Abstract
Context
Chronic pain is a major contributor to disability in older adults, however, the potential role of chronic pain as a risk factor for falls is poorly understood.
Objective
To determine whether chronic musculoskeletal pain is associated with an increased occurrence of falls in a cohort of community-living older adults.
Design, Setting, Participants
The MOBILIZE Boston Study is a population-based longitudinal study of falls in 749 adults aged 70 and older living in the Boston area. Participants were enrolled from September, 2005 through January, 2008.
Main Outcome Measure
Participants recorded falls on monthly calendar postcards mailed to the study center during an 18-month period.
Results
There were 1029 falls reported during the follow-up. Report of 2 or more locations of musculoskeletal pain at baseline was associated with greater occurrence of falls. The age-adjusted fall rates were: 1.18 (95%CI 1.13–1.23) falls per person-year(PPY), for participants with ≥2 sites of joint pain (n=300), 0.90 (95%CI 0.87–0.92) falls PPY for those with single site pain (n=181), and 0.78 (95%CI 0.74–0.81) falls PPY for persons reporting no joint pain (n=267). Similarly, more severe or disabling pain at baseline was associated with higher fall rates (p<0.05). The association persisted after adjusting for multiple confounders and fall risk factors. The greatest risk for falls was observed in persons who had ≥2 pain sites (adj. rate ratio (RR) =1.53, 95%CI 1.17–1.99), and those in the highest tertiles of pain severity (adj. RR=1.53, 95%CI 1.12–2.08) and pain interference with activities (adj. RR=1.53, 95%CI 1.15–2.05), compared to their peers with no pain or those in the lowest tertiles of pain subscales.
Conclusions
Chronic pain measured according to number of locations, severity or pain interference with daily activities was associated with greater risk for falls in older adults. A randomized controlled trial is needed to confirm whether improved pain control could reduce fall risk in older persons who have chronic pain.
Falls rank among the 10 leading causes of death in older adults in the U.S., resulting in over $19 billion in health care costs annually.1, 2 Despite a growing body of scientific evidence supporting associations between a number of risk factors and falls,3 efforts to translate these findings into effective fall prevention strategies have been limited.4 Perhaps one contributing factor to the limited success of multifactorial fall prevention efforts may be that some major causes of falls in older persons continue to elude us.
Few reports have examined chronic pain as a risk for falls in older adults5–7 and none have prospectively examined multiple pain sites in relation to fall risk in the general population of older adults living in the community. Pain contributes to functional decline and muscle weakness, and is associated with mobility limitations that could predispose to falls.8–10 In addition, neurocognitive deficits observed in elderly fallers11 are not unlike the mild cognitive deficits observed in older adults with chronic back pain,12 supporting the possibility of a central-mediated pathway whereby pain contributes to falls. Given the high prevalence of chronic pain coupled with the problem of under-treatment of chronic pain in older patients,13 it is reasonable to surmise that chronic pain could be an important contributor to falls. The MOBILIZE (Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly) Boston Study (MBS) used a longitudinal cohort design to explore a set of risk factors for falls that are generally more challenging to measure, in hopes of identifying new targets for fall prevention.
METHODS
Study participants were women and men aged 70 and older, living in the community in Boston and nearby suburbs. Recruitment and enrollment took place from September, 2005 to January, 2008, within a defined geographic area bounded by a 5-mile radius from the Institute for Aging Research at the Hebrew Rehabilitation Center (HRC) in Boston. The sampling area was chosen to capture a diverse urban and suburban population, to increase likelihood of recognition of the study center, and to minimize transportation burden. Details of the study methods were published previously.14, 15
Initial eligibility was based on age 70 years or older, ability to walk 20 feet without personal assistance, able to communicate in English, and the expectation of staying in the area for 2 years. Following the initial recruitment visit, study staff contacted prospective enrollees by telephone to confirm eligibility and schedule the baseline home and clinic visits. During the home visit, informed consent was obtained and participants were screened for moderate or severe cognitive impairment using the Mini-Mental State Examination (MMSE, score of <18).16, 17 All protocols for the study and consent procedures were approved by the Institutional Review Boards of the HRC and collaborating institutions.
Falls Assessments
A fall was defined as unintentionally coming to rest on the ground or other lower level not as a result of a major intrinsic event (e.g. myocardial infarction, stroke, or seizure) or an overwhelming external hazard (e.g. hit by a vehicle).18 During the home visit, participants were instructed to complete and return monthly falls calendar postcards. On the postcards, participants were to record an “F” for each fall on the day it occurred and an “N” on days when no fall occurred. This approach has been well-validated for use in epidemiologic cohort studies.19 Research staff monitored the return of the calendars and on any given month, approximately one-third of participants were called for missing or incomplete calendars. Falls were assessed for up to 18 months through April, 2009.
Chronic Pain Assessment
Pain was assessed according to location, overall pain severity and pain interference with daily activities, encompassing key dimensions for pain assessment recommended by the American Geriatrics Society.20 We used a 13-item joint pain questionnaire (JPQ) to assess chronic musculoskeletal pain in hands/wrists, shoulders, back, chest, hips, knees, and feet.21 This measure was previously associated with decline in physical function in older women.10, 22 Chronic pain in each site was based on participant’s report that pain was present in the previous month and present for at least 3 months in the previous year. Chest pain associated with angina was excluded, based on an algorithm used to classify angina from the Rose questionnaire23 and use of nitrates. We classified chronic joint pain as follows: (1) pain in 2 or more locations (referred to as polyarticular pain), (2) pain in a single location, and (3) no pain. We also developed a second set of pain location measures according to each specific joint site. For example, knee pain was classified as (1) pain in the knee(s) as well as 1 or more other joint locations, (2) pain in the knee(s) only, and (3) no knee pain. We used two subscales of the Brief Pain Inventory (BPI), the 4-item pain severity subscale and the 7-item BPI pain interference scale.24 The BPI, which measures pain in general without reference to location, was originally developed for use in cancer patients but has been validated for use in non-malignant pain.25, 26
Pain was also assessed monthly during follow-up using a single pain-rating question on the monthly fall postcards. The question, from the well-validated SF-36, was stated as follows, “In the past month, how much bodily pain have you had?” and response options were, “none, very mild, mild, moderate, severe, and very severe.”27
Sociodemographics, Chronic Conditions, and Fall Risk Factors
Sociodemographic characteristics assessed in the home interview included age, sex, race (self-identified), and years of education. Race was included because our prior work found Black race associated with polyarticular pain.22 Cognitive status was assessed using the MMSE, scored 0–30.17 We used the validated Physical Activity Scale for the Elderly (PASE) to measure physical activity in the previous week.28 Participants were asked about doctor-diagnosed major medical conditions. Heart disease included report of heart attack, congestive heart failure, angina, pacemaker or cardiac arrhythmia. Other self-reported diagnoses included stroke, Parkinson’s Disease, rheumatoid arthritis, and spinal stenosis/disc disease. Peripheral neuropathy was assessed using Semmes-Weinstein monofilament testing.29 Peripheral arterial disease was defined using an algorithm, based on an ankle-arm index <0.90 and the Rose Intermittent Claudication questionnaire.23 Diabetes was defined using an algorithm based on self-reported diabetes, use of antidiabetic medications, and laboratory measures from the baseline clinic visit including random glucose (≥200mg/dl) and hemoglobin A1c (>7%). American College of Rheumatology (ACR) clinical criteria for osteoarthritis(OA) of the hand and knee30, 31 were assessed in the clinic examination by experienced nurses trained by the study rheumatologist (R.H.S.). Depression was assessed using Eaton’s method based on a modification of the 20-item Centers for Epidemiologic Studies Depression (CESD) scale.32, 33 Distant vision was measured at 10-feet using a letter chart, the Good-Lite Chart Model 600A. Body mass index (BMI, height in cm2/weight in kilograms) was calculated from measured height and weight. Standing balance was scored using 4 timed tests (side-by-side, semi-tandem, tandem and one-leg stands).34 For the timed chair stands test, participants were asked to fold their arms across their chest and stand up and down from a chair 5 times as quickly as possible.34 Gait speed was based on the shortest time of 2 trials of a usual-paced 4-meter walk.
Medications
During the home visit, the interviewer recorded use of all prescription and over-the-counter medications taken in the previous 2 weeks. Active ingredients of medications were coded according to the Iowa Drug Information System (IDIS) ingredient codes.35 Analgesic medications included opioid and non-opioid analgesics and daily use was determined from dose and frequency information. Daily or less than daily use of 325mg or less of aspirin, probable anti-thrombotic therapy, was not included as an analgesic. Psychotherapeutic agents, including sedative, hypnotic, anxiolytic, antidepressant, and antipsychotic medications, were categorized as use of 2 or more daily, 1 daily, non-daily use, no use.
Analysis
We planned to enroll 800 participants in order to have 648 evaluable subjects at the end of follow-up, accounting for possible attrition. Assuming the annual occurrence of falls, estimated at 30%,36 follows a Poisson process, we expected to have 85% power to detect a difference as small as 20% between those with polyarticular pain compared to those with no pain, using a chi-square test with continuity correction and significance level 0.05.
In our analyses, we tested both the association between baseline pain measures and risk of falls over the 18-month follow-up and the short-term relationship between pain measured each month and risk for falls in the subsequent month. We used descriptive statistics and chi-square tests (1 d.f.) to describe prevalence of baseline characteristics and fall risk factors according to musculoskeletal pain categories (none, single site, polyarticular). Age-adjusted fall rates and 95% confidence intervals (CI) were calculated using the direct method, applying the crude age-specific rates to the age distribution of the cohort.37
Statistical models were performed using total number of falls (as a count variable) per total follow-up time for each participant, yielding multivariable-adjusted rate ratios (RR) and 95% CI. Using the Poisson distribution for fall counts assumes that the mean equals the variance and this assumption typically does not hold as the variance is often much higher than the mean. To correct for this overdispersion, which can result in underestimates of standard errors and overestimates of chi-square statistics, we used negative binomial regression models with an offset variable for log total years of follow-up. We examined 3 domains of baseline chronic pain in relation to fall risk: pain location (none, single joint site, polyarticular), severity (tertiles of the BPI pain severity subscale) and interference (tertiles of the BPI pain interference subscale). In addition, we performed a similar analysis using site-specific pain measures. There was very little missing information in the baseline measures and no single covariate had more than 2.4% missing. In the fully adjusted models that included all covariates, only 5.6% of records (n=42) were excluded for missing information. Analyses were performed using SAS version 9.1 (Cary, N.C.).
To evaluate the association between monthly pain ratings and risk for falls in the subsequent month during the 18-month follow-up, we performed pooled logistic regression models. Using an approach described previously, each month of follow-up for each subject is a separate observation in the dataset, which assumes within-subject observations are independent and risk for falls in relation to pain is unchanged over time.38, 39 The logistic regression models, generating odds ratios, were adjusted for baseline covariates used in the fully adjusted negative binomial models previously described. Because of the small numbers who reported very severe pain on the monthly pain rating, we grouped severe and very severe pain ratings.
RESULTS
From a random sample comprising 5,655 households within the target area, recruitment staff confirmed that 4,319 persons aged 70 years and older resided at the sampled addresses. Of these, 1,610 were ineligible, 1,916 were of unknown eligibility (including refusal to complete screening), 44 persons were eligible but did not complete the interview, and 749 persons were eligible and completed the baseline home interview and clinic examination. Ineligibility was most commonly related to language, poor health, mobility, and cognitive status.
To determine the response rate among those eligible to participate, which was 53%, we applied our observed eligibility rate (33%) to estimate the proportion of those we contacted whose eligibility was unknown would have been eligible to participate (American Association of Public Opinion Research40). Participants were younger than non-participants [mean in years (SD), 78 (5) and 79 (7) respectively, P < 0.001] and more likely to be white, non-Hispanic (81% vs. 77%, P = 0.02) but no more likely to be women (63% vs 64%, P = 0.81).
At baseline, 40% of participants reported chronic polyarticular pain. Another 24% reported chronic pain in only one joint area. The number of musculoskeletal pain locations was highly correlated with the tertile classifications of both BPI pain severity and pain interference (r = 0.55 for each). The two BPI subscales also were highly correlated (r = 0.70). Older adults who had polyarticular pain were more likely to be women, have fewer years of education, to be obese, have fallen in the previous year, and have poorer performance in tests of balance and mobility (Table 1). Medical conditions associated with chronic musculoskeletal pain included spinal stenosis/disc disease, hand and knee osteoarthritis, rheumatoid arthritis, depression, peripheral arterial disease, and heart disease (Table 2).
Table 1.
Baseline characteristicsa according to chronic musculoskeletal pain categories.
| Characteristics and fall risk factors | No Pain (n=267) | Single site (n=181) | Polyarticular pain (n=300) | p-value (trend)b |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Age in years | ||||
| 70–75 | 76 (28.5) | 49 (27.1) | 93 (31.0) | |
| 75–79 | 87 (32.6) | 64 (35.4) | 93 (31.0) | |
| 80–84 | 67 (25.1) | 45 (24.9) | 69 (23.0) | |
| ≥ 85 | 37 (13.9) | 23 (12.7) | 45 (15.0) | 0.78 |
| Women | 155 (58.1) | 106 (58.6) | 212 (70.7) | 0.002 |
| Education | ||||
| < high school | 17 (6.4) | 20 (11.0) | 48 (16.0) | |
| high school graduate | 64 (24.1) | 26 (14.4) | 84 (28.0) | |
| college graduate | 185 (69.5) | 135 (74.6) | 168 (56.0) | <0.001 |
| Race: | ||||
| White | 212 (79.4) | 142 (78.5) | 225 (75.3) | |
| Black | 37 (13.9) | 29 (16.0) | 57 (19.1) | |
| Other | 18 (6.7) | 10 (5.5) | 17 (5.7) | 0.51 |
| Body Mass Indexc | ||||
| <25 | 97 (37.0) | 50 (28.2) | 70 (24.0) | |
| 25–29.9 | 108 (41.2) | 82 (46.3) | 125 (43.0) | |
| >= 30 | 57 (21.8) | 45 (25.4) | 96 (33.0) | <0.001 |
| Visual deficitd | 73 (27.5) | 38 (21.0) | 75 (25.1) | 0.53 |
| Physical activity scoree | ||||
| 0 – 66 | 83 (31.1) | 54 (30.3) | 110 (37.3) | |
| 66.01 – 124 | 87 (32.6) | 68 (38.2) | 91 (30.8) | |
| 124.01 – 559 | 97 (36.3) | 56 (31.5) | 94 (31.9) | 0.12 |
| MMSE < 24f | 29 (10.9) | 19 (10.5) | 44 (14.7) | 0.16 |
| Fell in past year | 75 (28.3) | 69 (38.3) | 132 (44.2) | <0.001 |
| Psychotherapeutic medication useg | ||||
| None | 222 (83.5) | 139 (76.8) | 233 (77.7) | |
| Less than daily | 10 (3.8) | 17 (9.4) | 15 (5.0) | |
| Single drug daily | 26 (9.8) | 19 (10.5) | 36 (12.0) | |
| ≥Two drugs daily | 8 (3.0) | 6 (3.3) | 16 (5.3) | 0.07 |
| Daily analgesic useg | 31 (11.7) | 40 (22.1) | 114 (38.0) | <0.001 |
| Impaired balanceh (score <4 out of 7) | 67 (25.1) | 41 (22.7) | 115 (38.5) | <0.001 |
| Slow gait speed i (< 0.78m/sec) | 53 (19.9) | 39 (21.6) | 94 (31.4) | 0.001 |
| Slow chair standsj (> 16.37 sec) | 46 (17.2) | 31 (17.1) | 109 (36.5) | <0.001 |
One person of the original 749 was missing musculoskeletal pain information.
Mantel -Haenzel chi-square test for trend (1 d.f.), except for race comparisons, which used chi-square test for overall differences (6 d.f.).
Body Mass Index calculated as weight in kilograms divided by height in meters squared
Vision deficit assessed as lowest quartile in score of distant vision using Good Lite Box.
Physical activity tertiles measured using the Physical Activity Scale for the Elderly
Mini Mental State Examination cutpoint for cognitive impairment
Used one or more analgesic medications at least daily in the previous 2 weeks
Balance score was based on 4 progressively difficult stands: feet side-by-side, semi-tandem, tandem, and 1-leg stand.
Slow gait speed (meters/second) is slowest 25% based on time of fastest of 2 usual-paced 4 meter walks
Slowest 25% of timed performance of 5 repeated stands from a chair without using arms
Table 2.
Baseline medical conditions according to pain categories.
| Medical conditions | No Pain (n=267) | Single site (n=181) | Polyarticular pain (n=300) | p-value (trend)a |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Spinal Stenosis/Disc Diseaseb | 31 (11.6) | 29 (16.0) | 78 (26.0) | <0.001 |
| Arthritis c | ||||
| Neither site | 236 (88.4) | 116 (64.1) | 118 (39.5) | |
| Knee Only | 16 (6.0) | 36 (19.9) | 81 (27.1) | |
| Hand Only | 14 (5.2) | 26 (14.4) | 46 (15.4) | |
| Both | 1 (0.4) | 3 (1.7) | 54 (18.1) | <0.001 |
| Rheumatoid Arthritisb | 7 (2.6) | 7 (3.9) | 24 (8.0) | 0.003 |
| Depressiond | 11 (4.1) | 7 (3.9) | 37 (12.3) | <0.001 |
| Peripheral Neuropathye | 27 (10.2) | 21 (11.7) | 44 (15.1) | 0.08 |
| Peripheral Arterial Diseasef | 10 (3.8) | 13 (7.2) | 49 (16.3) | <0.001 |
| Heart Diseaseg | 94 (35.2) | 81 (44.8) | 139 (46.3) | 0.008 |
| Diabetesf | 44(16.5) | 40(22.1) | 67(22.3) | 0.09 |
| Parkinson’s Diseaseb | 0 (0) | 3 (1.7) | 3 (1.0) | 0.20 |
| Strokeb | 24 (9.0) | 15 (8.3) | 34 (11.3) | 0.34 |
Mantel -Haenzel chi-square test for trend (1 d.f.)
Assessed by self-report during home interview
Assessed in clinic exam using American College of Rheumatology clinical criteria
Mild to severe depression based on CESD-revised and DSM-IV criteria
Assessed using Semmes-Weinstein monofilament testing of great toes.
Based on disease algorithms (Peripheral arterial disease: using Rose claudication questionnaire and ankle-brachial index; Diabetes: using random glucose, HbA1c, antidiabetic medications or insulin, and self-report)
Assessed by self-report during home interview (heart disease included items about any heart disease, heart attack, irregular heart rhythm, pacemaker, angina, or heart failure).
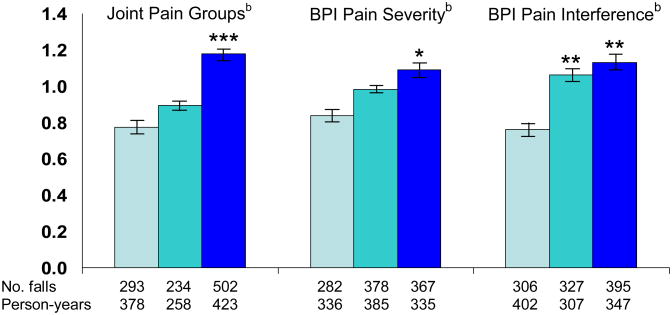
Overall, 76% of participants completed 18 monthly calendars, 90% completed 15 or more monthly calendars and 94% completed at least 12 monthly calendars. On average, 98% of falls calendar information was completed each month either by returned postcards or by telephone; specifically, the proportions of completed calendars at 6, 9, 12, and 18 months were 97%, 97%, 98% and 98%, respectively, among persons currently enrolled at each time point. A total of 1,029 falls were reported by the 749 participants on the monthly fall calendars during up to 18 months of follow-up. More than half of participants (n=409; 55%) fell at least once during the follow-up. Older persons who had chronic pain, whether measured by location, severity, or pain interference with activities, had higher rates of falls during follow-up compared to those who had no pain (p<0.05, Figure 1). After multivariable adjustment for chronic conditions and fall risk factors, each measure of chronic pain continued to be independently associated with increased occurrence of falls (Table 3). Adjustment for balance and mobility performance, use of psychotherapeutic medications, and, in subsequent models, adjustment for use of analgesics and clinical criteria for osteoarthritis of the hand and knee had little influence on the rate ratios (RR). When we adjusted for history of falls, the association with each pain measure was attenuated but remained significant (eTable 1 online). We found no evidence of an interaction between musculoskeletal pain and use of daily analgesics in relation to falls (test for interaction, p=0.78).
Figure 1. Age-adjusted fall rates according to pain measuresa.
a Age-adjusted rates and 95% confidence intervals derived using the direct method, adjusted to the age distribution of the study cohort.
bJoint pain groups: no pain, single site, multisite pain
BPI=Brief Pain Inventory, Pain Severity tertiles=0–0.99, 1.0–3.25, 3.26–10
Pain Interference tertiles= 0, 0.1–1.9, 2–10.
* compared to lowest category, p-value <0.05, ** <0.01, *** <0.001
Table 3.
Rate ratios for the occurrence of fallsa according to baseline pain measures.
| Pain categories | Nf | No. fallsf | Model 1b Adjusted for sociodemographic characteristics | Model 2c (+ chronic conditions, physical and cognitive status) | Model 3d (+physical performance and psychotherapeutic medications) | Model 4e (+ analgesic use and hand and knee arthritis clinical criteria) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Chronic Musculoskeletal Pain | ||||||||||
| None | 267 | 293 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Single site | 181 | 234 | 1.19 | 0.90, 1.56 | 1.15 | 0.86, 1.53 | 1.11 | 0.84, 1.47 | 1.11 | 0.84, 1.48 |
| Polyarticular pain | 300 | 502 | 1.70 | 1.34, 2.16 | 1.71 | 1.33, 2.20 | 1.60 | 1.23, 2.06 | 1.53 | 1.17, 1.99 |
| No. in model f | N=746 | N=709 | N=709 | N=709 | ||||||
| BPI Pain Severity Scoreg | ||||||||||
| Low severity tertile (0–0.99) | 237 | 282 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Middle tertile (1.0–3.25) | 267 | 378 | 1.19 | 0.92, 1.53 | 1.12 | 0.86, 1.46 | 1.12 | 0.86, 1.46 | 1.11 | 0.85, 1.44 |
| High severity tertile (3.26–10) | 242 | 367 | 1.54 | 1.18, 2.01 | 1.54 | 1.16, 2.05 | 1.50 | 1.12, 2.01 | 1.53 | 1.12, 2.08 |
| No. in model f | N=744 | N=708 | N=708 | N=708 | ||||||
| BPI Pain Interference Scoreg | ||||||||||
| Low interference tertile (0) | 284 | 306 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Middle tertile (0.1–1.9) | 211 | 327 | 1.44 | 1.11, 1.85 | 1.38 | 1.07, 1.80 | 1.33 | 1.02, 1.73 | 1.31 | 1.01, 1.71 |
| High interference tertile (2–10) | 251 | 395 | 1.67 | 1.31, 2.14 | 1.62 | 1.24, 2.10 | 1.52 | 1.16, 2.01 | 1.53 | 1.15, 2.05 |
| No. in model f | N=744 | N=707 | N=707 | N=707 | ||||||
Adjusted rate ratios and 95 % confidence intervals (C. I.) from negative binomial models predicting fall rate during up to 18 months of follow-up.
Model 1 covariates included age, sex, race, education
Model 2 included all variables from model 1 and heart disease, diabetes, Parkinson’s disease, history of stroke, vision score, BMI, cognitive function (MMSE), physical activity (PASE).
Model 3 included all variables from model 2 and balance score, repeated chair stand time, gait speed, and psychotherapeutic medications
Model 4 included all variables from model 3 and daily use of analgesic and non-pain clinical criteria for hand and knee osteoarthritis.
Totals vary between pain measures and between models due to missing information about pain and other covariates.
Pain severity and pain interferences subscales of the Brief Pain Inventory, each scored 0–10.
We considered individual musculoskeletal sites alone or in combination with other sites of pain in relation to falls. For each site of joint pain, risk for falls increased only when polyarticular pain was present (Table 4). The one exception was back pain, which was not associated with an increased rates of falls compared to persons without pain.
Table 4.
Rate ratios for the occurrence of fallsa according to pain sites.
| Pain categories | Nb | No. falls | RRc | 95% CI |
|---|---|---|---|---|
| Back and other joint pain | ||||
| None | 266 | 292 | 1.00 | |
| Pain other than back | 283 | 474 | 1.40 | 1.08, 1.79 |
| Back only | 23 | 35 | 1.37 | 0.75, 2.50 |
| Back and other pain | 175 | 227 | 1.22 | 0.90, 1.66 |
| No. in modelc | N = 708 | |||
| Hip and other joint pain | ||||
| None | 267 | 293 | 1.00 | |
| Pain other than hip | 352 | 540 | 1.31 | 1.03, 1.68 |
| Hip only | 14 | 24 | 1.23 | 0.56, 2.69 |
| Hip and other pain | 113 | 170 | 1.46 | 1.03, 2.07 |
| No. in modelc | N = 707 | |||
| Knee and other joint pain | ||||
| None | 267 | 293 | 1.00 | |
| Pain other than knee | 251 | 354 | 1.32 | 1.02, 1.72 |
| Knee only | 52 | 66 | 0.95 | 0.60, 1.49 |
| Knee and other pain | 176 | 315 | 1.51 | 1.12, 2.04 |
| No. in modelc | N = 708 | |||
| Feet and other joint pain | ||||
| None | 267 | 293 | 1.00 | |
| Pain other than feet | 297 | 433 | 1.24 | 0.97, 1.60 |
| Feet only | 30 | 36 | 1.07 | 0.62, 1.84 |
| Feet and other pain | 152 | 265 | 1.70 | 1.24, 2.32 |
| No. in modelc | N = 708 | |||
| Hands/wrist and other joint pain | ||||
| None | 266 | 293 | 1.00 | |
| Pain other than hands/wrist | 293 | 402 | 1.18 | 0.92, 1.53 |
| Hands/wrist only | 32 | 50 | 1.37 | 0.81, 2.32 |
| Hands/wrist and other pain | 156 | 284 | 1.65 | 1.22, 2.22 |
| No. in modelc | N = 708 | |||
| Shoulder and other joint pain | ||||
| None | 267 | 293 | 1.00 | |
| Pain other than shoulder | 325 | 471 | 1.23 | 0.96, 1.57 |
| Shoulder only | 20 | 14 | 0.82 | 0.36, 1.83 |
| Shoulder and other pain | 136 | 251 | 1.79 | 1.30, 2.46 |
| No. in modelc | N = 709 | |||
Rate ratios (RR) and 95 % confidence intervals (C. I.) from negative binomial models predicting fall rate during up to 18 months of follow-up; model covariates include age, sex, race, education, heart disease, diabetes, Parkinson’s disease, history of stroke, vision score, BMI, neuropathy, cognitive function (MMSE), physical activity (PASE), balance test score, repeated chair stand time, gait speed, daily use of psychotherapeutic medications, daily use of analgesic medications, hand and knee osteoarthritis clinical criteria excluding pain.
Totals vary slightly due to missing pain information for selected pain questions.
Sample sizes of models vary due to missing pain and covariate information.
In about one-third of the monthly postcards, participants rated their pain on average for the month as moderate to very severe. We observed a strong graded relationship in the short term between pain severity ratings each month with risk for falls in the subsequent month (Table 5). For example, among persons who reported severe or very severe pain for any given month on their calendar postcard, there was a 77% increased likelihood for a fall in the subsequent month, compared to those who reported no pain (multivariable adj. OR 1.77, 95%CI 1.32 – 2.38). Persons reporting even very mild pain also had an elevated risk for falls in any given month (adj. OR 1.36, 95%CI 1.08 – 1.71). Further adjustment for baseline pain status led to only a modest attenuation of the association with no change in the significance of the findings.
Table 5.
Adjusted odds ratiosa for falls in the subsequent month according to monthly pain ratings.
| Pain categories | No. falls | Months | Adj. OR | 95% CI |
|---|---|---|---|---|
| Bodily Pain Severity Ratingb | ||||
| None | 169 | 2983 | 1.00 | |
| Very mild | 254 | 3252 | 1.36 | 1.08, 1.71 |
| Mild | 228 | 2698 | 1.49 | 1.18, 1.89 |
| Moderate | 275 | 2906 | 1.59 | 1.26, 2.01 |
| Severe/Very severe | 122 | 1218 | 1.77 | 1.32, 2.38 |
Pooled logistic regression predicting one or more falls in the month subsequent to the monthly average pain severity rating, SF-36 bodily pain item on monthly calendar postcards; model adjusted for baseline covariates: age, sex, race, education heart disease, diabetes, Parkinson’s disease, history of stroke, vision score, neuropathy, BMI, cognitive function (MMSE), physical activity (PASE), balance score, gait speed, chair stands, analgesic use, psychoactive medication use, hand and knee osteoarthritis clinical criteria.
The severe and very severe categories were combined due to small numbers. Pain ratings were missing for 2% of the completed fall calendars.
COMMENT
Both chronic pain and falls were very common in our study population. Our results provide strong and consistent evidence that chronic musculoskeletal pain, regardless of the measure used, is associated with increased risk for falls in a general population of community-living older adults. The effect was observed using chronic pain assessed at baseline predicting falls over 18 months and, more immediately, in monthly pain ratings predicting falls in the subsequent month. Pain may be a marker for underlying pathology or treatments that could contribute to falls, such as spinal stenosis, osteoarthritis with deformities, or sedating medications. However, when we adjusted for these potentially confounding factors, pain remained a strong independent risk factor for falls.
Possible underlying mechanisms for the pain-falls relationship can be grouped into three categories, local joint pathology, neuromuscular effects of pain, and central mechanisms whereby pain interferes with cognition or executive function. Osteoarthritis is the main disease process contributing to joint pain in older adults. Polyarticular pain, as defined in our study, may represent a generalized arthritic process. Findings regarding risk for falls from arthritis are generally weak or inconclusive, possibly related to varying definitions of arthritis.41 Knee pain but not clinically diagnosed knee OA, was associated with increased fall risk in older trial participants.7 In our analyses, the association between pain and falls was independent of clinically assessed hand and knee OA, as well as mobility performance. However, we cannot be certain that unmeasured joint pathology could be a contributing factor to the observed associations.
Neuromuscular effects of pain could lead to leg muscle weakness or slowed neuromuscular responses to an impending fall. Muscle weakness could arise from lack of physical activity or from a direct effect of pain on muscle, referred to as reflex muscle inhibition.42 Another factor may be gait alterations or adaptations to chronic pain that lead to instability and subsequent balance impairments.
Chronic pain may serve as a distractor or, in some way, interfere with cognitive activity needed to prevent a fall. Successful avoidance or interruptions of a fall typically requires a cognitively-mediated physical maneuver. Recent imaging studies provide evidence that chronic pain patients exhibit changes in both structure and function of the brain consistent with changes observed through neuropsychological testing.43, 44 Patients with chronic pain show poorer executive function and decreased attentional resources compared to healthy controls.45 Attention has also been associated with gait changes and fall risk.46–48 A cognitively-mediated pathway would be consistent with our finding of similar fall risk with pain in the upper or lower extremities.
We did not observe a lower rate of falls among analgesic users, contrary to our previous study which found that analgesic users had lower fall risk than non-users among women with pain.6 Benefits of analgesic use may have been more evident among disabled women than in the higher functioning MBS cohort. Analgesic use is sometimes thought to contribute to falls, however, underuse of analgesics also could contribute to falls. This question deserves further study using an experimental design.
Mobility limitations and history of falls are among the strongest predictors of falls.3 The observed association between pain and falls was independent of mobility function. Including falls that occurred in the year before baseline in our models was likely an over-adjustment for chronic pain defined also in reference to the past year (lasting 3 or more months in the past year). Thus, according to our hypothesis, chronic pain in the previous year would likely contribute to falls in the previous year. We did not control for depression because pain and depression were highly correlated in the MOBILIZE Boston cohort, similar to other cohorts.12, 22 Nonetheless, this may be an important consideration for future investigations.
Although we studied fall risk prospectively, we cannot exclude the possibilities that baseline pain was a consequence of previous falls or that pain-related pathology was the underlying cause of the falls. We adjusted our models for comorbid conditions including clinical evidence of osteoarthritis without any substantive change in the pain-falls relationship. Strengths of this study include the population-based design, the extensive assessment of fall risk factors and possible confounders, the monthly falls ascertainment with little missing information, and the assessment of pain in several complimentary ways. Our results are likely generalizable to the population of mobile older adults living independently in the community without significant cognitive difficulties.
The findings provide evidence suggesting that the common complaint of the aches and pains of old age is related to a greater hazard than previously thought. Daily discomfort may accompany not only difficulties in performing daily activities but equally as important, may be a risk for falls and possibly fall-related injuries in the older population. The significance of this work is in the identification of chronic pain as an overlooked and potentially important risk factor for falls in older adults. A randomized controlled trial is needed to determine whether improved pain control could reduce risk for falls among older patients with chronic pain.
Supplementary Material
Acknowledgments
We thank the MOBILIZE Boston research team and study participants for their time, effort, and dedication. Dr. Leveille has full access to the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding and Support
This research was supported by the National Institute on Aging: Research Nursing Home Program Project Grant# P01AG004390. Dr. Guralnik’s time was funded by the Intramural Research Program, National Institute on Aging, NIH. The coding of the medication data for the MOBILIZE Boston Study was supported by an unrestricted grant from Pfizer Inc. None of the paper’s authors received any salary support, stipends or other funding from the Pfizer grant.
Role of the Sponsor
The Sponsor of this study is the National Institute on Aging. Dr. Guralnik is an employee of the National Institute on Aging, the sponsor of this study. Dr. Guralnik had no role in the decision to fund this research. He participated in the design of the study, interpretation of the analysis, and preparation of the manuscript. No employees of Pfizer Inc. had any role in the conduct of this research or in the coding of the medication data.
Footnotes
Financial Disclosures
Dr Kiel reports receiving grant support from Pfizer, Amgen, Merck, Novartis, and Hologic; consulting income from Amgen, Novartis, Merk, GlaxoSmithKline, Lilly, Procter & Gamble, Philips Lifeline, and Wyeth; and speakers’ honoraria from Novartis, Merck GlaxoSmithKline, and Lilly.
References
- 1.Gorina Y, Hoyert D, Lentzner H, Goulding M. Trends in causes of death among older persons in the United States. Aging Trends. 2005 Oct;6:1–12. [PubMed] [Google Scholar]
- 2.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006 Oct;12(5):290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006 Sep;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 4.Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. BMJ. 2008 Jan 19;336(7636):130–133. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyth FM, Cumming R, Mitchell P, Wang JJ. Pain and falls in older people. Eur J Pain. 2007 Jul;11(5):564–571. doi: 10.1016/j.ejpain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Leveille SG, Bean J, Bandeen-Roche K, Jones R, Hochberg M, Guralnik JM. Musculoskeletal pain and risk for falls in older disabled women living in the community. J Am Geriatr Soc. 2002;50(4):671–678. doi: 10.1046/j.1532-5415.2002.50161.x. [DOI] [PubMed] [Google Scholar]
- 7.Arden NK, Crozier S, Smith H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum. 2006 Aug 15;55(4):610–615. doi: 10.1002/art.22088. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont LH, Bean JF, Guralnik JM, Leveille SG. Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function. J Gerontol A Biol Sci Med Sci. 2009 Jul;64(7):763–770. doi: 10.1093/gerona/glp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb S, Guralnik J, Buchner D, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women’s Health and Aging Study. Ann Rheum Dis. 2000;59(5):331–337. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leveille SG, Bean J, Ngo L, McMullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007 Mar;128(1–2):69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006 Oct–Dec;32(4):411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006 Jan–Feb;7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Pahor M, Guralnik JM, Wan JY, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health. 1999;89(6):930–934. doi: 10.2105/ajph.89.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samelson EJ, Kelsey JL, Kiel DP, et al. Issues in conducting epidemiologic research among elders: lessons from the MOBILIZE Boston Study. Am J Epidemiol. 2008 Dec 15;168(12):1444–1451. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar J, Burnam A, Karno M, Forsythe A, Landsverk J, Golding J. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity. J Nerv Ment Dis. 1986;174:607–614. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg International Work Group on the Prevention of Falls by the Elderly. The prevention of falls in later life. Dan Med Bull. 1987 Apr;34 (Suppl 4):1–24. [PubMed] [Google Scholar]
- 19.Tinetti ME, Liu WL, Claus EB. Predictors and prognosis of inability to get up after falls among elderly persons. JAMA. 1993 Jan 6;269(1):65–70. [PubMed] [Google Scholar]
- 20.American Geriatrics Society Panel. The management of persistent pain in older persons. J Am Geriatr Soc. 2002 Jun;50(6 Suppl):S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, Maryland: National Institute on Aging; 1995. [Google Scholar]
- 22.Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135(12):1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 23.Rose G. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull WHO. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 24.Cleeland C. Measurement of pain by subjective report. In: Chapman C, Loeser J, editors. Advances in Pain Research and Therapy. Vol. 12. New york, NY: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 25.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004 Sep–Oct;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004 Mar;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988 Jul;26(7):724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 29.Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001 Feb;24(2):250–256. doi: 10.2337/diacare.24.2.250. [DOI] [PubMed] [Google Scholar]
- 30.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990 Nov;33(11):1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 31.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 32.Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD--R) In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Vol. 3. Mahwah NJ: Lawrence Erlbaum Assoc Inc; 2004. [Google Scholar]
- 33.Radloff L. The CES-D Scale: A self report depresion scale for research in teh general population. App Psych Meas. 1977;1:385–401. [Google Scholar]
- 34.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 36.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 37.Curtin LR, Klein RJ. Direct standardization (age-adjusted death rates) Healthy People 2000 Statistical Notes. 1995 Mar;6:1–10. [PubMed] [Google Scholar]
- 38.Cupples LA, D’Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1988 Jan–Feb;7(1–2):205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 39.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990 Dec;9(12):1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 40.American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys. 5. Lenexa, KS: AAPOR; 2008. [Google Scholar]
- 41.Leveille SG. Musculoskeletal Pain and Risk for Falls in Older Adults? Journal of Pain Management. 2009;1(4):329–338. [Google Scholar]
- 42.Graven-Nielsen T, Lund H, Arendt-Nielson L, Danneskiold-Samsoe B, Bliddal H. Inhibition of maximal voluntary contraction force by experimantal muscle pain: A centrally mediated mechanism. Muscle Nerve. 2002;26:708–712. doi: 10.1002/mus.10225. [DOI] [PubMed] [Google Scholar]
- 43.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009 Feb;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009 Apr;60(1):226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999 May;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Itzhak R, Giladi N, Gruendlinger L, Hausdorff JM. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008 Apr;56(4):695–700. doi: 10.1111/j.1532-5415.2007.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006 Jul;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 48.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005 Mar;53(3):410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.