Summary
Tagging the cell surface receptor with ubiquitin is believed to provide a signal for the endocytic pathway. E3 ubiquitin ligases such as Cbl-b and Itch have been implicated in T cell activation and tolerance induction. However, the underlying mechanisms remain unclear. Here we describe that in mice deficient in the E3 ubiquitin ligases Cbl-b and Itch, T cell activation was augmented, accompanied by spontaneous autoimmunity. The double mutant T cells exhibited increased phosphorylation of the T cell receptor-ζ (TCR-ζ) chain, whereas the endocytosis and stability of the TCR complex were not affected. TCR-ζ was polyubiquitinated via a K33-linkage, which affected its phosphorylation and association with the ζ chain-associated protein kinase Zap-70. The juxtamembrane K54 residue in TCR-ζ was identified to be a primary ubiquitin conjugation site, whose mutation increased its phosphorylation and association of TCR-ζ and Zap-70. Thus, the present study reveals unconventional K33-linked polyubiquitination in non-proteolytic regulation of cell surface receptor-mediated signal transduction.
Introduction
Protein ubiquitination represents a means of post-translational modulation by tagging the 76 amino acid small molecule ubiquitin (Ub) to a protein substrate. Ubiquitination involves a cascade of well-defined biochemical reactions through E1, E2 and E3 enzymes (Hershko and Ciechanover, 1998). The E3 Ub ligases are critical components in this system and specifically target the substrates. Based on their structural features, E3 ligases are generally classified into RING (really interesting new gene)-type and HECT (homologous to the E6 associated protein C-terminus)-type E3s (Kerscher et al., 2006; Pickart, 2001; Weissman, 2001). Ub conjugation is involved in diverse biological responses from yeast to man. Not surprisingly, the Ub system also plays an important role in the immune system, including lymphocyte development and activation, intracellular signal transduction, antigen presentation, and immune evasion (Liu, 2004). Although protein ubiquitination has been historically considered to be important for “garbage disposal” through proteasomal degradation, it is beginning to be appreciated as a means for functional modifications including receptor downmodulation, protein-protein interactions, or gene transcription (Chen and Sun, 2009).
One of the well-characterized RING-type E3 families in the immune system is the family of Cbl proteins including Cbl, Cbl-b and Cbl-c (Thien and Langdon, 2001). Loss of Cbl-b results in hyper-responsiveness and full T cell activation in the absence of CD28 co-stimulation (Bachmaier et al., 2000; Chiang et al., 2000). Cbl-b acts as an E3 ligase for a number of intracellular molecules such as phosphatidylinositol 3 (PI3)-kinase and affects the recruitment of PI3-kinase to the upstream molecules (Fang and Liu, 2001). Itch is an example of HECT-type E3 ligase whose mutation results in abnormal immunological and inflammatory responses, leading to skin-scratching itchy phenotype (Perry et al., 1998). Itch has been shown to target Jun family proteins, c-Jun and JunB and influence T cell proliferation and T helper type 2 (Th2) cell cytokine production (Fang et al., 2002). In addition, Itch is functionally regulated by upstream kinases via direct phosphorylation (Gao et al., 2004; Yang et al., 2006).
Previous studies have suggested that Itch and Cbl-b are involved in the process of T cell tolerance induction via inducing T cell anergy (Heissmeyer et al., 2004; Jeon et al., 2004; Venuprasad et al., 2006) and by regulating Foxp3 expression in induced regulatory T cells (Venuprasad et al., 2008). In addition, a previous biochemical study has shown that a Cbl family protein, Cbl-c, and perhaps other Cbl members, physically interact with Itch (Courbard et al., 2002). Intriguingly, it was also shown that HECT-type E3s like Nedd4 or Itch were shown to target Cbl or Cbl-b for ubiquitination and subsequent degradation (Magnifico et al., 2003; Yang et al., 2008). Thus, the exact biological function of the Itch and Cbl-b interaction remains to be established. Here we provide genetic evidence that deletion of both Cbl-b and Itch leads to augmented T cell activation and spontaneous autoimmunity. Surprisingly, the double-deficient T cells display augmented phosphorylation of T cell receptor-ζ (TCR-ζ) without affecting its down-modulation. The present study characterizes Ub K33-linked polyubiquitination of TCR-ζ, and points out the important role of unconventional cell surface receptor ubiquitination in regulating T cell activation in a proteolysis-independent manner.
Results
Spontaneous autoimmunity and augmented T cell activation in double-deficient mice
In this study, we crossed Cblb−/−C57BL/6 mice with itchy (Itch−/−) mice to generate wild-type (WT), Cblb−/−, Itch−/−, or Cbl-b and Itch double-deficient (Cblb−/−Itch−/−) mice. The most obvious observation was the enlarged spleen in 6 to 8 week-old double-deficient mice, which was 4~6 times bigger than the WT control littermates (Fig. S1A), whereas the spleen size of Cblb−/− mice was similar as WT mice, and the spleen of Itch−/− mice was about 2 times bigger than the control mice. The sizes of lymph nodes in double-deficient mice were also enlarged, as compared with the WT, Cblb−/−, or Itch−/− mice. The total cell numbers were counted in both spleens and lymph nodes and showed a proportional increases relative to the sizes of these lymphoid organs (Fig. S1B). We also examined the percentage of CD4+ or CD8+ T cells in the spleen of double-deficient mice and the ratio of CD4+ vs. CD8+ peripheral mature T cells remained largely unchanged in double-deficient mice, in comparison to the WT, Cblb−/−, or Itch−/− mice (Fig. S1C).
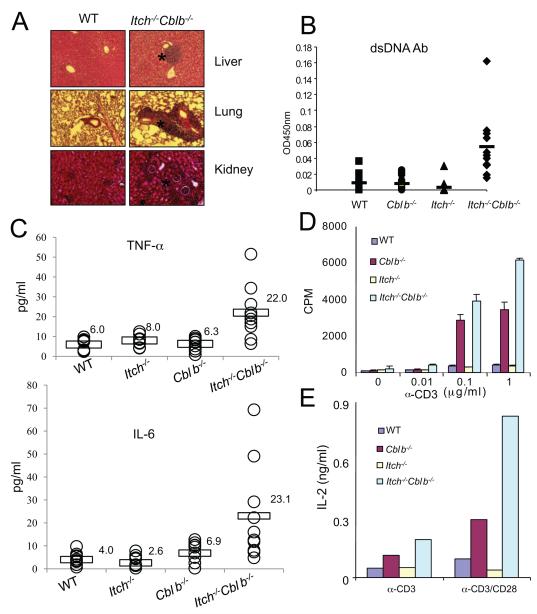
We next performed histological examination of various organs from WT and the mutant mice. Although not obvious in the single gene-targeted mice, there were massive lymphocyte infiltrations in multiple organs including the liver, lung, and kidney in double-deficient mice of 3 months of age (Fig. 1A). The double-deficient mice also exhibited higher concentrations of anti-double-stranded DNA antibodies in the sera compared with the WT, Cblb−/− or Itch−/− mice (Fig. 1B), suggesting the development of spontaneous autoimmunity in double-deficient mice. The serum titers of inflammatory cytokines such as TNF-α and IL-6 were augmented in double-deficient mice (Fig. 1C). In addition, we observed a shift from CD62LhiCD44lo naïve population to CD62LhiCD44hi memory or activated population and increased cell surface expression of CD25 and CD69 activation markers in double-deficient T cells (Fig. S1D to F).
Figure. 1.
Enhanced T cell activation and spontaneous autoimmunity in double-deficient mice.
(A) Histology of various tissue sections from wild-type (WT), Cblb−/−, Itch−/−, or Cblb−/−Itch−/− double-deficient mice (3-month old), visualized by HE staining (original magnification 10X). The sites with lymphocyte infiltration were marked (asterisk).
(B) Concentrations of serum anti-double-stranded (ds) DNA antibodies were determined by ELISA.
(C) Concentrations of serum TNF-α or IL-6 were determined by ELISA. The results in B and C were from ten mice of each group (from 6 to 14 weeks of age).
(D)Splenic T cells from WT or the mutant mice were isolated and stimulated with anti-CD3 for 24 h. Cell proliferation was measured by 3H-thymidine uptake.
(E) The supernatants from 48 h culture with anti-CD3 (1 μg/ml) or anti-CD3 plus anti-CD28 (10 μg/ml) were collected and measured for IL-2 concentrations by ELISA. Data are representatives of three repeated experiments.
Ablation of Cbl-b results in augmented proliferation of peripheral T cells in response to TCR stimulation (Bachmaier et al., 2000; Chiang et al., 2000), whereas Itch deficiency has only a marginal effect (Fang et al., 2002). To test whether loss of both Itch and Cbl-b affects the proliferative responses, we measured the thymidine incorporation of primary splenic T cells. The cell proliferation of double-deficient T cells was higher as compared with Cblb−/−, or Itch−/− T cells under anti-CD3 stimulated conditions (Fig. 1D). In addition, the double-deficient T cells also showed stronger proliferative responses under conditions stimulated with anti-CD3 plus anti-CD28 (Fig. S2A).
Due to the shift from naïve to memory or activated T cell phenotype in the double-deficient mice, we sorted the CD4+CD44lo naïve population and performed a similar proliferation experiment. We found that double-deficient T cells exhibited a higher proliferative response to anti-CD3 plus anti-CD28 stimulation (Fig. S2B). The double-deficient T cells produced more IL-2 than the WT, Cblb−/− or Itch−/− T cells in response to T cell stimulation measured by ELISA (Fig. 1E) or intracellular staining (Fig. S2C). Taken together, the results suggest that Cbl-b and Itch cooperate to regulate T cell proliferation and IL-2 production. In addition, we observed an increased production of IFN-γ and IL-4 cytokines in double-deficient T cells (Fig. S2D).
Increased T cell signaling but normal TCR downmodulation in double-deficient T cells
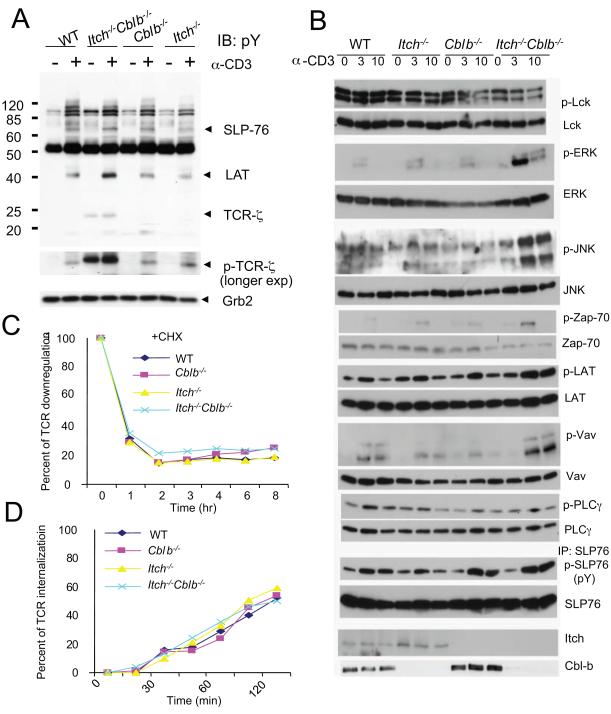
We then investigated the intracellular biochemical events in WT, single-deficient and double-deficient peripheral T cells. Anti-CD3 stimulation for a short period of time (5 min) slightly enhanced the overall tyrosine phosphorylation of intracellular signaling proteins (Fig. 2A). A longer exposure of the immunoblot exhibited a marked increase in TCR-ζ phosphorylation, which led to an increased migration at a molecular mass of ~23 kDa in double-deficient T cells whereas such an increase was not observed in Cblb−/− or Itch−/− T cells. Further analysis of the phosphorylation status of downstream intracellular signaling components revealed that phosphorylation of Zap-70, Erk, JNK, LAT, Vav, and SLP-76, but not Lck or PLC-γ1, was also enhanced in double-deficient T cells following stimulation (Fig. 2B). It should be mentioned that we also examined whether deficiency of both Cbl-b and Itch affects the protein stability of several signaling proteins. It seems that double-deficient T cells contain very similar protein amounts of PKCθ and Cbl as WT T cells (data not shown).
Figure 2.
Altered intracellular signaling and normal TCR downmodulation in double-deficient T cells.
(A) Purified CD4+ T cells were stimulated with anti-CD3 for 5 min and the cell lysates were subjected to immunoblotting with anti-phospho-tyrosine (pY) antibody (4G10). Molecular size markers are shown at left. The positions of several proteins were indicated. A longer exposure of the same blot was displayed to indicate the phosphorylation of TCR-ζ. The blot was reprobed with anti-Grb2 as an internal loading control of the samples.
(B) Purified CD4+ T cells were stimulated with anti-CD3 and the cell lysates were analyzed by probing with different antibodies. For the measurement of phospho-SLP-76, the cell lysates were immunoprecipiated with anti-SLP-76, and were subjected to probing with anti-pY antibody.
(C) TCR downregulation. Purified CD4+ T cells were stimulated with anti-CD3ε (2 μg/ml) for different time periods, followed by FACS analysis of the cell surface TCR expression. The percentage of TCR downmodulation was shown. T cells were pre-treated with cycloheximide (50 μM) to prevent new protein synthesis. Data are representatives of five repeated experiments.
(D) Ligand-independent internalization of TCR. Purified CD4+ T cells were first labeled with PE-anti-TCRβ and incubated at 37°C for different time periods. The surface un-internalized PE-anti-TCRβ was removed by brief stripping with acidic buffer. The intracellular fluorescence was measured by flow cytometry. The percentage of TCR internalization was shown. The mean of triplicate experiments from different mice was presented. Data are representatives of five repeated experiments.
See also Figure S3.
Ub conjugation of cell surface receptors has been implicated in endocytosis-dependent downregulation (Hicke and Dunn, 2003). Cbl proteins like Cbl and Cbl-b are involved in the internalization of the TCR (Myers et al., 2006; Naramura et al., 2002). The enhanced phosphorylation of TCR-ζ and increased intracellular signaling in double-deficient T cells prompted us to analyze whether loss of both E3 ligases affects the TCR endocytotic pathway. The cell surface expression of TCRβ or CD3ε was equivalent among the resting CD4+ T cells from WT, Itch−/−, Cblb−/− or double-deficient T cells (Fig. S3A). Stimulation of purified CD4+ T cells with anti-CD3 induced rapid down-modulation of the TCR, as measured by cell surface staining with anti-TCRβ in WT, Itch−/−, or Cblb−/− cells (Fig. S3B). Unexpectedly, the rate of TCR downmodulation in double-deficient T cells was quite similar, if not the same, as other types of T cells, even upon repeated experiments. To avoid the potential interference of newly synthesized protein, we pre-incubated the cells in the presence of a protein synthesis inhibitor, cycloheximide, and observed similar rate of downmodulation among all the examples (Fig. 2C). We also examined whether Cbl-b and Itch double-deficiency affected the ligand-independent internalization of the TCR using a previously described method (D’Oro et al., 2002) and found that T cells from double-deficient mice exhibited a similar rate of internalization as WT or single gene-targeted cells (Fig. 2D). Thus, we conclude that Cbl-b and Itch regulate the phosphorylation of TCR-ζ without affecting its downmodulation.
Cbl-b and Itch cooperate to regulate TCR-ζ ubiquitination and phosphorylation
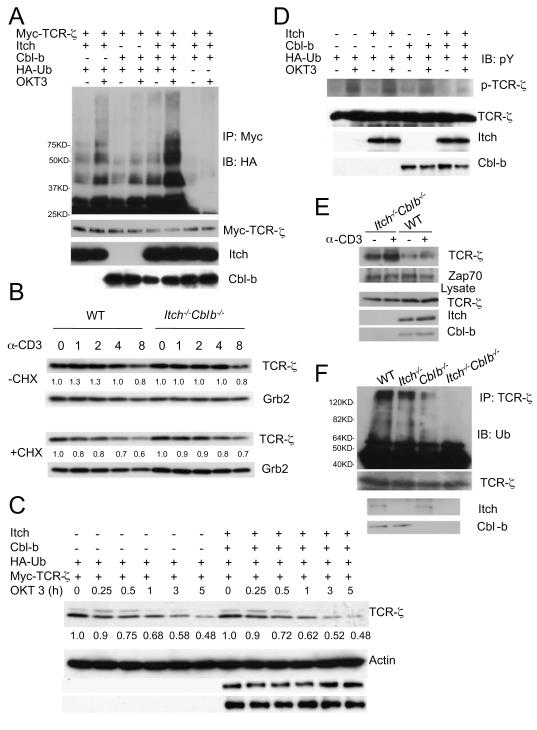
TCR-ζ is a well-characterized substrate for Ub conjugation (Cenciarelli et al., 1992; Wang et al., 2001). The increased T cell signaling, particularly the upregulated TCR-ζ phosphorylation, prompted us to examine Ub conjugation to TCR-ζ by Cbl-b and Itch in transiently transfected Jurkat T cells upon T cell stimulation. In the presence of Itch and Cbl-b, TCR-ζ ubiquitination was augmented when cells were stimulated with anti-CD3 (Fig. 3A), which caused a shift from lower molecular weight bands to higher molecular weight smears. To avoid the interference of other T cell-associated components in Jurkat T cells, we also used human embryonic kidney 293T cells for the transfection study and showed that TCR-ζ ubiquitination was augmented by co-expression of both Itch and Cbl-b, which was further increased by the treatment with a phosphatase inhibitor, pervanadate (Fig. S3C, D). In addition, a TCR-ζ mutant which lacks all lysine residues on its intracellular tail (TCR-ζ KR) was not ubiquitinated in the presence of both Cbl-b and Itch even under stimulated conditions (Fig. S3E). We therefore concluded that Itch and Cbl-b cooperate to induce TCR-ζ chain ubiquitination.
Figure 3.
Itch and Cbl-b cooperate to promote TCR-ζubiquitination without degradation.
(A)Plasmids as indicated were transfected into Jurkat T cells and the cells were unstimulated or stimulated with OKT3 for 5 min, followed by the analysis of TCR-ζ ubiquitination. Molecular size markers were labeled. The Ub ladders are indicated. The membrane was reprobed with anti-Myc, and the lysates was immunoblotted with indicated antibodies.
(B) Primary T cells were stimulated with anti-CD3ε for different time intervals in the absence or the presence of cycloheximide and the cell lysates were immunoblotted with anti-TCR-ζ and the same membrane was reprobed with anti-Grb2. The intensity of the protein bands was quantified and the relative amount of TCR-ζ at each starting point of stimulation (0) was given an arbitrary unit of 1.0.
(C)Jurkat T cells were transfected with plasmids as indicated, and the cells were stimulated with OKT3 for different time intervals in the presence of cycloheximide and the lysates were immunoblotted with anti-Myc and the same membrane was reprobed with indicated antibodies. The intensity of TCR-ζ was quantified and the relative amount of TCR-ζ at time 0 was given an arbitrary unit of 1.0.
(D)Transfected Jurakt T cells were stimulated with anti-CD3 for 2 min and the lysates were analyzed with the indicated antibodies.
(E) T cells from WT or double-deficient mice were left unstimulated or stimulated with anti-CD3 for 5 min and the cell lysates were immunoprecipitated with anti-Zap-70. The immunoprecipitates were immunoblotted with anti-pY. The position of phosphorylated TCR-ζ was indicated. The same blot was reprobed with anti-Zap-70.
(F) Splenocytes from 8 week-old WT, Cblb−/−, Itch−/− or double-deficient mice were stimulated with anti-CD3 for 5 min and lysates were immunoprecipitated with anti-TCR-ζ, followed by immunoblotting with anti-Ub. . Molecular size markers are indicated at left.
See also Figure S3.
The relatively normal amount of TCR downmodulation in double-deficient T cells led us to examine the degradation of TCR-ζ. Purified CD4+ T cells were stimulated with anti-CD3 in the absence or the presence of cycloheximide and TCR-ζ expression was analyzed by immunoblotting. Quantitative analysis suggested no obvious difference in TCR-ζ degradation between WT and double-deficient T cells in the absence of cycloheximide pretreatment (Fig. 3B, top panels). In the presence of the protein synthesis inhibitor, double-deficient T cells showed a slight increase of the TCR-ζ protein expression, which was not considered to be significant upon repeated experiments (Fig. 3B, bottom panels). We also performed a protein degradation assay in transiently transfected Jurkat T cells. Coexpression of Cbl-b and Itch did not lead an obvious change in the rate of TCR-ζ degradation upon TCR stimulation for a period of 5 h (Fig. 3C), further supporting our findings of normal TCR-ζ degradation in double-deficient T cells.
The increased phosphorylation of TCR-ζ in double-deficient T cells raised an issue of whether the two E3 ligases cooperate to affect the phoshorylation status of TCR-ζ without affecting TCR downmodulation. We found that coexpression of Cbl-b and Itch markedly reduced anti-CD3-induced tyrosine phosphorylation of TCR-ζ (Fig. 3D). Phosphorylated TCR-ζ recruits Zap-70 to initiate downstream TCR signaling upon TCR triggering (Weiss and Littman, 1994). To further confirm the early observation on TCR-ζ phosphorylation, we performed a co-immunoprecipitation assay with anti-Zap-70 and examined the amount of associated TCR-ζ. Consistent with the earlier results, we observed that more TCR-ζ was co-immunopreciated with anti-Zap-70 in anti-CD3 stimulated double-deficient T cells (Fig. 3E) compared to WT cells. We further investigated the ubiquitination status of endogenous TCR-ζ and found that ubiquitin conjugation to TCR-ζ was substantially reduced in double-deficient T cells (Fig. 3F) compared to WT, or single-deficient T cells. Taken together, these results indicate that Cbl-b and Itch act cooperatively to regulates TCR-ζ ubiquitination and phosphorylation in a proteolysis-independent manner.
K33-linked polyubiquitination of TCR-ζ
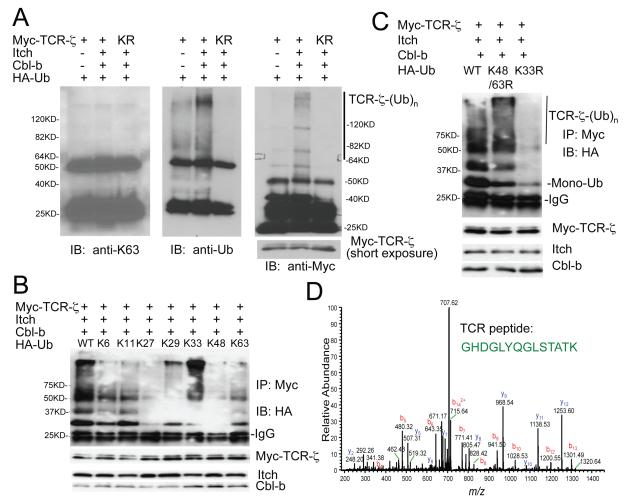
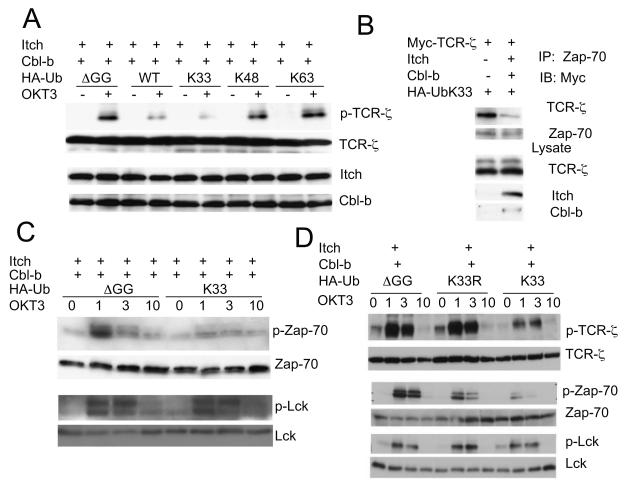
We next performed a detailed investigation of Cbl-b and Itch induced ubiquitination of TCR-ζ, and concluded that Cbl-b and Itch cooperate to induce TCR-ζ polyubiquitination, which depends on the E3 ligase activity of Itch and a C-terminal fragment of Cbl-b (Figs. S4, and Supplementary Results). We then examined TCR-ζ polyubiquitination and found that the polyubiquitinated TCR-ζ was not recognized by an Ub K63-specific antibody (Fig. 4A and S5A). To dissect which Ub lysine residue is responsible for the polyUb chain formation on TCR-ζ, we generated a series of Ub mutations on all 7 lysine residues by leaving only one lysine residue on the Ub molecule (Fig. S5B). Among all the Ub mutants, Ub K33-only mutant displayed the strongest polyUb chain formation on TCR-ζ (Fig. 4B). This finding was further confirmed by using a Ub K33R mutant, which completely abolished TCR-ζ polyubiquitination, whereas the K48-63R double mutant did not have an effect (Fig. 4C). The presence of Ub K33-linked chain formation on endogenous TCR-ζ was analyzed by mass spectrometry and both K33-linked peptide and TCR-ζ peptide were revealed in the trypsin-digested samples of K33 Ub affinity purification (Fig. 4D and S5C, D), thus providing further physical evidence of K33-linked TCR-ζ polyubiquitination. These results indicate that TCR-ζ is conjugated with a polyUb chain, which is linked by a previously not well-characterized K33 conjugation of Ub molecules.
Figure 4.
K33-linked polyubiquitination of TCR-ζ.
(A) Jurkat T cells were transfected with the indicated plasmids, and stimulated with anti-CD3, followed by immunoprecipitation and blotting with indicated antibodies. Molecular size markers are indicated. A short exposure of TCR-ζ blot is also shown.
(B) Jurkat T cells were coexpressed with individual Ub mutant and the TCR-ζ ubiquitination was analyzed by immunoprecipitation and blotting. Molecular size markers are shown at left.
(C) Effects of Ub K48-63R double, or K33R single mutation on TCRζ polyuniquitination. Molecular size markers are indicated at left.
(D) Tandem mass spectrum of a peptide derived from TCR-ζ. K33 Ub-linked mixture was purified using Ni-NTA beads and was subjected to mass spectrometry. The fully tryptic peptide unambiguously identified the peptide sequence corresponding to GHDGLYQGLSTATK of TCR-ζ.
K33-linkage affects TCR-ζ phosphorylation
The effect of Ub K33-linked TCR-ζ polyubiquitination on its phosphorylation was examined by co-transfection studies in Jurkat T cells. For this purpose, we tested the various Ub mutants with only one lysine residue (Fig. S5B). TCR-ζ phosphorylation was markedly reduced by co-expression with WT Ub or Ub K33-only mutant, whereas the K48-only or K63-only mutant did not have any effect (Fig. 5A). In addition, Ub K33-only mutant expression reduced the association between TCR-ζ and Zap-70 in stimulated Jurkat T cells in the presence of Cbl-b and Itch (Fig. 5B). Strikingly, co-expression of the Ub K33-only mutant almost completely abrogated anti-CD3-stimulated Zap-70 phosphorylation, without any alteration of Lck phosphorylation (Fig. 5C). To further substantiate these findings, we compared the Ub K33-only to K33R mutant, and found that Ub K33R did not have an effect on TCR-ζ phosphorylation (Fig. 5D). These biochemical data essentially recapitulate the enhanced TCR-ζ phosphorylation and its association with Zap-70 that is seen in double-deficient T cells. These events are most likely induced by K33-linked polyubiquitination of TCR-ζ.
Figure 5.
Effect of K33-linked polyubiquitination on TCR-ζ.
(A) K33-linked ubiquitination of TCR-ζ inhibits its phosphorylation. Jurkat T cells transfected with indicated plasmids were stimulated, lysed and blotted with indicated antibodies.
(B) Effect of Ub K33 on TCR-ζ interaction with Zap-70. Jurkat T cells transfected with indicated plasmids were stimulated with anti-CD3 for 10 min and the lysates were immunoprecipitated with anti-Zap-70 and blotted with anti-Myc. Aliquots of cell lysates were blotted with indicated antibodies in each panel.
(C) K33-linked ubiquitination of TCR-ζ inhibits Zap-70 phosphorylation. Jurkat T cells transfected with indicated plasmids were stimulated with OKT3 for 1 to 10 min and the lysates were blotted with indicated antibodies.
(D) K33 is the major linkage that is responsible for the inhibition of phosphorylation of TCR-ζ and Zap-70. Jurkat T cells transfected with indicated plasmids were stimulated with OKT3 for 1 to 10 min and the lysates were blotted with indicated antibodies.
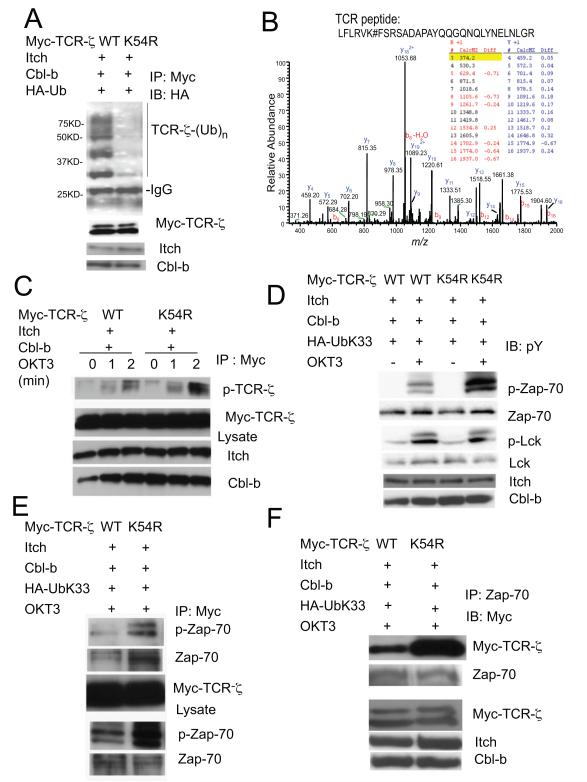
Identification of TCR-ζ K54 as an Ub conjugation site
There are seven lysine residues in the TCR-ζ intracellular domain which are conserved across different species. We generated a series of single or combinatory mutations in TCR-ζ to map the potential Ub conjugation site(s) in co-expression studies. Mutation of K54 within the TCR-ζ resulted in the most drastic reduction of TCR-ζ ubiquitination, particularly in the polyubiquitnated form in the presence of Cbl-b and Itch (Fig. 6A and S6A, B). To provide further biophysical evidence, we performed proteomics analysis using immunoprecipitated TCR-ζ, and found a modified TCR-ζ peptide sequence spanning the K54 residue, in which a mass increase of 114 dalton was detected (Figs. 6B, and S6C). This increase of molecular mass over the unmodified peptide corresponded to the two C-terminal glycine residues derived from Ub attached to K54. We went further to examine the effect of K54 mutation on its phosphorylation and complex formation. Compared with WT TCR-ζ, the TCR-ζ K54R mutant exhibited enhanced phosphorylation in response to anti-CD3 stimulation (Fig. 6C). In addition, this mutation increased the phosphorylation of Zap-70, but not that of Lck (Fig. 6D). More strikingly, the K54 to R mutation markedly enhanced anti-CD3-induced association between TCR-ζ and Zap-70 (Fig. 6E and F). The results suggest that K33-linked polyUb chain formation on TCR-ζ K54 residue affects its phosphorylation and association with Zap-70.
Figure 6.
The juxtamembrane K54 in TCR-ζ is the acceptor site for the K33-linked polyubiquitination.
(A) TCR-ζ K54R mutation reduces its ubiquitination by Itch and Cbl-b. TCR-ζ or its K54R mutant was coexpressed with Itch, Cbl-b and Ub plasmids, followed by the detection of TCR-ζ ubiquitination. Molecular size markers are indicated at left.
(B) Affinity-purified TCR-ζ mixtures were subjected to mass spectrometry. Tandem mass spectrum of ubiquitinated peptide derived from TCR: LFLRVK#FSRSADAPAYQQGQNQLYNELNLGR, with the precursor ion mass 1239.46. The half tryptic peptide identified TCR ubiquitination site at lysine 54 with a mass addition of 114. The existence of site localization ions on both side of K54, e.g., b6 and above, and neutral loss of y26, supports the modification event on K54. “#” denotes the residue on its left had a mass addition of 114.
(C) TCR-ζ K54R mutation reverses the inhibitory effect of K33 ubiquitination. TCR-ζor its K54R mutant was co-expressed with indicated plasmids and stimulated with anti-CD3. The lysates were immunoprecipitated with anti-Myc antibody and blotted with anti-pY to detect TCR-ζphosphorylation.
(D) The lysates as in C were blotted with phospho-specific antibodies for Zap-70 or Lck.
(E, F) TCR-ζK54R mutant shows increased interaction with Zap-70. Jurkat T cells transfected with indicated plasmids were stimulated with anti-CD3 for 10 min, followed by immunoprecipitation and blotting as indicated.
See also Figure S6.
Discussion
In this study, we provided both genetic and biochemical evidence to support the hypothesis that Itch and Cbl-b cooperate to regulate T cell activation and autoimmunity. We demonstrated that loss of both Cbl-b and Itch increases the phosphorylation of TCR-ζ without affecting TCR downmodulation and degradation. Further biochemical studies reveal that TCR-ζ is polyubiquitinated via an Ub K33-linkage, which reduces its association with Zap-70. Mutation at TCR-ζ K54 polyubiquitination site rescues the reduced TCR-ζ and Zap-70 phosphorylation and restores their association. The results suggest an unconventional polyubiquitination pathway that is critical for the regulation of cell surface receptor in a proteolysis-independent manner.
The present study clearly suggests that Cbl-b and Itch cooperate with each other to regulate T cell signaling transduction, as evidenced by the increased tyrosine phosphorylation of TCR-ζ and other downstream intracellular critical signal molecules upon TCR stimulation. The increased signal transduction events in the double-deficient T cells attribute to augmented T cell proliferation and IL-2 production. Excessive T cell activation in the double-deficient mice correlates with lymphocyte infiltration in multiple organs and the development of autoimmunity, as revealed by the presence of higher amounts of autoantibodies in the sera of double-deficient mice. It should be mentioned that such autoimmune phenotypes are observed in an early age when neither Cblb−/− nor Itch−/− mice exhibit any obvious alteration, thus consisting with a notion that the two proteins functions in a cooperative manner.
One surprising finding in this study is that TCR downmodulation was not affected by Cbl-b and Itch double deficiency. Although in our earlier preliminary studies using un-purified splenic T cells, a slight decrease in TCR downregulation was observed in double-deficient T cells (data not shown), repeated experiments using similar splenic T cells, or purified CD4+ or CD8+ T cells convincingly showed that T cell stimulation results in similar, if not the same, rate of TCR endocytosis among WT, single-deficient or double-deficient T cells. In addition, the ligand-independent TCR internalization in double-deficient T cells is the same as in WT or single-deficient T cells. Even though it is generally believed that Ub conjugation to the cell surface receptor leads to its endosome-lysosome-dependent downmodulation (Hicke and Dunn, 2003), our data clearly suggest that this is not the case for the ubiquitination of TCR-ζ promoted by a cooperative act of Cbl-b and Itch. It can be argued that TCR-ζ is polyubiquitinated, rather than mono-ubiquitinated, as revealed in our biochemical analysis, which may lead to proteasome-dependent degradation, instead of endosome-dependent endocytosis. This possibility has been ruled out, however, as both genetic and biochemical experiments showed that a combination of Cbl-b and Itch does not affect protein degradation of TCR-ζ. The results appear to differ from those obtained in Cbl−/− Cblb−/− T cells in which ligand-induced TCR downregulation is reduced (Naramura et al., 2002). However, Cbl (or Cbl-b) also regulates receptor internalization in an E3 ligase-independent mechanism (Petrelli et al., 2002; Soubeyran et al., 2002), or via interacting with other adaptor proteins (Myers et al., 2005; Myers et al., 2006), which may differ from the cooperative function between Cbl-b and Itch.
Importantly, we demonstrated that TCR-ζis conjugated with a polyUb chain via a K33 linkage, which affects its phosphorylation and association with Zap-70, as revealed in co-expression studies with a Ub K33 mutant. More importantly, the identification of TCR-ζ K54 as a potential Ub conjugation site and subsequent analysis further supports the hypothesis that K33-linked polyubiquitination of TCR-ζ critically regulates the status of its phosphorylation and Zap-70 association, without any effect on the upstream kinase Lck. These biochemical studies essentially recapitulate the increased tyrosine phosphorylation of TCR-ζ and Zap-70 binding observed in double-deficient T cells. It is possible that K33-linked Ub conjugation to TCR-ζ may cause a structural hindrance for the access of Zap-70 binding. This effect is selective, since neither double-deficient T cells nor transiently transfected T cells display any alteration in Lck phoshorylation. Support of this possibility is the observation that ubiquitination of the p85 subunit of PI3-kinase negatively affects its interaction with CD28 or the TCR complex (Fang and Liu, 2001). Similarly, TCR-ζ polyubiquitination interferes with recruitment of Zap-70, a direct binding partner for the phosphorylated TCR-ζ (Weiss and Littman, 1994). It has been demonstrated that the recruitment of Zap-70 to the TCR-ζ could protect TCR-ζ from dephosphorylation (van Oers et al., 1994), which most likely explain the increased TCR-ζ phosphorylation in double-deficient T cells.
Each of the seven lysine residues in Ub has been demonstrated to be used for Ub conjugation (Pickart and Fushman, 2004), which may lead to different biological outcomes. The classical K48-linked polyubiquitination targets the substrate for proteasomal degradation, whereas the K63-linked chains are involved in non-proteolytic regulation such as protein complex formation and kinase activation (Chen and Sun, 2009). An emerging mechanism for proteasome-dependent degradation involves the K11-linked polyUb chain in yeast, which targets Ubc6 for ER-associated degradation (Xu et al., 2009). Interestingly, profiling of the entire yeast proteome in this study reveals that the K33-linkage is relatively resistant to proteasomal degradation, only slightly weaker than the K63-linkage. A recent study implicated K29-K33-linked chain formation in regulating AMP-activated protein kinase-related kinases (Al-Hakim et al., 2008). These observations are thus consistent with our finding in T cells that K33-linked polyubiquitination of TCRζ is independent of proteasomal or endosomal degradation. Future proteomics studies will aim at the identification of more physiological substrates for K33-linked polyubiquitination, the elucidation of the molecular mechanisms underlying this unconventional modification, and the investigation of the implicated biological functions.
In conclusion, loss of a RING-type E3 ligase Cbl-b and a HECT-type E3 ligase Itch results in increased T cell activation and autoimmune responses. The two E3 ligases cooperate to induce K33-linked polyubiquitination of TCR-ζ, which functionally modifies the receptor phosphorylation and protein binding in a proteolysis-independent manner. Further elucidation of such Ub chain formation will help understand the molecular insights into the protein ubiquitination pathway in immune regulation
Experimental Procedures
Mice
Cblb−/− and Itch−/− mice on a C57BL/6 background were described previously (Fang et al., 2002; Fang and Liu, 2001). The mice were crossed to generate Itch+/−Cblb+/− double heterozygous mice that were intercrossed to obtain WT, heterozygous, single and double-deficient mice. Genotyping of the mice was performed by PCR for the detection of Itch mutation and by Southern blotting for the detection of Cbl-b mutation using tail DNA. Mice of 6 to 14 weeks old were used for the experiments, unless otherwise specified. The double-deficient mice did not develop any apparent abnormality at least at 4 months of age in the animal facility. Animal experiments were carried out according to the institutional guidelines approved by the institute ethical committee.
Cell proliferation and cytokine production
Single cell suspensions isolated from spleen or lymph nodes or purified CD4+ or CD8+ were cultured in the presence of anti-CD3 and/or anti-CD28. Purification of naïve CD4+ T cells was performed as described (Venuprasad et al., 2006). Proliferation of last 12 h of 48 h culture was detected by addition of 1 μCi of 3H-thymidine (ICN, Irvine, CA), and cell-incorporated radiation was monitored by β-counter (Wallac, Gaithersburg, MD). The culture supernatants were collected and assayed for the concentrations of IL-2 by standard ELISA.
Plasmids and cell transfection
Plasmids containing Xpress-tagged Cbl-b, Cbl-b WA, its N-terminal mutant encoding the N-terminal domain (Cbl-b N, amino acids 1 to 349), a C-terminal mutant encoding the RING finger plus the proline-rich region (Cbl-b C1, amino acids 341 to 982), or C-terminal mutant encoding only the distal proline-rich sequences (Cbl-b C2, amino acids 595-982), Itch, Itch ligase-inactive (Itch CA), Itch mutant lacking the HECT domain (Itch WW), Myc-TCR-ζ, or HA-Ub cDNA, were described previously (Fang et al., 2001; Wang et al., 2001). Ub mutants were generated by introducing point mutations from HA-Ub with all lysine mutated (K0) construct with Quikchange Mutagenesis kit (Qiagen) with the following primer sets: (UbK6-F: GCAGATCTTCGTCAAAACGTTAACCGGTAG; UbK6-R: CTACCGGTTAACGTTTTGAC GAAGATCTGC; UbK11-F: GAACGTTAACCGGTAAAACCATAACTCTAG; UbK11-R: CTA GAGTTATGGTTTTACCGGTTAACGTTC; UbK27-F: CCATCGAAAACGTTAAAGCTAGA ATTCAAGAC; UbK27-R: GTCTTGAATTCTAGCTTTAACGTTTTCGATGG; Ub29K-F: GA AAACGTTAGAGCTAAAATTCAAGACAGAGAAG; Ub29K-R: CTTCTCTGTCTTGAATTT TAGCTCTAACGTTTTC; UbK33-F: GCTAGAATTCAAGACAAAGAAGGCATTCCACCTG; UbK33-R: CAGGTGGAATGCCTTCTTTGTCTTGAATTCTAGC; UbK48-F: GATCTTTGC CGGTAAGCAGCTCGAGGACGG; UbK48-R: CCGTCCTCGAGCTGCTTACCGGCAAAG ATC; UbK63-F: GATTACAACATTCAGAAGGAGTCGACCTTAC; UbK63-R: GTAAG GTCGACTCCTTCTGAATGTTGTAATC). UbΔGG was mutated from HA-Ub with the following primmer set: (UbΔGG-F: GTCTTAAGACTAAGATGAGGTTGAACGCGTGGTACC; UbΔGG-R: GGTACCACGCGTTCAACCTCATCTTAGTCTTAAGAC). TCR-ζ K54R mutation was generated with the following primer set: (TCR-KR1-F: CTTGTTCCTGAG AGTGAGGTTCAGCAGGAGC; TCR-KR1-R: GCTCCTGCTGAACCTCACTCTCAGGAA CAAG). A TCR-ζ KR mutant in that all nine intracellular lysine residues were mutated to arginine was generated by multi-step site-directed mutagenesis. All the mutations were verified by DNA sequencing.
For protein expression in 293T cells or the human Jurkat T cells, cells were transfected with appropriate amounts of plasmids (usually 3~5 μg total) by electroporation (240V, 960 μF; Bio-Rad) or using Fugene 6 transfection reagent (Roche, Neutly, NJ).
Antibodies
Polyclonal antibodies specific to Cbl-b, Zap-70, PLC-γ1, Erk, JNK, Vav, Myc, Ub, and HA, and monoclonal antibody (mAb) specific to TCRζ (6B10) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A polyclonal antisera against TCR-ζ (551-ζ, was gift from David Wiest, Fox Chase Cancer Center, Philadelphia, PA. Antibodies against SLP-76, LAT, and phospho-LAT were from Upstate (Lake Placid, NY). mAbs specific to phospho-ERK, phospho-JNK, phospho-Zap-70, phospho-PLC-γ1, Itch, and Grb2 were purchased from Transduction Laboratories (Lexington, KY). Rabbit anti-Lck and anti-phospho-Src (Y418) were purchased from Cell Signaling. Anti-Xpress was from Invitrogen (Carlsbad, CA). Mouse anti-Ub K63 (HWA4C4) was purchased from Biomol (Plymouth Meeting, PA). Secondary anti-mouse Ig conjugated with FITC or Rhodamine and anti-rabbit Ig conjugated with FITC and Rhodamine were from Oraganon Teknika Corp (West Chester, PA). Phosphotyrosine specific mAb was purified from culture supernatant of 4G10 B-cell hybridoma. The T cell stimulatory mAb, anti-CD3ε was purified from the culture supernatant of B-cell hybridomas 2C11. Biotin-labeled TCRβ-specific mAb was purchased from PharMingen (San Diego, CA). Anti-TCR-ζ mAb H146-968 was provided by Dr. R. Kubo in our institute.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed by incubation the cell lysate with 1 μg of antibody at 4°C for 2 h, followed by addition of 30 μl of protein G-conjugated Sepharose beads (Santa Cruz Biotechnology). For displaying ubiquitinated protein, 1.0% SDS and 5 mM N-ethylmaleimide were added to lysis buffer to disrupt nonspecific protein interactions. Precipitated protein was denatured by boiling for 10 min and then diluted to 0.1% SDS before immunoprecipitation (Venuprasad et al., 2008). Samples were subjected to 8~10% SDS-PAGE separation, followed by electro-transferring to PVDF membrane (Millipore, Bedford, MA). Membrane was immunoblotted with the indicated antibodies, followed by horseradish peroxidase-conjugated second antibody, and visualized with enhanced chemiluminescence detection system (ECL, Amersham Pharmacia, Little Chalfont, UK). When necessary, the membrane was stripped as previously described (Yang et al., 2006).
Flow cytometry
Prior to FACS staining, 106 cells were incubated on ice for 30 min in 100 μl of 10% FCS media containing 2 μg/ml of anti-FcγR (2.4G2, PharMingen). Then, the cells were washed and stained on ice with 1:100 diluted labeled antibodies for 1 h, and analyzed on a FACScalibur or a FACS-LSR using the CellQuest software (Becton Dickinson, San Jose, CA). The following antibodies were used for staining: FITC-, PE-, APC-, or CyC-conjugated antibodies against murine CD4, CD8, TCRβ, CD3ε, CD5, CD25, CD44, CD62L or CD69 (PharMingen).
TCR downregulation and internalization
For TCR downregulation experiment, purified CD4+ or CD8+ T cells (106/sample) were cultured with 2 or 5 μg/ml of anti-CD3ε for various times in 96-well plate. To prevent the interference of newly synthesized proteins, T cells were pre-treated with cycloheximide for 2 h. The cell surface expression of TCRβ was analyzed by FACS analysis. For ligand-independent TCR internalization assay, we followed a previously published protocol (D’Oro et al., 2002). Briefly, 2 ×106 CD4+ or CD8+ T cells were first labeled with PE-conjugated anti-TCRβ antibody and the cells were then incubated at 37°C for different time intervals. The un-internalized TCRβ-PE was removed by a brief stripping with ice-cold acidic buffer. The intracellular fluorescence was measured by FACS analysis.
Purification of K33 ubiquitinated proteins
Jurkat T cells (2 ×108) overexpressing His-HA-Ub K33 ltch and Cbl-b (or Itch and Cbl-b only as control) were first lysed with a denaturing buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 0.5% NP-40, 8 M Urea, pH 8.0) by passing 21 gauge needle 15 times. Clarified lysates were added with 200 μl Ni-NTA agarose beads (Qiagen) and incubated at 4°C for 2 hr. Beads were washed with a washing buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 8 M Urea, pH 8.0) four times. Bound Ub conjugages were step-eluted by increase of imidazole concentrations (30, 40, 50, 250 mM) in washing buffer. Most polyubiquitinated products were eluted at 40 mM imidazole, which had minimum non-specific protein as shown in control sample. The samples were then directly preceded for trypsin digestion and Mass Spec analysis.
Proteomic identification of K33 ubiquitinated proteins
Protein samples were precipitated with 80% of acetone in -20 °C for 30 min and centrifuged at 14,000 × g for 15 min. The pellets were solubilized in 0.2% RapiGest/10 mM DTT at 60°C for 30 min. Iodoacetamide was added to a final concentration of 15 mM, and incubated at room temperature for 20 min in dark. The suspension was then diluted to a final RapiGest concentration of 0.1% with 100 mM Tris-HCl and 1 mM CaCl2. The proteins were digested with 1 μg of trypsin at 37 °C overnight. After digestion, the suspension was acidified by adding formic acid to 5%, and centrifuged. The solution containing peptides were then used for protein identification.
Peptides from each sample were pressure-loaded onto a 250 μm i.d. fused silica capillary column packed with a 3 cm, 5 μm Partisphere strong cation exchanger (SCX, Whatman, Clifton, NJ) and a 3 cm, 10 μm Jupiter reversed-phase C8 material (Phenomenex, Ventura, CA), with the SCX end fritted with immobilized Kasil 1624 (PQ Corperation, Valley forge, PA). After desalting, a 100 μm i.d. capillary with a 5 μm pulled tip packed with 10 cm, 4 μm Jupiter C8 material was attached to a zero dead volume (ZDV) union and the split-column was placed online with an Agilent 1100 quaternary HPLC system (Agilent, Santa Clara, CA); and analyzed using a modified, five-step multi-dimensional protein identification technology (MudPIT) described previously (Washburn et al., 2001). Briefly, three buffer solutions used were: 5% acetonitrile/0.1% formic acid (buffer A); 80% acetonitrile/0.1% formic acid (buffer B), and 500 mM ammonium acetate/5% acetonitrile/0.1% formic acid (buffer C). The first step consisted of a 100 min gradient from 0-100% buffer B. Steps 2-5 had the following profile: 3 min of 100% buffer A, 5 min of increasing buffer C concentration (10%, 30%, 75% or 100%), a 10 min gradient from 0-15% buffer B, and a 82 min gradient from 15-45% buffer B, followed by a 10 min gradient increase to 100% buffer B, and a 10 min reverse of gradient to 100% buffer A. As peptides were eluted from the microcapillary column they were electrosprayed directly into an LTQ linear ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) with the application of a distal 2.4 kV spray voltage. A cycle of one full-scan mass spectrum (400-1400 m/z) followed by 5 data-dependent MS/MS scan at a 35% normalized collision energy was repeated continuously throughout each step of the multidimensional separation.
The resulting MS/MS spectra were searched with the SEQUEST™ algorithm (Yates et al., 1995) against a human database from EBI (http://www.ebi.ac.uk/IPI) that was concatenated to a decoy database in which the sequence for each entry in the original database was reversed. The mutant Ub sequence and its reverse sequence were included in the database. The search parameters include a static cysteine modification of 57 amu, a di-glycine modification of lysine of 114 amu, and with half trypsin specificity. The database search results were assembled and filtered using the DTASelect program (Tabb et al., 2002) with a spectra level false discovery rate of less than 0.1%. Under such filtering conditions, no peptide hit from the reverse database was found. Because the Ub peptide with K33 ubiquitination did not pass the filtering criteria, we manually annotated the spectra and confirmed Ub conjugation at K33.
Supplementary Material
Acknowledgments
We thank former and current lab members Y. Bai, K. Cooper, D. Demydenko, J. Feng, Y. Harada, K. Iwanami, W. Piao, Y. Shao, A. Teng, K. Venuprasad, and W. Zhang for assistance. This work is supported by grants from NIH to Y-C. Liu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hakim AK, Zagorska A, Chapman L, Deak M, Peggie M, Alessi DR. Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem J. 2008;411:249–260. doi: 10.1042/BJ20080067. [DOI] [PubMed] [Google Scholar]
- Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Hou D, Hsu KC, Rellahan BL, Wiest DL, Smith HT, Fried VA, Weissman AM. Activation-induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- Courbard JR, Fiore F, Adelaide J, Borg JP, Birnbaum D, Ollendorff V. Interaction between two ubiquitin-protein isopeptide ligases of different classes, CBLC and AIP4/ITCH. J Biol Chem. 2002;277:45267–45275. doi: 10.1074/jbc.M206460200. [DOI] [PubMed] [Google Scholar]
- D’Oro U, Munitic I, Chacko G, Karpova T, McNally J, Ashwell JD. Regulation of constitutive TCR internalization by the zeta-chain. J Immunol. 2002;169:6269–6278. doi: 10.4049/jimmunol.169.11.6269. [DOI] [PubMed] [Google Scholar]
- Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol. 2002;3:281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- Fang D, Liu Y-C. Proteolysis-independent regulation of phosphatidylinositol 3-kinase by Cbl-b-mediated ubiquitination in T cells. Nature Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- Fang D, Wang H-Y, Fang N, Altman Y, Elly C, Liu Y-C. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J. Biol. Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, et al. Essential Role of the E3 Ubiquitin Ligase Cbl-b in T Cell Anergy Induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Liu YC. Ubiquitin ligases and the immune response. Annu Rev Immunol. 2004;22:81–127. doi: 10.1146/annurev.immunol.22.012703.104813. [DOI] [PubMed] [Google Scholar]
- Magnifico A, Ettenberg S, Yang C, Mariano J, Tiwari S, Fang S, Lipkowitz S, Weissman AM. WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. J Biol Chem. 2003;278:43169–43177. doi: 10.1074/jbc.M308009200. [DOI] [PubMed] [Google Scholar]
- Myers MD, Dragone LL, Weiss A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCRzeta for degradation. J Cell Biol. 2005;170:285–294. doi: 10.1083/jcb.200501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MD, Sosinowski T, Dragone LL, White C, Band H, Gu H, Weiss A. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- Perry WL, Hustad CM, Swing DA, O’Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [see comments] [DOI] [PubMed] [Google Scholar]
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Venuprasad K, Elly C, Gao M, Salek-Ardakani S, Harada Y, Luo JL, Yang C, Croft M, Inoue K, Karin M, Liu YC. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J Clin Invest. 2006;116:1117–1126. doi: 10.1172/JCI26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y, Liu YC. Cbl promotes ubiquitination of the T cell receptor{zeta} through an adaptor function of Zap-70. J Biol Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gay DL, MacLeod MK, Cao X, Hala T, Sweezer EM, Kappler J, Marrack P, Oliver PM. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat Immunol. 2008;9:1356–1363. doi: 10.1038/ni.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhou W, Jeon MS, Demydenko D, Harada Y, Zhou H, Liu YC. Negative Regulation of the E3 Ubiquitin Ligase Itch via Fyn-Mediated Tyrosine Phosphorylation. Mol Cell. 2006;21:135–141. doi: 10.1016/j.molcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.