Abstract
Here, we examined the status of stromal Cav-1 expression in patients with benign prostatic hypertrophy (BPH), primary prostate cancers (PCa), and prostate-cancer metastases (Mets). Interestingly, an absence of stromal Cav-1 directly correlated with prostate cancer disease progression. For example, virtually all BPH samples showed abundant stromal Cav-1 immunostaining. In contrast, in a subset of patients with primary prostate cancer, the stromal levels of Cav-1 were significantly decreased, and this correlated with a high Gleason score, indicative of a worse prognosis and poor clinical outcome. Remarkably, all metastatic tumors (either from lymph node or bone) were completely negative for stromal Cav-1 staining. Thus, stromal Cav-1 expression may be considered as a new biomarker of prostate cancer disease progression and metastasis. Mechanistically, stromal Cav-1 levels were inversely correlated with the epithelial expression levels of Cav-1 and epithelial phospho-Akt. Thus, loss of stromal Cav-1 is predictive of elevated levels of epithelial Cav-1 and epithelial Akt-activation. This provides important new clinical evidence for paracrine signaling between prostate cancer epithelial cells and the tumor stromal micro-environment, especially related to disease progression and metastasis.
Keywords: caveolin-1, prostate cancer, tumor progression, metastasis, Akt signaling
Introduction
Most tumors evolve in a stromal micro-enviroment that contains tissue fibroblasts, macrophages, smooth muscle and endothelial cells, among other cell types.1,2 In this epithelial-stromal context, is has now been recognized that tumor-associated fibroblasts play a major role in both tumor initiation and progression.3 However, markers of the cancer-associated fibroblast phenotype have been lacking and are difficult to define experimentally.
We and others have recently proposed that an absence of caveolin-1 (Cav-1) may be a marker of the cancer-associated fibroblast phenotype, as it relates to DCIS progression and breast cancer metastasis.4–9 For example, many different pro-proliferative and transforming stimuli (such as H-Ras (G12V), PDGF, FGF or p53 loss) all result in a loss of Cav-1 expression in stromal fibroblasts in culture.10–14 Similarly, cancer-associated fibroblasts prepared from primary breast cancers also show a significant reduction in Cav-1 expression (in 8 out of 11 patients examined).4 Finally, deletion of the Cav-1 gene is sufficient to confer the cancer-associated fibroblast phenotype upon mammary stromal fibroblasts in culture.5 Thus, a loss of Cav-1 expression appears to be a functional marker of the cancer-associated fibroblast phenotype, at least in breast cancers (reviewed in ref. 8).
To address the possible clinical relevance of stromal Cav-1 as a biomarker, we and others have now assessed its prognostic value in both breast cancer6,9 and DCIS patients.7 In breast cancer patients, an absence of stromal Cav-1 was associated with advanced tumor stage, increased nodal stage, early tumor recurrence, and metastasis, resulting in poor clinical outcome.6 In DCIS patients, an absence of stromal Cav-1 was specifically associated with progression to invasive breast cancer.7 Thus, an absence of stromal Cav-1 may be involved in both tumor initiation, and tumor progression/metastasis. However, these findings have not yet been applied to other types of cancer.
One hypothesis is that loss of stromal Cav-1 may be a “universal” or “widely-applicable” biomarker for many different types of cancer.8 Here, we assess the status of stromal Cav-1 in human prostate cancers. Our results indicate that a loss of stromal Cav-1 is associated with advanced prostate cancer and metastatic disease, as compared with benign patient samples processed in parallel using a tissue microarray (TMA) format.
Results
Stromal Cav-1 expression correlates with disease progression and metastasis in prostate cancer patients
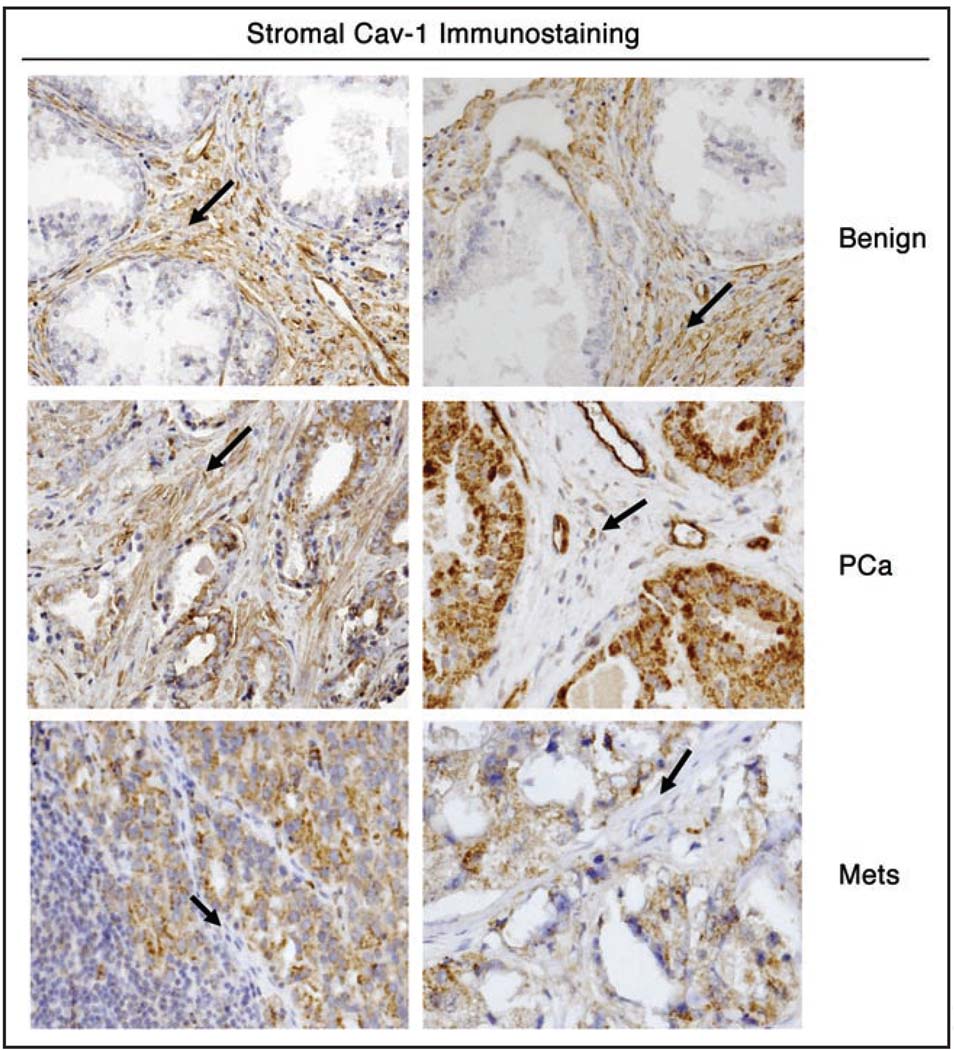
We established a stromal Cav-1 grading scale (0, 1 and 2), with 0 representing an absence of stromal Cav-1, and 1 and 2 representing moderate and high levels of stromal Cav-1, respectively. Representative examples of stromal Cav-1 immuno-staining in benign, primary, and metastatic prostate tumor sections are shown in Figure 1. Expression of stromal Cav-1 was significantly reduced in PCa in comparison with benign tissues, and in metastatic tumors (Mets) in comparison with PCa samples (Table 1).
Figure 1.
Expression of stromal Cav-1 in benign, primary prostate cancers and metastatic tumor samples. Two images are shown for each diagnostic category. Note that benign prostate samples abundantly express stromal Cav-1. Primary prostate cancers showed differential expression of stromal Cav-1, with either high (left), moderate (not shown), or absent/low expression (right). Finally, metastatic tumor samples [either from lymph node (at left) or bone (at right)] showed an absence of stromal Cav-1 staining. Note that in all cases, the vasculature remained Cav-1 positive. Arrowheads point at the tumor stroma in all six panels.
Table 1.
(A) Association of stromal Cav-1 with tumor progression; (B) Significant differences in stromal Cav-1 between different patient groups
| A | Stromal Cav-1 Level | |||
|---|---|---|---|---|
| 0 | 1 | 2 | p-value | |
| N = 53 | N = 31 | N = 13 | ||
| Tumor Progression | 3.11 × 10−17 | |||
| Benign | 4% (2) | 52% (16) | 92% (12) | |
| PCa | 32% (17) | 48% (15) | 8% (1) | |
| Mets | 64% (34) | 0% (0) | 0% (0) | |
| B | p-value |
|---|---|
| Benign vs PCa | 7.43 × 10−6 |
| Benign vs Mets | 3.89 × 10−16 |
| PCa vs Mets | 1.04 × 10−6 |
Test used: Fisher’s exact test
In fact, stromal Cav-1 was present in nearly all benign samples, with 12 cases showing high levels (score = 2) and 16 showing moderate levels (score = 1). Only 2 benign samples exhibited an absence (score = 0) of the protein in the stromal compartment. In contrast, stromal Cav-1 was present at high levels (score = 2) in only 1 localized prostate cancer sample (PCa), at moderate levels in 15 PCa samples, and was clearly absent in 17 PCa samples. Strikingly, stromal Cav-1 was absent in all of the 34 metastatic tumors examined (Table 1). These results provide strong evidence that an absence of stromal Cav-1 is specifically associated with prostate cancer progression and the spread of metastatic disease.
When we examined stromal Cav-1 expression exclusively in the organ-confined prostate cancer group, we observed a significant association with Gleason grading. Expression of stromal Cav-1 was significantly reduced in samples with a Gleason score of 4 + 3 or higher, in comparison with samples with Gleason scores of 3 + 4 or lower, suggesting that the levels of stromal Cav-1 might help identify aggressive PCa at a relatively early phase of the disease (Table 2). A high Gleason score is predictive of poor clinical outcome in prostate cancer patients.15 For example, if you compare patients with a Gleason score of 4 + 3, with patients with a Gleason score of 3 + 4, there is a 3-fold increase in the hazard ratio for lethal prostate cancer.15
Table 2.
Association of stromal Cav-1 with gleason score (GS)
| Stromal Cav-1 Status | ||
|---|---|---|
| GS | Absent | Present |
| 3+3 - 3+4 | 18% (3) | 69% (11) |
| 4+3 - 5+5 | 82% (14) | 31% (5) |
Test used: Fisher’s exact test
p-value = 4.9 × 10−3
Stromal Cav-1 levels are inversely correlated with epithelial Cav-1 expression and epithelial Akt activation, during prostate cancer disease progression and metastasis
Interestingly, we and others have previously demonstrated that the levels of epithelial Cav-1 and epithelial phospho-Akt (p-Akt), in the same patient cohort used here, are significantly increased with Gleason progression.16,17 To assess any potential correlations, these mean intensity values, available for epithelial Cav-1 and p-Akt, were dichotomized.
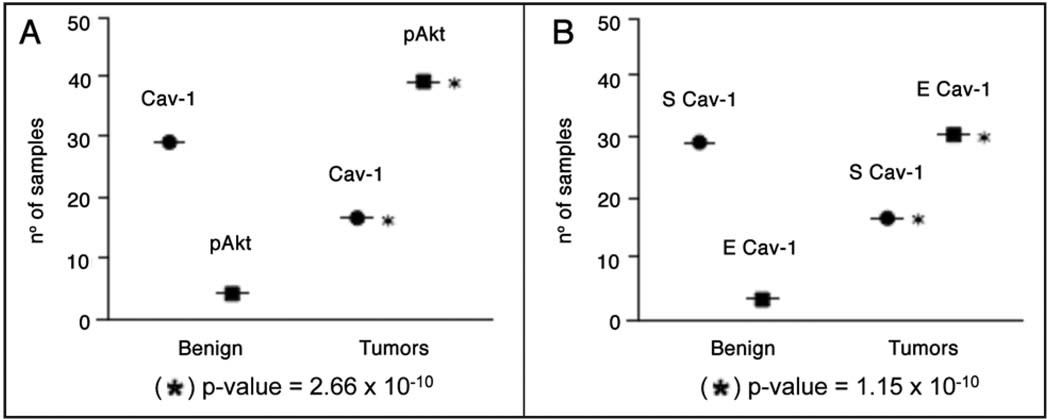
Remarkably, we found that the expression of stromal Cav-1 was inversely correlated with the levels of p-Akt in the prostate epithelium both in benign and tumor tissues (Table 3). There was also a significant inverse correlation between stromal Cav-1 and epithelial Cav-1 (Table 4). These results are depicted graphically in Figure 2.
Table 3.
Inverse correlation between stromal Cav-1 and epithelial p-Akt during tumor progression
| Stromal Cav-1 Status | p-Akt Status | |||
|---|---|---|---|---|
| Absent (0) | Present (1+2) | Absent (0) | Present (1) | |
| N = 53 | N = 44 | N = 58 | N = 43 | |
| Tumor Progression | ||||
| Benign | 4% (2) | 64% (28) | 45% (26) | 9% (4) |
| PCa | 32% (17) | 36% (16) | 26% (15) | 46% (20) |
| Mets | 64% (34) | 0% (0) | 29% (17) | 45% (19) |
Test used: Fisher’s exact test
Stromal Cav-1 status, absent versus present; p-value = 4.21 × 10−16
p-Akt status, absent versus present; p-value = 3.63 × 10−4
Stromal Cav-1 versus p-Akt; p-value = 2.66 × 10−10
Table 4.
Inverse correlation between stromal Cav-1 and epithelial Cav-1 during tumor progression
| Stromal Cav-1 Status | Epithelial Cav-1 Status | |||
|---|---|---|---|---|
| Absent (0) | Present (1+2) | Absent (0) | Present (1) | |
| N = 53 | N = 44 | N = 63 | N = 33 | |
| Tumor Progression | ||||
| Benign | 4% (2) | 64% (28) | 40% (25) | 9% (3) |
| PCa | 32% (17) | 36% (16) | 35% (22) | 33% (11) |
| Mets | 64% (34) | 0% (0) | 25% (16) | 58% (19) |
Test used: Fisher’s exact test
Stromal Cav-1 status, absent versus present; p-value = 4.21 × 10−16
Epithelial Cav-1 status, absent versus present; p-value = 1.14 × 10−3
Stromal Cav-1 versus Epithelial Cav-1; p-value = 1.15 × 10−10
Figure 2.
Stromal Cav-1 inversely correlates with epithelial Akt activation and epithelial Cav-1 overexpression in advanced prostate cancer and metastasis. Data presented in Table 3 (A) and Table 4 (B) are plotted here graphically. Only patients with positive expression are shown. For simplicity, primary prostate cancers and metastastic tumors are plotted as one group as “Tumors”, versus “Benign” lesions. In (A), Cav-1 refers to stromal Cav-1. In (B), E denotes epithelial, and S denotes stromal staining. Asterisks denote statistical significance.
Thus, a loss of stromal Cav-1 is predictive of elevated levels of epithelial Cav-1 and epithelial Akt-activation, in both primary prostate cancers and metastatic tumor tissue. This provides novel evidence for “heterotypic” signaling between epithelial cancer cells and the tumor stroma, specifically during prostate cancer disease progression and metastasis.
Discussion
Several distinct models have been proposed to explain the metastatic spread of primary tumor cells to both local and distant sites. Increasingly, it has become clear that the tumor micro-enviroment may play a critical role in this process of cancer cell dissemination. This may occur through epithelial-stromal interactions, via the paracrine secretion of growth factors by stromal cells, which then induce an epithelial-mesenchymal transition (EMT) in neighboring epithelial cells. This could then result in the increased migration of epithelial cancer cells, and may also enhance their invasive properties.
However, a completely new idea regarding epithelial cancer cell metastasis has recently been proposed.18,19 In this new mechanism, epithelial cancer cells would not be required to undergo an EMT, but instead would simply “hitch-a-ride” with fibroblasts.18,19 In this regard, Gaggioli et al. showed that epithelial cancer cells can attach themselves to migrating stromal fibroblasts, and these stromal fibroblasts can then carry epithelial cancer cells.18,19 While this new model of cancer cell metastasis is quite attractive, no clinical data has been presented to support such a model.
Here, we show that loss of stromal Cav-1 is a marker of advanced prostate cancer and metastatic disease spread. Based on these current findings, we would propose that “generic” cancer-associated fibroblasts lack caveolin-1 expression, and that these Cav-1 negative fibroblasts may represent the “metastatic fibroblasts” in the model proposed by Gaggioli et al.18,19
In support of this notion, we have previously shown that breast cancer-associated fibroblasts show significant downregulation of Cav-1.4 Furthermore, deletion of Cav-1 in mammary stromal fibroblasts is sufficient to mimic the cancer-associated fibroblast phenotype.5 Thus, future studies will be necessary to assess the status of Cav-1 expression in cancer-associated fibroblasts derived from human prostate cancers. However, our current clinical data would be consistent with a role for Cav-1 negative stromal fibroblasts in promoting prostate cancer cell metastasis, regardless of the exact mechanism.
An absence of stromal Cav-1 directly correlated with increased Gleason scores (GS), which is still one of the most important predictors of poor clinical outcome. Although the significance of the predominance of pattern 4 in GS 7 has been controversial, it is now clear that 4 + 3 cancers are associated with a 3-fold increase in lethal PCa in comparison with 3 + 4 cancers.15 Importantly, a significant reduction in stromal Cav-1 expression was observed in PCa with GS 4 + 3 in comparison with GS 3 + 4, strongly suggesting that an absence of stromal Cav-1 could help in identifying tumors with a potentially aggressive course.
Interestingly, all prostate-cancer derived metastatic tumor samples (from lymph node and bone) showed an absence of stromal Cav-1 expression. Mechanistically, an absence of stromal Cav-1 was inversely correlated with epithelial Cav-1 expression and epithelial Akt activation, in both primary and metastatic tumor samples. Thus, an absence of stromal Cav-1 is predictive of increased epithelial Cav-1 expression, and activated epithelial Akt signaling.
In this regard, it is important to note that increased levels of epithelial Cav-1 discriminated between localized and metastatic PCa in the same cohort of patients,17 and Cav-1 is a valuable serum marker of metastatic disease.20,21 Expression of activated Akt has also been shown to have significant predictive value for prostate cancer recurrence22,23 and to discriminate between hormone-naïve (HN) and hormone resistant (HR) metastases in the same cohort of patients.16
Thus, stromal Cav-1 expression may be considered as a new biomarker of prostate cancer disease progression and metastasis.
Materials and Methods
Case selection and tissue microarray construction
The human prostate tissue microarray (TMA)16,17 consists of benign and prostate tumor tissues from the Brigham and Women’s Hospital (Boston, MA), the University of Michigan (Ann Arbor, MI), and the University of Ulm (Ulm, Germany) and is composed of benign prostate (n = 30), localized PCa (n = 36), and metastases (n = 36). The metastatic cohort consists of 18 lymph-node hormone naïve and 18 distant metastases. For the tissue microarray construction, tissue cores (0.6 mm diameter) were sampled in quadruplicate from each block to account for tumor and tissue heterogeneity and transferred to the recipient block. One pathologist (Dolores Di Vizio, MD-PhD) excluded cases if adequate paraffin-embedded tumor tissue was not available; consequently a total of 30 benign, 33 organ-confined tumor and 34 metastates were included in the analysis.
Immunohistochemistry
Cav-1 expression in the normal and tumor stroma was assessed on 5 µm sections using a standard immuno-peroxidase method (DakoCytomation LSAB2 System-HRP, Carpinteria, CA). A rabbit polyclonal anti-Cav-1 IgG (N-20; directed against N-terminal residues 2–21 of human Cav-1; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:500) was chosen. The staining was scored semi-quantitatively as negative (0; no staining), moderate (1; either diffuse weak staining or strong staining in less than 30% of stromal cells per core), and strong (2; defined as strong staining of 30% or more of the stromal cells) metastasis.6–8 These were given numerical raw scores of 0, 1 and 2, respectively, and the median score was taken as the final score for the sample. Immunohistochemical analysis for epithelial p-Akt and epithelial Cav-1, analyzed using the ACIS System (ChromaVision Medical System, Inc.,) has been previously described.16,17
Statistical analysis
Results from IHC observation were grouped according to the presence of stromal Cav-1. Only cases with two or three cores containing tumor stromal cells were considered for statistical analysis in order to address possible heterogeneity of the staining in various tumor portions. Association between Stromal Cav-1 level and tumor progression was evaluated using Fisher’s exact test for categorical data. To assess potential correlations between Stromal Cav-1 and p-Akt, intensity mean values of p-Akt were dichotomized and Fisher exact test was applied on contingency tables. Summary data were expressed as percentage compared to the total. Distributions were considered 2-tailed and statistical significance was defined as p-value <0.001. Origin 8 software (OriginLab Corporation, Northampton, MA) was used for statistical analysis.
Acknowledgments
We would like to thank Dr. Abhijit Dasgupta for his critical review of the manuscript.
D.D.V. was supported by a K99 Award from the National Cancer Institute (NCI; CA-131472). M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-80250; R01-CA-098779; R01-CA-120876). M.R.F. was supported a grant from the NIH (NCI; R01-CA-112303). F.S. was supported by a Research Scholar Grant from the American Cancer Society (ACS). R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072, and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.).
This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronnov-Jessen L, Bissell MJ. Breast cancer by proxy: can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Allen KG, et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, et al. Caveolin-1−/− null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174:746–761. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1073–1081. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 8.Witkiewicz AK, Casimiro MC, Dasgupta A, Mercier I, Wang C, Bonuccelli G, et al. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009;8:1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 9.Sloan EK, Ciocca D, Pouliot N, Natoli A, Restall C, Henderson M, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 12.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, et al. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, et al. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: Role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J Biol Chem. 2004;279:24745–24756. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 15.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, et al. Gleason score and lethal prostate cancer: Does 3 + 4 = 4 + 3? J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.4669. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N, et al. The proapoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J. 2007;26:4523–4534. doi: 10.1038/sj.emboj.7601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, et al. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 18.Gaggioli C. Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell Adh Migr. 2008;2:45–47. doi: 10.4161/cam.2.1.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 20.Tahir SA, Frolov A, Hayes TG, Mims MP, Miles BJ, Lerner SP, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res. 2006;12:4872–4875. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 21.Tahir SA, Ren C, Timme TL, Gdor Y, Hoogeveen R, Morrisett JD, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9:3653–3659. [PubMed] [Google Scholar]
- 22.Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, et al. High levels of phosphorrylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 23.Shukla-Dave A, Hricak H, Ishill N, Moskowitz CS, Drobnjak M, Reuter VE, et al. Prediction of prostate cancer recurrence using magnetic resonance imaging and molecular profiles. Clin Cancer Res. 2009;15:3842–3849. doi: 10.1158/1078-0432.CCR-08-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]