Abstract
A high resolution, noninvasive approach to quantify atherosclerotic plaque in the peripheral vasculature could have significant clinical and research utility. Seventeen patients with peripheral arterial disease (PAD) were studied in a 1.5T CMR scanner. Atherosclerotic plaque volume in the superficial femoral artery was measured and interobserver, intraobserver, and test-retest variability determined. Nineteen vessels were studied with mean acquisition time of 13.1 minutes per vessel. Mean plaque volume was 7.27 ± 3.73 cm3. Intra-observer intraclass correlation was R = 0.997, inter-observer was R = 0.987, and test-retest reproducibility was R = 0.996. Thus, high resolution measurement of plaque volume in PAD is reliable and reproducible.
Keywords: Atherosclerosis, Cardiovascular Magnetic Resonance, Peripheral Arterial Disease, Peripheral Vascular Disease, Superficial Femoral Artery
INTRODUCTION
Peripheral arterial disease (PAD) is an increasingly common condition characterized by atherosclerotic obstruction of the arteries supplying the lower limbs. Although PAD is now recognized as an important manifestation of systemic atherosclerosis, it remains underdiagnosed and undertreated (1). While co-existing coronary and cerebrovascular disease is responsible for nearly 75% of deaths among PAD patients (2–4), morbidity directly attributable to peripheral vascular obstruction is substantial (5). Of the few therapies proven beneficial in the treatment of PAD, little is understood about their mechanisms of benefit (6–8).
The ankle-brachial index is an excellent test for identifying peripheral arterial obstruction (1) but limited in its utility for assessing disease severity and predicting clinical events (9). Direct measurement of plaque and peripheral arterial remodeling may be better suited for defining disease burden and predicting clinical progression. Because of the inherent limitations of lumenographic techniques in evaluating and assessing the extent of atheroma (10, 11), novel approaches for directly evaluating the vessel wall are needed. Intravascular ultrasound (IVUS) is currently the diagnostic standard for plaque quantification, but the technique is expensive, invasive, and poorly suited for the peripheral circulation (12).
With its safety record, flexibility, and excellent spatial resolution, cardiovascular magnetic resonance (CMR) could be an ideal approach for the noninvasive assessment of atherosclerotic plaque burden in the peripheral circulation. While CMR has been employed for measuring and characterizing plaque in the coronary (13, 14), carotid (15, 16), and aortic circulations (15–17), few studies have rigorously investigated its role in PAD (12, 18). The goal of this study was to develop and refine a practical technique for quantifying plaque in the superficial femoral artery (SFA) of patients with mild to moderate PAD that would allow for determination of plaque burden and noninvasive, serial monitoring of plaque progression in patients treated with both established and novel therapies.
METHODS
Study population
Patients between the ages of 30–85 years with symptoms of intermittent claudication without critical limb ischemia and an ankle-brachial index (ABI) between 0.4 and 0.9 were eligible for this study. The study was carried out in accordance with a protocol that was approved by the Human Investigation Committee at the University of Virginia Health System and all participants signed informed consent.
Protocol
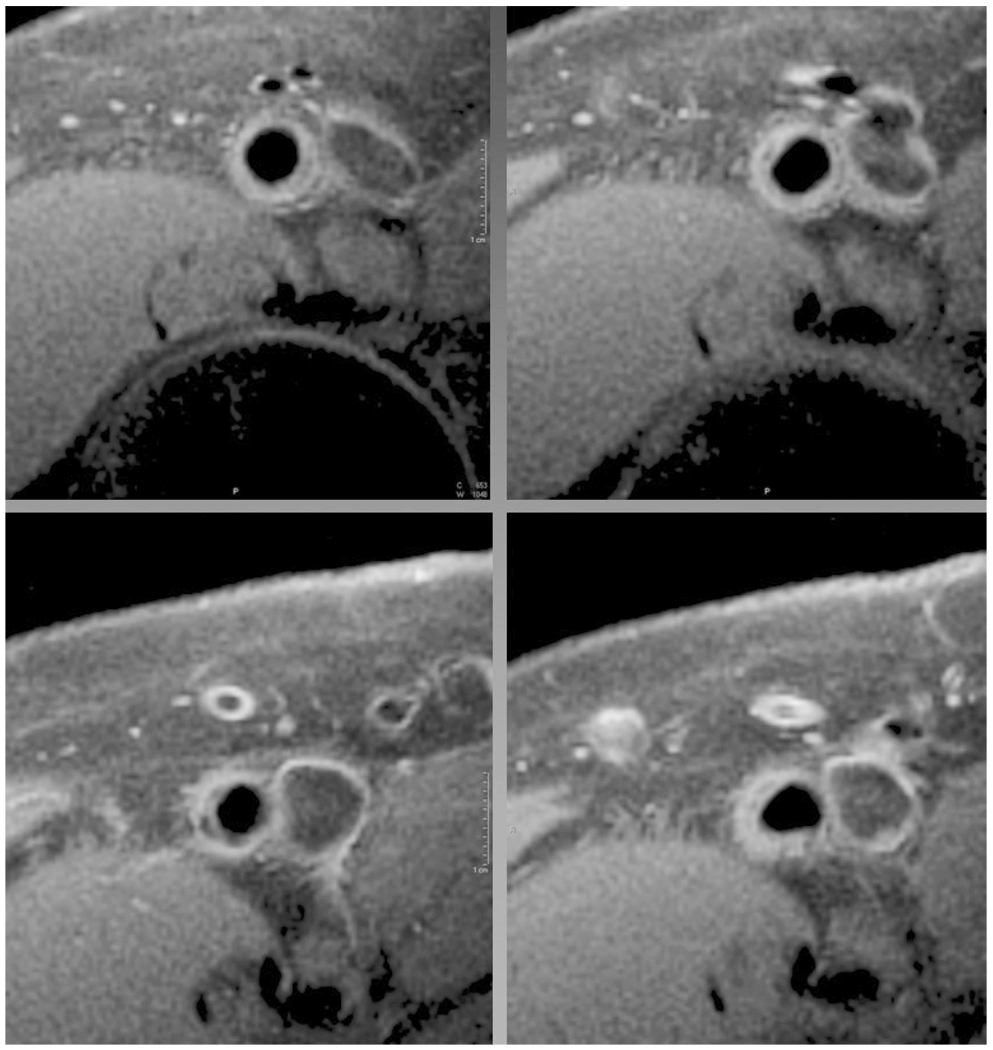
All subjects were placed supine in a 1.5T MR scanner (Siemens Medical Solutions, Erlangen, Germany) with the calf at the isocenter of the magnet. Monitoring of the electrocardiogram and blood pressure was performed with an InVivo 3155MVS (Intermagnetics Companies, Orlando, FL) throughout the study. A custom-built flexible, linear four-element (10 cm × 10 cm square element) surface coil array (Nova Medical, Wilmington, MA) was placed over the thigh and SFA. A multi-slice turbo-spin-echo pulse sequence with fat presaturation was used. Flowing blood was suppressed using spatial pre-saturation, with a combination of periodic excitation of upstream slices and spatial presaturation. Additional flow suppression for downstream slices was provided by the imaging excitations themselves, so that blood was suppressed throughout the multislice data set. Other imaging parameters included: repetition time 11 ms, echo time 7.6 ms, echo spacing 7.5 ms, turbo factor (9), voxel size 0.5 × 0.5 × 3 mm, 4 signal averages, with interleaved image sets used to cover the length of the SFA. Imaging began above the femoral bifurcation and continued through the adductor canal. Representative contiguous images of the femoral artery from a patient with PAD are shown (Fig. 1).
Figure 1.
Representative sequential images (upper left to lower right) from the femoral artery of a subject with mild to moderate peripheral arterial disease with both the luminal and adventitial border clearly delineated. Note the slice to slice variation in plaque morphology.
Image analysis
Before quantification of plaque volume, image quality was assessed and deemed adequate or inadequate for analysis. A total of 23 patients were imaged, and 17 had image quality suitable for analysis. In (4) of the (6) excluded data sets, complete occlusion of the SFA precluded analysis. Occlusion of flow was verified with velocity encoded imaging. In 1 subject, obesity (BMI = 33) and a metal artifact precluded analysis, and another subject’s obesity (BMI > 42) led to poor image quality.
In those subjects with a patent SFA and adequate image quality, atherosclerotic plaque volume (APV) of the SFA was contoured by two independent operators using VesseIMASS software (University of Leiden, Leiden, The Netherlands). For each individual slice, both the luminal and adventitial borders were manually delineated and cross-sectional area (CSA) of the vessel wall measured. Total vessel wall volume was calculated as:
where H is the slice thickness and n the number of slices in the 3-D data set. Volume measurements were initiated at the bifurcation of the common femoral artery, and total vessel distance covered for each analysis was predefined. Nine patients returned (at a mean of 24 days) for evaluation of test-retest reliability using anatomic landmarks and matched number of acquired slices. Imaging time was measured from acquisition of the first scouts to completion of the imaging protocol but does not include time for patient positioning, coil placement, and insertion and removal from the scanner.
Statistical analysis
Subject characteristics are summarized as mean and standard deviation. The intraclass correlation coefficients of reliability were calculated for the interobserver, intraobserver, and reproducibility data using the output of analysis of variance from SAS statistical software (version 9.0, Cary, NC). Reproducibility and both inter and intraobserver variability were also analyzed using the method of Bland and Altman (19). MedCalc software version 8.1.0.0, Mariakerke, Belgium) was used to generate Bland-Altman plots.
Using the test-retest reliability data, we estimated the sample sizes needed to detect the true difference between the means of APV with both 80% and 90% power. Assuming a two-tailed test, a significance level of 0.05, and using the estimated standard deviation, the sample sizes needed to detect the true mean differences ranging from 1% to 5% of the mean APV were established.
RESULTS
Seventeen patients (age 63 ± 10 yrs) with mild to moderate PAD (ankle brachial index 0.72 ± 0.11) were studied. Characteristics of patients are shown in Table 1. Nineteen SFA vessels in the 17 patients were evaluated (mean length 17.1 ± 5.2 cm) with total image acquisition time of 13.1 ± 2.7 minutes per vessel. Mean APV measured was 7.27 ± 3.73 cm3.
Table 1.
Patient characteristics
| ID | ABI | CAD | Stroke | DM | Tobacco | SBP (mmHg) |
BMI |
|---|---|---|---|---|---|---|---|
| 1 | 0.66 | Yes | No | Yes | Yes | 125 | 22.7 |
| 2 | 0.8 | Yes | No | No | Yes | 153 | 23.5 |
| 3 | 0.77 | Yes | No | Yes | Yes | 130 | 24.2 |
| 4 | 0.48 | No | No | No | Yes | 195 | N/A |
| 5 | 0.81 | Yes | No | Yes | Yes | 103 | 27.1 |
| 6 | 0.85 | No | No | No | Yes | 180 | 21.1 |
| 7 | 0.6 | Yes | No | No | Yes | 165 | 21 |
| 8 | 0.73 | Yes | Yes | Yes | Yes | 150 | 39.8 |
| 9 | 0.6 | Yes | No | Yes | Yes | 140 | 53.2 |
| 10 | 0.8 | Yes | No | Yes | Yes | 140 | 28.3 |
| 11 | 0.69 | Yes | Yes | No | Yes | 130 | 25.5 |
| 12 | 0.58 | Yes | No | Yes | Yes | 136 | 50.2 |
| 13 | 0.83 | No | No | No | Yes | 132 | 24 |
| 14 | 0.65 | No | No | Yes | Yes | 136 | 21.7 |
| 15 | 0.85 | No | No | No | Yes | 132 | 24.9 |
| 16 | 0.55 | No | No | No | Yes | 155 | 26.4 |
| 17 | 0.80 | No | No | No | Yes | 133 | 26.3 |
| Mean | 0.72 | 143 | 28.7 | ||||
| SD | 0.11 | 22 | 10.0 |
N/A indicates data not available
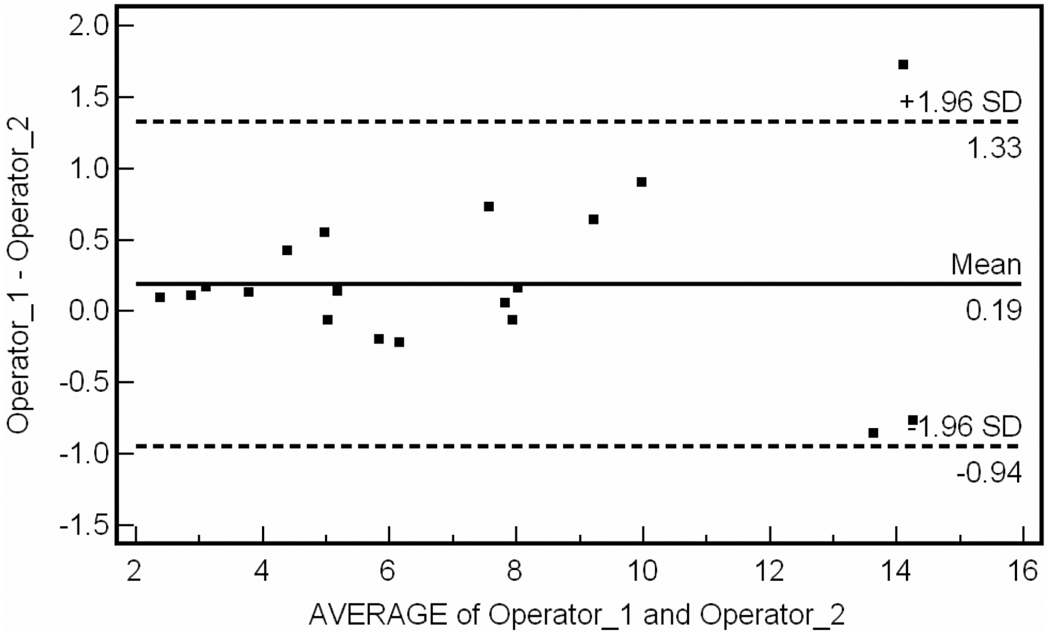
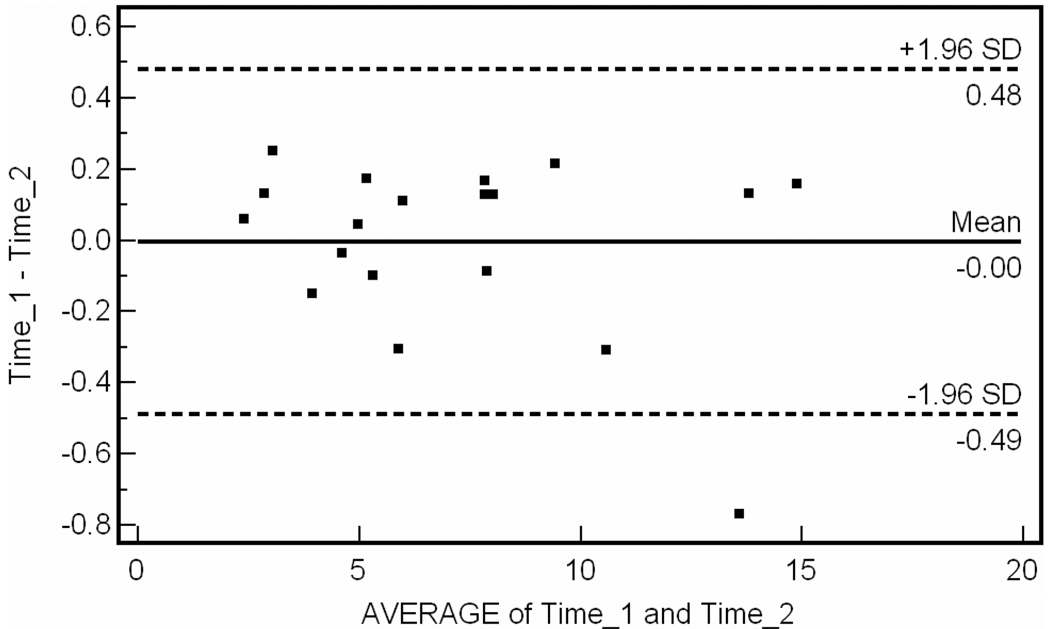
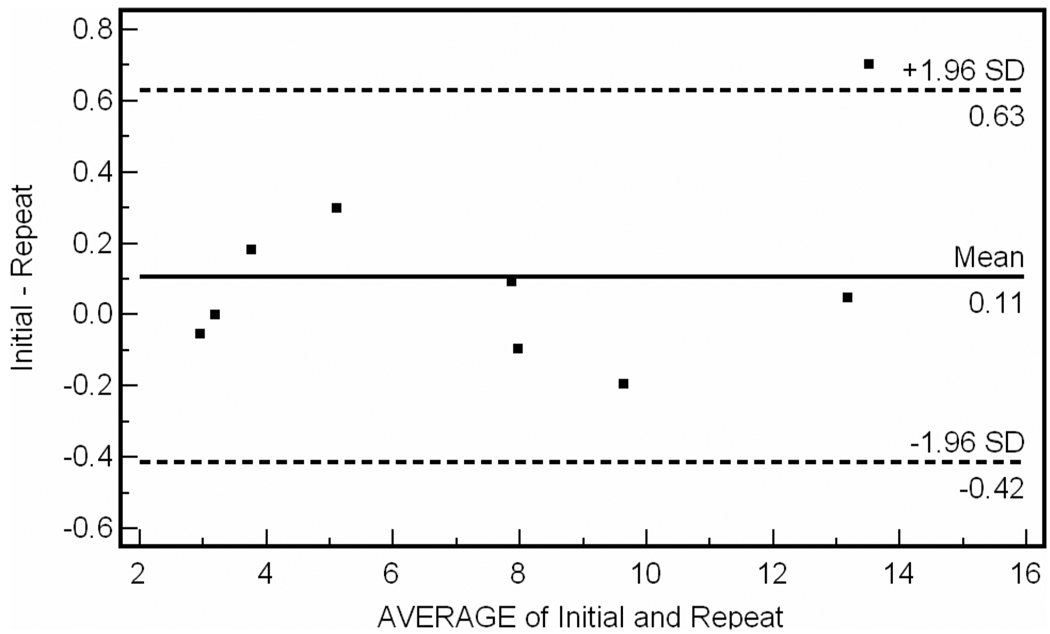
For the inter-observer data, intraclass correlation coefficient was R = 0.987 (95% CI 0.967–0.995). Bland-Altman plot for inter-observer variability is shown in Fig. 2. The percent difference between operator measurements was 5.2 ± 3.3% of the total plaque volume measured per patient. A single operator performed repeat analysis of the 19 vessel segments at a mean of 24 days later. For intra-observer data, intraclass correlation coefficient was R = 0.997 (95% CI 0.993 to 0.998). Bland-Altman plot for intra-observer data is shown in Fig. 3. For reproducibility data, intraclass correlation coefficient was R = 0.996 (95% CI 0.991 to 0.999). Bland-Altman analysis of test-retest reliability (n = 9) is shown in Fig. 4.
Figure 2.
Bland-Altman plot of inter-observer variability. Mean difference between different operator measurements is 0.19cm3.
Figure 3.
Bland-Altman plot of intra-observer variability. Mean difference between same operator measurements on single data-set is 0.01 cm3.
Figure 4.
Bland-Altman plot of test-retest reproducibility. Mean difference between test-retest is 0.11 cm3.
Sample size estimates for a study evaluating APV progression/regression were made using a paired t-test and standard deviation = 0.267 obtained from the test-retest reliability data (Table 2). These estimates assume all patients enrolled will have adequate image quality for analysis.
Table 2.
Sample size estimates
| Mean Δ APV | N (power = 0.80) | N (power = 0.90) |
|---|---|---|
| 1% | 107 | 142 |
| 2% | 27 | 36 |
| 3% | 12 | 16 |
| 5% | 5 | 6 |
DISCUSSION
The present study demonstrates that high resolution, high volume MR measurement of atherosclerotic plaque in the superficial femoral artery in peripheral arterial disease is feasible and highly reproducible and reliable. Potential applications include diagnosing preclinical vascular disease, assessing disease severity in those with established PAD, and monitoring atherosclerotic plaque progression in response to both established and novel therapies. Furthermore, this noninvasive approach could reduce required sample sizes for clinical studies using atherosclerotic plaque progression rate as a surrogate efficacy endpoint.
Contrast x-ray angiography remains the most commonly used clinical tool for diagnosing atherosclerosis and assessing its severity. The technique has also been used extensively in studies evaluating plaque progression rate (20–22) a dynamic parameter now established as an important surrogate marker of clinical risk (23). However, angiography is associated with the risk of procedural complications and is hampered by its inability to visualize the vessel wall, a pitfall that can lead to systematic underestimation of plaque burden (11). It is now recognized that atherosclerotic plaque in coronary arteries may not encroach the lumen until the lesion occupies up to 40% of the combined arterial wall and lumen volume, a phenomenon commonly referred to as positive remodeling (10). Positive remodeling also appears to play an important role in disease progression in the peripheral vasculature (24), suggesting that a technique capable of visualizing the vessel wall will be most accurate for the assessment of atherosclerotic disease burden in the periphery.
In recent years, IVUS has emerged as the diagnostic gold standard for imaging vessel wall pathology. IVUS is a catheter based imaging technique that provides cross-sectional images of both the vessel lumen and wall with excellent spatial resolution, but it is invasive and can be associated with vessel spasm, dissection, and guide wire entrapment (25, 26). Although increasingly employed to evaluate plaque burden and vascular remodeling as a surrogate efficacy endpoint in randomized clinical studies (23, 27, 28), IVUS is not an ideal strategy for making serial measurements in humans, prompting investigators to seek out novel noninvasive approaches for quantifying atheromatous plaque.
There are a number of advantages to using CMR in the study of human peripheral atherosclerosis. In addition to its excellent safety profile, recent technological advances in sequence design, imaging speed, and detection coils have made possible rapid, high-resolution imaging of atherosclerosis in-vivo (29). Furthermore, its sensitivity to different chemical components of tissue can be exploited to noninvasively characterize plaque composition (30–32). Enthusiasm for the MR approach has already led to several studies evaluating atherosclerotic plaque progression in humans (15–17), but few studies have evaluated the utility of CMR in measuring plaque in the peripheral circulation among PAD. In the most rigorously performed study to date, 7 patients with peripheral arterial occlusive disease were examined by both high-resolution MR imaging and IVUS (12). The MR approach consisted of a three-dimensional time-of-flight sequence used to measure vessel wall parameters with an in-plane resolution of 0.78 × 0.49 mm2 and slice thickness of 2 mm. High-resolution MR using this bright blood technique consistently overestimated plaque area in the peripheral vessels compared to IVUS measurements. Moreover, posterior echo shadowing precluded reliable IVUS analysis in nearly half of the segments assessed, also highlighting the limitations of IVUS in PAD.
We sought to develop an approach to high resolution assessment of plaque volume in the peripheral circulation of patients with mild to moderate PAD. To facilitate this, a flexible, linear four-element surface array coil was designed and built specifically for this protocol. In order to clearly delineate both the luminal and adventitial border of the vessel wall, a black blood technique with fat saturation was chosen. Rather than using double inversion recovery sequences to generate black blood, flow saturation bands were applied in order to reduce acquisition times. With this protocol, nearly 17 cm of the SFA was imaged in just over 13 minutes with excellent in-plane spatial resolution.
There are several potential advantages of imaging plaque volume in the SFA. The lack of SFA movement can significantly limit artifacts compared to imaging in other vascular territories such as the carotids, coronaries, and aorta which can be limited by swallowing or respiratory motion. Also, the relative proximity of the arteries in the leg to the body surface allows the utilization of high-resolution surface coils like the one custom-built for this protocol. Perhaps the biggest advantage of imaging plaque in the SFA is the sheer volume of atheroma that can be measured, 7.27 ± 3.73 cm3 in this study, several orders of magnitude greater than volumes reported in either the carotid or coronary circulations. The large volume of plaque that can be quantified and excellent reproducibility of the measurements would reduce sample sizes needed to adequately power clinical studies using atherosclerotic plaque progression as a surrogate endpoint (Table 2).
When estimating the potential impact of this technique on sample size estimates for clinical studies, one must consider the other noninvasive strategies currently being employed. Using carotid intimal medial thickness measurements as a surrogate efficacy end-point, Bots et al. reported that 468 patients would be required to detect a treatment effect of 30% (power 0.80) (33). Three-dimensional ultrasound of plaque in the carotids would require fewer patients to demonstrate efficacy, yet a recent study reported that 203 patients would still be needed to detect a 10% change in plaque volume (power = 0.90) measured over a three month period (34). Measuring the progression of coronary calcium with cardiac computed tomography has been proposed as yet another noninvasive alternative to evaluating disease progression, yet sample sizes required for these studies remain large, and there is the strong suggestion that calcification represents a fundamentally distinct biological process which may correlate less with clinical events than plaque volume progression (35–37). Sample size estimates for our noninvasive approach are similar to that reported using coronary IVUS (27).
Limitations
Using the techniques described, the ability to accurately define the luminal border of the vessel was predicated on the generation of black blood. Whether using inversion recovery or flow saturation bands, as done in this study, black blood contrast requires adequate inflow from outside of the selected slice. Even in this population of patients with mild to moderate disease, 17% had near-complete or complete occlusion of the SFA making images impossible to analyze. The reliability and reproducibility of this imaging strategy may suffer further when performed in patients with more advanced vascular disease and significantly reduced flow with potentially higher rates of SFA occlusion. While techniques that do not rely on the generation of black blood (steady-state free precession) to define the lumen may be adequate in patients with advanced disease and very slow blood flow, these techniques will still be insufficient in the setting of complete occlusions.
Because we utilized a small-element surface coil, vessel image quality suffered in more obese patients. However, only one patient in this study could not be analyzed as a consequence of obesity, and two individuals with BMI > 50 were included in the analysis. A larger coil array and longer scan times with more signal averages would improve image quality in larger patients. We also found that image quality diminished in the distal 1/4 of the SFA in some patients, typically as the vessel coursed deeper into the tissue and posteriorly into the popliteal fossa. This could be problematic as the adductor canal region of the SFA has a known predilection for atherosclerosis and is a common site of disease progression (38, 39). In the coronary vasculature, even modest changes in plaque progression may be associated with large differences in vascular outcomes (20, 21, 23). Whether this relationship between plaque progression and vascular events holds true in the periphery remains undetermined.
Sample size estimates are based on plaque volumes measured in patients with established PAD. In clinical studies evaluating therapeutic efficacy among patients with subclinical atherosclerosis, larger total sample sizes may be required using our approach because of smaller total plaque burden in this population. While required sample sizes may be reduced with measurement of plaque volume progression in the SFA, total imaging costs may be similar to that of other techniques because of the increased expense of CMR. However, these imaging costs would be small compared to the potential savings realized by having a substantially smaller patient cohort to enroll, image, analyze, and follow.
Future directions
We have demonstrated that accurate noninvasive quantification of peripheral plaque burden is feasible in PAD. This approach could be used to better understand the natural history of PAD and characterize plaque volume and distribution in different PAD patient subsets, including those who are asymptomatic, have atypical symptoms, or are diabetic. We also hope to identify plaque characteristics that can be used to recognize PAD at highest risk for adverse clinical outcomes in the lower extremities. Furthermore, serial noninvasive measurement of plaque volume with CMR could allow for the study and validation of both established and novel therapies for PAD, including behavioral, pharmacologic, genetic, stem cell, and percutaneous interventions. Correlation of plaque progression in the peripheral circulation with vascular events in the coronary, cerebral, and peripheral circulation is also warranted.
Acknowledgments
Supported by NIH RO1 HL075792 (CMK) and AHA 0425674U (DCI).
REFERENCES
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH. Systemic atherosclerosis risk and the mandate for intervention in atherosclerotic peripheral arterial disease. Am J Cardiol. 2001;88:43J–47J. doi: 10.1016/s0002-9149(01)01881-1. [DOI] [PubMed] [Google Scholar]
- 3.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death:a cohort study. BMJ. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis:the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 6.Mohler ER, III, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 7.Nehler MR, Hiatt WR. Exercise therapy for claudication. Ann Vasc Surg. 1999;13:109–114. doi: 10.1007/s100169900228. [DOI] [PubMed] [Google Scholar]
- 8.Regensteiner JG, Ware JE, Jr, McCarthy WJ, Zhang P, Forbes WP, Heckman J, Hiatt WR. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease:meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002;50:1939–1946. doi: 10.1046/j.1532-5415.2002.50604.x. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease:associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 10.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 11.McPherson DD, Hiratzka LF, Lamberth WC, Brandt B, Hunt M, Kieso RA, Marcus ML, Kerber RE. Delineation of the extent of coronary atherosclerosis by high-frequency epicardial echocardiography. N Engl J Med. 1987;316:304–309. doi: 10.1056/NEJM198702053160604. [DOI] [PubMed] [Google Scholar]
- 12.Meissner OA, Rieger J, Rieber J, Klauss V, Siebert U, Tato F, Pfeifer KJ, Reiser M, Hoffmann U. High-resolution MR imaging of human atherosclerotic femoral arteries in vivo:validation with intravascular ultrasound. J Vasc Interv Radiol. 2003;14:227–231. doi: 10.1097/01.rvi.0000058325.82956.63. [DOI] [PubMed] [Google Scholar]
- 13.Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, Aguinaldo JG, Badimon JJ, Sharma SK. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2002;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 14.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106:296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 15.Corti R, Fayad ZA, Fuster V, Worthley SG, Helft G, Chesebro J, Mercuri M, Badimon JJ. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions:a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 16.Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Chaplin WF, Muntwyler J, Viles-Gonzalez JF, Weinberger J, Smith DA, Mizsei G, Badimon JJ. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions:a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2005;46:106–112. doi: 10.1016/j.jacc.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Lima JA, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336–2341. doi: 10.1161/01.CIR.0000145170.22652.51. [DOI] [PubMed] [Google Scholar]
- 18.Wyttenbach R, Gallino A, Alerci M, Mahler F, Cozzi L, Di Valentino M, Badimon JJ, Fuster V, Corti R. Effects of percutaneous trans-luminal angioplasty and endovascular brachytherapy on vascular remodeling of human femoropopliteal artery by noninvasive magnetic resonance imaging. Circulation. 2004;110:1156–1161. doi: 10.1161/01.CIR.0000140672.70862.5B. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 20.Azen SP, Mack WJ, Cashin-Hemphill L, LaBree L, Shircore AM, Selzer RH, Blankenhorn DH, Hodis HN. Progression of coronary artery disease predicts clinical coronary events. Long term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 21.Waters D, Craven TE, Lesperance J. Prognostic significance of progression of coronary atherosclerosis. Circulation. 1993;87:1067–1075. doi: 10.1161/01.cir.87.4.1067. [DOI] [PubMed] [Google Scholar]
- 22.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 23.Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis:a randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 24.Vink A, Schoneveld AH, Borst C, Pasterkamp G. The contribution of plaque and arterial remodeling to de novo atherosclerotic luminal narrowing in the femoral artery. J Vasc Surg. 2002;36:1194–1198. doi: 10.1067/mva.2002.128300. [DOI] [PubMed] [Google Scholar]
- 25.Batkoff BW, Linker DT. Safety of intracoronary ultrasound:data from a Multicenter European Registry. Cathet Cardiovasc Diagn. 1996;38:238–241. doi: 10.1002/(SICI)1097-0304(199607)38:3<238::AID-CCD3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Hausmann D, Erbel R, Alibelli-Chemarin MJ, Boksch W, Caracciolo E, Cohn JM, Clup SC, Daniel WG, De S I DiMario C. The safety of intracoronary ultrasound. A multicener survey of 2207 examinations. Circulation. 1995;91:623–630. doi: 10.1161/01.cir.91.3.623. [DOI] [PubMed] [Google Scholar]
- 27.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes:a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 28.Tardif JC, Gregoire J, Lesperance J, Lambert J, L'Allier PL, Rodes J, Anderson T, Blue JW, Imus J, Heinonen T. Design features of the Avasimibe and Progression of coronary Lesions assessed by intravascular UltraSound (A-PLUS) clinical trial. Am Heart J. 2002;144:589–596. doi: 10.1067/mhj.2002.125329. [DOI] [PubMed] [Google Scholar]
- 29.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque:Part II:approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 30.Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001;89:305–316. doi: 10.1161/hh1601.095596. [DOI] [PubMed] [Google Scholar]
- 31.Kramer CM, Cerilli LA, Hagspiel K, Dimaria JM, Epstein FH, Kern JA. Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation. 2004;109:1016–1021. doi: 10.1161/01.CIR.0000116767.95046.C2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers WJ, Prichard JW, Hu YL, Olson PR, Benckart DH, Kramer CM, Vido DA, Reichek N. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler Thromb Vasc Biol. 2000;20:1824–1830. doi: 10.1161/01.atv.20.7.1824. [DOI] [PubMed] [Google Scholar]
- 33.Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies:design options, progression rates, and sample size considerations:a point of view. Stroke. 2003;34:2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke. 2005;36:1904–1909. doi: 10.1161/01.STR.0000178543.19433.20. [DOI] [PubMed] [Google Scholar]
- 35.James G, Raggi P. Electron beam tomography as a non invasive method to monitor effectiveness of antiatherosclerotic therapy. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:177–181. doi: 10.2174/1568006043336366. [DOI] [PubMed] [Google Scholar]
- 36.Demer LL, Tintut Y. Mineral exploration:search for the mechanism of vascular calcification and beyond:the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–1743. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 37.Schmermund A, Achenbach S, Budde T, Buziashvili Y, Forster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V, Lahiri A, Leischik R, Moshage W, Schartl M, Siffert W, Steinhagen-Thiessen E, Sinitsyn V, Vogt A, Wiedeking B, Erbel R. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months:a multicenter, randomized, double-blind trial. Circulation. 2006;113:427–437. doi: 10.1161/CIRCULATIONAHA.105.568147. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DB, Powell RJ, Stukel TA, Henderson EL, Cronenwett JL. Superficial femoral artery stenoses:characteristics of progressing lesions. J Vasc Surg. 1997;25:512–521. doi: 10.1016/s0741-5214(97)70262-3. [DOI] [PubMed] [Google Scholar]
- 39.Watt JK. Origin of femoro-popliteal occlusions. Br Med J. 1965;5476:1455–1459. doi: 10.1136/bmj.2.5476.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]