Abstract
NR4A nuclear receptors are a diverse group of orphan nuclear receptors with critical roles in regulating cell proliferation and cell differentiation. The ortholog of the NR4A nuclear receptor in C. elegans, NHR-6, also has a role in cell proliferation and cell differentiation during organogenesis of the spermatheca. Here we show that NHR-6 is able to bind the canonical NR4A monomer response element and can transactivate from this site in mammalian HEK293 cells. Using a functional GFP-tagged NHR-6 fusion, we also demonstrate that NHR-6 is nuclear localized during development of the spermatheca. Mutation of the DNA-binding domain of NHR-6 abolishes its activity in genetic rescue assays, demonstrating a requirement for the DNA-binding domain. This study represents the first genetic demonstration of an in vivo requirement for an NR4A nuclear receptor DNA-binding domain in a whole organism.
Keywords: NHR-6, NR4A, C. elegans, DNA-binding domain, spermatheca, organogenesis
The NR4A orphan nuclear receptors (NRs) are rapidly emerging as key regulators of a diverse array of physiological and developmental processes (Maxwell and Muscat, 2006). One of the critical biological functions of the NR4A NRs is the regulation of cell proliferation and cell differentiation. All three vertebrate NR4A paralogs (NR4A1–3) have described cell proliferation and cell differentiation functions in a variety of cellular contexts, including atherosclerosis, endothelial cell growth, myeloid homeostasis, and differentiation of dopaminergic neurons (Bonta et al., 2007; Castro et al., 2001; Kolluri et al., 2003; Martorell et al., 2008; Mullican et al., 2007). How NR4A NRs can mediate opposing cell proliferation and cell differentiation processes is unknown. Structural studies demonstrate that NR4A NRs lack a ligand-binding pocket and thus are not regulated by the same canonical mechanism as other NRs (Baker et al., 2003; Wang et al., 2003). It is also apparent that NR4A NR activity is regulated through diverse interactions with signal transduction pathways (Wansa et al., 2003) and these differential interactions are likely the key to understanding the diverse cellular functions of NR4A NRs. NR4A NRs are also known to have both nuclear and cytoplasmic functions in regulating cellular processes, such as apoptosis (Chintharlapalli et al., 2005). It is not known whether NR4A NRs regulate cell proliferation and cell differentiation through a DNA-binding mechanism in vivo. In this study, we assessed the activity and in vivo function of the DNA-binding domain from the C. elegans ortholog of the NR4A NR, NHR-6. Previous analyses of nhr-6 null mutants demonstrated a requirement for NHR-6 in the organogenesis of the spermatheca, a somatic gonad organ that regulates oocyte ovulation and fertilization (Gissendanner et al., 2008). nhr-6 mutants have low brood sizes and sterility due to defective spermatheca development. NHR-6 is necessary for both cell proliferation and cell differentiation during development of this organ, indicating a striking conservation of biological function between NHR-6 and its mammalian homologs.
Vertebrate NR4A NRs bind DNA as a monomer to the canonical sequence: 5’-AAAGGTCA-3’ (also known as the NGFI-B response element, NBRE) (Wilson et al., 1992). We investigated if NHR-6 can bind the NBRE in vitro and in a cellular context. A GST-NHR-6 fusion protein containing the DNA-binding domain (DBD) and ligand-binding domain (LBD) binds the NBRE in a dose-dependent manner in electrophoretic mobility shift assays (Fig. 1a). The binding to a radiolabeled NBRE probe is competed using molar excess of cold NBRE but not by NBRE with two nucleotide substitutions (5’-CCAGGTCA-3’), indicating specificity of binding to the NBRE sequence. To determine if the NHR-6 DBD is necessary for the in vitro binding, we generated a cysteine to serine substitution (C288S; see Fig. 4a) in the first zinc finger of the DBD. The C288S mutation is located within the P-box, the region of the DBD important for NR binding to specific DNA sequences. Mutation of this cysteine to the similar sized serine is known to eliminate DNA binding activity in other NRs (Severne et al., 1988). Binding to the NBRE was abolished by the C288S mutation, demonstrating a requirement for a functional NHR-6 DBD in the gel-shift experiments. We next asked if NHR-6 can activate transcription from the NBRE in a cellular context. NR4A NRs can activate transcription in a variety of mammalian cell lines when co-transfected with an NBRE-luc reporter that contains three copies of the NBRE sequence (Forman et al., 1995; Perlmann and Jansson, 1995). Co-transfection of nhr-6 cDNA and the 3XNBRE-luc reporter into mammalian HEK 293 cells resulted in 3-5-fold activation of luciferase expression over empty vector controls in multiple independent experiments (Fig. 1b, c). As a comparison, we also performed co-transfection of mammalian NR4A3 and 3XNBRE-luc in parallel. In our experiments, the level of activation by NR4A3 from a 3XNBRE-luc reporter in HEK293 was similar to previously published observations (Castro et al., 1999). Consistent with previous studies (Wilson et al., 1991), the level of activation over empty vector controls was dependent on the number of NBRE sites as reporters with 2XNBRE and 4XNBRE exhibited different levels of activation by both NHR-6 and NR4A3 (Supplemental Fig. 1). NHR-6 exhibited less activity compared to NR4A3, a result that is not surprising considering the evolutionary divergence of nematodes and mammals. Nonetheless, NHR-6 is able to recognize the NR4A response element and recruit the mammalian transcriptional machinery, indicating that there is structural conservation between NHR-6 and mammalian NR4A NRs. A functional DBD of NHR-6 was required for the activation as NHR-6 with the C288S mutation in the DBD failed to activate transcription of the reporter (Fig. 1b, c).
FIG. 1. NHR-6 binds to the canonical NR4A response element and can transactivate in mammalian HEK293 cells.
(a) Electrophoretic mobility assay of GST-NHR-6. GST-NHR-6 (lanes 1-5) and GST-NHR-6 (C288S) (lanes 6, 7) incubated with radiolabeled 26-mer oligonucleotide containing the NBRE (5’-AAAGGTCA-3’). An increase in intensity of the shifted oligonucleotide is seen with increasing amounts of protein (lanes 1–3, 140ng, 280ng, and 560ng of input protein, respectively). Two major shifted bands are observed (arrows). The two bands could be due to dimerization of the protein in the EMSA reaction. Both shifted bands are competed by 50X cold NBRE (lane 4, 280ng of input protein). The shifted bands were not competed by 50X cold mutant NBRE (5’-CCAGGTCA-3’) (lane 5, 280ng of input protein). GST-NHR-6 (C288S) did not cause a shift (lanes 6 and 7, 280ng and 560ng of input protein, respectively. Coomassie stained gel of the GST-NHR-6 and GST-NHR-6 (C288S) protein preparations is shown below the EMSA, indicating equal amounts of protein in the preparations (arrow). Both proteins are ~68KDa in predicted size. 15 μl of protein preparation were loaded for each lane. (b) Representative graph showing transactivation by NHR-6 from the 3XNBRE in HEK293. Cells were co-transfected with pCMV-nhr-6, pCMV-nhr-6(C288S), pCMV-NR4A3, or empty pCMV vector (100ng each), along with 100ng of 3XNBRE-luc. Experiments were performed in triplicate and luciferase values (± SD) were determined 24 hours post-transfection. Luciferase values were normalized to Renilla luciferase (RLU). (c) Western blot analysis indicating expression of NHR-6 and NHR-6 (C288S) in HEK293 cells 24-hours post-transfection, as detected by anti-NHR-6 LBD polyclonal antibodies. Two specific bands are detected (arrows) as well as a non-specific band (*) that is also detected in cells transfected with empty vector. Detection of α-tubulin is used as a loading control.
FIG. 4. Genetic rescue by NHR-6::GFP requires a wild-type DNA-binding domain.
(a) Diagram of the two zinc fingers of the NHR-6 DNA binding domain showing the C288S mutation (blue circles indicate the zinc ions). (b) Brood size analysis of transgenic nematodes expressing NHR-6::GFP (C288S). See legend to Fig. 2 for description of genotypes. Arrays sgEx20-23 fail to rescue the brood size phenotype of nhr-6(lg6001) homozygotes (compare to −/−, p>0.8). Array sgEx21 does not express GFP (*). sgEx22 (genotype +/−; sgEx22) does not exhibit dominant effects in a heterozygous background (compare to +/−, p>0.4). n=15 for −/−, −/−; sgEx21-22; n=8 for −/−; sgEx20; n=12 for −/−; sgEx23; n=5 for +/+, +/−, and +/−; sgEX22. (c, d) Epifluorescence (c) and Nomarski (d) of NHR-6::GFP (C288S) expression during the mid-L4 stage from the sgEx23 array in nhr-6 (lg6001) homozygous animals. Arrow indicates nucleus in focal plane.
Previous studies of NHR-6 expression utilized either transcriptional reporter fusions to green fluorescent protein (GFP) or translational fusions to GFP that did not encode the complete NHR-6 protein (Gissendanner et al., 2008). Thus, cellular localization could not be addressed in those studies. To assess the localization of NHR-6 protein during spermatheca development, we constructed an nhr-6::GFP reporter transgene (pLG0097) to express full-length NHR-6 with a C-terminal GFP fusion (Fig. 2a). Transgenic nematodes bearing this transgene were generated in an nhr-6(lg6001) homozygous null mutant background. Six transgenic nematode lines that expressed NHR-6::GFP exhibited near wild-type brood sizes and did not exhibit any nhr-6 mutant phenotypes of low brood size/sterility, abnormal egg morphology, or defective spermatheca morphogenesis (Fig. 2b and Fig. 5). Thus, the GFP-tagged NHR-6 is fully functional for spermatheca development. A transgenic nhr-6 mutant line, sgEx19, that did not express GFP (see Methods) was not rescued, indicating that rescue requires expression of NHR-6::GFP (Fig. 2b and Fig. 3k, l). The expression pattern of NHR-6::GFP was nearly identical to the previously reported expression of transcriptional and partial translational fusions of nhr-6::GFP (Fig. 3). However, we did notice a few differences in the current study, including expression of NHR-6::GFP in precursor cells of the spermatheca-sheath lineages (SS cells) and in the precursors and descendents of the dorsal-uterine lineage (DU cells) (Figure 3a–j). Another difference was sheath cell expression in late L4 animals (data not shown). The GFP expression in both dorsal uterine and sheath cells was less intense than spermathecal cell expression. The slightly dissimilar expression pattern could reflect differences in the regulatory regions between the two reporter transgenes (see Methods) and/or differences in expression intensity as the GFP expression intensities of the reporter transgenes in the previous study were comparatively weaker.
FIG. 2. Rescue of nhr-6 (lg6001) mutants by transgenes expressing NHR-6::GFP.
(a) Diagram showing the fusion of GFP to NHR-6. Domains of NHR-6 are indicated. The numbers above the diagram indicate amino acid positions of NHR-6. EGFP is fused at the C-terminus at amino acid 608 of NHR-6. NHR-6 has 619 amino acids so the final 11 amino acids of the LBD are not included in the fusion. (b) Brood size analysis of transgenic and non-transgenic nematodes. “+/+” refers to animals homozygous for the wild-type nhr-6 allele (C. elegans N2 strain). “−/− “refers to animals homozygous for the nhr-6 (lg6001) allele, and “+/− “refers to nhr-6(lg6001)/qC1 dpy-19(e1259) glp-1(q339) heterozygotes (strain IP1001, see Methods). Animals bearing independent transgenic arrays containing the nhr-6::GFP transgene are indicated by the sgEx designation. Five or six animals were scored for +/+, +/−, and −/−; sgEx13; 15-18 genotypes. Ten animals were scored for the −/−; sgEx19 genotype and fifteen animals were scored for the −/−genotype. Nematodes bearing arrays sgEx15-18 exhibited rescue of the brood size phenotype with brood sizes that were not significantly different from heterozygotes (p>0.2). −/−; sgEx19 animals were not rescued and these animals do not express GFP presumably due to the lack of the nhr-6::GFP transgene in the array (*).
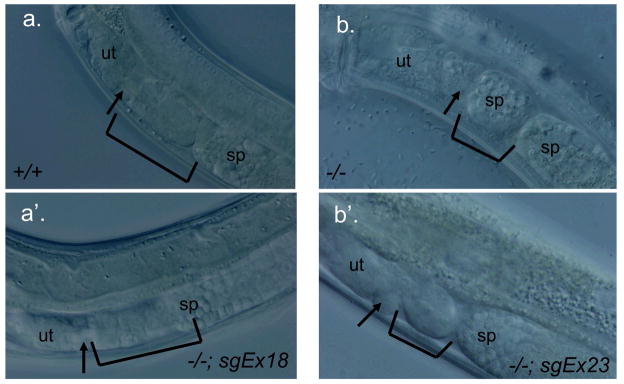
FIG. 5. Spermatheca morphogenesis phenotypes of rescued and non-rescued animals.
(a, a’) Wild-type (+/+) and nhr-6(lg6001)/nhr-6(lg6001); sgEx18 young adult hermaphrodites. The spermatheca-uterine valve at the distal end of the spermatheca is formed properly (arrow) and the spermatheca has a normal size and cell number (bracket). In (a’), most of the distal constriction in the proximal end of the spermatheca is out of the plane of focus. (b, b’) nhr-6(lg6001)/nhr-6(lg6001); sgEx23 young adult hermaphrodites. A spermatheca-uterine valve is absent (arrow) and the spermatheca is smaller and abnormally formed (bracket). The animal in (b) is slightly older and sperm has entered the spermatheca through the defective distal constriction. sp=sperm in proximal gonad; ut=uterus.
FIG. 3. Expression and nuclear localization of NHR-6::GFP in cells of the developing spermatheca.
Epifluorescence micrographs (a, c, e, g, i, k) and Nomarski micrographs (b, d, f, h, j, l) of nematodes bearing the sgEx13 array. White arrow indicates spermatheca nuclei and the pink arrow indicates dorsal uterine nuclei. Only nuclei in the plane of focus are indicated. C. elegans nuclei have a button appearance and contain a prominent nucleolus. Expression of GFP is restricted to the nucleus at all stages. (a, b) Expression of NHR-6::GFP in the SS and DU precursor cells in early L3 larval stage. (c, d) Expression in SS and DU descendents at mid-L3. (e, f) Expression in developing spermatheca and dorsal uterine cells during late L3. (g, h) Expression in early L4 spermatheca and dorsal uterus. (i, j) Expression in the spermatheca and dorsal uterus at mid-L4. Dorsal uterine expression has become very faint. Brackets indicate the regions of the developing anterior and posterior spermathecae in L4. (k, l) Absence of GFP expression in the spermatheca of −/−; sgEx19 animals (white bracket). The image in (k) was digitally enhanced and the diffuse fluorescence signal observed is due to autofluorescence of the gut.
Expression of NHR-6::GFP was predominantly localized to the nuclei of spermathecal cells throughout somatic gonad development (Fig. 3). Diffuse expression in non-dividing cells, which would be indicative of both nuclear and cytoplasmic localization, was never observed at any stage. As observed in previous studies, adult animals did not express NHR-6::GFP in the spermatheca. The nuclear localization of NHR-6::GFP suggests that NHR-6 has a nuclear function in the regulation of spermatheca development.
Since NHR-6::GFP is localized to the nucleus an important question is whether NHR-6 functions through a DNA-binding mechanism. The functional NHR-6::GFP allowed us to use this reporter in a genetic rescue assay to determine if a functional DBD is necessary for NHR-6 activity. We generated the same C288S mutation in NHR-6::GFP that abolished activity of NHR-6 in the gel shift and transcriptional reporter assays. We then generated transgenic nhr-6 (lg6001) homozygous mutant nematodes under the same conditions as with the wild-type transgene. We generated four transgenic lines, three of which expressed GFP. None of the GFP expressing lines exhibited any level of rescue, as brood sizes were not statistically different from those for nhr-6(lg6001) homozygous animals (Fig. 4b). Additionally, all transgenic lines exhibited a highly penetrant (>99%) abnormal egg morphology phenotype. As was the case with the wild-type fusion protein, GFP was expressed throughout spermatheca development despite the disruption of the DNA binding activity. The expression intensity of NHR-6(C288S)::GFP in the transgenic lines was qualitatively equivalent to the range of expression intensities in transgenics expressing wild-type NHR-6::GFP. Nuclear localization was also not affected by the DBD mutation (Fig. 4c, d). The C288S mutation did not have any dominant effects as nhr-6(lg6001)/+ heterozygous animals bearing the transgene were phenotypically the same as non-transgene bearing heterozygotes (Fig. 4b). Morphological analysis of these animals also revealed a lack of spermatheca-uterine valve and distal constriction development (Fig. 5). Therefore, we conclude that a wild-type DBD is necessary for spermathecal development, with the C288S mutation likely disrupting the ability of NHR-6 to bind NR4A response elements in vivo.
An important question with respect to nuclear receptor biology is the extent by which NRs function through a DNA-binding mechanism. Few studies have been performed in whole animal systems demonstrating an in vivo requirement for NR DNA binding. Mice bearing mutations in the glucocorticoid receptor (GR) that renders it unable to bind DNA are largely phenotypically normal, demonstrating that GR DNA binding is not essential for viability (Reichardt et al., 1998). Conversely, deletion of the germ cell nuclear factor (GCNF) DBD in mice does demonstrate a requirement for DNA binding in vivo for this NR during embryonic development (Lan et al., 2002). NR4A NRs have been documented to have both genomic and non-genomic functions, the latter being important in T-cell apoptosis (Li et al., 2000). While NR4A2 has been shown by chromatin immunoprecipitation to bind DNA in the ventral mid-brains of mouse embryos (Jacobs et al., 2009), no genetic studies have been performed in a whole animal system that demonstrates a requirement for the DNA-binding domain of NR4A NRs. The null-like phenotype of nhr-6 (lg6001) animals expressing NHR-6(C288S) suggests that DNA-binding activities of NHR-6 are required for spermatheca development. Therefore, based on these results, a likely mechanism of NHR-6 activity during spermatheca development is direct binding to promoter regions to regulate target gene transcription. More importantly, the genetic rescue assay developed in this study will provide a powerful animal-based system to further investigate the molecular mechanisms of NHR-6 activity, allowing an opportunity to identify, with in vivo relevance, downstream effectors of NHR-6 as well as the use of mutational studies to identify other regions of NHR-6 necessary for its activity.
METHODS
Plasmid construction
To generate an nhr-6::GST fusion, nhr-6 cDNA was PCR amplified and cloned into the NotI/BamHI site of the pGEX5-1 vector. The encoded protein has GST fused to amino acids 260-619 of NHR-6 which includes both the DBD and LBD. For expression of NHR-6 in HEK293, full-length nhr-6 cDNA was PCR amplified and cloned into the KpnI/XbaI of pCMV6-XL5 vector (OriGene) to place nhr-6 expression under control of the CMV promoter. There are two mRNA isoforms of nhr-6, alpha and beta (Gissendanner et al., 2008). The shorter beta isoform encodes an NR4A protein with a shortened N-terminal A/B domain. We chose to use the full-length alpha isoform for the cell transfection assays described here.
pLG0097 is a genomic clone of nhr-6 that contains the last ~2.4 kb of intron 1 and all coding sequences with sequence in the last exon fused in frame to EGFP (the last 11 amino acids of NHR-6 are not included in the fusion). Primers LG29.1 (5’-AGTCTGCAGTGCGGTGTTTTCTCTCAAAA-3’) and LG29.2 (5’-AGTGGATCCGACGAGGAAGATGGTCCAAA-3’) were used to amplify a 3108 bp PCR-fragment from C. elegans genomic DNA (containing the nhr-6 promoter (intron 1) and exon 2 (which contains the start codon)) that was cloned into the PstI/BamHI site of plasmid pBY1153 (gift from Ralf Baumeister, containing EGFP) resulting in plasmid pLG0029. Primers LG97.3 (5’-AGTGGATCCTCGCAAAGGCTCC-3’) and LG97.4 (5’-AGTCCATGGAACTTCCAGATGGGGCAAC-3’) were then used to amplify a 4040 bp PCR-fragment (containing nhr-6 genomic sequence downstream of exon 2) from C. elegans genomic DNA and cloned into the BamHI/NcoI site of plasmid pLG0029 resulting in plasmid pLG0097.
Site-directed mutagenesis to generate the C288S mutation was performed using the QuickChange II XL Site-Directed Mutagenesis Kit (Stratagene). All three mutagenesis reactions utilized the same primer combinations (5’-CCAGAACTTGCGAAGGAAGCAAAGGATTCTTCAAGC-3’and 5’-GCTTGAAGAATCCTTTGCTTCCTTCGCAAGTTCTGG-3’) and the same general reaction conditions according to manufacturer’s protocols. All constructs that were generated were sequenced to verify the C288S mutation and that no other mutations were present. For pLG0097, only the coding regions (with splice junctions) of the construct were sequenced.
Electrophoretic Mobility Shift Assays (EMSA)
GST-NHR-6 protein expression was induced at 37°C with 100mM IPTG in NEB Express E. coli (New England Biolabs). The GST-NHR-6 was purified using GST SpinTrap columns (GE Healthcare) according to manufacturer’s instructions.
For the EMSA assays primers 5’-GAGTTTTAAAAGGTCATGCTCAATTT-3’ and 5’AAATTGAGCATGACCTTTTAAAACTC-3’ , 2.5 pmoles each, were labeled with γ33P ATP in a 20 ml reaction using T4 Polynucleotide Kinase (New England Biolabs) according to manufacturer's instructions. Kinase was inactivated and primers were annealed by heating to 95°C for 5 minutes then allowing the reaction to cool to room temperature.
Purified GST-NHR6 protein, wild-type and mutant, was incubated in EMSA Buffer (10 mM Tris pH 7.5, 50 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 4% glycerol) containing 50 mg/ml poly dI/dC, 50 ng/ml ssDNA, and 0.25 pmoles of labeled annealed primers in a 10 μl reaction at room temperature for 5 minutes. 12.5 pmoles of unlabeled annealed primers (above) or annealed mutant primers (5’-GAGTTTTACCAGGTCATGCTCAATTT-3’ and 5’-AAATTGAGCATGACCTGGTAAAACTC-3’) were added as competitor in some reactions. Samples were then electrophoresed on a 6% polyacrylamide gel in 0.5X TBE Buffer at 100 volts for 75 minutes. Gels were subsequently fixed in 5% methanol, 5% acetic acid, dried and exposed to film.
Transient cell transfection assays
For the 3XNBRE-luc transfections, low passage HEK293 cells were seeded in 24 well plates and cultured in routine growth media for 24 hours or until cells reached approximately 60–70% confluency. Before transfection, old growth media was replaced with fresh routine growth media containing no antibiotics and 1% FBS. Transfection of plasmid constructs was performed using Lipofectamine LXT (Invitrogen). Each well received 100ng of experimental DNA, 100ng NBRE reporter (a gift from Thomas Perlmann), and 1 μl of Lipofectamine LXT (Invitrogen) diluted into 100ul of 1x DMEM. Each well also received 3ng of pRL-CMV vector (Renilla luciferase vector) (Promega) to serve as a control for transfection efficiency. Cells were incubated in normal conditions for 24 hours. As a positive control, cells were transfected with a pCMV-NR4A3 expression plasmid (OriGene). As a negative control, cells were transfected with the empty pCMV vector. Luciferase activity was determined using the Dual Glo Luciferase Assay System (Promega). All luciferase values were normalized to Renilla luciferase values.
For the 2XNBRE and 4XNBRE Gaussia luciferase reporter transfections, HEK293 cells at 80% confluency were transfected with 100ng experimental DNA, 100ng NBRE reporter, and 20ng pCMV-lacZ. Cells were harvested the next day and assayed for luciferase using Luciferase Assay Reagent (Promega). β-galactosidase was assayed using the β-galactosidase Assay Reagent (Tropix).
For Western analysis of transfected cells, cell pellets were lysed with 150 μl of cold RIPA buffer (Sigma) and incubated on ice for 20 minutes. Blots were incubated for 18 hours at 4°C with 10μl of primary chicken polyclonal NHR-6 antibody or monoclonal α-tubulin antibody diluted in 10ml of blocking buffer. Membranes were washed three times in TBS-T (0.1% Tween 20) and incubated at room temperature in 10μl of alkaline phosphate-linked secondary antibody diluted in 10ml of blocking buffer for three hours. Blots were washed 3 times with TBS-T buffer. The color reaction was observed by incubating the membrane with 5 ml of Western Blue Substrate (Promega).
Generation and analysis of nematode transgenics
C. elegans was cultured at 20°C under standard conditions (Brenner, 1974). Generation of transgenic nematodes was performed by microinjection as described (Mello and Fire, 1995). Constructs (either 5ng/μl or 1ng/μl) were co-injected with 100 ng/μl of pRF4 (rol-6 (su1006)) dominant mutation) as a transformation marker. Injections were performed on young, gravid IP1001 (nhr-6(lg6001)/qC1 dpy-19(e1259) glp-1(q339) hermaphrodites. Stable transgenic lines were isolated in both nhr-6(lg6001)/qC1 dpy-19(e1259) glp-1(q339) heterozygous and nhr-6(lg6001) homozygous backgrounds (qC1 dpy-19(e1259) glp-1(q339) homozygotes are sterile). nhr-6(lg6001) homozygotes bearing transgenic arrays were recovered by segregation from heterozygous transgenic lines. Initial injections were performed with 5ng/μl of pLG0097. Four lines (two in heterozygous backgrounds and two in homozygous backgrounds) were recovered. The two nhr-6(lg6001) homozygous lines bearing the transgenic arrays sgEx13 and sgEx14 were rescued for brood size (236±27 and 172±63, respectively, n=6). The other two lines failed to transmit the transgenic array in subsequent generations indicating dominant negative effects of the transgenic array in these lines. This has been previously observed for transgenics bearing other nhr-6 coding sequences (Gissendanner et al., 2008). Therefore, all subsequent injections were performed with 1ng/μL of wild-type and mutant constructs. From the wild-type construct injections, five lines were isolated (bearing arrays sgEx15-19). sgEx15-18 bearing lines expressed GFP and rescued nhr-6(lg6001) homozygotes (Fig. 2). The line bearing sgEx19 did not express GFP and did not rescue. Given the nature of transgenic array formation in C. elegans (Mello and Fire, 1995) the sgEx19 array likely did not incorporate the pLG0097 plasmid due to the low concentration of DNA injected. For the pLG0097 (C288S) construct, four transgenic lines bearing the arrays sgEx20-23 were recovered. Lines bearing the array sgEx21 did not express GFP. None of the four arrays rescued nhr-6(lg6001) homozygotes (Fig. 4). Brood size and other phenotypic assessments (abnormal egg morphology and spermatheca morphogenesis) of individual animals were performed as previously described (Gissendanner et al., 2008). Statistical analyses were performed using two-tailed t-tests assuming unequal variance.
Supplementary Material
Acknowledgments
We thank Tim Lindblom and Elias Fernandez for critical reading of the manuscript and Kris Kelley, Riddhi Bhatt, and Jianping Xiao for technical assistance. The project described was supported by Grant Number P20RR016456 from the National Center for Research Resources.
References
- Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, Thummel CS, Willson TM, Mangelsdorf DJ. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell. 2003;113:731–742. doi: 10.1016/s0092-8674(03)00420-3. [DOI] [PubMed] [Google Scholar]
- Bonta PI, Pols TW, de Vries CJ. NR4A nuclear receptors in atherosclerosis and vein-graft disease. Trends Cardiovasc Med. 2007;17:105–111. doi: 10.1016/j.tcm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DS, Arvidsson M, Bondesson Bolin M, Perlmann T. Activity of the Nurr1 carboxyl-terminal domain depends on cell type and integrity of the activation function 2. J Biol Chem. 1999;274:37483–37490. doi: 10.1074/jbc.274.52.37483. [DOI] [PubMed] [Google Scholar]
- Castro DS, Hermanson E, Joseph B, Wallen A, Aarnisalo P, Heller A, Perlmann T. Induction of cell cycle arrest and morphological differentiation by Nurr1 and retinoids in dopamine MN9D cells. J Biol Chem. 2001;276:43277–43284. doi: 10.1074/jbc.M107013200. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Gissendanner CR, Kelley K, Nguyen TQ, Hoener MC, Sluder AE, Maina CV. The Caenorhabditis elegans NR4A nuclear receptor is required for spermatheca morphogenesis. Dev Biol. 2008;313:767–786. doi: 10.1016/j.ydbio.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs FM, van der Linden AJ, Wang Y, von Oerthel L, Sul HS, Burbach JP, Smidt MP. Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons. Development. 2009;136:2363–2373. doi: 10.1242/dev.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Chung AC, Xu X, DeMayo FJ, Cooney AJ. The embryonic function of germ cell nuclear factor is dependent on the DNA binding domain. J Biol Chem. 2002;277:50660–50667. doi: 10.1074/jbc.M209586200. [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Martorell L, Rodriguez C, Calvayrac O, Gentile M, Badimon L, Martinez-Gonzalez J. Vascular effects of thrombin: involvement of NOR-1 in thrombin-induced mitogenic stimulus in vascular cells. Front Biosci. 2008;13:2909–2915. doi: 10.2741/2895. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. San Diego, CA: Academic Press; 1995. pp. 451–482. [Google Scholar]
- Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Severne Y, Wieland S, Schaffner W, Rusconi S. Metal binding 'finger' structures in the glucocorticoid receptor defined by site-directed mutagenesis. Embo J. 1988;7:2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278:24776–24790. doi: 10.1074/jbc.M300088200. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992;256:107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.