The identification and study of autoimmune diseases has taught us that recognition of self-antigens can have devastating consequences. Yet there is a paradox to autoreactivity: when correctly balanced, it is at the heart of robust self-tolerance. This concept gives rise to several questions. Can this balance be manipulated? And if so, by what means and through which mechanisms? What are the rules that govern this opportunity to restore homeostasis?
Studies in a variety of animal models, which act as replicas of the major chronic inflammatory diseases that affect humans, have offered many answers to these questions. One of the clearer outcomes is that delivery of autoantigens, administered at different disease stages via a variety of routes, can provide robust, sustained health and protection from inflammatory autoimmune disease. The most appealing element to this approach, termed antigen-specific immunotherapy (ASI), has been that it not only provides an effective means of controlling the autoimmune response via induction or restoration of β-cell–specific tolerance, but that it may achieve these goals without major concerns over safety and certainly without the specter of immune suppression. Yet significant questions remain. Are we doing enough to realize the potential of this sacred cow? How do we move from concept to reality?
In this article, we provide an update on the mechanisms through which ASI is currently thought to operate. We discuss why, despite this body of knowledge, alternative, non-ASI approaches have emerged as the current vogue. We argue that more should be done to counter this trend and realize the potential of ASI, including strategies that combine its strengths with those of other complementary ways forward.
Mechanisms of therapeutic effect including evidence from human trials.
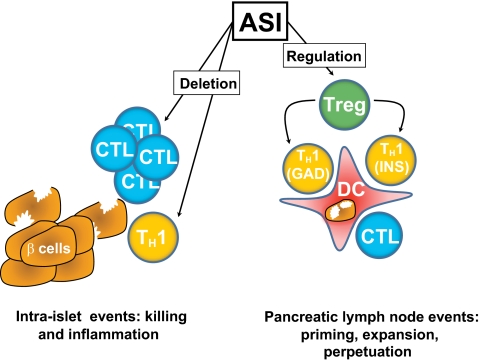
Predicting the outcome of immunization with islet antigens is complex. The resulting immune response depends not only on the dose, frequency, and route of administration but also on the precise context, in which the use of suitable adjuvants and inflammation can profoundly influence the resulting immune response or lack thereof. In addition, one should expect interindividual variations in the autoreactive repertoire of T-cells: some islet-reactive T-cells might already be activated at the time of immunization and their avidities can be expected to vary depending on central (thymic) and peripheral tuning events, which in turn will influence the character (magnitude and cytokine production) of the resulting antigen-specific response. The underlying mechanisms are more apparent in murine studies, but evidence is also beginning to emerge from human studies in vivo, both in autoimmunity and comparable inflammatory settings. The two main outcomes involve the induction or augmentation of beneficial (regulatory) immune responses (1–3) and the elimination (deletion) of deleterious islet–specific effector responses (4–6), both of which are context-dependent and will be discussed below (Table 1). It is self-evident that both outcomes could be beneficial in type 1 diabetes (Fig. 1) where there may be both a regulatory T-cell (Treg) defect and effector cells that are relatively resistant to regulation (7).
TABLE 1.
Mechanisms of action of antigen-specific immunotherapy and predicted nature of response
| Mechanism | Predicted outcomes | Tolerance induction | Durability |
|---|---|---|---|
| Immune regulation induced against β-cell antigen (typically associated with aTregs, IL-10, TGF-β induction) | Responses should be detectable (e.g., by cytokine production or functional read-out) | Operational tolerance should be achieved | Responses durable in the range of months up to 1 year |
| Should offer benefit of linked suppression of response to other β-cell antigens | |||
| Immune deviation associated with change of dominant cellular phenotype (e.g., from TH1 to TH2) | Responses should be detectable (e.g., by cytokine production or functional read-out) | Operational tolerance should be achieved | Responses durable in the range of months |
| May offer benefit of linked suppression | |||
| Immune deletion of β-cell antigen-specific T-cells | Difficult to detect deletion | Operational tolerance not guaranteed | Transient (weeks/months) |
| No benefit of linked suppression |
FIG. 1.
Beneficial effects of antigen-specific immunotherapy (ASI) on the pathological immune responses that result in type 1 diabetes. β-cell damage is a result of the combined actions of proinflammatory helper T cells (TH1) and cytotoxic T lymphocytes (CTL), which are primed against islet autoantigens such as GAD65 (GAD) and preproinsulin (INS) by inflammatory dendritic cells (DCs). ASI has two predominant beneficial effects, namely deletion of T-cells and induction of regulation, either via priming of regulatory T-cells (Tregs) or immune deviation. The major benefit of Treg induction is linked suppression, the process by which Tregs induced to regulate in response to one autoantigen (e.g., GAD) can also regulate responses to other autoantigens (e.g., INS) presented by the same DC.
Studies in mice have clearly documented the enhancement of Tregs and the skewing of cytokine responses (immune deviation) after mucosal (oral, nasal) or peripheral (peptide with and without adjuvant, DNA vaccination) insulin, proinsulin, or other autoantigenic peptide administration (8,9). In these experiments, repeated administration of β-cell autoantigens, most notably insulin or its peptides, led to increased interleukin (IL)-4, IL-10, and transforming growth factor (TGF)-β production by insulin-specific polyclonal T-cell populations isolated from the spleen, pancreatic lymph nodes, and, in some cases, the islets. Unfortunately the numbers of autoreactive T-cells in the blood are low and, therefore, little information is available about their frequency and function in peripheral blood, which would be very helpful to guide human biomarker efforts. The antigen-induced or enhanced cell populations can actively suppress effector immune responses as evidenced by adoptive transfer studies. Unless they are enabled by certain chemokines or chemokine receptors or integrins to home to solid organs, the antigen-induced modulating cells are usually found in lymph nodes and spleens following transfer into pre-diabetic recipient mice. Thus, antigenic immunization can endow islet-reactive T-cells with the ability to regulate and suppress deleterious effector responses, which entitles them to be called adaptive regulatory T-cells (aTregs). In some cases, the transcription factor forkhead box P3 (FoxP3), a good marker for naturally occurring Tregs (nTregs), shows increased expression. FoxP3 is of value as a bona fide Treg marker in the mouse, but its utility in humans is much more limited because it is also expressed on recently activated effector T-cells. Thus, successful ASIs that prevent type 1 diabetes in animal models are associated with the induction of cytokines, which can be considered as protective from type 1 diabetes (immune deviation) and are produced by CD4+ T-cells that can function as aTregs. In this context, the difference between immune deviation and Treg induction is mainly a semantic argument. The control of effector responses of various specificities (bystander suppression) likely occurs through modulation of antigen-presenting cells (APCs) resulting in a lack of anti-islet effector T-cell expansion, but not their deletion. New alternative models have also been described recently in which APCs are perhaps incapacitated from their role of antigen presentation to autoreactive effector T-cells through a direct APC-lytic process mediated by Tregs both in vitro in man and in vivo in murine studies (10,11).
Similarly, immune deviation indicative of Treg generation has been documented in humans (for example, the induction of a proinsulin peptide–specific IL-10 response after low dose intradermal administration of the peptide in type 1 diabetic patients) (12), reminiscent of observations in studies of peptide immunotherapy in clinical allergy (13,14). Other examples are the significant increases in GAD-specific γ-interferon, IL-5,-13,-10,-17,-6, tumor necrosis factor-α, FoxP3, and TGF-β mRNA responses as well as autoantibody induction (15) following subcutaneous administration of 20 μg of recombinant human GAD65 adsorbed onto alum (in a classic prime-boost regimen, n = 35/group). In other clinical trials where the limited effects of ASI have been documented, for example after daily dosing of oral insulin (16) or after a proinsulin-expressing DNA vaccine (17), no such clear effects as of yet have been documented and will have to await future biomarker studies.
The strong clinical advantage of modulating the β-cell–specific immune response and redirecting its aggressive nature to a more regulatory function is that the resulting aTregs can suppress heterologous islet–specific effector responses, and they can do so in a site-specific manner because they are predicted to become active only at sites where islet antigens are being presented. Thus, in a sense, ASI offers a site-specific immune modulatory drug. The clinical disadvantage is that antigen-specific therapies may have less potency than directly immunosuppressive strategies, as evidenced by the fact that they tend only to work prior to onset of diabetes in preclinical models. Translation to man may therefore require deployment at the early stages of pre-diabetes or enhancement with other complementary strategies (see below). Thus, optimization of dosing and delivery regimens for ASI in parallel with the development of suitable adjunct therapies needs to be considered as a major priority area (see the detailed discussion below).
The current clinical trial landscape in type 1 diabetes: a question of balance.
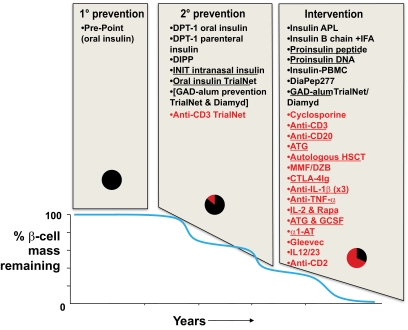
Given the many potential advantages of ASI over non-ASI discussed heretofore (safety, prospect of tolerance, site-specific regulation), it might be expected that clinical strategies based around antigens would be pursued vigorously and in many quarters. Paradoxically, however, this is far from being the case (Fig. 2). A snapshot view of major studies (as opposed to small-scale pilots) that have been completed and published, are currently in progress, or are at an advanced stage of planning indicates that at the stages of disease that are often referred to as primary and secondary prevention, ASI is indeed the dominant modality under investigation. However, there is much more clinical trial activity in the intervention arena (i.e., tertiary prevention, very close to diagnosis), and many more of the agents under evaluation are non-ASIs, especially when one considers the trials that are currently active. In general the ASI studies are low-risk and dominated by a single antigen, insulin. There is a sense that the desire to conduct studies in the prevention arena has led to trials of the very safest of drugs (e.g., injectable insulin), but these trials have not necessarily been fully optimized for efficacy.
FIG. 2.
Schematic representation of the balance of clinical trial activity in type 1 diabetes. Data are modeled onto a graphical representation of diabetes progression (adapted from reference [38]; reprinted with permission from Atkinson). Data are separated in two dimensions. First, according to stage of disease (primary prevention in the genetically at-risk before autoimmunity is apparent; secondary prevention when autoimmunity is present but no disease; and tertiary prevention or intervention when diabetes has been diagnosed but there is the opportunity to preserve C-peptide secretion); second, according to whether the therapy is antigen-specific (in black) or nonantigen-specific (in red). Underlined therapies are currently actively recruiting. The pie charts indicate the relative proportions of antigen-specific (black) and nonantigen-specific immunotherapy (red) in use at the different disease stages. DIPP, Diabetes Prediction and Prevention (39); APL, altered peptide ligand (40); ATG, anti-thymocyte globulin; CTLA-4Ig, cytotoxic T lymphocyte antigen-4 immunoglobulin; GCSF, granulocyte colony stimulating factor; HSCT, hematopoietic stem cell transplant; IFA, incomplete Freund's adjuvant; IL-1β, interleukin-1β; MMF/DZB, mycophenolate mofetil and daclizumab (41); α1-AT, α-1 antitrypsin; PBMC, peripheral blood mononuclear cell; TNF-α, tumor necrosis factor-α.
There are numerous explanations for this evident bias toward evaluation of novel non-ASI reagents at the intervention stage (Table 2).
TABLE 2.
Explanations for the paucity of antigen-specific immunotherapy studies in the intervention setting
| “Biologics” and other nonantigen-specific approaches | Antigen-specific immunotherapy | |
|---|---|---|
| Biomarkers | Facile (e.g., reduction in B-cells during anti-CD20 therapy) | Emerging but remain typically site and study-specific; lack of consensus |
| Dosing and route of administration | Clear treatment pathways from Phase I studies and/or other diseases | Often difficult and complex in ASI; issues over use of adjuvants unresolved; optimal routes remain to be determined |
| Preclinical models | Generally robust and informative | Translation not always straightforward (e.g., is the intranasal route appropriate in humans; antigen or peptide choice; timing of therapy in relation to natural history) |
| Success in other autoimmune diseases | Yes | Not yet (but whole allergen and allergen peptide immunotherapy are effective) |
| Target population | All patients with type 1 diabetes | Inclusion criteria may require staging to presence of selected autoantibodies and their titre, and to HLA type for peptides |
| Efficacy | Often effective as interventions | Intervention is a tough arena for trials with metabolic outcomes (i.e., C-peptide preservation) |
| Safety | Variable but generally predictable | Good |
| Biotechnology/Pharmaceutical involvement | High | Variable; e.g., there is still no Good Manufacturing Practice (GMP) grade proinsulin; new Intellectual Property (IP) relies upon novel modes of delivery |
Perhaps most worrying are 1) the limited involvement of the biotechnology and pharmaceutical industries in developing ASI and 2) the fact that assessing ASI at the intervention stage and expecting favorable metabolic outcomes in order that a full program of development can be progressed is a very hostile environment for these “weaker” therapies. The upshot is that this important treatment modality is not being evaluated in sufficient depth at the safest stage of disease (because the patients already have diabetes) when the acquisition of subjects is the least expensive (because screening is not required). Given that the collective ability to conduct rationally designed biomarker analyses has improved markedly in the last 10 years or so (18), it would make sense for ASI to be evaluated in the intervention setting more on the basis of its effect on biomarkers than on metabolic outcomes.
Risk analysis for antigen-specific immunotherapy.
There are three major areas of concern for the use of ASI in type 1 diabetes: acceleration of disease, leading to more rapid β-cell loss; induction of life-threatening hypersensitivity; and induction of “off-target” autoimmunity. The first two of these will need to be discussed with prospective subjects being enrolled into any prevention or intervention study that uses an antigen-based approach, whereas the third area of concern is antigen-dependent and will therefore depend upon the nature of the trial.
The picture in relation to ASI and disease acceleration in nonclinical studies is generally reassuring. For example, an extensive analysis of the literature in relation to the nonobese diabetic (NOD) model of spontaneous autoimmune diabetes (in which, for example, approximately 100 published studies since 1996 have involved injection or ingestion of whole or peptide autoantigens, either as simple solutions or in conjunction with powerful adjuvants) has revealed that it is extremely unusual to accelerate disease; in most cases the maneuvers are protective or have no effect. In one reported study, disease was accelerated. In that case, two peptides of the β-cell autoantigen GAD65 were administered intrathymically and caused a mild acceleration of diabetes onset in NOD mice (19). The authors pointed out that immunization with whole GAD65 does not induce CD4 T-cells reactive with either peptide used, implying that these are cryptic epitopes not naturally processed and presented by NOD mouse APCs. The route of administration may also be important in this outcome—our own studies injecting a single dose of one of these cryptic GAD65 epitopes intraperitoneally gave a strong protective effect (20). The use of naturally processed and presented epitopes or whole antigens may be an important safeguard against the danger of priming additional T-cells with cryptic peptides. Other examples of disease exacerbation by autoantigen administration in models of autoimmune demyelination (21) and autoimmune diabetes (22) have also been reported, but these appear to be rare occurrences. In clinical studies, the experience is obviously much less extensive. It is notable that in the study of oral insulin administration to first-degree relatives with insulin autoantibodies, the subgroup analysis of those who did not have confirmed insulin autoantibodies ≥80 nU/ml suggested a trend toward a detrimental effect of the treatment (16), reminding us that we may need to develop carefully argued selection algorithms for trial entry. The examples of disease exacerbation in multiple sclerosis (MS) using altered peptide ligands of the autoantigen myelin basic protein is yet another strong argument for using native peptide sequences or whole antigens (23,24). Apart from these examples, there has been no evidence of disease exacerbation from ASI trials in rheumatoid arthritis (25,26) or more recent studies of MS patients using native sequence peptides (27).
A second safety consideration raised by published nonclinical studies is the risk that antigen injection can result in hypersensitivity, systemic allergy, and anaphylaxis. The most relevant report here was of fatal anaphylaxis in NOD mice after repeated injections of an immunodominant peptide of insulin (residues B9–23). However, relatively large quantities (total >1 mg) and repeated (seven times) dosing of peptide were used in these studies (28). These responses may have been idiosyncratic to this strain and were alleviated by the alteration of the peptide's isoelectric point (29). Such responses have not been seen to date in the setting of autoimmune disease in man (apart from the altered peptide ligand studies in MS discussed above, in which hypersensitivity responses were also observed). It may be that the induction of T helper 2 (TH2)-like responses requires the achievement of a fine balance between those that may be beneficial (see the comments on immune deviation above) and those that are dangerous.
The final safety issue relates to the induction de novo of an autoimmune process, for example, when an ASI study uses a β-cell autoantigen that is expressed in other tissues as in the case of GAD65, which is expressed in the peripheral and central nervous systems. To date, the nonclinical and clinical experiences suggest that such a complication remains only a theoretical risk; notably there was no induction of neurological disease despite administration of GAD65 with adjuvant in a regime that boosted GAD-specific autoantibody titers to levels more typically seen in patients with stiff-man syndrome (15).
Synthesizing these comments, there are theoretical risks of ASI and for some of these risks, there are nonclinical studies to indicate that theory can become reality; but this is the exception rather than the rule, and clinical studies have proved extremely safe.
Optimizing antigen-specific immunotherapy for the clinic.
Based on preclinical models, several factors are emerging as critical in determining the outcome of antigen-specific immunizations, most notably dose, route, adjuvant, and frequency of administration. Studies have shown that too frequent antigen administrations, as well as very high dosages, do not result in optimal induction of immune regulation and tolerance. In addition, there is no evidence that there is such a thing as “regulatory memory,” and in most prevention studies so far, repeated administration of the antigen has been required. Exceptions arise, for example when certain adjuvants such as alum or incomplete Freund's adjuvant are used. Here, at least in animal models, a one-time administration of antigen with adjuvant is sufficient to prevent diabetes. In these cases, rather than augmentation of nTregs or de novo induction of iTregs or aTregs, an immune deviation to TH2 and induction of TH2 memory cells that produce IL-5, -4, and -13 might have occurred. This in itself might be very beneficial and the desired outcome of antigen-specific immunization in type 1 diabetes. We might not need to induce bona fide Tregs after all (i.e., the FoxP3+CD127lowCD25high type), but a mere TH2 deviation could be sufficient. IL-4 is exquisitely protective in various animal models for type 1 diabetes and has a large therapeutic range, especially when delivered locally to the pancreatic islets or lymph nodes via β-cell antigen–specific Tregs (30). Thus, choosing the correct adjuvant (i.e., a TH2-deviating compound) might be the key to antigenic immunization and long-term tolerance in type 1 diabetes (15). Tolerance-inducing adjuvants are not an area of large-scale research and are probably not in the development pipelines of many pharmaceutical companies.
In addition, several other factors need to be resolved. The most pressing issue is the precise dosing regimen. From the studies in various animal models, it is known that too high dosages might not be effective by leading to the deletion of Tregs rather than their augmentation. In murine models, for example, only oral insulin dosages between 0.2 and 2 mg are effective when given twice per week by oral gavage (31). Higher and lower dosages have no strong effect on preventing diabetes, therefore there is a strong need to translate dose regimens used in these animal models to humans as accurately as possible. This has not been achieved to date, at least in part because there is no fully validated formula. However, the currently utilized 7.5-mg dose in the oral insulin study of Diabetes TrialNet, which mirrors that used in the Diabetes Prevention Trial (DPT)-1 (16), is most likely too low comparatively and, based on animal models, less frequent dosing with a higher dose should greatly increase efficacy. This factor could also explain the lack of efficacy in the Finnish nasal insulin diabetes prevention trial (39).
New strategies and creative approaches will be required to more rationally and rapidly translate from mouse studies to human trials, as we will discuss in the next section.
Emerging strategies for antigen-specific immunotherapy: implementation and bold steps.
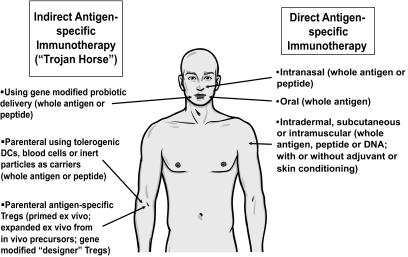
The future is not all bleak: several new strategies are emerging that may well achieve success in enhancing the delivery and potency of ASI and when assembled together, the ASI portfolio offers a number of appealing options (Fig. 3). These options include strategies for the delivery of multiple epitopes from multiple antigens to mirror the approach that is proving successful in clinical allergy; the use of steroid hormone adjuvants (glucocorticoids and vitamin D) to modulate APCs presenting autoantigens both in vitro for adoptive transfer (32,33) and in vivo to enhance tolerance induction in the skin; new methods for the delivery of antigens to the gut using Lactococcus lactis gene modified to deliver islet autoantigens and cytokines; using soluble T-cell receptors specific for islet peptides (for details, see http://naimit.eu/); and antigens coupled to inert cells. As discussed above, these approaches tend to center on novel modes of antigen delivery, perhaps partially as a means to generate funding interest or intellectual property and thus to sustain the effort. Additional strategies will also be useful if conducted in parallel; notable among these strategies is the emerging interest in analyzing immune responses, tolerance, and ASI through investigation of disease models generated in silico (34).
FIG. 3.
Approaches currently under evaluation for delivery of antigen-specific immunotherapy. DCs, dendritic cells.
Notwithstanding these fertile new areas, there is a sense that the progress made in getting antigens into the clinic has stalled and requires renewed invigoration. There is nothing intrinsically flawed about oral or nasal antigen administration or antigen injection. They have simply not been fully evaluated in a staged developmental program. As discussed above, much remains to be understood about dose, regimen, and route. We would advocate a return to these questions, addressed in the context of small clinical studies with the emphasis on mechanistic outcomes. A second line approach will be the development of suitable combinations of antigens with immune modulators that have been specifically selected to foster Treg function and expansion while reducing the effector cell load. Such an initiative could be very beneficial in overcoming some of these issues and, most importantly, enabling antigen-specific Treg induction to be effective later during the disease process, for example in individuals at high risk of developing type 1 diabetes or in recently diagnosed patients (35). Animal studies using a combination of anti-CD3 and nasal proinsulin peptide strongly support this concept (36).
As a final comment, one advance that could facilitate rapid advances in these key areas relates to trial design. Perhaps, for example, the emphasis in relation to outcomes should shift away from metabolic recovery toward an intense focus on immunological biomarkers. This could be done in the context of small optimization trials. These trials, in turn, would be considerably better informed if another knowledge gap—namely the natural history of such immunological biomarkers in the 1- to 2-year period after diagnosis—were adequately mapped through longitudinal studies. Going forward, these efforts should enable the type 1 diabetes community to make informed decisions about the merits of ASI and hopefully realize its potential for “negotiating” with the immune system (37).
ACKNOWLEDGMENTS
M.P. acknowledges financial support from the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy's and St Thomas' National Health Service Foundation Trust in partnership with King's College London from the Juvenile Diabetes Research Foundation and via the European Union's Seventh Framework Programme (FP7) Large-scale Focused Collaborative Research Project on Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes. M.v.H. is supported by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the American Diabetes Association, and the Brehm Coalition.
No potential conflicts of interest relevant to this article were reported.
The authors thank Ezio Bonifacio, Centre for Regenerative Therapies, Dresden and Damien Bresson, and the La Jolla Institute for Allergy and Immunology, for useful discussions.
REFERENCES
- 1.Petersen JS, Bregenholt S, Apostolopolous V, Homann D, Wolfe T, Hughes A, De Jongh K, Wang M, Dyrberg T, Von Herrath MG. Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes. Clin Exp Immunol 2003;134:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 2008;29:464–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol 1999;11:1625–1634 [DOI] [PubMed] [Google Scholar]
- 4.Mori F, Bianchi L, Pucci N, Azzari C, De Martino M, Novembre E. CD4+CD25+Foxp3+ T regulatory cells are not involved in oral desensitization. Int J Immunopathol Pharmacol 2010;23:359–361 [DOI] [PubMed] [Google Scholar]
- 5.Falb D, Briner TJ, Sunshine GH, Bourque CR, Luqman M, Gefter ML, Kamradt T. Peripheral tolerance in T cell receptor-transgenic mice: evidence for T cell anergy. Eur J Immunol 1996;26:130–135 [DOI] [PubMed] [Google Scholar]
- 6.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1994;1:327–339 [DOI] [PubMed] [Google Scholar]
- 7.Lawson JM, Tremble J, Dayan C, Beyan H, Leslie RD, Peakman M, Tree TI. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin Exp Immunol 2008;154:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, Arita S, Evans C, Atkinson MA, Mullen Y, Sarvetnick N, Tobin AJ, Lehmann PV, Kaufman DL. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med 1996;2:1348–1353 [DOI] [PubMed] [Google Scholar]
- 9.Tisch R, Wang B, Serreze DV. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol 1999;163:1178–1187 [PubMed] [Google Scholar]
- 10.Tree TIM, Lawson J, Edwards H, Skowera A, Arif S, Roep BO, Peakman M. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes 2010;59:1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity 2010;32:266–278 [DOI] [PubMed] [Google Scholar]
- 12.Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, Van-Krinks C, Lozanoska-Ochser B, Marquesini L, Brown S, Wong FS, Dayan CM, Peakman M. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol 2009;155:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Grönlund H, van Hage M, Reynolds CJ, Boyton RJ, Cobbold SP, Kay AB, Altmann DM, Lloyd CM, Larché M. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med 2009;206:1535–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med 2005;2:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 16.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 17.Solvason N, Lou YP, Peters W, Evans E, Martinez J, Ramirez U, Ocampo A, Yun R, Ahmad S, Liu E, Yu L, Eisenbarth G, Leviten M, Steinman L, Garren H. Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J Immunol 2008;181:8298–8307 [DOI] [PubMed] [Google Scholar]
- 18.Roep BO, Peakman M. Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol 2010;10:145–152 [DOI] [PubMed] [Google Scholar]
- 19.Cetkovic-Cvrlje M, Gerling IC, Muir A, Atkinson MA, Elliott JF, Leiter EH. Retardation or acceleration of diabetes in NOD/Lt mice mediated by intrathymic administration of candidate beta-cell antigens. Diabetes 1997;46:1975–1982 [DOI] [PubMed] [Google Scholar]
- 20.Xu XJ, Gearon C, Stevens E, Vergani D, Baum H, Peakman M. Spontaneous T-cell proliferation in the non-obese diabetic mouse to a peptide from the unique class II MHC molecule, I-Ag7, which is also protective against the development of autoimmune diabetes. Diabetologia 1999;42:560–565 [DOI] [PubMed] [Google Scholar]
- 21.Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science 1996;274:2054–2057 [DOI] [PubMed] [Google Scholar]
- 22.Bellmann K, Kolb H, Rastegar S, Jee P, Scott FW. Potential risk of oral insulin with adjuvant for the prevention of type I diabetes: a protocol effective in NOD mice may exacerbate disease in BB rats. Diabetologia 1998;41:844–847 [DOI] [PubMed] [Google Scholar]
- 23.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000;6:1167–1175 [DOI] [PubMed] [Google Scholar]
- 24.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial: the Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 2000;6:1176–1182 [DOI] [PubMed] [Google Scholar]
- 25.Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, de Kleer I, Bonnin D, Lanza P, Berry C, Massa M, Billetta R, Albani S. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A 2004;101:4228–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL, Kavanaugh A, Molitor JA, Schiff MH, Posever JO, Bathon JM, Kivitz AJ, Samodal R, Belardi F, Dennehey C, van den Broek T, van Wijk F, Zhang X, Zieseniss P, Le T, Prakken BA, Cutter GC, Albani S. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum 2009;60:3207–3216 [DOI] [PubMed] [Google Scholar]
- 27.Streeter HB, Pillai S, Scolding NJ, Wraith D. ATX-MS1467 a therapeutic peptide vaccine for treatment of multiple sclerosis (Abstract). Mult Scler 2008;14:S185 [Google Scholar]
- 28.Liu E, Moriyama H, Abiru N, Miao D, Yu L, Taylor RM, Finkelman FD, Eisenbarth GS. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9–23 and B:13–23. J Clin Invest 2002;110:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu E, Moriyama H, Abiru N, Paronen J, Devendra D, Finkelman FD, Eisenbarth GS. Preventing peptide-induced anaphylaxis: addition of C-terminal amino acids to produce a neutral isoelectric point. J Allergy Clin Immunol 2004;114:607–613 [DOI] [PubMed] [Google Scholar]
- 30.King C, Mueller Hoenger R, Malo Cleary M, Murali-Krishna K, Ahmed R, King E, Sarvetnick N. Interleukin-4 acts at the locus of the antigen-presenting dendritic cell to counter-regulate cytotoxic CD8+ T-cell responses. Nat Med 2001;7:206–214 [DOI] [PubMed] [Google Scholar]
- 31.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007;148:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haase C, Yu L, Eisenbarth G, Markholst H. Antigen-dependent immunotherapy of non-obese diabetic mice with immature dendritic cells. Clin Exp Immunol 2010;160:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin-Gallen S, Clemente-Casares X, Planas R, Pujol-Autonell I, Carrascal J, Carrillo J, Ampudia R, Verdaguer J, Pujol-Borrell R, Borras FE, Vives-Pi M. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol 2010;160:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoda LK, Kreuwel HT, Gadkar KG, Zheng Y, Whiting CC, Atkinson MA, Bluestone J, Mathis D, Young DL, Ramanujan S. The Type 1 Diabetes PhysioLab Platform: a validated physiologically based mathematical model of pathogenesis in the non-obese diabetic mouse. Clin Exp Immunol 2010;161:250–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews JB, Staeva T, Bernstein P, Peakman M, Von Herrath M. Developing combination immunotherapies for type 1 diabetes: recommendations from the Immune Tolerance Network–Juvenile Diabetes Research Foundation Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 2010;160:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 2006;116:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gale EA. Can we change the course of beta-cell destruction in type 1 diabetes? N Engl J Med 2002;346:1740–1742 [DOI] [PubMed] [Google Scholar]
- 38.Atkinson MA. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can't we prevent or reverse this disease? Diabetes 2005;54:1253–1263 [DOI] [PubMed] [Google Scholar]
- 39.Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 40.Alleva DG, Maki RA, Putnam AL, Robinson JM, Kipnes MS, Dandona P, Marks JB, Simmons DL, Greenbaum CJ, Jimenez RG, Conlon PJ, Gottlieb PA. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol 2006;63:59–69 [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, Schatz DA, Moran AM, Lachin JM, Skyler JSType 1 Diabetes TrialNet MMF/DZB Study Group Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 2010;33:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]