Abstract
There is increasing evidence that human pregnancy outcome can be significantly compromised by suboptimal maternal nutritional status. Poor diet results in a maternal-fetal environment in which the teratogenicity of other insults such as alcohol might be amplified. As an example, there is evidence that zinc (Zn) can interact with maternal alcohol exposure to influence the risk for fetal alcohol spectrum disorders (FASD). Studies with experimental animals have shown that the teratogenicity of alcohol is increased under conditions of Zn deficiency, while its teratogenicity is lessened when animals are given Zn supplemented diets or Zn injections prior to the alcohol exposure. Alcohol can precipitate an acute phase response resulting in a subsequent increase in maternal liver metallothionein, which can sequester Zn and lead to decreased Zn transfer to the fetus. Importantly, the teratogenicity of acute alcohol exposure is reduced in metallothionein knockout mice, which can have improved Zn transfer to the conceptus relative to wild-type mice. Consistent with the above, Zn status has been reported to be low in alcoholic women at delivery. Preliminary data from two basic science and clinical nutritional studies that are ongoing as part of the international Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) support the potential role of Zn, among other nutritional factors, relative to risk for FASD. Importantly, the nutrient levels being examined in these studies are relevant to general clinical populations and represent suboptimal levels rather than severe deficiencies. These data suggest that moderate deficiencies in single nutrients can act as permissive factors for FASD, and that adequate nutritional status or intervention through supplementation may provide protection for some of the effects of prenatal alcohol exposure.
Keywords: Alcohol, FAS, FASD, zinc, micronutrients, pregnancy
1. Introduction
It is estimated that, conservatively, 50% of human concepti are lost before or during implantation, and of those that successfully implant, an additional 15–20% are lost prior to delivery [1,2]. With respect to completed pregnancies, approximately 3% result in a child with one or more severe malformations. These numbers, as discouraging as they are, do not take into account the increased risk many children can have for chronic diseases later in life, including obesity, diabetes, hypertension and vascular disease, as a consequence of select prenatal or early postnatal insults [3,4]. While a diversity of factors can contribute to the occurrence of developmental abnormalities, there is increasing evidence that human pregnancy outcome can be significantly compromised by suboptimal maternal nutritional status, and that poor nutrition may be a leading cause of preventable birth defects. A number of investigators since the early 1940’s have reported that women who consume diets that can be classified as “poor” have an increased risk for pregnancy complications compared to women who consume diets that can be classified as “good” (Table 1). It is worth noting that this association has been reported over a period of time where many would argue as to what constitutes a good versus a poor diet; however, a consistent theme over the past seven decades has been that “good” diets are characterized by high micronutrient content. Importantly, data from a variety of nutrition intervention trials, ranging from the provision of whole foods, to multivitamin-mineral supplements, to single nutrients such as folate and iodine, provide evidence that improvements in a mother’s diet can result in marked reductions in her risk for a complicated pregnancy [5–11]. When viewed in its totality, the data supporting the concept that maternal nutritional status is a key predictor of human pregnancy outcome seem overwhelming; however, a complication in the story is that while the diets of women who consume “poor’ diets may not be ideal, pronounced essential nutrient deficiencies are rare. This observation has led to the idea that the increased risk for pregnancy complications observed in this group of women may, in many cases, be more due to the fact that the poor diet results in a maternal-fetal environment in which the teratogenicity of other insults is amplified, than due to a simple deficit of one or more essential nutrients in the diet.
Table 1.
Maternal Diet and Pregnancy Outcome: “Good” vs. “Poor” Diet
| Author | Study |
|---|---|
| Ebbs et al., 1941 | Intervention |
| Burke et al., 1943 | Observational |
| Jeans et al., 1955 | Observational |
| Primrose and Higgins, 1971 | Intervention |
| Laurence et al., 1983 | Intervention |
| Friel et al., 1995 | Observational |
| Wright, 1995 | Observational |
| Torfs et al., 1998 | Observational |
| Velie et al., 1999 | Observational |
| Di Cintio et al., 2001 | Observational |
| Krapels et al., 2004 | Observational |
| Rees et al., 2005 | Observational |
| Lam and Torfs, 2006 | Observational |
| Knudsen et al., 2008 | Observational |
| Yang et al., 2008 | Observational |
| Baker et al., 2009 | Observational |
| Haggarty et al., 2009 | Observational |
In the current paper, we explore this concept using alcohol as an example of a developmental insult whose teratogenicity can be modulated by maternal diet. It is now widely accepted that excessive maternal alcohol intake during pregnancy can result in a number of developmental abnormalities commonly referred to as the Fetal Alcohol Syndrome (FAS), or more recently, the Fetal Alcohol Spectrum Disorder (FASD) [12]. FASD is now thought to be one of the most common causes of developmental mental retardation in the human population. Due to space constraints we will focus our discussion on the ability of zinc (Zn) to modulate the teratogenic expression of alcohol. In the last section of this paper, preliminary data from an ongoing multi-nutrient supplementation trial in Russia and the Ukraine with women who are at high risk for having a child with FASD will be discussed. We would like to emphasize that while our comments are focused on Zn, there is good evidence that numerous other nutrients, including copper (Cu), iron (Fe), magnesium (Mg), selenium (Se), methionine, choline, vitamin B12 and folate, can modulate alcohol’s developmental toxicity. Owing to space constraints, review articles are cited in several instances and the reader is directed to them for additional references.
2. Alcohol-Zn Interactions
Although the concomitant effects of alcohol and specific nutrient deficiencies on the developing brain are not well understood, animal studies have shown that compromised nutrition can exacerbate ethanol’s teratogenic effects. Many adverse effects of prenatal alcohol exposure including low birth weight [13], physical anomalies [14], brain damage [15] and reduced IGF levels [16] have been reported to be more severe when the alcohol is consumed along with poor diets; however, a complication of this finding is that blood alcohol levels are often higher among malnourished subjects than in subjects thought to be characterized by similar alcohol intakes, but better diets. On the positive side, results from early animal studies suggest that select nutritional supplements can attenuate some of the effects of prenatal alcohol exposure, although the effectiveness depends on many factors, including the level of alcohol exposure and the outcome measure [17]. The contribution of select nutritional factors to the risk for FASD in humans has not been well characterized, however, the overall nutritional status of heavy drinkers is generally recognized to be poor [18]. It is critically important to define the role that malnutrition or under-nutrition plays in the risk for FASD. Of particular interest in the pregnant heavy drinker is assessing the status for those micronutrients that are critical for normal neurulation and development of the central nervous system (CNS). From work with experimental animals, it is well documented that deficiencies of certain nutrients, including folate, vitamin B12, Zn, Fe and Cu, during pregnancy can result in abnormal CNS development, and deficiencies of these nutrients are commonly noted in alcoholics [18–23].
With respect to the above nutrients, the hypothesis that maternal Zn status is an important predictor of the risk for FASD has received particular attention. Over 25 years ago, Flynn et al reported that maternal plasma Zn and fetal cord plasma Zn were lower in pregnant women who consumed alcohol versus non-alcohol drinking women [24]. Importantly, these investigators reported that there were negative correlations between maternal plasma Zn concentrations and the severity and frequency of birth defects in the infants, suggesting an etiologic role for Zn deficiency in human FASD. Consistent with the idea that maternal Zn status might be a predictor of the risk for FASD are the early data of Miller et al [25]; these investigators reported that the reproductive toxicity of alcohol in rats was elevated in dams fed marginal Zn diets (10 µg Zn/g diet) compared to that in dams fed diets with control amounts of Zn (45 µg/g) [25]. It is important to note that in the above study by Miller et al, the amount of Zn that was provided in the diet of the marginal Zn group would not be viewed as “deficient”, as the consumption of this diet throughout pregnancy would not typically result in marked developmental anomalies. Consistent with the data of Miller et al, the teratogenicity of alcohol has been reported to be amplified in pregnant rats and mice fed Zn deficient diets (1 µg Zn/g) [26–28].
The relevance of the above work by Miller et al is underscored by the observation that in certain human populations, dietary Zn intake during pregnancy can be well below current recommended dietary intakes [29–32], suggesting that suboptimal Zn status is common in the human population. The concept that a deficit of Zn during early development presents a risk to the human conceptus is reasonable, given that in experimental animals, the teratogenicity of severe Zn deficiency is well documented, resulting in malformations that affect multiple organ systems including the CNS [33]. Even moderate deficiencies of this nutrient during development can result in persistent adverse effects on the immune system and neurobehavioral abnormalities [2,34,35]. Mechanistically, Zn deficiency is thought to influence embryonic and fetal development through multiple mechanisms including abnormal nucleic acid metabolism, reduced protein synthesis, impaired cell migration and cell signaling due to alterations in the cytoskeleton secondary to impaired tubulin polymerization, excessive cellular oxidative stress, reduced binding of transcription factors and hormones that are dependent on Zn-finger regions, and reductions in insulin and IGF-signaling [2,23,34,36–40] (Table 2; Figure 1).
Table 2.
Potential mechanisms of Zn deficiency teratogenicity
| Indirect |
| Zinc deficiency-induced changes in maternal metabolism |
| Direct |
| Impaired DNA replication and transcription |
| Chromosomal damage |
| Decreased binding of growth factors and hormones to their receptors |
| Excessive oxidative damage |
| Altered patterns of cell death |
| Altered cell signaling |
| Impaired cell migration |
| Low activities of numerous zinc enzymes |
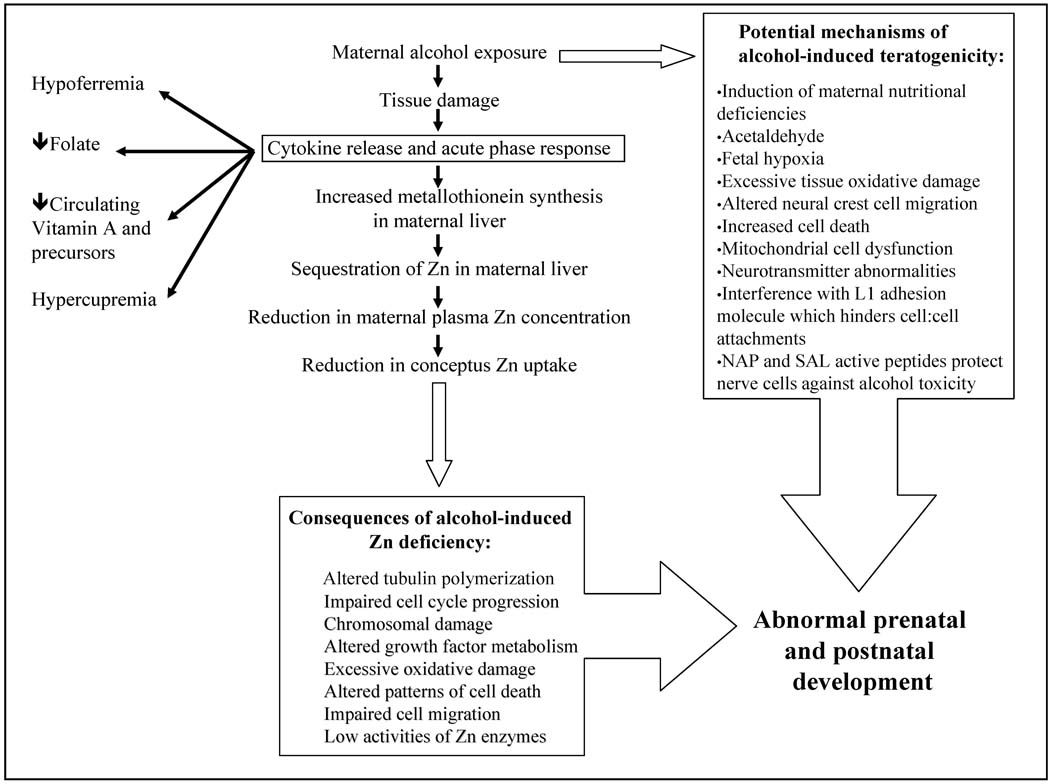
Figure 1.
Alcohol-induced acute phase response (APR) and precipitation of conceptal Zn deficiency. An APR can also lead to changes in the metabolism of other essential nutrients. Multiple mechanisms underlie the teratogenicity of alcohol.
It is important to note that mechanistically, one would predict multiple synergistic interactions between an alcohol insult and a condition of marginal Zn deficiency. As an example of the above, it is well documented that Zn contributes to the oxidant defense system through multiple means, including through its ability to: (1) regulate Cu-Zn superoxide dismutase (CuZn SOD) activity; (2) regulate metallothionein levels; (3) protect sulfhydryl groups from oxidation; (4) modulate intracellular thiol groups; and (5) inhibit the binding of redox active metals, such as Fe and Cu, to intracellular sites where they can generate free radical reactions (e.g., Fenton-type reactions). Given the above, it is evident that one functional consequence of Zn deficiency is an increased susceptibility to exogenous oxidative stressors, such as smoking, endotoxin challenge, and, particularly germane to this paper, alcohol [27,39,41]. The consequences of excessive tissue oxidative stress in the embryo can include lipid, protein and DNA oxidative damage, and an increase in apoptosis, all of which can trigger abnormal development. It is important to note that all of the above are common findings in animal models for FASD [23].
Another potential point of interaction between Zn deficiency and an alcohol challenge involves sonic hedgehog (Shh) signaling. Shh signaling is critical for polarizing activity, and Shh null fetuses are characterized by a postaxial forelimb ectrodactyly in mouse models [42]. Shh is a Zn-dependent developmental trigger [43], and reduced Shh expression has been implicated in ethanol-induced postaxial forelimb ectrodactyly in the mouse [44]. Schreiner and co-workers [45] have suggested that a state of embryonic Zn deficiency secondary to an alcohol-induced acute-phase response (see below; [46–48]) in the mother results in reduced Shh signaling with subsequent dysmorphology [45]. This is an interesting hypothesis that merits further investigation. Importantly, if it is shown to be correct, there are numerous other Zn-dependent developmental proteins, many in the hedgehog signaling pathway (e.g., Gli Zn finger transcription regulators), that might also be affected through the mechanism described above.
A third finding that is common to experimental Zn deficiency, and alcohol challenges during pregnancy, is a reduction in IGF levels and action [49,50]. Zn deficiency can alter IGF-1 and IGF-binding protein metabolism in maternal and fetal blood [49] and may contribute to growth retardation of the offspring. Growth deficit is also commonly noted in offspring of alcohol-exposed women. Administration of ethanol by intubation (5.25 g/kg/day) on postnatal days 4–9 results in motor coordination impairments that are significantly improved with intranasal administration of IGF on postnatal days 10–13 [50].
Finally, a common finding in animal models for developmental Zn deficiency and FASD is an elevated occurrence of apoptosis in the embryo and fetus. Multiple mechanisms have been implicated in the inducing-effects of alcohol and Zn deficiency on apoptosis including disruptions in growth factor signal transduction pathways mediated by receptor tyrosine kinases, an increase in the expression of caspase-3, a down-regulation of NF-κB-dependent anti-apoptotic genes, and an increase in cellular oxidative stress. [36,38,51].
Evidence that severe Zn deficiency presents a reproductive risk in humans is provided by the observation that women with a congenital disorder in Zn absorption (acrodermatitis enteropathica; AE) have a very high risk for pregnancy complications unless they are given dietary Zn supplements [2]. It is now recognized that the AE is due to a defective Zn transporter. The analogue of this transporter in mice is Zip4, and consistent with the human literature, homozygous Zip4-knockout mouse embryos die during early embryogenesis and are characterized by multiple defects [52]. As is depicted in Table 3, a number of studies have reported that low plasma Zn concentrations in the first or third trimester is associated with an increased risk for several pregnancy complications, including birth defects and growth retardation. In further support of the concept that maternal Zn status can be a predictor of pregnancy complications, numerous investigators have reported that even in non-AE populations, the provision of dietary Zn supplements during pregnancy results in a reduced risk for pregnancy complications (Table 3). However, as is also provided in Table 3, there are several studies in which the provision of Zn supplements during pregnancy had no measurable positive effects. Indeed, in a recent Cochrane Review on the effects of Zn supplementation on pregnancy outcome [53], the authors concluded that with the exception of the risk for prematurity, there was no consistent positive effect of Zn supplements during pregnancy. In our opinion, the somewhat negative finding by Mahomed et al [53] is due to the fact that in many cases, the Zn supplementation trials have been done with relatively healthy, non-stressed populations. This may be important since, in addition to low dietary Zn intake, stressor-induced changes in the metabolism of Zn and other nutrients are often secondary to an acute phase response (APR), which can be triggered by a diverse set of cytokines that are released following tissue injury.
Table 3.
Evidence for an influence of Zn on human pregnancy outcome
| Poor pregnancy outcome in women with acrodermatitis enteropathica |
| Hambidge et al., 1975 |
|
Low Zn status (low blood and hair Zn concentrations) is associated with an increased risk for complications |
| Jameson |
| Meadows et al. |
| Cavdar et al. |
| Neggers et al. |
| Srinivas et al. |
| Scheplyagina |
| Golalipour et al. |
| Zn supplements are associated with improved pregnancy outcome |
| Kynast and Saling, 1986 |
| Cherry et al., 1989 |
| Cavdar et al., 1991 |
| Goldenberg et al., 1995 |
| Osendarp et al., 2001 |
| Saaka et al., 2009 |
| Hozyasz et al., 2009 |
| Danesh et al., 2009 |
| Zn supplements do not reduce pregnancy complications |
| Hunt et al., 1985 |
| Mahomed et al., 1989 |
| Jonsson et al., 1996 |
| Caulfield et al., 1999 |
| Hafeez et al., 2005 |
2.1. Acute Phase Response-Induced Fetal Zn Deficiency
It has been hypothesized that the developmental toxicity of a wide variety of toxicants and environmental insults is mediated in part through the induction of the APR, which increases maternal hepatic metallothionein synthesis and Zn sequestration, leading to reduced Zn transfer to the conceptus. If severe enough, this can result in abnormal development [2,46,54–56] (Figure 1). The potential human relevance of the above work with experimental animals is illustrated by the finding that pregnant women who are infected with cytomegalovirus, a common etiologic agent of intrauterine infection, are characterized by high cytokine levels including TNF-α and IL-6, and lower than normal plasma Zn concentrations [57]. Underscoring the potential significance of the above finding is the recent report by Collier et al [58] that maternal infections during pregnancy are common. In rats, disease and environmental factors that reduce maternal serum Zn concentrations can disrupt Zn-dependent processes in the embryo and produce developmental defects, even when the mother consumes a Zn “adequate” diet [54,59]. Significantly, the teratogenic effects of the APR-induced hypozincemia can be amplified by marginal Zn diets, and reduced with Zn supplementation [46,54]. Critical to the current paper, in rodent models, acute alcohol exposure is associated with APR-induced reductions in fetal Zn uptake [48,54–56,60,61]. That the above reductions in fetal Zn uptake are functionally significant is suggested by the observation that the teratogenicity of alcohol is lessened when animals are given Zn prior to the alcohol exposure [60–63]. Moreover, the teratogenicity of acute alcohol exposure is reduced in metallothionein knockout mice compared to metallothionein wild-type mice [55].
It is important to note that in the above discussion, the focus has been on the potential positive effects of Zn supplementation on the expression of FASD in the offspring. Zn supplementation has also been reported to be of value in attenuating ethanol-induced liver damage in the adult [64–66] and mitigating lung epithelial and macrophage dysfunction induced by chronic alcohol intake [67]. Thus, it can be speculated that Zn supplementation of the high risk patient could directly benefit the mother, as well as her child.
In addition to pregnant women who are exposed to alcohol, infants with FASD have been reported to have low plasma Zn concentrations and higher twenty-four hour urinary Zn excretions compared to normal controls, indicating altered Zn homeostasis [68]. Interestingly Murillo-Fuentes and coworkers [69] reported that rat pups whose mothers were given alcohol during the period of lactation were also characterized by higher than normal levels of urinary Zn excretion. It is important to stress that the above reports are preliminary in nature. However, if the finding can be replicated, it represents a critical observation as it could provide one explanation for the persistent immunological abnormalities that have been reported for some children with FASD [70,71]. If these persistent immunological abnormalities are secondary in part to persistent abnormalities in Zn metabolism, they could be responsive to Zn supplements. In support of this concept, Zn given by injection [72], as well as the feeding of diets high in Zn [73], have been reported to reduce neurobehavioral abnormalities in mouse and rat offspring of ethanol-exposed dams. While these data are compelling, others, using a rat model, did not observe a protective effect of Zn supplementation on alcohol-induced developmental brain abnormalities [74]. In addition, it is important to note that there can be adverse interactions among nutrients. For example, supplementation of an alcohol-containing liquid diet with 300 µg/ml of Zn resulted in severe fetal Cu deficiencies relative to controls [75]. Cu deficiency during pregnancy is also teratogenic, affecting cardiac, vascular, neurological, pulmonary, skeletal, and immune systems [21,76,77].
Ethanol exposure has also been shown to alter Fe regulation and homeostasis. Chronic ethanol consumption increases body stores of Fe and is associated with a significant risk of Fe overload [78–81]. Levels of non-transferrin-bound Fe are higher in alcohol abusers [78], which could contribute to reactive oxygen species formation via its involvement in Fe-catalyzed Fenton reactions [82]. When antioxidants, Fe chelators or sulfhydryl compounds that increase cellular glutathione are administered, ethanol toxicity is significantly reduced [83]. Miller and coworkers [84] reported that ethanol consumption by rat dams perturbs the temporal patterns between Fe concentrations and Fe-regulatory proteins in brain regions of offspring. That fetal alcohol exposure can result in low Fe stores in the human infant has been reported by Carter et al [85].
It is critical to note that the metabolism of Fe, Zn and Cu are interrelated, and it has been demonstrated in experimental animal models that a maternal deficit of any one of the above elements can result in alterations in the metabolism of the other elements in the mother as well as the fetus [86]. The above observation underscores the potential risk of focusing on a signal nutrient when one is studying alcohol-nutrient interactions with respect to the risk for FASD. In this regard, it is important to note that while hypozincemia is a hallmark of an APR, as is depicted in Figure 1, an APR can also result in marked disturbances in the metabolism of Fe, Cu, folate and vitamins, as well as other essential nutrients. The extent to which APR-induced changes in theses nutrients might affect fetal development has been largely unexplored.
3. Testing of the Zn/Alcohol Hypothesis: The Need for Large-Scale Prospective Trials
There is a need for multiple prospective studies investigating whether alterations in maternal Zn status can affect fetal outcome in alcohol-exposed women. These studies should be designed in a way that they could later be analyzed via meta-analysis as is done in Cochrane reviews to determine whether suboptimal Zn status is a predictor of adverse fetal outcomes. Moreover, maternal nutrient supplementation could be instituted as this is easily modifiable. Towards this goal, a longitudinal prospective study that aims to examine maternal nutritional status and its contribution to risk for FASD, as well as to test a multi-micronutrient intervention with or without choline supplementation, is currently underway at sites in Ukraine, as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). The study protocol was approved by institutional review boards in Russia and Ukraine and institutional review boards at the University of California, San Diego and the University of California, Davis; all study participants provided informed consent.
In the above study, alcohol-exposed and comparison group subjects are selected from prenatal patients at each of the two sites who were screened by prenatal care providers at first prenatal visit, using a short standardized screening tool. Subjects are considered eligible for the alcohol-exposed group based on quantity and frequency of alcohol consumption during pregnancy. A positive screen for quantity and frequency of alcohol consumption is defined as at least four episodes of five or more standard drinks, at least five episodes of 3–4 standard drinks, or at least 10 episodes of 1–2 standard drinks either in the month around the time of conception, or the most recent month of pregnancy. Subjects are considered eligible for the comparison group based on minimal to no alcohol consumption during either time period, as reported at the time of the initial screening.
All eligible alcohol-exposed women are invited to enroll, and comparison group women are recruited in an approximate 1:1 ratio. This is accomplished following enrollment of each alcohol-exposed woman by approaching the next pregnant woman presenting for prenatal care who reports low to no alcohol exposure in response to the screening questionnaire and who agrees to participate in the study.
As part of the pregnancy follow-up procedure, enrolled women participate in comprehensive standardized interviews, newborn physical examinations, and standard neurobehavioral testing of infants. For the nutrition component of this study, all subjects are randomized to receive a nutritional intervention involving three arms: multi-micronutrient supplement provided from time of enrollment to delivery, multi-micronutrient supplement with choline supplement provided from the time of enrollment to delivery, or no treatment (current standard of care in Russia and Ukraine). Maternal blood samples are drawn at the time of enrollment to establish baseline nutritional status, and again in the 3rd trimester to evaluate change in status, and to validate the impact of treatment group on change in nutritional status.
The samples collected to date in Russia have been analyzed in the laboratory of one of the authors (AS) at the Institute for Biotech Medicine in Moscow. The samples collected to date from the Ukraine have been analyzed by three of the authors in the U.S. at the University of California, Davis (CLK, JYUA, KG). Due to potential variability in the laboratory procedures, and to differences in the characteristics of subjects, preliminary data from these two sites are presented separately. Baseline maternal blood samples were collected in heparinized tubes and analyzed for Zn, Cu, Mg, Fe and Ca by inductively coupled plasma optical emission spectrometry (ICP) (Trace scan; Thermo Elemental, Franklin, MA) [87]. Certified reference solutions (QC 21, Spec Centri Prep, Metuchen, NJ) were used to generate standard curves for each element. National Bureau of Standards reference samples were included with each run to ensure accuracy and reproducibility.
Preliminary data on analysis of mineral status at the time of enrollment is available for a total of 69 subjects from the two sites. Women in the alcohol-exposed groups at the two sites were similar in age, primigravidity, and years of education to their respective no or low-exposed groups, but more likely to be single mothers and to be current smokers relative to their comparison groups (Table 4).
Table 4.
Characteristics of pregnant women enrolled in nutrition and FASD study in Russia and Ukraine
| Russian Sample | Ukraine Sample | |||
|---|---|---|---|---|
| Maternal Characteristic |
Alcohol Exposed N = 10 |
Alcohol Unexposed N = 10 |
Alcohol Exposed N = 28 |
Alcohol Unexposed N = 21 |
| Maternal Age – years, mean (SD) |
27.7 (8.0) | 27.5 (6.0) | 23.9 (4.6) | 24.2 (4.9) |
| Primigravid n (%) | 5 (50.0) | 5 (50.0) | 11 (52.4) | 19 (67.9) |
| Education – years, mean (SD) |
14.7 (2.2) | 16.4 (2.0) | 13.0 (2.4) | 14.1 (1.6) |
| Marital Status n (%) | ||||
| Single | 4 (40.0) | 1 (10.0) | 7 (25.0) | 2 (9.5) |
| Married | 2 (20.0) | 8 (80.0) | 21 (75.0) | 19 (90.5) |
| Separated | 3 (40.0) | 1 (10.0) | 0 | 0 |
| Smokers n (%) | 8 (80.0) | 2 (20.0) | 7 (25.0) | 1 (4.8) |
Table 5 describes the characteristics of maternal alcohol use as reported at the time of enrollment regarding the month around the time of conception. Consistent with the group selection criteria, women in the alcohol-exposed groups were predominately binge drinkers, with almost no women reporting daily drinking, even in small amounts.
Table 5.
Reported alcohol consumption in month around time of conception among pregnant women enrolled in nutrition and FASD study in Russia and Ukraine
| Russian Sample | Ukraine Sample | |||
|---|---|---|---|---|
| # Drinks per Occasion in Month |
Alcohol Exposed N = 10 |
Alcohol Unexposed N = 10 |
Alcohol Exposed N = 28 |
Alcohol Unexposed N = 21 |
| 5 or more n (%) # of occasions |
4 (40.0) 0–5 |
0 | 12 (42.9) 0–3 |
0 |
| 3 or 4 n (%) # of occasions |
10 (100) 2–5 |
0 | 24 (85.7) 0–10 |
0 |
| 1 or 2 n (%) # of occasions |
9 (90.0) 0–7 |
7 (70.0) 0–3 |
28 (100) 1–28 |
7 (33.3) 0–2 |
| Daily n (%) | 0 | 0 | 2 (7.1) | 0 |
As shown in Table 6, although numbers are small in each sample, for most of the minerals, consistently lower mean values were observed in the alcohol-exposed groups than in the controls; these differences were statistically significant for Zn at both sites, and for Cu at the Ukraine site.
Table 6.
Maternal plasma mineral concentrations at enrollment among pregnant women participating in nutrition and FASD study in Russia and Ukraine
| Mineral Mean (SD) |
Russia Sample | Ukraine Sample | ||||
|---|---|---|---|---|---|---|
| Alcohol Exposed N = 10 |
Alcohol Unexposed N = 10 |
p-value* | Alcohol Exposed N = 28 |
Alcohol Unexposed N = 21 |
p-value* | |
| Ca (µg/mL) | 88.5 ± 3.3 | 93.8 ± 4.5 | 0.348 | 83.4 ± 1.2 | 83.5 ± 0.7 | 0.915 |
| Cu (µg/mL) | 2.2 ± 0.2 | 2.4 ± 0.2 | 0.409 | 1.7 ±0.1 | 1.9 ± 0.1 | 0.043 |
| Fe (µg/mL) | 1.4 ± 0.3 | 1.1 ±0.1 | 0.342 | 0.78 ± 0.06 | 0.82 ± 0.05 | 0.637 |
| Mg (µg/mL) | 18.1 ± 0.7 | 19.0 ±1.0 | 0.489 | 15.9 ± 0.02 | 16.2 ± 0.2 | 0.187 |
| Zn (µg/mL) | 0.59 ± 0.04 | 0.73 ± 0.06 | 0.073 | 0.57 ± 0.02 | 0.64 ± 0.05 | 0.022 |
t-test appropriate for equal or unequal variances
4. Summary and Concluding Comments
The above comments have been aimed at the overarching hypothesis that the occurrence of numerous features of FASD, including growth deficiency, structural features, persistent immunological defects and neurobehavioral impairment, is influenced by the nutritional status of the mother, as well as the conceptus. Current data support the concept that select micronutrient deficiencies increase the risk for the occurrence of FASD in high-risk populations. In theory, these nutritional deficiencies can arise as a consequence of poor diets, as well as a consequence of tissue injury-induced alterations in the metabolism of select nutrients. If the above concepts are correct, it is reasonable to predict that the use of select micronutrient supplements could reduce the frequency and severity of FASD in these populations.
Consistent with this hypothesis, preliminary data from the ongoing studies in Ukraine and Russia show that plasma Zn and Cu concentrations are low in pregnant women who report high alcohol intakes. In addition to the mounting evidence that certain micronutrients can affect normal structural development, it is evident that prenatal nutrition is an important factor both in prenatal growth and in postnatal cognitive performance [88,89]. As described above, Zn, as well as Cu, is involved in multiple biochemical pathways that are critical for brain growth and function.
As the toxicity of alcohol is thought to be due, in part, to free radical-induced oxidative damage [83], deficits of Zn and Cu would both be predicted to increase the sensitivity of the developing conceptus to alcohol, given that these nutrients contribute to the oxidative defense system [90]. The reported blunting of alcohol’s adverse effects by folate, vitamin B12, choline and Zn supplementation indicates that there may be some commonalities among these nutrients [22,91–93]. For example, all of these nutrients can affect redox stress, as well as gene methylation patterns, underscoring the fact that none of these nutrients should be looked at in isolation. Important future research directions include determination of the mechanisms underlying the developmental effects of the “suboptimal” nutritional status that can occur with alcohol exposure as well as delineation of how specific, persistent, and important these effects are.
A major aim of the ongoing CIFASD trial is to evaluate nutritional risk modifiers for FASD and to determine if maternal micronutrient supplementation during pregnancy in drinking women has substantial promise as an easily accomplished environmental manipulation that should substantially improve pregnancy outcome for both the mother and infant. Large prospective FASD studies that utilize well-“validated” biomarkers for maternal nutritional status (acute and chronic) prior to and during early pregnancy are a critical research need. In these studies, maternal nutritional status should be evaluated at regular intervals to capture environment-induced changes. These large prospective nutrient supplementation trials should be implemented with populations of pregnant women who are at a high risk for FASD. Moreover, nutrient supplementation trials should also be done with FASD children to determine whether nutritional intervention can alleviate long-term adverse outcomes in the offspring.
In closing, it should be noted that the concept that women who abuse alcohol during pregnancy should be encouraged to use multivitamin, multimineral supplements is not new. Indeed, in 1990, the American Institute of Medicine identified this group of women who should be particularly encouraged to use these supplements during pregnancy [94]. To date, our experience in Russia, as well as in Ukraine, is that women at high risk for having a FASD child are very receptive to using supplements. Obviously, the most appropriate message to these women is to stop drinking. However, on a practical level, combining this message with one that is aimed at improving their overall nutritional status would seem to be an appropriate public health strategy.
Acknowledgments
These studies were funded by NIAAA Grant numbers U01AA014835 and U24AA014811, and NIH Grant numbers HD 01743 and HD 26777. Part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
References
- 1.MACDP: 40th Anniversary Edition Surveillance Report. Birth Defects Res. A Clin. Mol. Teratol. 2007;79:66–186.
- 2.Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, Oteiza P, Uriu-Adams JY. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J. Nutr. 2003;133:1597S–1605S. doi: 10.1093/jn/133.5.1597S. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian P, Stewart CP. Maternal Micronutrient Deficiency, Fetal Development, and the Risk of Chronic Disease. J. Nutr. 2010 doi: 10.3945/jn.109.116327. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Ebbs JH, Tisdall FF, Scott WA. The influence of prenatal diet on the mother and child. J. Nutr. 1941;22:515–526. [Google Scholar]
- 6.Keen CL, Zidenberg-Cherr S. Should vitamin-mineral supplements be recommended for all women with childbearing potential? Am. J. Clin. Nutr. 1994;59:532S–538S. doi: 10.1093/ajcn/59.2.532S. discussion 538S–539S. [DOI] [PubMed] [Google Scholar]
- 7.Laurence KM, Campbell H, James NE. The role of improvement in the maternal diet and preconceptional folic acid supplementation in the prevention of neural tube defects. In: Dobbing J, editor. Prevention of Spina Bifida and Other Neural Tube Defects. New York: Academic Press; 1983. pp. 85–125. [Google Scholar]
- 8.Primrose T, Higgins A. A study in human antepartum nutrition. J. Reprod. Med. 1971;7:257–264. [PubMed] [Google Scholar]
- 9.Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis. J. Obstet. Gynaecol. Can. 2006;28:680–689. doi: 10.1016/S1701-2163(16)32227-7. [DOI] [PubMed] [Google Scholar]
- 10.Picciano MF, McGuire MK. Use of dietary supplements by pregnant and lactating women in North America. Am. J. Clin. Nutr. 2009;89:663S–667S. doi: 10.3945/ajcn.2008.26811B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. Am. J. Epidemiol. 2006;164:470–477. doi: 10.1093/aje/kwj218. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen SA, Erickson JD, Reef SE, Ross DS. Teratology: from science to birth defects prevention. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- 13.Wiener SG, Shoemaker WJ, Koda LY, Bloom FE. Interaction of ethanol and nutrition during gestation: influence on maternal and offspring development in the rat. J. Pharmacol. Exp. Ther. 1981;216:572–579. [PubMed] [Google Scholar]
- 14.Weinberg J, D'Alquen G, Bezio S. Interactive effects of ethanol intake and maternal nutritional status on skeletal development of fetal rats. Alcohol. 1990;7:383–388. doi: 10.1016/0741-8329(90)90020-d. [DOI] [PubMed] [Google Scholar]
- 15.Wainwright P, Fritz G. Effect of moderate prenatal ethanol exposure on postnatal brain and behavioral development in BALB/c mice. Exp. Neurol. 1985;89:237–249. doi: 10.1016/0014-4886(85)90279-1. [DOI] [PubMed] [Google Scholar]
- 16.Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FA, Badger TM, Ronis MJ. Physiologic and genomic analyses of nutrition-ethanol interactions during gestation: Implications for fetal ethanol toxicity. Exp. Biol. Med. (Maywood) 2006;231:1379–1397. doi: 10.1177/153537020623100812. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcohol Clin. Exp. Res. 1985;9:49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 18.Gloria L, Cravo M, Camilo ME, Resende M, Cardoso JN, Oliveira AG, Leitao CN, Mira FC. Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am. J. Gastroenterol. 1997;92:485–489. [PubMed] [Google Scholar]
- 19.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 20.Keen CL, Taubeneck MW, Daston GP, Rogers JM, Gershwin ME. Primary and secondary zinc deficiency as factors underlying abnormal CNS development. Ann. N.Y. Acad. Sci. 1993;678:37–47. doi: 10.1111/j.1749-6632.1993.tb26108.x. [DOI] [PubMed] [Google Scholar]
- 21.Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, Rucker RB. Effect of copper deficiency on prenatal development and pregnancy outcome. Am. J. Clin. Nutr. 1998;67:1003S–1011S. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Li L, Zhang Z, Li Y. Effects of folinic acid and Vitamin B12 on ethanol-induced developmental toxicity in mouse. Toxicol. Lett. 2006;167:167–172. doi: 10.1016/j.toxlet.2006.07.341. [DOI] [PubMed] [Google Scholar]
- 23.Chen CP. Syndromes, disorders and maternal risk factors associated with neural tube defects (VI) Taiwan J. Obstet. Gynecol. 2008;47:267–275. doi: 10.1016/S1028-4559(08)60123-0. [DOI] [PubMed] [Google Scholar]
- 24.Flynn A, Miller SI, Martier SS, Golden NL, Sokol RJ, Del Villano BC. Zinc status of pregnant alcoholic women: a determinant of fetal outcome. Lancet. 1981;1:572–551. doi: 10.1016/s0140-6736(81)92029-8. [DOI] [PubMed] [Google Scholar]
- 25.Miller SI, Del Villano BC, Flynn A, Krumhansl M. Interaction of alcohol and zinc in fetal dysmorphogenesis. Pharmacol. Biochem. Behav. 1983;18 Suppl 1:311–315. doi: 10.1016/0091-3057(83)90192-2. [DOI] [PubMed] [Google Scholar]
- 26.Ruth RE, Goldsmith SK. Interaction between zinc deprivation and acute ethanol intoxication during pregnancy in rats. J. Nutr. 1981;111:2034–2038. doi: 10.1093/jn/111.11.2034. [DOI] [PubMed] [Google Scholar]
- 27.Dreosti IE, Partick EJ. Zinc, ethanol, and lipid peroxidation in adult and fetal rats. Biol. Trace Elem. Res. 1987;14:179–191. doi: 10.1007/BF02795685. [DOI] [PubMed] [Google Scholar]
- 28.Keppen LD, Pysher T, Rennert OM. Zinc deficiency acts as a co-teratogen with alcohol in fetal alcohol syndrome. Pediatr. Res. 1985;19:944–947. doi: 10.1203/00006450-198509000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2000;130:1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- 30.Harley K, Eskenazi B, Block G. The association of time in the US and diet during pregnancy in low-income women of Mexican descent. Paediatr. Perinat. Epidemiol. 2005;19:125–134. doi: 10.1111/j.1365-3016.2005.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah D, Sachdev HP. Effect of gestational zinc deficiency on pregnancy outcomes: summary of observation studies and zinc supplementation trials. Br. J. Nutr. 2001;85 Suppl 2:S101–S108. doi: 10.1079/bjn2000301. [DOI] [PubMed] [Google Scholar]
- 32.Hess SY, Lonnerdal B, Hotz C, Rivera JA, Brown KH. Recent advances in knowledge of zinc nutrition and human health. Food Nutr. Bull. 2009;30:S5–S11. doi: 10.1177/15648265090301S102. [DOI] [PubMed] [Google Scholar]
- 33.Keen CL, Taubeneck MW, Daston GP, Gershwin ME, Ansari A, Rogers JM. Primary and secondary zinc deficiency as factors contributing to abnormal central nervous system development. Dev. Brain Dysfunct. 1995;8:79–89. [Google Scholar]
- 34.Maret W, Sandstead HH. Possible roles of zinc nutriture in the fetal origins of disease. Exp. Gerontol. 2008;43:378–381. doi: 10.1016/j.exger.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Golub MS, Keen CL, Gershwin ME. Moderate zinc-iron deprivation influences behavior but not growth in adolescent rhesus monkeys. J. Nutr. 2000;130:354S–357S. doi: 10.1093/jn/130.2.354S. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JE, Kovacic JP. The ubiquitous role of zinc in health and disease. J. Vet. Emerg. Crit. Care (San Antonio) 2009;19:215–240. doi: 10.1111/j.1476-4431.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 37.Duffy JY, Overmann GJ, Keen CL, Clegg MS, Daston GP. Cardiac abnormalities induced by zinc deficiency are associated with alterations in the expression of genes regulated by the zinc-finger transcription factor GATA-4. Birth Defects Res. B Dev. Reprod. Toxicol. 2004;71:102–109. doi: 10.1002/bdrb.20004. [DOI] [PubMed] [Google Scholar]
- 38.Clegg MS, Hanna LA, Niles BJ, Momma TY, Keen CL. Zinc deficiency-induced cell death. IUBMB Life. 2005;57:661–669. doi: 10.1080/15216540500264554. [DOI] [PubMed] [Google Scholar]
- 39.Oteiza PI, Mackenzie GG, Verstraeten SV. Metals in neurodegeneration: involvement of oxidants and oxidant-sensitive transcription factors. Mol. Aspects Med. 2004;25:103–115. doi: 10.1016/j.mam.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Jansen J, Karges W, Rink L. Zinc and diabetes--clinical links and molecular mechanisms. J. Nutr. Biochem. 2009;20:399–417. doi: 10.1016/j.jnutbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Keen CL, Uriu-Adams JY. Assessment of zinc, copper and magnesium status: Current approaches and promising new directions. In: Institute of Medicine, editor. Mineral Requirements for Military Personnel. Washington, D.C: The National Academies Press; 2006. pp. 304–315. [Google Scholar]
- 42.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev. Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 43.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Chrisman K, Kenney R, Comin J, Thal T, Suchocki L, Yueh YG, Gardner DP. Gestational ethanol exposure disrupts the expression of FGF8 and Sonic hedgehog during limb patterning. Birth Defects Res. A Clin. Mol. Teratol. 2004;70:163–171. doi: 10.1002/bdra.20019. [DOI] [PubMed] [Google Scholar]
- 45.Schreiner CM, Bell SM, Scott WJ., Jr Microarray analysis of murine limb bud ectoderm and mesoderm after exposure to cadmium or acetazolamide. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:588–598. doi: 10.1002/bdra.20577. [DOI] [PubMed] [Google Scholar]
- 46.Daston GP, Overmann GJ, Baines D, Taubeneck MW, Lehman-McKeeman LD, Rogers JM, Keen CL. Altered Zn status by alpha-hederin in the pregnant rat and its relationship to adverse developmental outcome. Reprod. Toxicol. 1994;8:15–24. doi: 10.1016/0890-6238(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 47.Keen CL, Peters JM, Hurley LS. The effect of valproic acid on 65Zn distribution in the pregnant rat. J. Nutr. 1989;119:607–611. doi: 10.1093/jn/119.4.607. [DOI] [PubMed] [Google Scholar]
- 48.Taubeneck MW, Daston GP, Rogers JM, Keen CL. Altered maternal zinc metabolism following exposure to diverse developmental toxicants. Reprod. Toxicol. 1994;8:25–40. doi: 10.1016/0890-6238(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 49.Hanna LA, Clegg MS, Ellis-Hutchings RG, Niles BJ, Keen CL. The influence of gestational zinc deficiency on the fetal insulin-like growth factor axis in the rat. Exp. Biol. Med. 2010 doi: 10.1258/ebm.2009.009018. In press. [DOI] [PubMed] [Google Scholar]
- 50.McGough NN, Thomas JD, Dominguez HD, Riley EP. Insulin-like growth factor-I mitigates motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2009;31:40–48. doi: 10.1016/j.ntt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, Keenan A, Oteiza PI. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox. Res. 17:1–14. doi: 10.1007/s12640-009-9067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem. Soc. Trans. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahomed K, Bhutta Z, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD000230.pub3. CD000230. [DOI] [PubMed] [Google Scholar]
- 54.Taubeneck MW, Daston GP, Rogers JM, Gershwin ME, Ansari A, Keen CL. Tumor necrosis factor-alpha alters maternal and embryonic zinc metabolism and is developmentally toxic in mice. J. Nutr. 1995;125:908–919. doi: 10.1093/jn/125.4.908. [DOI] [PubMed] [Google Scholar]
- 55.Carey LC, Coyle P, Philcox JC, Rofe AM. Ethanol decreases zinc transfer to the fetus in normal but not metallothionein-null mice. Alcohol Clin. Exp. Res. 2000;24:1236–1240. [PubMed] [Google Scholar]
- 56.Carey LC, Coyle P, Philcox JC, Rofe AM. Maternal ethanol exposure is associated with decreased plasma zinc and increased fetal abnormalities in normal but not metallothionein-null mice. Alcohol Clin. Exp. Res. 2000;24:213–219. [PubMed] [Google Scholar]
- 57.Zhang JP, Li F, Yu XW, Sheng Q, Shi XW, Zhang XW. Trace elements and cytokine profile in cytomegalovirus-infected pregnancies: a controlled study. Gynecol. Obstet. Invest. 2008;65:128–132. doi: 10.1159/000110013. [DOI] [PubMed] [Google Scholar]
- 58.Collier SA, Rasmussen SA, Feldkamp ML, Honein MA. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:193–201. doi: 10.1002/bdra.20540. [DOI] [PubMed] [Google Scholar]
- 59.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell. Mol. Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey LC, Coyle P, Philcox JC, Rofe AM. Zinc supplementation at the time of ethanol exposure ameliorates teratogenicity in mice. Alcohol Clin. Exp. Res. 2003;27:107–110. doi: 10.1097/01.ALC.0000046337.19144.7D. [DOI] [PubMed] [Google Scholar]
- 61.Summers BL, Rofe AM, Coyle P. Dietary zinc supplementation throughout pregnancy protects against fetal dysmorphology and improves postnatal survival after prenatal ethanol exposure in mice. Alcohol Clin. Exp. Res. 2009;33:591–600. doi: 10.1111/j.1530-0277.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 62.Coyle P, Martin SA, Carey LC, Summers BL, Rofe AM. Ethanol-mediated fetal dysmorphology and its relationship to the ontogeny of maternal liver metallothionein. Alcohol Clin. Exp. Res. 2009;33:1051–1058. doi: 10.1111/j.1530-0277.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 63.Seyoum G, Persaud TV. Protective influence of zinc against the deleterious effects of ethanol in postimplantation rat embryos in vivo. Exp. Toxicol. Pathol. 1995;47:75–79. doi: 10.1016/S0940-2993(11)80290-3. [DOI] [PubMed] [Google Scholar]
- 64.Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M. Zinc supplementation attenuates ethanol- and acetaldehyde-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS) production and by influencing intracellular signaling. Biochem. Pharmacol. 2009;78:301–314. doi: 10.1016/j.bcp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol. Aspects Med. 2005;26:391–404. doi: 10.1016/j.mam.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell. Mol. Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Assadi FK, Ziai M. Zinc status of infants with fetal alcohol syndrome. Pediatr. Res. 1986;20:551–554. doi: 10.1203/00006450-198606000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Murillo-Fuentes ML, Artillo R, Ojeda ML, Delgado MJ, Murillo ML, Carreras O. Effects of prenatal or postnatal ethanol consumption on zinc intestinal absorption and excretion in rats. Alcohol Alcohol. 2007;42:3–10. doi: 10.1093/alcalc/agl084. [DOI] [PubMed] [Google Scholar]
- 70.McGill J, Meyerholz DK, Edsen-Moore M, Young B, Coleman RA, Schlueter AJ, Waldschmidt TJ, Cook RT, Legge KL. Fetal exposure to ethanol has long-term effects on the severity of influenza virus infections. J. Immunol. 2009;182:7803–7808. doi: 10.4049/jimmunol.0803881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp. Biol. Med. (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- 72.Summers BL, Rofe AM, Coyle P. Prenatal zinc treatment at the time of acute ethanol exposure limits spatial memory impairments in mouse offspring. Pediatr. Res. 2006;59:66–71. doi: 10.1203/01.pdr.0000190573.23893.13. [DOI] [PubMed] [Google Scholar]
- 73.Summers BL, Henry CM, Rofe AM, Coyle P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behav. Brain Res. 2008;186:230–238. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Chen WJ, Berryhill EC, West JR. Zinc supplementation does not attenuate alcoholinduced cerebellar Purkinje cell loss during the brain growth spurt period. Alcohol Clin. Exp. Res. 2001;25:600–605. [PubMed] [Google Scholar]
- 75.Zidenberg-Cherr S, Benak PA, Hurley LS, Keen CL. Altered mineral metabolism: a mechanism underlying the fetal alcohol syndrome in rats. Drug Nutr. Interact. 1988;5:257–274. [PubMed] [Google Scholar]
- 76.Gambling L, McArdle HJ. Iron, copper and fetal development. Proc. Nutr. Soc. 2004;63:553–562. doi: 10.1079/pns2004385. [DOI] [PubMed] [Google Scholar]
- 77.Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J. Nutr. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- 78.De Feo TM, Fargion S, Duca L, Cesana BM, Boncinelli L, Lozza P, Cappellini MD, Fiorelli G. Non-transferrin-bound iron in alcohol abusers. Alcohol Clin. Exp. Res. 2001;25:1494–1499. doi: 10.1097/00000374-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 79.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 80.Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Whitfield JB, Zhu G, Heath AC, Powell Lw, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin. Exp. Res. 2001;25:1037–1045. [PubMed] [Google Scholar]
- 82.Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- 83.Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic. Biol. Med. 2001;31:1524–1526. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 84.Miller MW, Roskams AJ, Connor JR. Iron regulation in the developing rat brain: effect of in utero ethanol exposure. J. Neurochem. 1995;65:373–380. doi: 10.1046/j.1471-4159.1995.65010373.x. [DOI] [PubMed] [Google Scholar]
- 85.Carter RC, Jacobson SW, Molteno CD, Jacobson JL. Fetal alcohol exposure, irondeficiency anemia, and infant growth. Pediatrics. 2007;120:559–567. doi: 10.1542/peds.2007-0151. [DOI] [PubMed] [Google Scholar]
- 86.Gambling L, Andersen HS, McArdle HJ. Iron and copper, and their interactions during development. Biochem. Soc. Trans. 2008;36:1258–1261. doi: 10.1042/BST0361258. [DOI] [PubMed] [Google Scholar]
- 87.Mackenzie GG, Zago MP, Keen CL, Oteiza PI. Low intracellular zinc impairs the translocation of activated NF-kappa B to the nuclei in human neuroblastoma IMR-32 cells. J. Biol. Chem. 2002;277:34610–34617. doi: 10.1074/jbc.M203616200. [DOI] [PubMed] [Google Scholar]
- 88.Avchen RN, Scott KG, Mason CA. Birth weight and school-age disabilities: a population-based study. Am. J. Epidemiol. 2001;154:895–901. doi: 10.1093/aje/154.10.895. [DOI] [PubMed] [Google Scholar]
- 89.Faden VB, Hanna E, Graubard BI. The effect of positive and negative health behavior during gestation on pregnancy outcome. J. Subst. Abuse. 1997;9:63–76. doi: 10.1016/s0899-3289(97)90006-7. [DOI] [PubMed] [Google Scholar]
- 90.Keen CL. Teratogenic effects of essential trace metals: deficiencies and excesses. In: Chang LW, Magos L, Suzuki T, editors. Toxicology of Metals. New York: CRC Press; 1996. pp. 977–1001. [Google Scholar]
- 91.Xu Y, Li Y, Tang Y, Wang J, Shen X, Long Z, Zheng X. The maternal combined supplementation of folic acid and Vitamin B(12) suppresses ethanol-induced developmental toxicity in mouse fetuses. Reprod. Toxicol. 2006;22:56–61. doi: 10.1016/j.reprotox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav. Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 94.Subcommittee on Nutritional Status and Weight Gain During Pregnancy, editor. Institute of Medicine. Nutrition During Pregnancy: Part I: Weight Gain, Part II: Nutrient Supplements. Washington, D.C: National Academy Press; 1990. [Google Scholar]
- 95.Burke BS, Beal VA, Kirkwood SB, Stuart HC. The Influence of nutrition during pregnancy upon the condition of the infant at birth: Two figures. J. Nutr. 1943;26:569–583. [Google Scholar]
- 96.Jeans PC, Smith MB, Stearns G. Incidence of prematurity in relation to maternal nutrition. J. Am. Diet. Assoc. 1955;31:576–581. [PubMed] [Google Scholar]
- 97.Friel JK, Frecker M, Fraser FC. Nutritional patterns of mothers of children with neural tube defects in Newfoundland. Am. J. Med. Genet. 1995;55:195–199. doi: 10.1002/ajmg.1320550209. [DOI] [PubMed] [Google Scholar]
- 98.Wright ME. A case-control study of maternal nutrition and neural tube defects in Northern Ireland. Midwifery. 1995;11:146–152. doi: 10.1016/0266-6138(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 99.Torfs CP, Lam PK, Schaffer DM, Brand RJ. Association between mothers' nutrient intake and their offspring's risk of gastroschisis. Teratology. 1998;58:241–250. doi: 10.1002/(SICI)1096-9926(199812)58:6<241::AID-TERA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 100.Velie EM, Block G, Shaw GM, Samuels SJ, Schaffer DM, Kulldorff M. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. Am. J. Epidemiol. 1999;150:605–616. doi: 10.1093/oxfordjournals.aje.a010059. [DOI] [PubMed] [Google Scholar]
- 101.Di Cintio E, Parazzini F, Chatenoud L, Surace M, Benzi G, Zanconato G, La Vecchia C. Dietary factors and risk of spontaneous abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;95:132–136. doi: 10.1016/s0301-2115(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 102.Krapels IP, van Rooij IA, Ocke MC, West CE, van der Horst CM, Steegers-Theunissen RP. Maternal nutritional status and the risk for orofacial cleft offspring in humans. J. Nutr. 2004;134:3106–3113. doi: 10.1093/jn/134.11.3106. [DOI] [PubMed] [Google Scholar]
- 103.Rees GA, Doyle W, Srivastava A, Brooke ZM, Crawford MA, Costeloe KL. The nutrient intakes of mothers of low birth weight babies - a comparison of ethnic groups in East London, UK. Matern. Child. Nutr. 2005;1:91–99. doi: 10.1111/j.1740-8709.2005.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lam PK, Torfs CP. Interaction between maternal smoking and malnutrition in infant risk of gastroschisis. Birth Defects Res. A Clin. Mol. Teratol. 2006;76:182–186. doi: 10.1002/bdra.20238. [DOI] [PubMed] [Google Scholar]
- 105.Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF. Major dietary patterns in pregnancy and fetal growth. Eur. J. Clin. Nutr. 2008;62:463–470. doi: 10.1038/sj.ejcn.1602745. [DOI] [PubMed] [Google Scholar]
- 106.Yang W, Shaw GM, Carmichael SL, Rasmussen SA, Waller DK, Pober BR, Anderka M. Nutrient intakes in women and congenital diaphragmatic hernia in their offspring. Birth Defects Res. A Clin. Mol. Teratol. 2008;82:131–138. doi: 10.1002/bdra.20436. [DOI] [PubMed] [Google Scholar]
- 107.Hambidge KM, Neldner KH, Walravens PA. Letter: Zinc,acrodermatitis enteropathica, and congenital malformations. Lancet. 1975;1:577–578. doi: 10.1016/s0140-6736(75)91601-3. [DOI] [PubMed] [Google Scholar]
- 108.Jameson S. Variations in maternal serum zinc during pregnancy and correlation to congenital malformations, dysmaturity, and abnormal parturition. Acta Med. Scand. Suppl. 1976;593:21–37. doi: 10.1111/j.0954-6820.1976.tb12824.x. [DOI] [PubMed] [Google Scholar]
- 109.Meadows NJ, Ruse W, Smith MF, Day J, Keeling PW, Scopes JW, Thompson RP, Bloxam DL. Zinc and small babies. Lancet. 1981;2:1135–1137. doi: 10.1016/s0140-6736(81)90587-0. [DOI] [PubMed] [Google Scholar]
- 110.Cavdar AO, Bahceci M, Akar N, Erten J, Bahceci G, Babacan E, Arcasoy A, Yavuz H. Zinc status in pregnancy and the occurrence of anencephaly in Turkey. J. Trace Elem. Electrolytes Health Dis. 1988;2:9–14. [PubMed] [Google Scholar]
- 111.Neggers YH, Cutter GR, Alvarez JO, Goldenberg RL, Acton R, Go RC, Roseman JM. The relationship between maternal serum zinc levels during pregnancy and birthweight. Early Hum. Dev. 1991;25:75–85. doi: 10.1016/0378-3782(91)90186-7. [DOI] [PubMed] [Google Scholar]
- 112.Srinivas M, Gupta DK, Rathi SS, Grover JK, Vats V, Sharma JD, Mitra DK. Association between lower hair zinc levels and neural tube defects. Indian J. Pediatr. 2001;68:519–522. doi: 10.1007/BF02723245. [DOI] [PubMed] [Google Scholar]
- 113.Scheplyagina LA. Impact of the mother's zinc deficiency on the woman's and newborn's health status. J. Trace Elem. Med. Biol. 2005;19:29–35. doi: 10.1016/j.jtemb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 114.Kynast G, Saling E. Effect of oral zinc application during pregnancy. Gynecol. Obstet. Invest. 1986;21:117–123. doi: 10.1159/000298940. [DOI] [PubMed] [Google Scholar]
- 115.Cherry FF, Sandstead HH, Rojas P, Johnson LK, Batson HK, Wang XB. Adolescent pregnancy: associations among body weight, zinc nutriture, and pregnancy outcome. Am. J. Clin. Nutr. 1989;50:945–954. doi: 10.1093/ajcn/50.5.945. [DOI] [PubMed] [Google Scholar]
- 116.Cavdar AO, Bahceci M, Akar N, Erten J, Yavuz H. Effect of zinc supplementation in a Turkish woman with two previous anencephalic infants. Gynecol. Obstet. Invest. 1991;32:123–125. doi: 10.1159/000293011. [DOI] [PubMed] [Google Scholar]
- 117.Goldenberg RL, Tamura T, Neggers Y, Copper RL, Johnston KE, DuBard MB, Hauth JC. The effect of zinc supplementation on pregnancy outcome. J.A.M.A. 1995;274:463–468. doi: 10.1001/jama.1995.03530060037030. [DOI] [PubMed] [Google Scholar]
- 118.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet. 2001;357:1080–1085. doi: 10.1016/s0140-6736(00)04260-4. [DOI] [PubMed] [Google Scholar]
- 119.Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Browdy BL, Coulson AH, Clark VA, Settlage RH, Smith JC., Jr Zinc supplementation during pregnancy in low-income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. Am. J. Clin. Nutr. 1985;42:815–828. doi: 10.1093/ajcn/42.5.815. [DOI] [PubMed] [Google Scholar]
- 120.Mahomed K, James DK, Golding J, McCabe R. Zinc supplementation during pregnancy: a double blind randomised controlled trial. B.M.J. 1989;299:826–830. doi: 10.1136/bmj.299.6703.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jonsson B, Hauge B, Larsen MF, Hald F. Zinc supplementation during pregnancy: a double blind randomised controlled trial. Acta Obstet. Gynecol. Scand. 1996;75:725–729. doi: 10.3109/00016349609065735. [DOI] [PubMed] [Google Scholar]
- 122.Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. J. Nutr. 1999;129:1563–1568. doi: 10.1093/jn/129.8.1563. [DOI] [PubMed] [Google Scholar]
- 123.Hafeez A, Mehmood G, Mazhar F. Oral zinc supplementation in pregnant women and its effect on birth weight: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2005;90:F170–F171. doi: 10.1136/adc.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]