Abstract
Seizures induced by fever (febrile seizures) are the most frequent seizures affecting infants and children; however, their impact on the developing hippocampal formation is not completely understood. Such understanding is highly important because of the potential relationship of prolonged febrile seizures to temporal lobe epilepsy. Using an immature rat model, we have previously demonstrated that prolonged experimental febrile seizures render the hippocampus hyperexcitable throughout life. Here we examined whether (1) neuronal loss, (2) altered neurogenesis, or (3) mossy fiber sprouting, all implicated in epileptogenesis in both animal models and humans, were involved in the generation of a pro-epileptic, hyperexcitable hippocampus by these seizures. The results demonstrated that prolonged experimental febrile seizures did not result in appreciable loss of any vulnerable hippocampal cell population, though causing strikingly enhanced sensitivity to hippocampal excitants later in life. In addition, experimental febrile seizures on postnatal day 10 did not enhance proliferation of granule cells, whereas seizures generated by kainic acid during the same developmental age increased neurogenesis in the immature hippocampus. However, prolonged febrile seizures resulted in long-term axonal reorganization in the immature hippocampal formation: Mossy fiber densities in granule cell- and molecular layers were significantly increased by 3 months (but not 10 days) after the seizures. Thus, the data indicate that prolonged febrile seizures influence connectivity of the immature hippocampus long-term, and this process requires neither significant neuronal loss nor altered neurogenesis. In addition, the temporal course of the augmented mossy fiber invasion of the granule cell and molecular layers suggests that it is a consequence, rather than the cause, of the hyperexcitable hippocampal network resulting from these seizures.
Keywords: hippocampus, dentate gyrus, epilepsy, epileptogenesis, development, interneurons
INTRODUCTION
Febrile seizures (seizures induced by fever) have generated intense research efforts because of their high incidence (2–5% of children between the ages of 6 months and 5 years) and their potential relationship to limbic (temporal lobe) epilepsy (TLE) later in life (for review, see Lewis, 1999; Cendes and Andermann, 2002). Epidemiological data indicate that the risk of developing epilepsy later in life is only slightly enhanced after a single, short (<15 min) febrile seizure. However, prolonged or recurrent febrile seizures constitute a more serious risk factor (Hesdorffer and Hauser, 2002; Shinnar, 2002). The critical question is whether these febrile seizures only unmask preexisting abnormalities in the developing brain, or do they, in themselves, cause injury that may then evolve into TLE. Recent results obtained from an immature animal model (Toth et al., 1998; Dubé et al., 2000) indicate that prolonged febrile seizures can indeed have long-lasting consequences, even in an otherwise normally developing brain. Specifically, hyperthermic seizures of only 20 min duration caused acute hippocampal neuronal injury (Toth et al., 1998) and enhanced susceptibility to further limbic seizures throughout life (Dubé et al., 2000). This higher susceptibility to seizures was at least partially due to hyperexcitability of the hippocampal formation itself, that emerged within a week of the seizures (Dubé et al., 2000; K. Chen et al., 2001). However, the mechanisms that cause and sustain this enhanced hippocampal excitability have remained elusive.
An established, common mechanism for seizure-induced alteration of brain excitability and for the consequent development of TLE in mature hippocampus involves death of specific populations of hippocampal neurons. The loss of these neurons and the resulting changes in hippocampal circuitry are considered the principal neuroanatomical basis of epileptogenesis (for review, see Houser, 1999). Because seizures in animal models can lead to both epilepsy and to neuroanatomical changes highly reminiscent of these observed in hippocampus of patients with TLE (deLanerolle et al., 1989; Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991; Sloviter, 1991; Mathern et al., 1995; Mikkonen et al., 1998), it has been widely hypothesized that (1) hippocampal cell loss in the human is a consequence of previous seizure-induced injury, and (2) this seizure-induced injury plays a key role in the development of TLE. These hypotheses have been particularly important in considering the relationship of prolonged febrile seizures and subsequent temporal lobe epilepsy (Cendes et al., 1993; French et al., 1993).
Altered neurogenesis of dentate gyrus (DG) granule cells (GCs) has recently been proposed as an additional mechanism by which seizures can modulate the hippocampal network (Parent et al., 1997). Several experimental seizure paradigms increase GC production in the adult rat dentate gyrus (Bengzon et al., 1997; Parent et al., 1997; Scott et al., 1998; Gray and Sundstrom, 1998). These newly produced GCs and their aberrant innervation might contribute to the structural and functional network abnormalities in the epileptic hippocampal formation of adult rodents (Parent et al., 1997). Seizure-induced alteration of GC production might be particularly disruptive during hippocampal development, since neurogenesis in the dentate gyrus peaks during the first and second postnatal weeks (Schlessinger et al., 1975; Altman and Bayer, 1990).
We have previously demonstrated that prolonged experimental febrile seizures cause transient neuronal injury, but do not result in acute hippocampal cell death (Toth et al., 1998). However, these prolonged experimental febrile seizures led to long-term functional hyperexcitability of the hippocampal network. Whether the long-term functional changes may, in turn, be associated with chronic delayed cell death has not been resolved. Therefore, in the current studies we investigated whether prolonged febrile seizures early in life produce long-term structural changes in the hippocampus that may underlie or contribute to the augmented seizure susceptibility found later in life. Specifically, we asked the questions: (1) do prolonged febrile seizures result in delayed, chronic hippocampal neuronal loss?, (2) do prolonged febrile seizures affect GC neurogenesis in the developing dentate gyrus?, and (3) do prolonged febrile seizures cause axonal reorganization of the mossy fiber pathway?
MATERIALS AND METHODS
Generation of Developmental Seizures
Sprague-Dawley-derived rats, born and maintained in quiet facilities with controlled temperature (21–22°C), 12-hr light schedule, and unlimited access to food and water were used for the experiments (a total of 120). All experiments, aimed to minimize pain and discomfort, were approved by the UCI Animal Care Committee and conformed to National Institutes of Health (NIH) guidelines. Prolonged experimental febrile seizures were provoked as described previously (Toth et al., 1998; Chen et al., 1999; Dubé et al., 2000; Dubé, 2002), using immature rats during a hippocampal and cortical developmental age generally equivalent to that of the human infant (Gottlieb et al., 1977; Hershkowitz et al., 1997). Hyperthermia, i.e., increased body and brain temperature, was induced in 10–11-day-old Sprague-Dawley rats (HT group) using a warmed air stream directed ~50 cm above the animals. Core temperatures were measured before hyperthermia induction, at 2-min intervals and at the onset of hyperthermia-provoked seizures. These core temperatures have been correlated with brain temperatures under the precise experimental conditions used (Dubé, 2002). After 30 min of hyperthermia, calibrated to evoke ~20 min of seizures, animals were moved to a cool surface then returned to their mothers. Little evidence of dehydration was observed from this mild procedure (loss of body weight <3%), and animals rapidly regained normal activity including suckling (Dubé, 2002).
Longer hippocampal seizures (lasting 120–180 min) were induced in immature rats of the same age, using the glutamate receptor agonist kainic acid (KA), as described elsewhere (Brunson et al., 1998). The dose used (1.2 mg/kg, i. p.), resulted in limbic hyper-excitability and electrographic as well as behavioral seizures (Tremblay et al., 1984; Brunson et al., 1998). With a latency of ~20 min, KA-injected pups developed seizures recognizable first as behavioral arrest, scratching and constant chewing, then proceeding to generalized, tonic/clonic seizures, which have previously been correlated with electrographic seizures (Brunson et al., 1998). Behavioral phenotype and seizure duration were carefully monitored and noted at 5-min intervals. Control rats from the same litter, injected with saline, did not develop seizures.
Electrographic and Behavioral Analyses of Hippocampal Sensitivity to KA in Adult Rats That Experienced Experimental Febrile Seizures Early in Life
At 3 months of age, rats were implanted unilaterally in the dorsal hippocampus with bipolar twisted wire electrodes as described previously (Brunson et al., 1998; Dubé et al., 2000). Animals were allowed a 1-week recovery period and were then subjected to hippocampal recording to determine the baseline hippocampal activity. Hippocampal recordings were carried out via long flexible cables in freely moving rats using a GRASS 78E Polygraph as described in detail elsewhere (Dubé et al., 2000). To determine whether early-life experimental febrile seizures influenced susceptibility to limbic convulsants, control and experimental adult animals (n = 8 and n = 9, respectively) were subjected to subthreshold doses of KA. A dose of 5 mg/kg, shown not to induce major seizures in normal adult rats, was given to hippocampal-electrode-carrying animals. Latency to the onset of electrographic and behavioral seizures, as well as the duration of these seizures, was measured. Specifically, the duration of spike-and-wave discharges on the hippocampal tracings (i.e., total spikes duration per hour recording) was determined. The total duration of recording was 180 or 60 min from the onset of status epilepticus, when this occurred.
Tissue Harvesting and Processing
At the appropriate ages after seizures (for BrdU analysis: 5, 9, and 30 days; for cell counts: 3 months), rats (n = 5 for each age and experimental group) were deeply anesthetized with sodium pentobarbital and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). Brains were removed, postfixed in 4% PFA (2 h), cryoprotected in 25% sucrose/PB (48 h), and then frozen in −50°C isopentane. Coronal serial sections (50 μm) were cut throughout the hippocampus using a cryostat. For cell counts, every sixth section of the series was counterstained with 1% cresyl violet acetate. Sections immediately adjacent to those stained with cresyl violet were processed for immunocytochemistry (glutamate receptor subunits 2/3, [GluR2/3]) or in situ hybridization (glutamate decarboxylase 67 [GAD67] mRNA), respectively. For analysis of GC neurogenesis, every fourth section of the hippocampal series was processed for BrdU detection. Adjacent sections were subjected to immunocytochemistry for glial fibrillary acidic protein (GFAP), to identify glial cells in the DG.
Immunocytochemistry
Standard avidin-biotin complex methods were used to perform immunocytochemistry (ICC) on free-floating sections (Yan et al., 1998; Bender et al., 2001; Y. Chen et al., 2001). Sections were washed several times in 0.1 M PB (pH 7.4) and immersed in 3% normal goat serum/PB for 2 h, to block unspecific binding sites. They were then incubated for 48 h at 4°C with the primary antisera: monoclonal mouse anti-GFAP, 1:1,000, or polyclonal rabbit anti-GluR2/3, 1:500; (Chemicon, Temecula, CA). After repeated washes (4 × 5 min) in 0.1 M PB, sections were exposed for 3 h at room temperature to a secondary antiserum (biotinylated goat anti-mouse IgG or goat anti-rabbit IgG, respectively; 1:250; Vector Laboratories Burlingame, CA). Antibody binding was visualized by an avidin-biotin-peroxidase reaction (Vectastain ABC-Kit, Vector Laboratories) using hydrogen peroxide (0.002%), and 3,3′ diaminobenzidine (0.05%; Sigma, St. Louis, MO) as chromogen.
In Situ Hybridization
Sections were processed for in situ hybridization (ISH) according to a protocol described in detail previously (Bender et al., 2000; Y. Chen et al., 2001). Briefly, free-floating sections were collected and washed in 2× SSC (i.e., 0.3 M sodium chloride, 0.03 M sodium citrate; 2× 10 min); they were then incubated in a mixture (1:1) of 2× SSC and hybridization buffer (15 min) and prehybridized (50°C, 2 h) in hybridization buffer (50% formamide, 4× SSC, 250 μg/ml denatured salmon sperm DNA, 100 μg/ml yeast tRNA, 5% dextran sulfate, 1× Denhardt's solution). For hybridization, digoxigenin-labeled GAD67 riboprobes were added to the solution, and sections were incubated for 12–16 h (50°C). For all steps, RNase-free solutions and sterile plates were used. After hybridization, sections were subjected to washes of increasing stringency, including 2× SSC (room temperature, 2× 15 min), 50% formamide/2× SSC (60°C, 1 h), 50% formamide/0.1× SSC (60°C, 1 h) and 0.1× SSC (60°C, 30 min). Hybrid molecules were detected using an anti-digoxigenin antibody tagged with alkaline phosphatase (Roche, Indianapolis, IN). Staining was carried out using 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Roche) as chromogens. Color reaction was stopped when distinct, blue cytoplasmic in situ hybridization signals were clearly recognizable (after 2–4 h). Control experiments, substituting labeled sense probes for the antisense probes revealed no signal. Digoxigenin-labeled antisense and sense probes were generated from a pBluescript transcription vector containing rat GAD67 cDNA (Erlander et al., 1991).
BrdU Detection
For analysis of GC neurogenesis, rats were injected with a single dose of 5-bromo-2′-deoxyuridine (BrdU, Roche, 50 μg/g body weight, i. p.) either 3, 7, or 28 days after febrile or KA-induced seizures. Rats were perfused 48 h after the injection, and brains were then processed as described above. BrdU incorporation was detected as follows. Sections were first immersed in 50% form-amide/2× SSC (65°C, 2 h) and were then incubated in 2 M HCl (37°C, 30 min) to denature DNA. After neutralization with 0.1 M sodium borate (pH 8.5; room temperature, 10 min), sections were preincubated with 2% normal goat serum in 0.1 PB supplemented with 0.25% Triton X100 (room temperature, 1 h). Primary antiserum was then added (monoclonal rat anti-BrdU, Accurate Chemical, Westbury, NY; 1:1,000); sections were incubated for 24 h (4°C). Binding of the primary antibodies was visualized using the avidin-biotin-peroxidase-technique described above, except that 0.01% NiCl2 and 0.01% CoCl2 were added as signal enhancers to the peroxidase-catalyzed reaction. This reaction produces a dark black reaction product in immunopositive nuclei. Omission of the primary or secondary antibodies abolished the nuclear signal.
Timm Histochemistry
Mossy fiber sprouting was analyzed at 10 days or 3 months after the febrile seizures. Rats (10 days, n = 5 per group; 3 months, n = 10 per experimental group, with none of this group receiving KA) were transcardially perfused with the following series of solutions (modified from Schwegler and Lipp, 1983): 150 ml of 0.4% sodium sulfide, then 200 ml of 2.5% glutaraldehyde (in 0.15 M PB, pH 7.3) then 150 ml of 0.4% sodium sulfide. Brains were dissected out, the subcortical tissue was removed, and the remaining cortex and hippocampus were postfixed in 2.5% glutaraldehyde for 12 h, followed by cryoprotection as described above. Serial sections (50 μm) were cut throughout the hippocampus in either the coronal (n = 5, each experimental group) or the horizontal plane (n = 5, for the 3 months experimental groups only). Sections were mounted onto glass slides and were air dried. For Timm staining, slides were exposed to a solution containing 5.1 g citric acid, 4.7 g sodium citrate, 3.4 g hydroquinone, 0.17 g silver nitrate, and 120 ml 50% gum arabic, in a total of 200 ml of double distilled water. Slides were incubated in the dark at 27°C and were monitored at 30-min intervals. When the desired staining intensity was reached, the reaction was stopped by rinsing the slides with water. Finally, slides were dipped for 1 min into 1% sodium thiosulfate, washed again with distilled water, counterstained with cresyl violet, dehydrated through a series of ethanol concentrations, cleared with xylene, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). Sections from HT and control groups were processed simultaneously.
Quantitative Analysis and Statistical Considerations
For each analysis, five representative sections per animal were used. These sections were selected from the septal hippocampus (region between bregma −3.3 mm and bregma −4.8 mm; Paxinos and Watson, 1982) and from the temporal hippocampus (region between interaural 3.9 mm and interaural 5.9 mm; Paxinos and Watson, 1982). Counts were performed in both the left and right hippocampus of each section. Care was taken to match the selected sections, and all analyses were carried out by an observer blinded to the experimental-group status of the sections.
Cell Counts
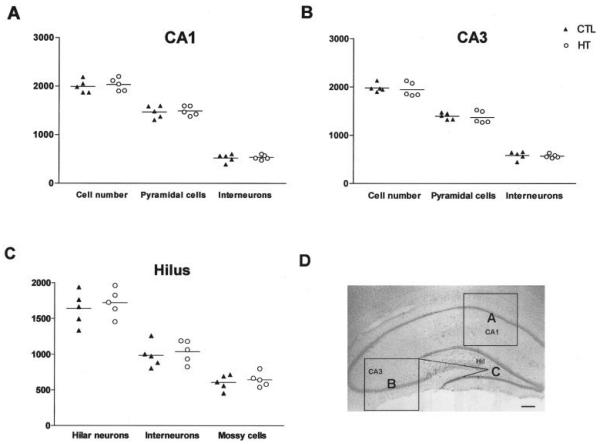
Neuronal numbers were determined in sections from the septal hippocampus. Areas for analysis were defined as follows (see Fig. 2D). First, for CA1, a counting frame (1 × 1 mm) was used. The frame was positioned with its upper margin at the stratum oriens/ alveus border, its orientation parallel to the vertical axis of the coronal section, and its horizontal midpoint positioned above the midpoint of the GC layer suprapyramidal blade (frame A in Fig. 2D). All neuronal perikarya with >50% of their surface within the resulting square were then counted. Second, for CA3, the same counting frame was used. Here, one upper corner was positioned at the tip of the GC layer suprapyramidal blade and its upper margin was positioned along a virtual line connecting the tip and the CA3/CA2 junction of the pyramidal cell layer (frame B, Fig. 2D). Third, for the hilus, borders were defined by drawing virtual lines connecting each tip of the GC layer with the proximal end of CA3c pyramidal layer (frame C, Fig. 2D). Neurons located between these imaginary lines and the GC layer were counted. Counts were performed under 400× magnification (40× objective, 10× ocular), focusing throughout the depth of the section. Neurons were included in the analysis only when the nucleus was recognizable. The number of neurons within the counting frame as well as specific subpopulations were determined, using cresyl violet-stained sections for the former and GAD67 mRNA-positive γ-aminobutyric acid (GABA)ergic interneurons (Houser and Esclapez, 1994) or GluR2/3-immunoreactive hilar mossy cells (Leránth et al., 1996) for the latter.
FIGURE 2.
Comparison of neuronal numbers in defined regions of the hippocampal formation of five rats with developmental febrile seizures (HT) and five age-matched controls (CTL) 3 months after the seizures. Data represent cell counts pooled from analyses of five sections (left and right hippocampus = 10 analyses per rat and subfield). A: CA1. B: CA3. C: Hilus. Note that in all analyzed subfields, neuronal numbers were not significantly different between the HT and control groups. In addition, the proportional relationships of these subpopulations were preserved: interneurons constituted ~26% of the neuronal population in CA1, ~29% in CA3 and ~60% in the hilus, mossy cells constituted ~37% of hilar neurons in both the HT and the control group. D: Demonstration of the areas analyzed: CA1 (frame A), CA3 (frame B), and hilus (frame C). Scale bar = 250 μm in D.
BrdU Analysis
BrdU-immunoreactive nuclei were analyzed in sections from the septal hippocampus. Nuclei located in or directly adjacent to the GC layer were counted.
Mossy Fiber Analysis
Timm-positive mossy fibers in GC- and molecular layers were analyzed in the septal hippocampus of the younger rat group (10 days after seizures). For the 3-month-old rats, sections from both the septal and the temporal hippocampus were analyzed, to account for potential differences between these regions (Masukawa et al., 1995). For this analysis, the suprapyramidal blade of GC layer was subdivided into three parts of equal length and only the middle part was used for analysis to exclude regions (crest and tip) with abundance of ‘normal’ mossy fibers (Sutula et al., 1988; Baram and Ribak, 1995; Nissinen et al., 2001). Mossy fibers traversing the GC layer or entering the molecular layer (ML) were counted throughout the entire length of the defined area using 400× magnification and focusing through the whole depth of the section at each position. A fiber was included only if clearly demarcated by a chain of Timm-stained boutons. Results are presented as numbers of fibers per unit length (linear mm) of the GC layer.
Volume Analysis
To account for potential volume change of the hippocampal formation after the experimental febrile seizures, the hippocampal volume was estimated in both animal groups. Specifically, cresyl violet-stained sections of each series were captured digitally, and the hippocampal surface area was measured with the aid of an Image analysis system (ImageTool, UTHSC, San Antonio, TX). The average value (total surface area/number of analyzed sections) was then multiplied with the distance from the septal to the temporal pole, calculated from the total number of sections in the series.
Statistical Analysis
Data were evaluated using the Mann-Whitney u-test and are presented as means with standard errors. Significance levels were set at P < 0.05.
RESULTS
Prolonged Experimental Febrile Seizures Early in Life Increase the Sensitivity of the Mature Hippocampal Formation to Excitatory Agents
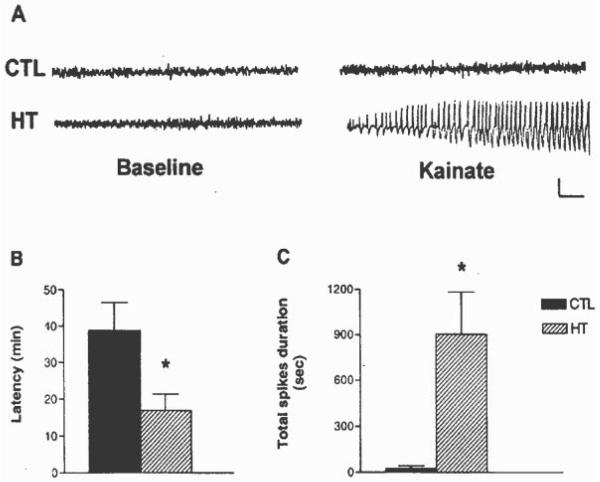
Whereas overt, spontaneous hippocampal seizures were not evident in adult rats that had sustained hippocampal seizures provoked by hyperthermia during the second postnatal week, the hippocampal network of these rats was fundamentally modified. When challenged with a small (5-mg/kg) dose of KA, all these animals rapidly developed striking and sustained trains of hippocampal spikes, whereas hippocampal recordings from littermate controls demonstrated only isolated or fragmentary spike trains. Only two of these control animals eventually developed short seizures, lasting <1 min each. Figure 1A shows typical in vivo hippocampal tracings obtained via bipolar electrodes in behaving animals. Baseline traces are similar in both experimental (HT) and control records; after administration of KA, the tracings on the right side of Figure 1A show little change in the encephalogram (EEG) in the controls, whereas spike trains were prevalent in the HT group. The behavioral seizures associated with the electrographic ones consisted of a sequence of hyperactivity, immobility, and motor seizures including rearing (stage 4) and loss of balance with falling (stage 5).
FIGURE 1.
A: Hippocampal tracings (EEGs) obtained from adult rats before and after administration of a low-dose of kainic acid (KA). The normal tracings on the left are those from control (CTL) rats, as well as from those that had experienced experimental febrile seizures early in life (HT). The tracings on the right were obtained after KA administration. While hippocampal EEG activity remained normal in most controls (only two developed short seizures), high amplitude epileptiform spike-wave trains, and associated behavioral seizures, occurred in the HT rats. Calibration: vertical, 1 mV; horizontal, 1 s. B: Differential latency to the onset of KA-induced seizures in adult rats, depending on their previous seizure history: KA led to prolonged seizures in all HT rats (n = 9), whereas only 2 of 8 controls developed brief seizures. The latencies to the development of seizures were significantly shorter in HT group in comparison with latencies to the rare seizures of the control group. Values depict means ±SEM; *Significant difference: P < 0.05). C: Quantitative analysis of KA-induced total spikes duration (total duration of spike-and-wave discharges per hour recording) in rats that had sustained experimental febrile seizures early in life compared with littermate controls. After KA administration, hippocampi of HT animals exhibited spike-and-wave discharges of significantly longer total duration, compared with the CTL group (means ±SEM; *P < 0.05). Note: recording lasted for 180 min, or for 60 min after onset of status epilepticus.
Figure 1B demonstrates the markedly reduced latency to the onset of hippocampal spike trains and seizure onset after KA in HT versus the two control animals that did develop seizures. Reduced latency to seizures onset is a hallmark of functionally higher convulsant doses; the reduced latency in the HT animals thus indicates their enhanced sensitivity to the low KA dose used (5 mg/kg). Indeed, the latency to seizures onset in these animals was consistent with latencies observed after KA doses of 15–18 mg/kg in normal adult Sprague-Dawley rats (Dubé et al., unpublished observations). Figure 1C compares total spikes duration after KA administration in the animals that had sustained experimental febrile seizures in early life and in the controls. The significant longer total spikes duration in the HT group provides further support for the persisting modulation of the hippocampal circuit in these animals, with increased susceptibility to pro-convulsant stimuli. Taken together, these data indicate that although the hippocampal formation of adult rats experiencing febrile seizures early in life did not generate demonstrable spontaneous seizures, the balance of excitation/inhibition in the network was fundamentally altered. A limited increase of excitatory input, derived from modest activation of non-NMDA-type glutamate receptors was sufficient to provoke profound repetitive firing and electrographic and behavioral seizures in the experimental group. These in vivo data are in line with earlier in vitro studies demonstrating that experimental febrile seizures lead to intrinsic hippocampal hyperexcitability, manifest as increased probability of neuronal firing in CA1 pyramidal cells in response to stimulation (Dubé et al., 2000, see Fig. 4; K. Chen et al., 2001, see Fig. 3).
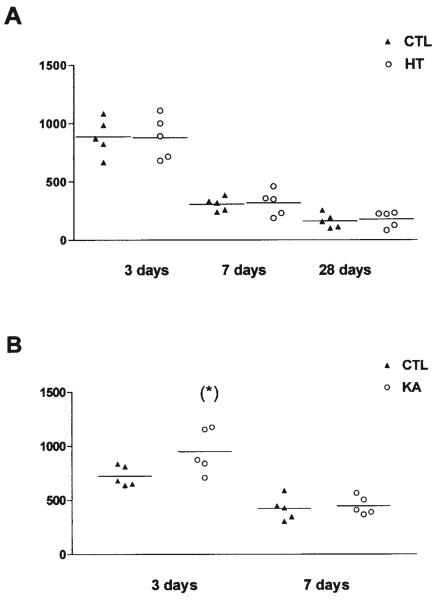
FIGURE 4.
Quantitative analysis of 5-bromo-2′-deoxyuridine (BrdU)-immunoreactive nuclei in the granule cell (GC) layer after (A) experimental febrile seizures (HT) lasting ~20 min, or (B) kainic acid (KA)-induced status epilepticus lasting ~120 min. A: Rats were injected with BrdU at 3, 7, or 28 days after the febrile seizures and perfused 48 h later. Data represent BrdU-positive nuclei counted in five sections (left and right hippocampus = 10 analyses per rat). The numbers of BrdU-labeled cells declined with age, due to the established developmental reduction in GC neurogenesis. However, no differences were found between HT and control animals at any age or time point. B: Rats were injected with BrdU at 3 or 7 days after KA-induced seizures and were perfused 48 h later. In contrast to the HT-induced seizures, KA-induced seizures significantly increased the number of BrdU-labeled cells at the 3-day time point (asterisk). The seizure-induced neurogenesis was transient and was no longer evident when rats were sacrificed 1 week after the seizures.
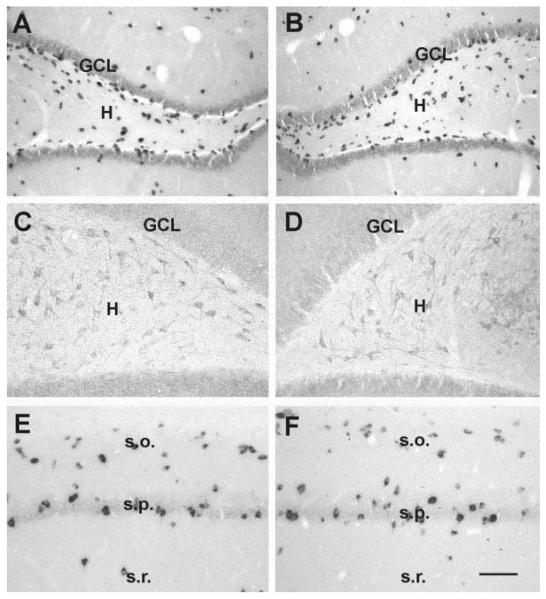
FIGURE 3.
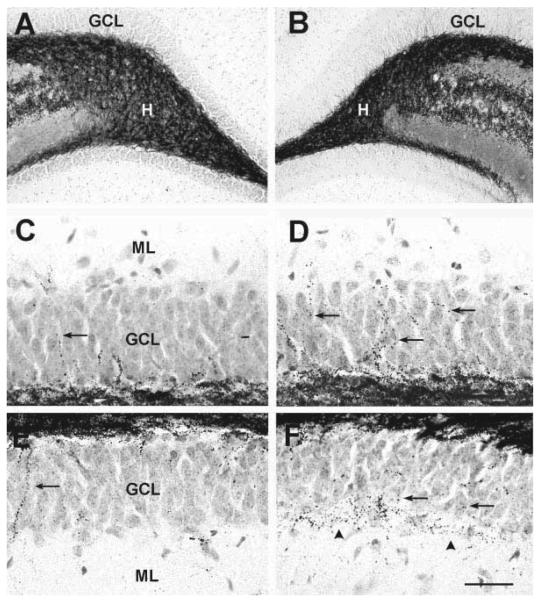
Neuronal populations which are typically vulnerable to seizure-induced excitotoxicity are not reduced in rats studied 3 months after developmental febrile seizures (B,D,F) when compared with age-matched controls (A,C,E). A,B: Hilar γ-aminobutyric acid (GABA)ergic interneurons visualized using in situ hybridization (ISH) for GAD67 mRNA. C,D: Hilar mossy cells, demonstrated using immunocytochemistry for AMPA-receptor subunits GluR2/3. E,F: CA1 interneurons, visualized using GAD67-ISH. GCL, granule cell layer; H, hilus; s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum. Scale bars = 80 μm in A,B; 50 μ min C–F.
Prolonged Febrile Seizures Do Not Lead to Delayed Death of Vulnerable Hippocampal Neuronal Populations
Selective neuronal cell death as a result of seizures has been demonstrated in a number of adult animal models of hippocampal epilepsy, with some variation depending on the experimental paradigm. In remarkable similarity to the situation in human TLE, neurons in the hilus and in the CA3 and CA1 pyramidal cell layers have been particularly vulnerable in these models (Nadler, 1981; Ben-Ari, 1985; Freund et al., 1991; Sloviter, 1991; Houser and Esclapez, 1996; Buckmaster and Dudek, 1997). Therefore, the analyses here focused on these hippocampal subfields.
The overall numbers of cells, as well as of seizure-sensitive neuronal subpopulations were determined in HT and control animals in defined areas of hippocampal CA1 and CA3 and in the hilus of the dentate gyrus (see diagram, Fig. 2D). In all regions examined, numbers of neurons were not significantly different in experimental animals compared with controls (Fig. 2A–C). Specifically, in CA1 and CA3, both pyramidal and interneuronal cell numbers in seizure-experiencing and control groups were indistinguishable (Fig. 2A,B). The maintained proportional relationship of seizure-sensitive cell sub-populations further supports the notion that none of these populations was affected by the seizures: Thus, GABAergic interneurons in HT and control groups constituted 26.1% and 26.5% of the neuronal population in CA1 (Fig. 2A), and 29.5% (HT) and 29.3% (control) in CA3 (Fig. 2B). In the hilus, mossy cells constituted 37.6% (HT) and 37.5% (control) of the hilar neurons (Figs. 2C, 3C,D). The corresponding numbers for hilar GABAergic interneurons were 60.4% (HT) and 60.9% (control; Figs. 2C, 3A,B). Hippocampal volume did not differ in animals which had sustained experimental febrile seizures 3 month earlier, compared with controls (total hippocampal volume: 247 ± 20 mm3 in controls, 252 ± 21 mm3 in the HT group; n = 5/group). Therefore, these results indicate that prolonged experimental febrile seizures do not cause acute or long-term neuronal loss in hippocampus, in concordance with previous findings in the amygdala (Toth et al., 1998).
Prolonged Febrile Seizures Do Not Alter Neurogenesis in Immature DG
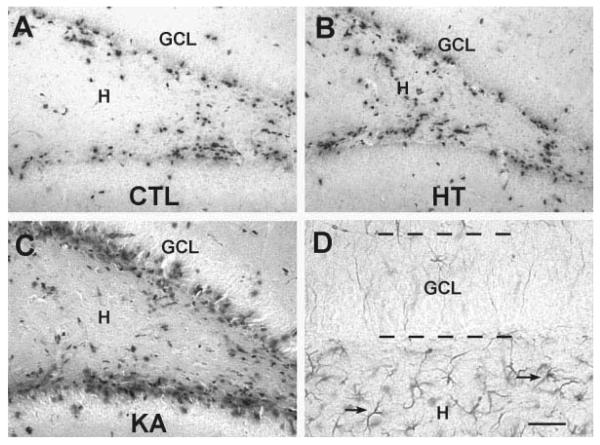
Because seizure-induced neurogenesis may promote aberrant, excitatory connectivity in the hippocampal formation, the influence of prolonged febrile seizures on GC proliferation was examined. Animals experiencing experimental febrile seizures and age-matched controls were injected with a single dose of BrdU, 3, 7, or 28 days later, to determine the early, intermediate and long-term influence of the seizures on the birthrate of neurons in the hippocampal formation. The numbers of BrdU-labeled cells were determined 48 h after the administration of this modified DNA base, a marker of dividing neurons (Nowakowski et al., 1989). The analysis was confined to BrdU-labeled cells that were located in, or closely adjacent to, the GC layer (Figs. 4, 5). In this area, glial cells are uncommon (Fig. 5D), and therefore, differences among groups would likely reflect changes in GC neurogenesis. As shown in Figure 4A, the total numbers of BrdU-labeled cells declined with age, due to the established developmental reduction in GC neurogenesis. No differences were found between HT and control animals at any age or time point (Figs. 4A, 5A,B), suggesting that prolonged experimental febrile seizures did not significantly influence neurogenesis in the immature rat hippocampal formation.
FIGURE 5.
Photomicrographs of the dentate gyrus of immature rats subjected to experimental seizures at P10 (HT, B), kainic acid (KA)-induced prolonged seizures (KA, C) compared with controls (CTL, A). All animals were injected with 5-bromo-2′-deoxyuridine (BrdU) on P13. Sections were processed for BrdU immunocytochemistry following the experimental procedures described in Fig. 4. The numbers of immunoreactive nuclei did not differ between the HT and control groups (see also Fig. 4A). Increased neurogenesis was evident after KA seizures. The analysis included only BrdU-immunopositive nuclei within or subjacent to the granule cell layer (GCL). These were probably not astrocytes, because GFAP immunocytochemistry (D) demonstrated the presence of the astrocyte marker only well within the hilus. Scale bars = 50 μm in A–C; 25 μminD.
The absence of altered neurogenesis after experimental febrile seizures might derive from their relatively short duration or other model-specific properties of these seizures. Alternatively, neurogenesis rate in the immature hippocampus may not be regulated by hippocampal seizures of any duration or intensity, perhaps due to immaturity of the circuit (Ribak and Navetta, 1994). To distinguish between these alternatives, we induced longer hippocampal seizures (hippocampal status epilepticus), using KA (seizure duration >2 h). Prolonged, KA-induced seizures significantly increased the number of BrdU-labeled cells 3 days later (Figs. 4B, 5A vs 5C). This enhanced proliferation rate was transient: by 7 days after the seizures, GC neurogenesis had returned to control levels (Fig. 4B). These results indicate that prolonged, intense developmental seizures are capable of influencing the proliferation rate of GC progenitors in the developing DG. They suggest that the relatively shorter hyperthermic seizures may not reach the threshold necessary to alter this process, or that other model-specific factors act to prevent increase of neurogenesis.
Mossy Fiber Collateral Density in GC and Molecular Layers Is Increased 3 Months After Prolonged Experimental Febrile Seizures
While the projection of GC axons (mossy fibers) to the ML is quite sparse in normal rodents (Claiborne et al., 1986; Ribak and Peterson, 1991; Sutula et al., 1998), it is remarkably augmented in animal models of TLE (reviewed in Houser, 1999), as well as in humans with this hippocampal epilepsy (deLanerolle et al., 1989; Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991, Mikkonen et al., 1998). Because the majority of these “sprouted” supragranular mossy fibers terminate on GC dendrites (Okazaki et al., 1995; Wenzel et al., 2000; Buckmaster et al., 2002), they may increase the excitability of GCs and thus promote epileptogenesis (but see Longo and Mello, 1998; Nissinen et al., 2001). Therefore, in the current study we queried whether prolonged febrile seizures caused mossy fiber sprouting, altering hippocampal connectivity in a manner favoring enhanced hippocampal excitability, and carried out quantitative analyses of the mossy fiber projections in mature (3-month-old) animals which had sustained febrile seizures early in life, compared with a control group (Figs. 6, 7).
FIGURE 6.
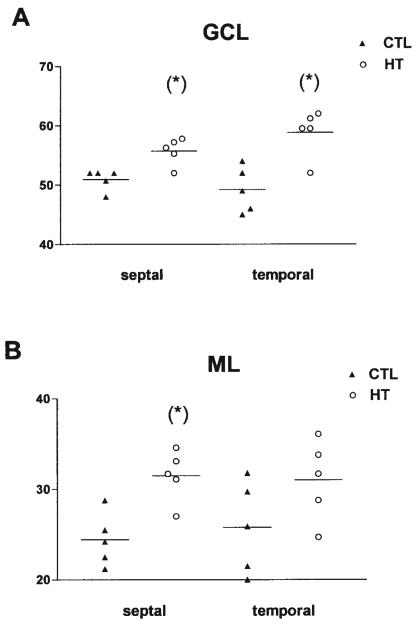
Quantitative analysis of mossy fiber innervation of the granule cell (GCL, A) and molecular (ML, B) layers of the dentate gyrus in septal and temporal hippocampus (n = 5, each group). The hippocampal formation of naive 3-month-old rats (CTL) was compared with those from animals sustaining experimental febrile seizures early in life (HT). Sections were processed for Timm's stain as described in the methods, and all analyses were carried out without knowledge of treatment group. Significantly increased numbers of mossy fibers traversed the GCL in the HT animals (asterisks, A). More fibers also penetrated the ML in the septal (but not temporal) hippocampus (B). The y-axis denotes the numbers of fibers per linear millimeter (mm) of the suprapyramidal blade of the GCL.
FIGURE 7.
Photomicrographs of the dentate gyrus in Timm-stained sections from adult rats subjected to experimental febrile seizures 3 months earlier (B,D,F), or from controls (A,C,E). Top: Low-magnification views, to demonstrate similar technical processing of samples. Middle: Suprapyramidal blade of the granule cell layer (GCL) from controls, C), or from experimental animals (D). Bottom: analogous set of photographs from the infrapyramidal blade (E,F). The increased numbers of Timm-stained chains of boutons (arrows) are clearly visible in the sections from the experimental group. In addition to increased fiber number, an almost continuous band of Timm-positive boutons (arrowheads) in the GCL- molecular layer (ML) boundary can be seen in experimental (F), but not in control rats (E). H, hilus. Scale bars = 150 μm in A,B; 25 μm in C–F.
In the control group, mossy fibers innervated the hilus densely (Fig. 7A) and extended into CA3. Occasionally, recurrent mossy fiber collaterals branched off from the main axons in the hilus and traversed the GC layer to innervate targets in ML (Fig. 7C,E). However, in none of the control rats was an abnormal mossy fiber innervation of the GC or molecular layers recognizable. The rare supragranular fibers never formed dense patches or a confluent band in ML.
In HT rats, the overall pattern of the mossy fibers projection did not differ from controls: the hilus (Fig. 7B) and the CA3 were densely innervated. However, closer inspection revealed that mossy fibers traversing GC layer and innervating ML were significantly more frequent in HT rats (Figs. 6, 7D,F) compared with controls (Fig. 7C,E). In some HT rats (4 out of 10), the density of Timm granules in ML was so high that they periodically formed patches or confluent “bands” composed of Timm-stained boutons (Fig. 7F; Timm score 1, according to Cavazos et al., 1991). In addition to the Timm-stained boutons, quantitative analyses of the number of fibers confirmed the abnormal mossy fiber innervation in HT rats: When the course of mossy fibers was determined, by following the chain of synaptic boutons formed by these fibers (Fig. 7C,D), numbers of mossy fiber collaterals innervating GC layer and ML were found to be significantly higher (~15%) in HT animals compared with controls in both the septal and temporal hippocampus (Fig. 6).
Density of Mossy Fiber Collaterals in GC Layer and ML Is Not Increased 10 Days After Prolonged Febrile Seizures
The results of the previous paragraph indicate that prolonged febrile seizures can influence mossy fiber connectivity in the rat hippocampus long-term. However, hyperexcitability of the hippocampal network was detected in HT rats as early as 1 week after seizures (Dubé et al., 2000; K. Chen et al., 2001). We therefore queried whether mossy fiber sprouting was an early consequence of the seizures which could perhaps be causal in the generation of the observed hyperexcitability.
However, a quantitative analysis of mossy fiber density in GC and molecular layers conducted in rats sacrificed 10 days after seizures, did not reveal differences between the HT group (61.2 fibers/mm in GC and 41.7 fibers/mm in the molecular layer) and the controls (65.7 fibers/mm and 43.4 fibers/mm in GC and molecular layers, respectively). The absence of appreciable difference in the mossy fiber innervation of these two layers at the time when functional seizure-induced hippocampal hyperexcitability was already established argues against a mechanistic role for mossy fiber sprouting in the generation of this alteration of the hippocampal network.
DISCUSSION
There were several major findings of these studies: (1) prolonged experimental febrile seizures enhanced hippocampal excitability long-term, with marked potentiation of the effects of a subsequently administered chemical convulsant; (2) this long-lasting alteration of hippocampal excitability did not require significant loss of any seizure-sensitive neuronal population; (3) neurogenesis was not enhanced by these prolonged febrile seizures, whereas it was increased by KA-provoked status epilepticus during the same developmental period; and (4) prolonged febrile seizures resulted in long-term axonal reorganization, which followed, rather than preceded the functional alterations in hippocampal excitability. Taken together, these findings indicate that experimental prolonged febrile seizures change the hippocampal circuit long-term. However, the mechanisms underlying these long-lasting changes are distinct from structural seizure-induced alterations which contribute to the process of epileptogenesis in the mature hippocampal network.
Because of the high incidence of developmental seizures in humans, an understanding of the impact of these seizures on the immature brain has become a focus of intense research. The studies reported here utilized an animal model that closely reproduces prolonged febrile seizures, the most frequent seizure type in humans (Dubé et al., 2000; Dubé, 2002). In the human, the majority of febrile seizures are quite short (lasting <10 min, Berg and Shinnar, 1996). However, it is the longer seizures that are statistically correlated with subsequent hippocampal epilepsy. Therefore, we took advantage of the fact that in the animal model seizure duration can be tightly controlled to generate seizures of defined duration and to estimate the effect of seizures as a function of their duration. Studies using this model revealed that experimental febrile seizures lasting ~20 min (i.e., not status epilepticus, which is defined as a seizure >30 min) provoke long-lasting modification of the hippocampal network. Specifically, in vivo data showed that the seizures result in a reduced threshold for further limbic seizures throughout life (Dubé et al., 2000). In vitro studies, using the hippocampal slice preparation, clearly demonstrated that the cause of this increased susceptibility to seizures is an intrinsic hyperexcitability of the hippocampal circuit (Dubé et al., 2000; K. Chen et al., 2001). What might the mechanisms for such a long-lasting modification be?
The results reported exclude neuronal death as a significant factor. This is not surprising in light of previous studies which almost unanimously showed that the immature hippocampus is remarkably resistant to seizure-induced neuronal death (Nitecka et al., 1984; Sperber et al., 1991; Nehlig and Pereira de Vasconcelos, 1996; Liu et al., 1996; Toth et al., 1998; Sarkisian et al., 1999; Haas et al., 2001), and that strong, persistent stimulation (prolonged status epilepticus) is required to cause neuronal degeneration (Sankar et al., 1998; Thompson et al., 1998). These cited studies, as well as our earlier publications focused on acute/subchronic time frames (24 h to 4 weeks after the seizures). However, long-term functional changes may result from, or be associated with slow degeneration processes and delayed neuronal death. Therefore, the current study considered such potential long-term effects and focused on time frames extending to 3 months after the seizures. As shown here, no neuronal loss was detected in the hippocampus of HT animals during the time period when these rats were highly susceptible to provocation of hippocampal seizures (i.e., at 3–4 months of age, Fig. 1), This neuronal sparing involved neuronal populations shown to be vulnerable to seizure-induced death in a large number of models of both limbic/ hippocampal and generalized seizures. Thus, neither mossy cells (Sloviter, 1994) nor specific subpopulations of interneurons (deLanerolle et al., 1989; Houser and Esclapez et al., 1996; Buckmaster and Dudek, 1997) were depleted.
The current results also suggest that altered GC neurogenesis is unlikely to contribute to the long-term hyperexcitability of the hippocampal network: Febrile seizures of ~20 min duration did not affect this process in the immature hippocampus. The lack of impact on GC proliferation might be due to several issues: First, in the immature (P10) hippocampus, neurogenesis may not be responsive to any seizure stimulus. This is unlikely, because in contrast to the febrile seizures, those induced in animals of the same age by KA (and lasting >120 min) led to robust enhancement of granule cell proliferation. Alternatively, the duration of the febrile seizures was insufficient to provoke GC neurogenesis. This hypothesis is consistent with the data, and would suggest that neurogenesis in the immature hippocampus is far less sensitive to circuit activation compared with that of the adult, because even brief stimuli have been suggested to influence GC proliferation rate in the mature hippocampal formation (Bengzon et al., 1997).
The impact of developmental seizures on GC neurogenesis has not been fully resolved. Sankar et al. (2000) reported increased proliferation of these cells after lithium-pilocarpine-induced status-epilepticus, consistent with the data reported for KA-induced status epilepticus. Interestingly, McCabe et al. (2001) reported decreased GC proliferation after recurrent flurothyl-induced seizures. These authors documented the need for several (~10) seizures for this effect. While the precise mechanism of the discrepant effects on neurogenesis—including potential differences in age, stress hormone levels, hypoxia, or the neuronal circuits activated—is unknown, all these studies are consistent with the notion that much more protracted seizures are required to alter GC neurogenesis in immature vs. adult hippocampus.
The finding of an increased, though modest, projection of mossy fiber collaterals to the GC and molecular layers was surprising, particularly because neither significant neuronal death nor altered GC neurogenesis, often considered major causes for mossy fiber sprouting, was detectable. Given the limitations of morphological analyses, we cannot entirely exclude the possibility that minor cell loss had escaped detection (e.g., Sutula et al., 1988; Cavazos and Sutula, 1990). However, an alternative explanation is provided by recent studies demonstrating that mossy fiber sprouting does not always require neuronal degeneration (Stringer et al., 1997; Holmes et al., 1998), but can also result from continuous neuronal activation (Adams et al., 1997; Escobar et al., 1997; Hassan et al., 2000). The protracted time course of the mossy fiber sprouting after experimental febrile seizures suggests that the mechanisms involved might be chronic: the pattern observed here was that of a slow increase of mossy fibers in GC layer and ML. Indeed, we found little sprouting 10 days after the febrile seizures, consistent with other short-term studies in immature hippocampus (Sperber et al., 1991; Haas et al., 2001). A similar slow increase of supragranular mossy fibers, associated with aging rather than with neuronal death or seizures, has been observed in rat (Gaarskjaer, 1978), mouse (Qiao and Noebels, 1993), guinea pig (Wolfer and Lipp, 1995), and human hippocampus (Cassell and Brown, 1984). Thus, this age-dependent mossy fiber sprouting may well reflect the cumulative history of synchronized neuronal activity in hippocampal pathways (Qiao and Noebels, 1993). In the current study, chronic hyperexcitability of the hippocampal network, even below the level of overt seizures (Dubé et al., 2000; K. Chen et al., 2001), might be responsible for accelerated mossy fiber sprouting.
Could the increased number of mossy fibers in GC and molecular layers contribute to the reduced seizure threshold in HT animals? In the normal hippocampus, the sparse supragranular mossy fiber projection terminates mainly on basket cell dendrites (Ribak and Peterson, 1991). However, seizure-induced, newly sprouted mossy fibers terminate predominantly on GC dendrites (Okazaki et al., 1995; Wenzel et al., 2000; Buckmaster et al., 2002), forming recurrent excitatory circuits. These new excitatory circuits, if not counterbalanced (e.g., by concurrent sprouting of GABAergic fibers; Davenport et al., 1990; Deller et al., 1995; André et al., 2001), can indeed facilitate GC excitation and thus render the hippocampal network hyperexcitable (Tauck and Nadler, 1985; Wuarin and Dudek, 1996; Patrylo and Dudek, 1998; Okazaki et al., 1999; but see Sloviter, 1992). Thus, a causal contribution of the sprouted fibers to hippocampal hyperexcitability in HT animals is theoretically possible. However, it is considered unlikely: hippocampal hyperexcitability has been detected in HT rats as early as 1 week after the seizures (Dubé et al., 2000; K. Chen et al., 2001), at a time when no increase of mossy fibers was detectable. This temporal relationship strongly suggests that the mossy fiber sprouting observed here constitutes a consequence, rather than a cause of the hyperexcitable hippocampal network in the seizure-experiencing immature rat.
CONCLUSIONS
These studies indicate that prolonged febrile seizures in the immature rat model induce long-term structural and functional alteration of the hippocampal network. Increased hippocampal excitability and modest but significant axonal reorganization processes occur concurrently, without significant cell loss or altered neurogenesis. The time course of the mossy fiber sprouting does not favor the notion that this process is a key mechanism of the functional hippocampal changes. In the virtual absence of cell death, the search for the mechanisms underlying the profound and long-lasting modulation of the hippocampal network by experimental febrile seizures should target distinct molecular alterations such as neurotransmitter release (Chen et al., 1999), or the expression and function of specific receptors (Eghbal-Ahmadi et al., 2001) and channels (K. Chen et al., 2001; Brewster et al., 2002).
Acknowledgments
The authors thank Dr. H.J. Wenzel for helpful comments and M. Hinojosa for excellent editorial help. This work was supported by NIH grants NS 35439, NS 28912 (to T.Z.B). and by a postdoctoral research fellowship from the Epilepsy Foundation of America and the Milken Foundation (to R.A.B.).
Grant sponsor: National Institutes of Health; Grant number: NS 35439; Grant number: NS 28912; Grant sponsor: Epilepsy Foundation of America.
REFERENCES
- Adams B, Lee M, Fahnestock M, Racine RJ. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997;775:193–197. doi: 10.1016/s0006-8993(97)01061-5. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Bender R, Hofmann MC, Frotscher M, Nitsch C. Species-specific expression of parvalbumin in the entorhinal cortex of the mongolian gerbil: dependence on local activity but not extrinsic afferents. Neuroscience. 2000;99:423–431. doi: 10.1016/s0306-4522(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Bender RA, Lauterborn JC, Gall CM, Cariage W, Baram TZ. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur J Neurosci. 2001;13:679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37:126–133. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dubé C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Schultz L, Baram TZ. The in vivo proconvulsant effects of corticotropin releasing hormone in the developing rat are independent of ionotropic glutamate receptor activation. Dev Brain Res. 1998;111:119–128. doi: 10.1016/s0165-3806(98)00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Brown MW. The distribution of Timm's stain in the nonsulphide-perfused human hippocampal formation. J Comp Neurol. 1984;222:461–471. doi: 10.1002/cne.902220311. [DOI] [PubMed] [Google Scholar]
- Cavazos J, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- Cavazos J, Golarai G, Sutula T. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;15:774–789. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendes F, Andermann F. Do febrile seizures promote temporal lobe epilepsy? In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 78–86. [Google Scholar]
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-Cendes I, Peters T, Melanson D. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy. An MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- deLanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Deller T, Frotscher M, Nitsch R. Morphological evidence for the sprouting of inhibitory commissural fibers in response to the lesion of the excitatory entorhinal input to the rat dentate gyrus. J Neurosci. 1995;15:6868–6878. doi: 10.1523/JNEUROSCI.15-10-06868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé C. Do prolonged febrile seizures in an immature rat model cause epilepsy? In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 215–229. [Google Scholar]
- Dubé C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Yin H, Stafstrom CE, Tran K, Weiss JH, Baram TZ. Altered expression of specific AMPA type glutamate receptor subunits after prolonged experimental febrile seizures in CA3 of immature rat hippocampus. Soc Neurosci Abs. 2001;31:684.6. [Google Scholar]
- Erlander MG, Tillakaratne NJK, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;1:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Escobar ML, Barea-Rodriguez EJ, Derrick BE, Reyes JA, Martinez JL. Opioid receptor modulation of mossy fiber synaptogenesis: independence from long-term potentiation. Brain Res. 1997;751:330–335. doi: 10.1016/s0006-8993(96)01373-x. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy. I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Freund TF, Ylinen A, Miettinen R, Pitkänen A, Lahtinen H, Baimbridge KG, Riekkinen PJ. Pattern and neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull. 1991;28:27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. I. Terminal area related to number of granule and pyramidal cells. J Comp Neurol. 1978;178:49–72. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Hassan H, Pohle W, Rüthrich H, Brödermann R, Krug M. Repeated long-term potentiation induces mossy fibre sprouting and changes the sensibility of hippocampal granule cells to subconvulsive doses of pentylenetetrazol. Eur J Neurosci. 2000;12:1509–1515. doi: 10.1046/j.1460-9568.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- Hershkowitz N, Kagan J, Zilles K. Neurobiological bases of behavioral development in the first year. Neuropediatrics. 1997;28:296–306. doi: 10.1055/s-2007-973720. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA. Febrile seizures and the risk for epilepsy. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 63–76. [Google Scholar]
- Holmes GL, Gaiarsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–557. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;5:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leránth C, Szeidemann Z, Hsu M, Buzsáki G. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR2/3 in most interneurons. Neuroscience. 1996;70:631–652. doi: 10.1016/s0306-4522(96)83003-x. [DOI] [PubMed] [Google Scholar]
- Lewis DV. Febrile convulsions and mesial temporal lobe sclerosis. Curr Opin Neurol. 1999;12:197–201. doi: 10.1097/00019052-199904000-00011. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian M, Tandon P, Yang Y, Hori A, Holmes GL. Age-dependent effects of glutamate toxicity in the hippocampus. Dev Brain Res. 1996;97:178–184. doi: 10.1016/s0165-3806(96)00141-1. [DOI] [PubMed] [Google Scholar]
- Longo BM, Mello LEAM. Supragranular mossy fiber sprouting is not necessary for spontaneous seizures in the intrahippocampal kainate model of epilepsy in the rat. Epilepsy Res. 1998;32:172–182. doi: 10.1016/s0920-1211(98)00049-7. [DOI] [PubMed] [Google Scholar]
- Masukawa LM, O'Connor WM, Lynott J, Burdette LJ, Uruno K, McGonigle P, O'Connor MJ. Longitudinal variation in cell density and mossy fiber reorganization in the dentate gyrus from temporal lobe epileptic patients. Brain Res. 1995;678:65–75. doi: 10.1016/0006-8993(95)00167-o. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kälviänen R, Tapiola T, Ylinen A, Vapalahti M, Paljärvi L, Pitkänen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A. The model of pentylenetetrazol-induced status epilepticus in the immature rat: short- and long-term effects. Epilepsy Res. 1996;26:93–103. doi: 10.1016/s0920-1211(96)00045-9. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Lukasiuk K, Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Molnar P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81:1645–1660. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol. 1998;79:418–429. doi: 10.1152/jn.1998.79.1.418. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1982. [Google Scholar]
- Qiao X, Noebels JL. Developmental analysis of hippocampal mossy fiber outgrowth in a mutant mouse with inherited spike-wave seizures. J Neurosci. 1993;13:4622–4635. doi: 10.1523/JNEUROSCI.13-11-04622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Navetta MS. An immature mossy fiber innervation of hilar neurons may explain their resistance to kainate-induced cell death in 15-day old rats. Dev Brain Res. 1994;79:47–62. doi: 10.1016/0165-3806(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Peterson GM. Intragranular mossy fibers in rats and gerbils form synapses with the somata and proximal dendrites of basket cells in the dentate gyrus. Hippocampus. 1991;1:355–364. doi: 10.1002/hipo.450010403. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;15:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;7(suppl):53–56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Holmes GL, Carmant L, Liu Z, Yang Y, Stafstrom CE. Effects of hyperthermia and continuous hippocampal stimulation on the immature and adult brain. Brain Dev. 1999;21:318–325. doi: 10.1016/s0387-7604(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb ID. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schwegler H, Lipp HP. Hereditary covariations of neuronal circuitry and behavior: correlations between the proportions of hippocampal synaptic fields in the region inferior and two-way avoidance in mice and rats. Behav Brain Res. 1983;7:1–38. doi: 10.1016/0166-4328(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wang S, Burnham WM, De Boni U, Wojtowicz JM. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett. 1998;248:73–76. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- Shinnar S. Do febrile seizures lead to temporal lobe epilepsy? Prospective and epidemiological studies. In: Baram TZ, Shinnar S, editors. Febrile seizures. Academic Press; San Diego, CA: 2002. pp. 88–101. [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell hypothesis” and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Stanton PK, Moshe SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Dev Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Agarwal KS, Dure LS. Is cell death necessary for hippocampal mossy fiber sprouting? Epilepsy Res. 1997;27:67–76. doi: 10.1016/s0920-1211(97)01025-5. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula T, Zhang P, Lynch M, Sayin Ü , Golarai G, Rod R. Synaptic and axonal remodeling of mossy fibers in the hilus and supragranular region of the dentate gyrus in kainate-treated rats. J Comp Neurol. 1998;390:578–594. doi: 10.1002/(sici)1096-9861(19980126)390:4<578::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Holm AM, Schousboe A, Popper P, Micevych P, Wasterlain C. Hippocampal stimulation produces neuronal death in the immature brain. Neuroscience. 1998;82:337–348. doi: 10.1016/s0306-4522(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience. 1984;13:1051–1072. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Woolley CS, Robbins CA, Schwartzkroin PA. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of the rat. Hippocampus. 2000;10:244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP. Evidence for physiological growth of hippocampal mossy fiber collaterals in the guinea pig during puberty and adulthood. Hippocampus. 1995;5:329–340. doi: 10.1002/hipo.450050406. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rat. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]