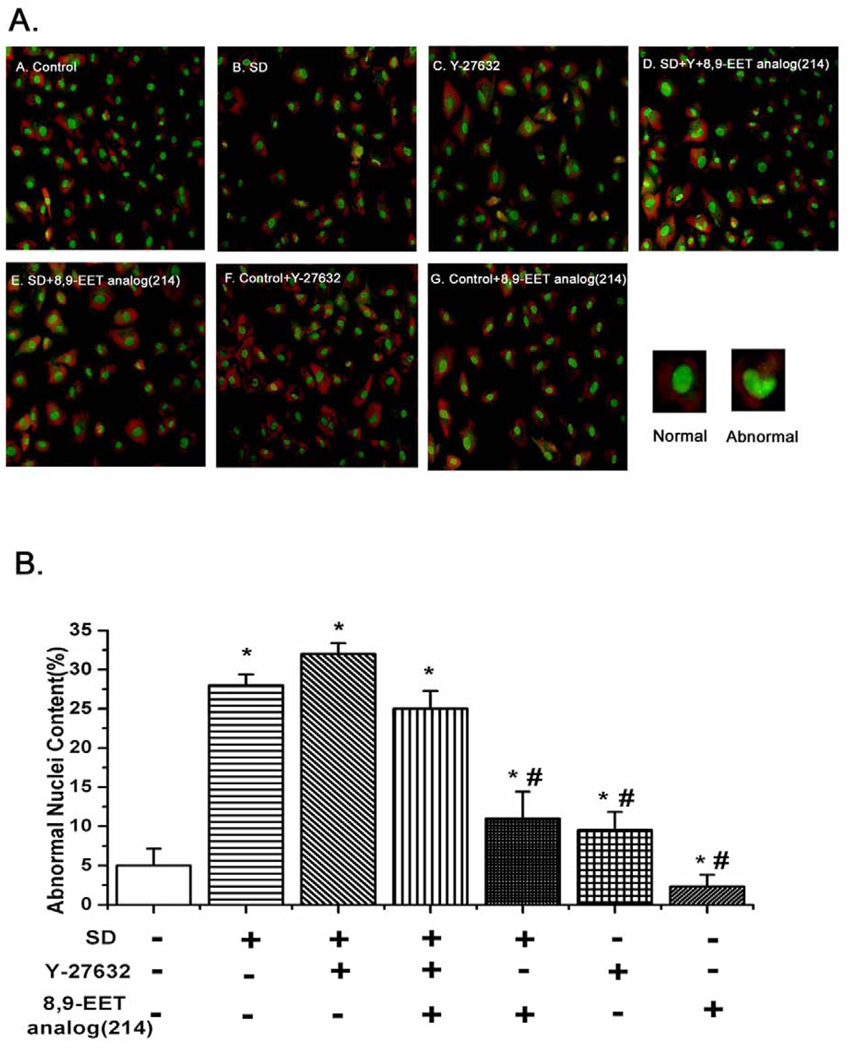
To explore whether 8,9-EET analog(214) prevented PASMCs from nuclear shrinkage via ROCK, we assessed nuclear conformation by staining PASMCs with acridine orange. We found that serum deprivation enhanced abnormal nuclei content cells (crenation, condensation and fractionation) in a manner which was reversed by treatment with the 8,9-EET analog(214). However, after inhibition of ROCK by Y-27632, 8,9-EET analog(214) no longer protected against serum deprivation induced alterations in nuclei conformation (Fig. 7, n=10, P<0.05).
Fig. 7.
8,9-EET analog(214) suppresses the nuclear deformation and chromatin condensation via ROCK. A: PASMCs were treated the same way as in Fig. 5A, and then stained with AO (acridine orange). The percentage of cells with abnormal nuclear contour was quantified by fluorescent microscope after staining with AO. Representative images from each of the 7 groups of cells studies are shown as labeled. B: Quantitative analysis of abnormal nuclei content (crenation, condensation and fractionation) in different groups. The percentage of abnormal nuclei from images of ten randomly selected fields in each group of cells is shown. All values are denoted as means ± S.E.M. from ten independent photographs shot in each group (*p<0.05 compared with Control; #p<0.05 compared with SD).
8,9-EET analog(214) suppressed DNA fragmentation through ROCK activation
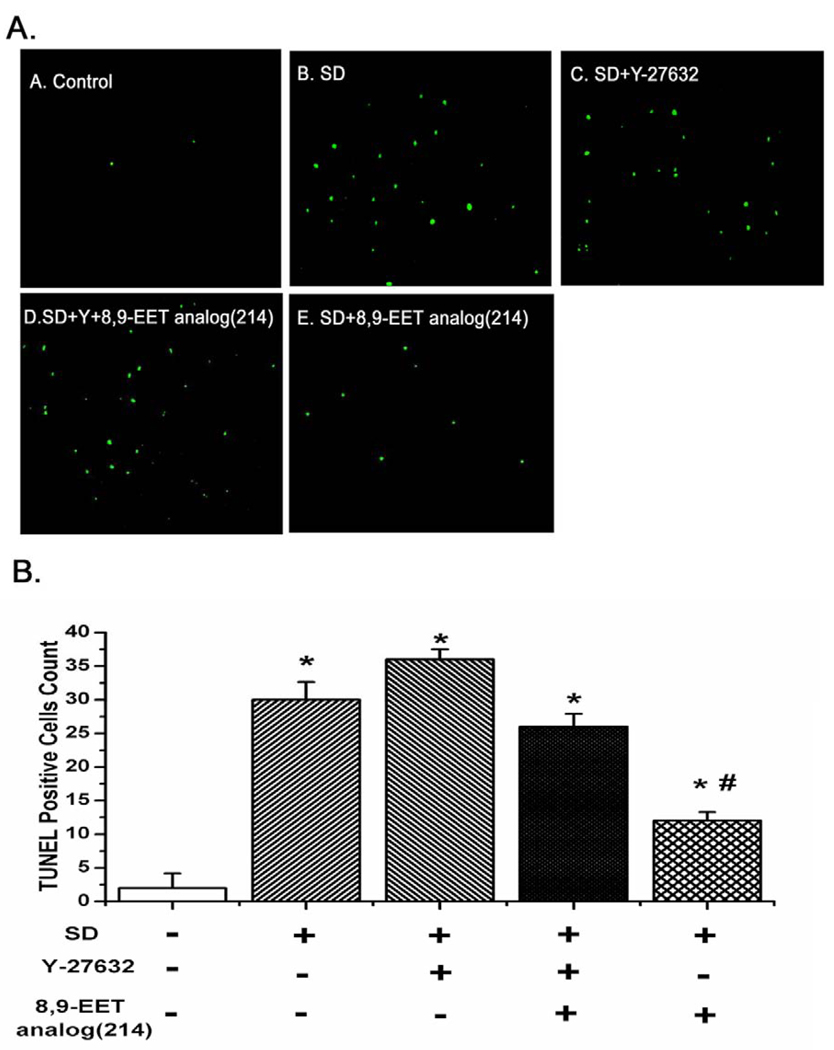
TUNEL assay was undertaken to determine whether ROCK participated in the 8,9-EET analog(214)-inhibited DNA fragmentation of PASMCs. As shown in Fig. 8B, the number of TUNEL-positive cells was increased after serum deprivation for 48 h; 8,9-EET analog(214) decreased the number of TUNEL-positive cells induced by SD. However, the protective effect of 8,9-EET analog(214) was lost after blocking ROCK with Y-27632 (Fig. 8, n=10, P<0.05).
Fig. 8.
ROCK pathway mediates the inhibitory effect of 8,9-EET analog(214) on the increase of TUNEL-positive cells induced by serum deprivation. PASMCs were treated the same way as in Fig. 5A before TUNEL staining and fluorescent imaging. Cells undergoing apoptosis were positively stained with TUNEL reagent and were shown in green. A: Representative photographs of TUNEL staining in different groups. B: Quantitative analysis of TUNEL positive cells content among groups. All values are denoted as means ± S.E.M. of green cells from ten images obtained in randomly selected fields from each group (*P<0.05 compared with Control; #P<0.05 compared with SD).
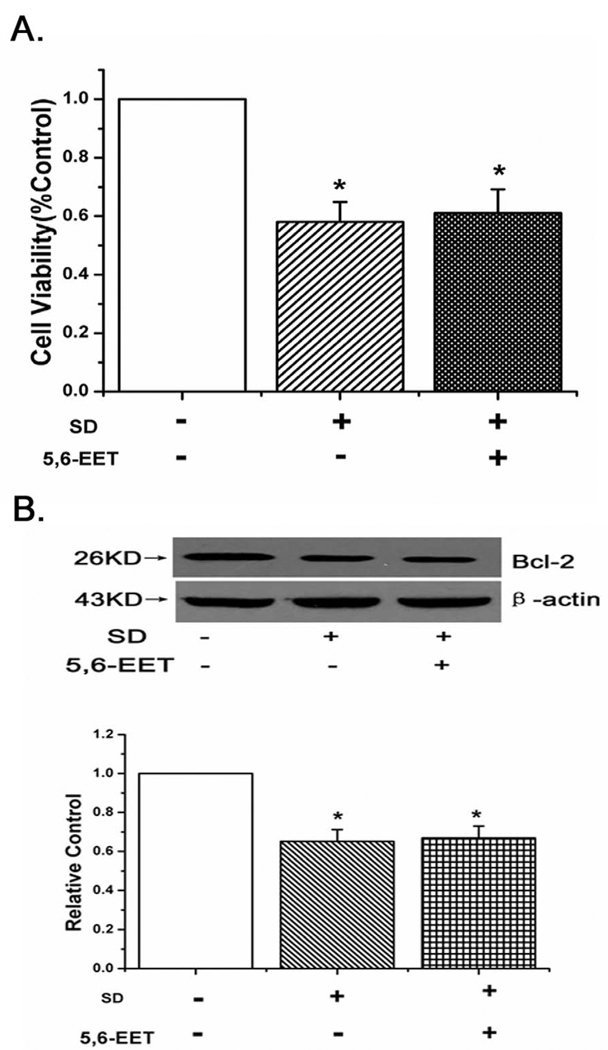
5,6-EET had no effects on improving the cell viability and protecting against apoptosis caused by serum deprivation in PASMCs
To demonstrate the specificity of 8,9-EET analog(214) in inhibiting PASMCs apoptosis, 5,6-EET was tested in rat PASMCs. We found 5,6-EET could not improve the cell viability and affect Bcl-2 expression in PASMCs (Fig. 9A,B, n=6, p<0.05). The results show that 5,6-EET do not block the apoptosis induced by serum deprivation in PASMCs.
Fig. 9.
Effects of 5,6-EET in serum-starved PASMCs. A: The effect of 5,6-EET on cell viability in PASMCs. 5,6-EET had no effects on improving PASMCs viability. B: 5,6-EET did not induce the expression of Bcl-2. All values are denoted as means ± S.E.M. from three or more independent batches of cells (*p<0.05 compared with Control).
Discussion
Our first novel finding is protection of serum deprived PASMCs by an 8,9-EET analog, but not native 8,9-EET itself or several other analogs. Protective and pro-angiogeneic effects of EETs in a variety of cell types have been well described by us as well as other groups [4,16–18]. Human lung microvascular endothelial cells and human coronary endothelial cells are protected against apoptosis evoked by activation of both intrinsic and extrinsic pathways by 8,9-EET [3]. 8,9-EET promotes survival and angiogenesis of murine lung microvascular endothelial cells [1]. Cardiomyocytes are protected from doxorubicin toxicity by over-expression of the epoxygenase isoform CYP2J2 or from hypoxia/reperfusion by 8,9-EETs [4,19]. Interestingly, pro-apoptotic effects of 8,9-EETs in a PDGF stimulated fibroblast cell line have also been reported [20]. Thus our findings of no survival benefit from 8,9-EET in PASMCs fits with the interpretation that protection from apoptosis by 8,9-EET is cell type dependent. Natural or exogenously supplemented lipids which promote survival or apoptosis in selective cell types may be therapeutically advantageous. For example central venous infusion of 8,9-EETs in a patient with necrotizing pneumonia may have protective effects on pulmonary endothelium without promoting overgrowth of vascular smooth muscle cells.
One of the interesting phenomena found in this study is that there are differences between PASMCs and PAECs in response to native 8,9-EET and 8,9-EET analog in protection against starvation-induced apoptosis. We find that native 8,9-EET is not effective in protection from starvation, but the 8,9-EET analog(214) do increase the survival of PASMCs. In contrast, native 8,9-EET protects pulmonary artery endothelial cells from starvation induced apoptosis, whereas the 8,9-EET analog(214) do not. As EETs are mainly further metabolized by soluble epoxide hydrolase (sEH) [21–25], we suspect that the lack of response to native 8,9-EET in PASMCs is due to the quick hydroxylation and inaction of lipid before it reacted with PASMCs. The hypothesis is proved by the measurement of sEH activity, which is higher in PASMCs than that in PAECs. In addition, after inhibiting the sEH activity of PASMCs with AUDA, the protective effect of 8,9-EET against starvation-induced apoptosis is observed in PASMCs. It is possible that the responses provided by 8,9-EET in different cells are influenced by the intra-cellular content of sEH.
Which structural requirements account for the protective effects of the 8,9-EET analog in PASMCs? The analog which protects PASMCs in our studies has a single double bond between carbons 11 and 12. Analogs with a single bond between carbons 5 and 6 are not effective, nor are compounds with 2 or more double bonds. The double bonds between carbons 5,6 and 14,15 of 8,9-EET are not needed for the pro-survival effects of 8,9-EET analog in PASMCs. These data stand in contrast to 8,9-EET analog protection of bovine pulmonary artery endothelial cells from staurosporine induced apoptosis; 8,9-EET variants containing at least 2 double bonds protected endothelial cells from apoptosis in this model. Derivatives containing only a single double bond do not afford a survival advantage. Additional studies to examine the contribution of species or model of injury to the different effect of EET analogs on survival are important in this regard. EETs are known to stimulate a number of prosurvival pathways [3,6,26]. For example, EETs activate EGFR, MAP kinase, and PI3 kinase signaling pathways in endothelial and epithelial cells [27–29]. RhoA /ROCK is reported to mediate EET induced pulmonary artery vasoconstriction [30], as well as hypoxic vasoconstriction [13]. However, the role of RhoA/ROCK in 8, 9-EET induced protection from apotosis in any vascular cell type has not been reported to our knowledge. Our studies provide the first evidence that an 8,9-EET analog affords protection of PASMCs from starvation induced apoptosis in a manner which depends upon activation of ROCK. Specifically we find that the 8,9-EET analog(214) improves cell viability, and decreases nuclear condensation and TUNE positive cells-all in a ROCK dependent manner.
Our model of injury is serum deprivation in PASMC. Serum deprivation often induces cells death via apoptosis, and many regulating factors are responsible for this process. An increase generation of reactive oxygen species (ROS) contributes greatly to serum starved cells apoptosis [31]. Reports show that serum starvation makes cell growth arrested in G0/G1 phases of the cell cycle, inhibits the transition from G1 phase to S phase and decreases the numbers of cells in the S phases of the cell cycle [32–34]. And also serum starvation inhibits cell survival through blocking the activation of survival pathways, like PI3 kinase-Akt and Insulin/insulin-like growth factor (IGF-I) [35,36]. There are reports that serum deprivation triggers apoptotic responses through the mitochondrial pathway, leading to the decreases in the mitochondrial membrane potential. Disruption of the mitochondrion leads to activation of caspase-9 and caspase-3 [37,38]. Therefore, reduction of mitochondrial membrane potential and activation of caspase-3 and caspase-9 are considered key indicators for mitochondria-dependent apoptosis [39,40]. We find that serum deprivation in PASMCs is associated with loss of mitochondrial membrane potential, and treatment with the 8,9-EET analog(214) prevents the same in a ROCK dependent manner. Treatment of SD PASMCs with 8,9-EET analog(214) enhances the expression of Bcl-2, which is localized to the outer mitochondrial membranes and controls the stabilization of mitochondrial membranes. Moreover, the 8,9-EET analog(214) inhibits the cleavage of procaspase-3 and the activation of caspase-3 and caspase-9, all via the ROCK pathway. On the basis of these results, we believe that the 8,9-EET analog(214) inhibits apoptosis through ROCK pathways by promoting mitochondrial-dependent prosurvival pathways in PASMCs.
Recent studies have indicated that RhoA/ROCK signaling contributes to the pathogenesis of some cardiovascular diseases. Thus ROCK is now regarded as an important therapeutic target [41,42]. In this regard, inhibition of the ROCK pathway may limit remodeling through promoting apoptosis of vascular smooth muscle cells. For this reason the effect of naturally occurring 8,9-EET and analogs such as those we tested in this communication on the expression of ROCK in PASMCs and vascular smooth muscle cells from key circulations should be carefully examined.
As productions of CYP2J2 family, not only 8,9-EET is related to cell apoptosis, but also 8,9-EET regioisomers (14,15-EET and 11,12-EET) attenuate apoptosis or promote proliferation in carcinoma cells or cardiac ischemia/reperfusion process [3,4,20,43]. However, 5,6-EET is different from others and provides no protective effect in cell survival in human endothelial cells from the pulmonary and coronary vasculature, which is consistent with our results that 5,6-EET can not inhibit the apoptosis induced by serum deprivation in PASMCs [3].
In conclusion, we have shown that an 8, 9-EET analog, but not native 8,9-EET, inhibits apoptosis of serum deprived PASMCs via ROCK pathway. The opposite profile of protection is observed for pulmonary artery endothelial cells: native 8,9-EET but not 8,9-EET analog increases survival of starved cells. These observations raise new therapeutic opportunities for these compounds with the potential targeting of smooth muscle or endothelial cells from a particular vascular bed. Since activation of Rho-kinase signaling has been associated pathophysiologically with cardiovascular diseases including pulmonary hypertension and coronary artery vasospasm, careful studies evaluating the effects of EETs and their analogs on RhoA activation in vascular smooth muscle cells from other circulations, particularly coronary and renal, are needed.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 30470752), NIH GM31278 and the Robert A. Welch Foundation (J. Falck), NIH HL069996 (M Medhora), HL49294 (ER Jacobs).
Abbreviations
- EETs
Epoxyeicosatrienoic acids
- PAECs
Pulmonary artery endothelial cells
- ROCK
Rho-kinase
- PASMCs
Pulmonary artery smooth muscle cells
- SD
Serum deprivation
- AUDA
12-(3-adamantan-1-yl-ureido)-dodecanoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280(29):27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 2.Benoit C, Renaudon B, Salvail D, Rousseau E. EETs relax airway smooth muscle via an EpDHF effect: BK(Ca) channel activation and hyperpolarization. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L965–L973. doi: 10.1152/ajplung.2001.280.5.L965. [DOI] [PubMed] [Google Scholar]
- 3.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291(2):H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 4.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294(2):H724–H735. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 6.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 7.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98(3):322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 8.Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T, Todoroki N-Ikeda, Mogami K, Mizukami Y, Kuriyama S, Haze K, Suzuki M, Kobayashi S. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7(4):399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 11.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24(10):1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171(5):494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 13.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 14.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70(2):174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 15.Dhanasekaran A, Bodiga S, Gruenloh S, Gao Y, Dunn L, Falck JR, Buonaccorsi JN, Medhora M, Jacobs ER. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2009;296(3):H777–H786. doi: 10.1152/ajpheart.01087.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278(4):H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33(12):2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- 18.Medhora M, Dhanasekaran A, Gruenloh SK, Dunn LK, Gabrilovich M, Falck JR, Harder DR, Jacobs ER, Pratt PF. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 2007;82(1–4):19–29. doi: 10.1016/j.prostaglandins.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, El-Sikhry H, Chaudhary KR, Batchu SN, Shayeganpour A, Jukar TO, Bradbury JA, Graves JP, DeGraff LM, Myers P, Rouse DC, Foley J, Nyska A, Zeldin DC, Seubert JM. Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2009;297(1):H37–H46. doi: 10.1152/ajpheart.00983.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieves D, Moreno JJ. Epoxyeicosatrienoic acids induce growth inhibition and calpain/caspase-12 dependent apoptosis in PDGF cultured 3T6 fibroblast. Apoptosis. 2007;12(11):1979–1988. doi: 10.1007/s10495-007-0123-3. [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Kaduce TL, Weintraub NL, VanRollins M, Spector AA. Functional implications of newly characterized pathway of 11,12-epoxyeicosatrienoic acid metabolism in arterial smooth muscle. Circ Res. 1996;79:784–793. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- 22.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells: implication for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 24.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 25.Fang X, VanRollins M, Kaduce TL, Spector AA. Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. J. Lipid Res. 1995;36:1236–1246. [PubMed] [Google Scholar]
- 26.Baron A, Frieden M, Beny JL. Epoxyeicosatrienoic acids activate a high-conductance, Ca(2+)-dependent K + channel on pig coronary artery endothelial cells. J Physiol. 1997;504(Pt 3):537–543. doi: 10.1111/j.1469-7793.1997.537bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 28.Chen Chen JK, Wang DW, Falck JR, Capdevila J, Harris RC. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J Biol Chem. 1999;274:4764–4769. doi: 10.1074/jbc.274.8.4764. [DOI] [PubMed] [Google Scholar]
- 29.Fleming, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 30.Losapio JL, Sprague RS, Lonigro AJ, Stephenson AH. 5,6-EET-induced contraction of intralobar pulmonary arteries depends on the activation of Rho-kinase. J Appl Physiol. 2005;99(4):1391–1396. doi: 10.1152/japplphysiol.00473.2005. [DOI] [PubMed] [Google Scholar]
- 31.Jung SH, Kang KD, Ji D, Fawcett RJ, Safa R, Kamalden TA, Osborne NN. The flavonoid baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes. Neurochem Int. 2008;53(6–8):25–37. doi: 10.1016/j.neuint.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva MYu, Obeid LM, Hannun YA. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270(5):2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 33.Howard MK, Burke LC, Mailhos C, Pizzey A, Gilbert CS, Lawson WD, Collins MK, Thomas NS, Latchman DS. Cell cycle arrest of proliferating neuronal cells by serum deprivation can result in either apoptosis or differentiation. J Neurochem. 1993;60(5):1783–1791. doi: 10.1111/j.1471-4159.1993.tb13404.x. [DOI] [PubMed] [Google Scholar]
- 34.Bai J, Cederbaum AI. Cycloheximide protects HepG2 cells from serum withdrawal-induced apoptosis by decreasing p53 and phosphorylated p53 levels. J Pharmacol Exp Ther. 2006;319(3):1435–1443. doi: 10.1124/jpet.106.110007. [DOI] [PubMed] [Google Scholar]
- 35.Navarro P, Valverde AM, Benito M, Lorenzo M. Insulin/IGF-I rescues immortalized brown adipocytes from apoptosis down-regulating Bcl-xS expression, in a PI 3-kinase- and map kinase-dependent manner. Exp Cell Res. 1998;243(2):213–221. doi: 10.1006/excr.1998.4168. [DOI] [PubMed] [Google Scholar]
- 36.Kang KD, Andrade da Costa BL, Osborne NN. Stimulation of Prostaglandin EP2 Receptors on RGC-5 Cells in Culture Blunts the Negative Effect of Serum Withdrawal. Neurochem Res. 2010;35(5):820–829. doi: 10.1007/s11064-010-0140-4. [DOI] [PubMed] [Google Scholar]
- 37.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 38.Samraj AK, Sohn D, Schulze-Osthoff K, Schmitz I. Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell. 2007;18(1):84–93. doi: 10.1091/mbc.E06-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Li Q, Wang Z, Liang D, Liang S, Tang X, Guo L, Zhang R, Zhu D. 15-HETE suppresses K(+) channel activity and inhibits apoptosis in pulmonary artery smooth muscle cells. Apoptosis. 2009;14(1):42–51. doi: 10.1007/s10495-008-0286-6. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Tang X, Li Y, Leu C, Guo L, Zheng X, Zhu D. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmacol. 2008;588(1):9–17. doi: 10.1016/j.ejphar.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 41.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51(1):2–17. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Nossaman BD, Kadowitz PJ. The role of the RhoA/rho-kinase pathway in pulmonary hypertension. Curr Drug Discov Technol. 2009;6(1):59–71. doi: 10.2174/157016309787581057. [DOI] [PubMed] [Google Scholar]
- 43.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65(11):4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]