Abstract
OBJECTIVE
Type 2 diabetes and insulin resistance have been associated with mitochondrial dysfunction, but it is debated whether this is a primary factor in the pathogenesis of the disease. To test the concept that mitochondrial dysfunction is secondary to the development of insulin resistance, we employed the unique model of prolonged fasting in humans. Prolonged fasting is a physiologic condition in which muscular insulin resistance develops in the presence of increased free fatty acid (FFA) levels, increased fat oxidation and low glucose and insulin levels. It is therefore anticipated that skeletal muscle mitochondrial function is maintained to accommodate increased fat oxidation unless factors secondary to insulin resistance exert negative effects on mitochondrial function.
RESEARCH DESIGN AND METHODS
While in a respiration chamber, twelve healthy males were subjected to a 60 h fast and a 60 h normal fed condition in a randomized crossover design. Afterward, insulin sensitivity was assessed using a hyperinsulinemic-euglycemic clamp, and mitochondrial function was quantified ex vivo in permeabilized muscle fibers using high-resolution respirometry.
RESULTS
Indeed, FFA levels were increased approximately ninefold after 60 h of fasting in healthy male subjects, leading to elevated intramuscular lipid levels and decreased muscular insulin sensitivity. Despite an increase in whole-body fat oxidation, we observed an overall reduction in both coupled state 3 respiration and maximally uncoupled respiration in permeabilized skeletal muscle fibers, which could not be explained by changes in mitochondrial density.
CONCLUSIONS
These findings confirm that the insulin-resistant state has secondary negative effects on mitochondrial function. Given the low insulin and glucose levels after prolonged fasting, hyperglycemia and insulin action per se can be excluded as underlying mechanisms, pointing toward elevated plasma FFA and/or intramuscular fat accumulation as possible causes for the observed reduction in mitochondrial capacity.
Although the existence of mitochondrial abnormalities in patients with type 2 diabetes has been extensively reported during the last decade (1–5), there is no evidence that a reduced mitochondrial function is a primary factor in the pathophysiology of this disease. In fact, alternative theories state that impaired mitochondrial capacity is secondary to the insulin-resistant or diabetic state. In this context, it has been shown that insulin can stimulate mitochondrial biogenesis and increases ATP synthesis in skeletal muscle (6,7). A reduced insulin action in skeletal muscle, as observed in type 2 diabetic patients, could therefore contribute to the origin of mitochondrial dysfunction. Additionally, the increased exposure of skeletal muscle mitochondria to elevated levels of free fatty acids (FFA), seen in insulin resistance and type 2 diabetes, has been suggested to interfere with proper mitochondrial function. Thus, Szendroedi et al. (8) showed that plasma FFA levels negatively correlated with mitochondrial function measured by magnetic resonance spectroscopy. Furthermore, we showed that the acute elevation of plasma FFA by lipid infusion is accompanied by downregulation of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1α (PGC1α) and other genes involved in mitochondrial metabolism (9). Moreover, it was shown in a comparable study that short-term elevation of lipid availability reduces insulin-stimulated increase in ATP synthase flux in skeletal muscle (10), although this may mainly reflect an effect of muscular insulin resistance on ATP flux.
Prolonged fasting (>48 h) in humans is accompanied by a reduction in insulin sensitivity, elevated plasma FFA levels, elevated intramuscular fat levels, but also an increase in whole-body fat oxidative capacity (11,12). Furthermore, prolonged fasting-induced insulin resistance is not accompanied by hyperglycemia or hyperinsulinemia, factors that have been suggested to cause mitochondrial dysfunction in diabetes (7,13). In fact, prolonged fasting is a physiologic condition in which insulin resistance develops to spare glucose for utilization by the brain, and increased FFA levels are accompanied by increased fat oxidation. It could therefore be anticipated that despite the development of insulin resistance, mitochondrial function is maintained to accommodate increased fat oxidation during prolonged fasting. Alternatively, if (lipid-induced) insulin resistance or factors associated with the insulin-resistant state indeed cause mitochondrial dysfunction, we anticipate a reduction in mitochondrial function with prolonged fasting. Therefore, we aim to test the concept that mitochondrial dysfunction originates secondary to the development of insulin resistance by employing the physiologic model of prolonged fasting-induced insulin resistance.
RESEARCH DESIGN AND METHODS
Twelve healthy, lean, male volunteers who had no family history of diabetes or any other endocrine disorder participated in this study (Table 1). None of the subjects engaged in sports activities for more than 2 h per week. Body composition (14) and maximal aerobic capacity (15) were measured as described previously. The study protocol was reviewed and approved by the Medical Ethical Committee of Maastricht University Medical Centre and all subjects gave their written informed consent before participating in the study.
TABLE 1.
Subject characteristics
| Parameter | Mean ± SE |
|---|---|
| Age (years) | 23.6 ± 1.0 |
| Body weight (kg) | 78.5 ± 2.5 |
| Fat-free mass (kg) | 65.9 ± 1.8 |
| Height (m) | 1.86 ± 0.02 |
| BMI (kg/m2) | 22.6 ± 0.5 |
| Maximal aerobic capacity (ml O2/kgFFM/min) | 57.5 ± 1.5 |
Experimental design.
Subjects participated in two experimental trials: a 60 h fast and a 60 h normal fed condition, in a randomized crossover design with a 2-week washout period. In the fast condition, subjects were fasted for 60 h (calorie-free drinks only), whereas in the second condition, subjects were fed in energy balance (50–35–15% of energy as carbohydrates, fat, and protein, respectively). Before the start of each experimental period, a standardized evening meal was provided. Subject stayed in a respiration chamber during the entire 60 h to ensure compliance to the dietary regime and to allow the measurement of 24 h substrate oxidation and energy expenditure (16). In the respiration chamber, subjects followed an activity protocol as previously described (17). During the intervention, blood samples were taken after 12, 36, and 60 h after an overnight fast in case of the fed condition.
Hyperinsulinemic-euglycemic clamp.
After leaving the respiration chamber on the morning of the third day, a muscle biopsy was taken (18) and a hyperinsulinemic-euglycemic clamp procedure (4) was performed. Insulin-stimulated plasma glucose rate of disappearance (Rd), endogenous glucose production (EGP), and nonoxidative glucose disposal (mainly reflecting glycogen synthesis) were calculated as by Phielix et al. (4). Substrate oxidation in the basal and the insulin-stimulated state was measured using indirect calorimetry (Omnical, Maastricht, the Netherlands) and calculated according to Frayn (19).
Muscle biopsy.
After taking the muscle biopsy, a portion of the muscle tissue was directly frozen in melting isopentane and stored at −80°C until assayed. Another portion (∼30 mg) was immediately placed in ice cold preservation medium (4).
Blood analyses.
Plasma nonesterified fatty acids (Wako Nefa C test kit; Wako Chemicals, Neuss, Germany) and glucose (hexokinase method; LaRoche, Basel, Switzerland) were measured with enzymatic assays automated on a Cobas Fara/Mira. Insulin concentration was determined using a radioimmunoassay (Linco Reseach, St. Charles, MO).
Intramuscular triacylglycerols.
Fresh cryosections (5 μm) were stained for intramuscular triacylglycerols (IMTG) by Oil Red O staining combined with fibertyping and immunolabeling of the basal membrane marker laminin to allow quantification of IMTG, as described previously (20,21).
Mitochondrial DNA copy number and citrate synthase activity.
Mitochondrial DNA (mtDNA) copy number, the ratio of NADH dehydrogenase subunit one (ND1) to lipoprotein lipase (LPL) (mtDNA/nuclear DNA) was determined as described previously (4). Citrate synthase (CS) activity was measured spectrophotometrically as described previously (22).
High resolution respirometry.
Permeabilized skeletal muscle fibers were immediately prepared from the muscle tissue collected in the preservation medium, as described elsewhere (4,23). Subsequently, the permeabilized muscle fibers (∼2.5 mg wet weight) were analyzed for mitochondrial function using an oxygraph (OROBOROS Instruments, Innsbruck, Austria), in essence according to Phielix et al. (4). To prevent oxygen limitation, the respiration chambers were hyperoxygenated up to ∼500 μmol/l O2. Subsequently, two different multisubstrate/inhibition protocols were used in which substrates and inhibitors were added consecutively in saturating concentrations. State 2 respiration was measured after the addition of malate (4 mmol/l) plus octanoyl-carnitine (50 μmol/l) or malate (4 mmol/l) plus glutamate (10 mmol/l). Subsequently, an excess of 2 mmol/l of ADP was added to determine coupled (state 3) respiration. Coupled respiration was then maximized with convergent electron input through Complex I and Complex II by adding saturating concentrations of succinate (10 mmol/l). Finally, the chemical uncoupler carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP) was titrated or oligomycin (2 μg/ml) was added to evaluate the maximal capacity of the electron transport chain and the respiration not coupled to ATP synthesis (state 4o respiration), respectively. The integrity of the outer mitochondrial membrane was assessed by the addition of cytochrome C (10 μmol/l) upon maximal coupled respiration. All measurements were performed in duplicate.
Western blotting.
Oxidative phosphorylation (OXPHOS) protein levels and mitochondrial uncoupling protein-3 (UCP3) content were measured in whole muscle by Western blotting as described previously (24). UCP3 was expressed as a ratio over the sum of OXPHOS complexes to correct for differences in mitochondrial density.
Statistics.
Data are reported as means ± SE. Statistical analyses were performed using the statistical computer program SPSS 16.0 for Mac OS X. Statistical comparisons between the two conditions (fed versus fast) were performed using the paired Student t test. Plasma FFA, glucose, and insulin were compared over time by two-way repeated measures ANOVA for investigation of treatment and time (treatment*time) interactions. When the interaction was significant, we performed post hoc testing to determine the exact location of the difference. Differences were considered statistically significant if P < 0.05.
RESULTS
Plasma parameters.
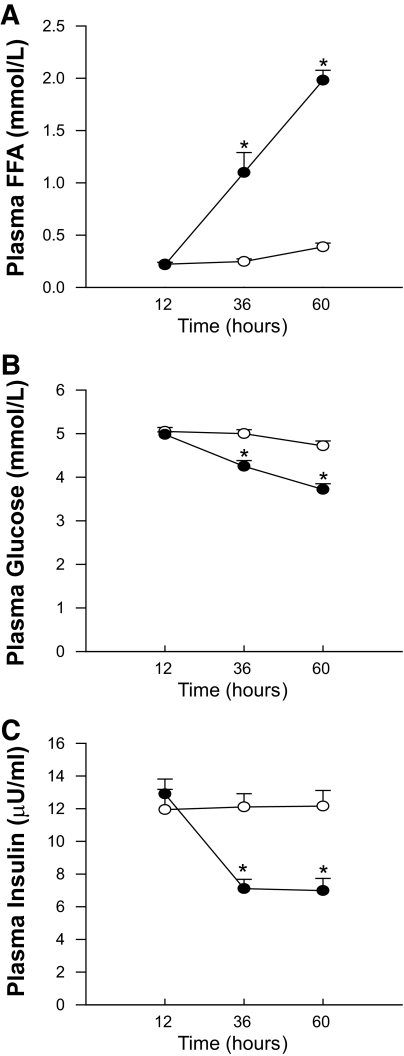
A significant treatment*time interaction (P < 0.001) was observed for all plasma parameters (Fig. 1). At the start of the intervention (t = 12 h, after an overnight fast) plasma FFA levels (Fig. 1A) were similar between both conditions (221 ± 18 vs. 215 ± 27 μmol/l, respectively, P = 0.82). Upon 60 h of fasting, plasma FFA increased dramatically, up to 1,981 ± 95 μmol/l vs. 387 ± 37 in the fed condition (P < 0.001).
FIG. 1.
Plasma free fatty acids (A), plasma glucose (B), and plasma insulin (C) levels after 12, 36, and 60 h of fasting. Open circles represent the fed condition; closed circles represent the fasted condition. Values are mean ± SE. *P < 0.05.
Plasma glucose values (Fig. 1B) were similar at baseline, averaging 5.05 ± 0.09 and 4.98 ± 0.06 mmol/l in the fed and the fasted state, respectively (P = 0.28), and remained unchanged throughout the fed condition. During fasting, however, plasma glucose levels gradually decreased to 3.72 ± 0.13 mmol/l at t = 60 h (P < 0.001).
Baseline plasma insulin levels (Fig. 1C) were similar in both conditions (12.9 ± 0.9 vs. 11.9 ± 1.2 μU/ml in fed versus fasted, respectively, P = 0.25) and did not change in the fed condition. In the fasted condition however, plasma insulin levels were markedly reduced to 7.1 ± 0.6 μU/ml at t = 36 h (P < 0.001) and were maintained at this lower level (7.0 μU/ml ± 0.74) at t = 60 h (P < 0.001).
Indirect calorimetry.
Twenty-four hour energy expenditure during the last 24 h of the 60 h intervention was slightly but significantly reduced upon prolonged fasting (10.88 ± 0.33 vs. 10.30 ± 0.30 MJ/day, in fed versus fasted, respectively, P = 0.02). The difference was mainly caused by a reduction in diet-induced thermogenesis, and not caused by a decrease in resting metabolic rate (data not shown). Additionally, whole-body 24-h fat oxidation was increased upon prolonged fasting as evidenced by a significant reduction in 24-h respiratory exchange ratio (RER) (0.91 ± 0.009 vs. 0.77 ± 0.003 in fed versus fasted, respectively, P < 0.001).
Insulin sensitivity.
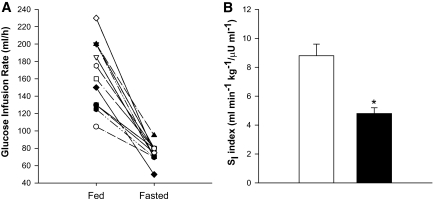
All subjects displayed a decrease in glucose infusion rate upon 60 h of fasting (Fig. 2A). We also calculated the insulin sensitivity index, an index that takes into account the variation in insulin and glucose levels during the clamp (25). Insulin sensitivity index was reduced by ∼45% upon 60 h of fasting, as compared with the fed condition (Fig. 2B, P < 0.001).
FIG. 2.
Assessment of insulin sensitivity by hyperinsulinemic-euglycemic clamping after 60 h of fasting. A represents the individual data for the glucose infusion rate. B displays the group results of the insulin sensitivity index, i.e., the glucose infusion rate corrected for body weight, glucose, and plasma insulin levels during the clamp procedure. The white bar represents the fed condition whereas the black bar depicts the fasted condition. Values are mean ± SE. *P < 0.05. SI, insulin sensitivity index.
The reduction in whole-body insulin sensitivity was mainly accounted for by a reduction in insulin-stimulated glucose disposal (ΔRd, P < 0.001, Table 2). The reduced insulin-stimulated glucose disposal after fasting, mainly reflecting muscle glucose uptake, was due to both reduced insulin-stimulation of glucose oxidation and reduced nonoxidative glucose disposal (Table 2). However, insulin-stimulated glucose oxidation seemed to be more severely suppressed by 60 h of fasting (Table 2). Also, baseline endogenous glucose production was reduced after 60 h of fasting. However, insulin-induced suppression of endogenous glucose production, reflecting hepatic insulin sensitivity, was only marginally affected by fasting and was almost complete in both conditions (Table 2).
TABLE 2.
Substrate kinetics
| Fed | Fasted | |
|---|---|---|
| Rd Glucose (μmol/kg/min) | ||
| Basal | 11.3 ± 0.6 | 7.6 ± 0.4* |
| Clamp | 38.0 ± 2.8 | 18.8 ± 0.9* |
| Delta | 26.7 ± 2.6 | 11.2 ± 1.0* |
| EGP (μmol/kg/min) | ||
| Basal | 10.7 ± 0.6 | 6.8 ± 0.3* |
| Clamp | −1.0 ± 0.4 | 0.7 ± 0.4* |
| Delta absolute | −11.7 ± 0.6 | −6.07 ± 0.5* |
| Delta % | 111.6 ± 5.9 | 90.0 ± 6.7* |
| CHO oxidation (μmol/kg/min) | ||
| Basal | 9.7 ± 0.8 | 3.4 ± 0.6* |
| Clamp | 20.8 ± 1.1 | 6.6 ± 0.8* |
| Delta | 11.1 ± 0.9 | 3.4 ± 0.7* |
| NOGD (μmol/kg/min) | ||
| Basal | 1.6 ± 0.5 | 4.2 ± 0.8* |
| Clamp | 17.2 ± 2.2 | 12.6 ± 0.8* |
| Delta | 15.6 ± 2.4 | 8.2 ± 1.1* |
| Lipid oxidation (μmol/kg/min) | ||
| Basal | 1.2 ± 0.0 | 1.8 ± 0.2* |
| Clamp | 0.3 ± 0.1 | 1.52 ± 0.1* |
| Delta | −0.8 ± 0.1 | −0.31 ± 0.1* |
Values are mean ± SE.
*P < 0.05. EGP, endogenous glucose production; CHO, carbohydrate; NOGD, nonoxidative glucose disposal.
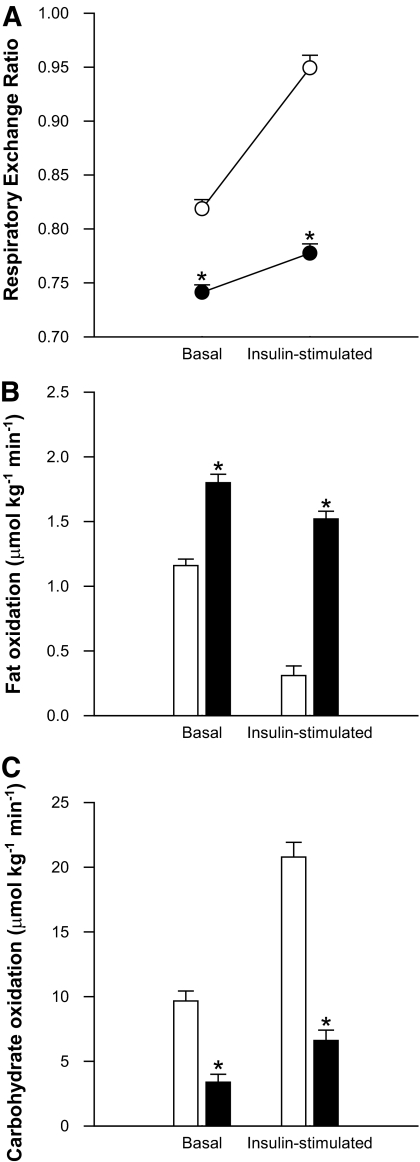
Metabolic flexibility.
Metabolic flexibility was blunted in the fasted condition when compared with the fed condition (Fig. 3A, P < 0.001). Basal whole-body fat oxidation was increased by 1.5-fold upon prolonged fasting (Fig. 3B, P < 0.001). During the glucose clamp, fat oxidation significantly decreased in both conditions (P ≤ 0.001), but the suppression was significantly less in the fasted condition (Fig. 3B, P < 0.001). Basal carbohydrate oxidation after fasting was only ∼35% of the value obtained in the fed situation (P < 0.001), but increased in both conditions during the glucose clamp (Fig. 3C). However, this insulin-induced change in carbohydrate oxidation was blunted upon fasting (P < 0.001).
FIG. 3.
Indirect calorimetry results after 60 h of fasting in the basal state and upon the hyperinsulinemic-euglycemic clamp. A shows that metabolic flexibility, defined as the change in respiratory exchange ratio upon insulin stimulation, is blunted upon prolonged fasting. B and C display whole-body lipid and carbohydrate oxidation, respectively. White bars/circles represent the fed condition; black bars/circles represent the fasted condition. Values are mean ± SE. *P < 0.05.
Intramuscular triacylglycerols.
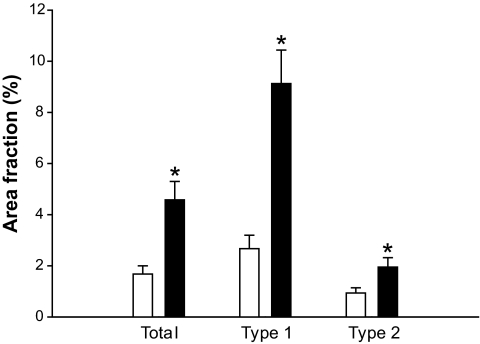
The mean intramuscular IMTG area fraction (Fig. 4), was ∼2.7-fold higher after 60 h of fasting in comparison with the fed condition (P = 0.001). The increase in lipid accumulation was more pronounced (∼3.5 fold, P < 0.001) in fibers identified as slow, oxidative (type 1) fibers. Within type 2 muscle fibers, IMTG levels increased by approximately twofold after 60 h of fasting (P = 0.015).
FIG. 4.
Intramuscular triacylglycerols measured by Oil Red O staining combined with an immunofluorescence staining against slow myosin heavy chain (sMHC), to determine fibertyping. White bars represent the fed condition; black bars represent the fasted condition. Values are mean ± SE. *P < 0.05.
Mitochondrial function.
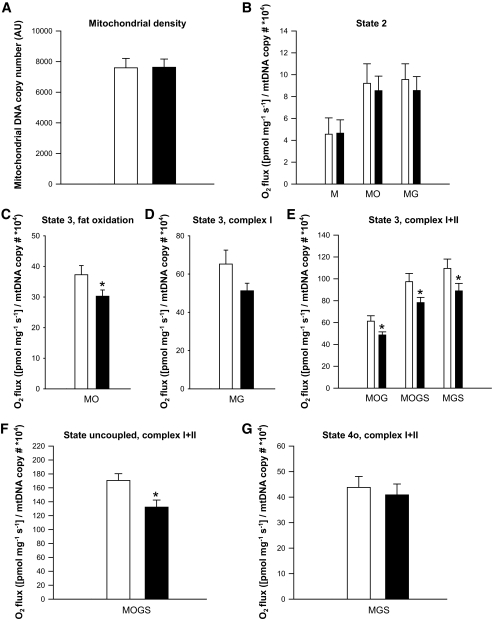
Mitochondrial DNA copy number was similar in both the fed and the fasting condition (Fig. 5A, P = 0.96). Also, OXPHOS protein levels (Complex I: 21.2 ± 6.4 vs. 17.7 ± 5.7 arbitrary units, P = 0.50; Complex II: 48.7 ± 14.6 vs. 48.3 ± 16.2 AU, P = 0.97; Complex III: 11.6 ± 1.5 vs. 11.9 ± 1.1 AU, P = 0.88; Complex IV: 92.8 ± 7.0 vs. 93.7 ± 9.1 AU, P = 0.85; Complex V: 4.4 ± 0.7 vs. 4.8 ± 0.9, P = 0.66) and CS activity (76.2 ± 7.3 vs. 70.2 ± 6.6 μmol/min/g protein, P = 0.41) were similar in the fed versus the fasted state, respectively, confirming an equal mitochondrial mass in both conditions.
FIG. 5.
Evaluation of mitochondrial function. A: Mitochondrial density. B: Mitochondrial respiration upon substrates only (state 2). C: ADP-stimulated respiration (state 3) upon a lipid substrate. D: State 3 respiration fueled by Complex I-linked substrates. E: State 3 respiration upon parallel electron input into Complex I and II. F: Maximally uncoupled respiration upon FCCP. G: Mitochondrial respiration uncoupled from ATP synthesis (state 4o). White bars represent the fed condition, black bars represent the fasted condition. Values are mean ± SE, n = 10. *P < 0.05. M, malate; O, octanoyl-carnitine; G, glutamate; S, succinate.
Nonetheless, we adjusted the oxygen fluxes for individual differences in mtDNA copy number. However, similar results were obtained without this correction (see supplemental Fig. 1 in the online appendix available at http://diabetes.diabetesjournals.org). State 2 respiration (i.e., respiration in the presence of substrate alone) was not different between conditions on any of the substrate combinations studied (Fig. 5B). However, ADP-stimulated (state 3) respiration on a lipid substrate (malate + octanoyl-carnitine, MO) was significantly reduced upon fasting, as compared with the fed situation (P = 0.03, Fig. 5C). Similarly, state 3 upon the Complex I substrates malate + glutamate (MG) was ∼22% lower after fasting, although this did not reach statistical significance (P = 0.12, Fig. 5D).
Additionally, respiration upon parallel electron input to both Complex I and II was reduced by ∼20% upon prolonged fasting. Thus, state 3 respiration upon malate + octanoyl-carnitine + glutamate (MOG) was significantly lower in the fasted state compared with the fed condition (P = 0.01, Fig. 5E). Similar differences were observed for state 3 respiration upon malate + octanoyl-carnitine + glutamate + succinate (MOGS, P = 0.02, Fig. 5E) and malate + glutamate + succinate (MGS, P = 0.04, Fig. 5E). Maximal FCCP-induced uncoupled respiration, reflecting the maximal capacity of the electron transport chain, was also reduced by ∼23% after 60 h of fasting (P = 0.008, Fig. 5F). Finally, state 4o respiration (reflecting mitochondrial proton leak) was similar between the fed and fasted state (P = 0.48, Fig. 5G). The average increase in oxygen consumption upon cytochrome C was less than 10% (underscoring the viability of the muscle fibers) and similar in both conditions (P = 0.54).
Mitochondrial UCP3 did not change upon 60 h of fasting in humans and averaged 2.21 ± 0.38 vs. 2.20 ± 0.41 AU in the fed versus fasted condition (P = 0.96).
DISCUSSION
In this study, we evaluated the effect of prolonged fasting on skeletal muscle mitochondrial functional capacity in humans to examine whether the mitochondrial dysfunction that is frequently reported in insulin resistance and type 2 diabetes can be a consequence of lipid-induced insulin resistance, rather than a cause. In contrast to the hyperglycemia and hyperinsulinaemia accompanying “energy excess”-induced insulin resistance (lipid infusion, high-fat diets), prolonged fasting-induced insulin resistance is associated with hypoglycemia and hypoinsulinemia. Moreover, prolonged fasting-induced lipid accumulation and insulin resistance are considered to be a functional physiologic response. Thus, reduced insulin sensitivity saves carbohydrates for the central nervous system, being obligate for glucose and not requiring insulin for its uptake, whereas increased lipid availability at the same time can serve as a direct available energy source for the muscles and is paralleled by an enhanced fat oxidative capacity (12). Therefore, we anticipated that skeletal muscle mitochondrial function would not be impaired in this model unless mitochondrial function is impaired by factors that are secondary to the lipid-induced insulin-resistant state. Intriguingly, we found that only 60 h of fasting in humans was accompanied by an overall reduction in skeletal muscle mitochondrial capacity, which was not explained by changes in mitochondrial density.
We assessed mitochondrial functional capacity in detail (Fig. 5) by using a wide variety of substrates and substrate combinations to determine the maximum ADP-stimulated respiration (state 3) fueled by a lipid substrate, by Complex I substrates, and upon parallel electron input into Complex I (NADH) and II (FADH2). Interestingly, state 3 respiration was reduced by ∼20% upon all substrates, which reduces the possibility that the decline is caused by substrate-specific alterations such as substrate uptake into the mitochondria. Therefore, both a reduction in the activity of the electron transport chain and the oxidative phosphorylation system could underlie the reduced state 3 respiration. Using the chemical uncoupler FCCP, control over respiration by the oxidative phosphorylation system is bypassed; thus, FCCP-induced respiration reflects the maximal capacity of the electron transport chain. Irrespective of the intervention, FCCP was able to enhance mitochondrial respiration considerably over state 3 values, indicating that the electron transport chain is not rate-limiting in state 3. However, FCCP-induced respiration in itself was reduced by ∼23% upon prolonged fasting (Fig. 5F), indicating that a combined reduction of both the capacity of the electron transport chain and oxidative phosphorylation underlies the reduced mitochondrial oxidative capacity upon fasting.
The reduced mitochondrial capacity was not accounted for by a reduction in mitochondrial density. Thus, mtDNA copy number, CS activity, and OXPHOS protein levels remained unaffected by fasting. Although this does not exclude the possibility that prolonged exposure to high FFA and/or insulin resistance may lead to a reduced mitochondrial function as observed in type 2 diabetes via reduced mitochondrial biogenesis (e.g., via PGC-1α), this finding indicates that fasting interferes with intrinsic mitochondrial capacity in skeletal muscle. Interestingly, a similar reduction in intrinsic mitochondrial capacity, without differences in mitochondrial content, was recently reported by us in type 2 diabetic patients and first degree relatives when compared with BMI- and age-matched obese control subjects (4). Thus, the reduction in mitochondrial function upon fasting mimics the situation as observed in the diabetic state, and suggests that similar (secondary) effects are involved in causing mitochondrial dysfunction. However, it should be noted that fasting is not a direct model for type 2 diabetes. Furthermore, although the available data in the literature underpin the notion that fasting-induced lipid accumulation is responsible for reduced insulin sensitivity upon fasting, we cannot exclude the possibility that the reduced mitochondrial function upon prolonged fasting triggers the insulin resistance observed.
Resistance of skeletal muscle to insulin action per se has been suggested to explain the reduction in mitochondrial functional capacity observed in diabetes. Thus, in healthy individuals, it was shown that a 7 h insulin infusion increased mitochondrial protein synthesis, cytochrome C oxidase (COX), and citrate synthase (CS) enzyme activities and ATP production (26). Moreover, it has been reported that exposing human primary muscle cells to insulin upregulates the expression of PGC-1α (27). Therefore, the reduction in insulin action in type 2 diabetes may underlie the observed mitochondrial defects. In agreement with this hypothesis, it was demonstrated that at low doses of insulin (reflecting postabsorptive levels) the skeletal mitochondrial ATP synthesis rate was not different between diabetic patients and controls, indicating that there is no intrinsic muscle mitochondrial defect in type 2 diabetic patients (7). On the other hand, high (postprandial) levels of insulin increased the mitochondrial ATP production rate in nondiabetic subjects, whereas this increase was absent in type 2 diabetic patients (7). Furthermore, the lack of response in the diabetic patients was accompanied by a reduced expression of PGC-1α, CS, and COX.
Besides reduced insulin action, the hyperglycemia associated with insulin resistance and type 2 diabetes has also been suggested to exert harmful effects on mitochondrial functional capacity via induction of oxidative stress. Indeed, hyperglycemia has been shown to increase mitochondrial ROS production in endothelial cells (28), as well as in other cell types (29). In addition, it was reported that severe hyperglycemia inhibited respiration in human skeletal muscle, which was restored upon insulin treatment (13).
It should be noted that the insulin-resistant state after prolonged fasting was accompanied by hypoinsulinemia and hypoglycemia. Mitochondrial function was thus assessed after exposure to low insulin and glucose concentrations. It is therefore unlikely that the reduced mitochondrial functional capacity observed here is caused by hyperinsulinemia and/or hyperglycemia associated with reduced insulin action. However, it remains possible that chronic hyperinsulinemia and hyperglycemia may negatively affect mitochondrial function in type 2 diabetes patients.
An alternative candidate to explain the observed reduction in mitochondrial capacity upon fasting is prolonged exposure to elevated plasma FFA levels. This is underscored by previous findings in isolated mouse and human skeletal muscle mitochondria showing a dosage-dependent inhibition of ATP synthesis upon incubation with high but physiologic levels of FFA metabolites (30). Furthermore, it was shown in mice that prolonged consumption of a high-fat diet for the duration of 16 weeks reduced mitochondrial function (31). Also in human in vivo studies, negative associations between high fatty acid availability and markers for mitochondrial function have been reported. Thus, PGC-1α expression (9) and the insulin-stimulated increase in skeletal muscle ATP synthesis (10) were reduced upon lipid infusion. Furthermore, it was recently shown that mitochondrial membrane potential was impaired upon short-term lipid infusion in healthy individuals, although several other markers of mitochondrial function remained unaffected (32). Despite the reported negative associations between mitochondrial function and (plasma) FFA, there are also several lines of evidence suggesting the opposite. Thus, raising plasma FFA by high-fat feeding combined with daily heparin injections for 4 weeks in rats increased skeletal muscle mitochondrial biogenesis and mitochondrial enzymes involved in fat oxidation, the citric acid cycle, and the respiratory chain (33). Furthermore, we previously showed that high-fat feeding for 8 weeks in rats resulted in a twofold increase of PGC-1α protein levels (34).
Despite the obvious species differences between these studies, the explanation for the discrepancy in these results remains unclear. Adding to the complexity is the fact that several approaches (high-fat feeding and lipid infusion combined with a hyperinsulinemic-euglycemic clamp) to elevate plasma FFA levels are accompanied by hyperinsulinemia and/or hyperglycemia and insulin resistance, all factors that have also been suggested to interfere with mitochondrial capacity (26,29). Finally, differences in absolute levels of plasma FFA achieved in the different studies may contribute to (part of) the variation.
Within the context of mitochondrial lipotoxicity, we and others have previously postulated that mitochondrial UCP3 may be involved in protecting mitochondria against (lipid-induced) oxidative damage (35). Therefore, we determined protein levels of UCP3 and found that UCP3 content was similar between the fed and the fasted condition. This is a surprising finding since fasting has been quite convincingly shown to increase UCP3 protein levels in animal studies (36,37). Moreover, UCP3 mRNA levels were also elevated after 15 h (∼5-fold) and 40 h (∼10-fold) of fasting in humans (38). It should be noted, however, that this is the first study to evaluate UCP3 protein content upon prolonged fasting in humans.
The impressive increase in plasma FFA upon prolonged fasting is in line with previous findings in humans (12,39), although the absolute values achieved in this study (∼2.0 mol/l) are high. Also, the ∼2.7-fold increase in IMTG levels after 60 h of fasting is slightly higher in comparison with previous reports (12,39). The high plasma FFA and IMTG levels might be caused by complete compliance to the fasting regimen in the present study since, in contrast to other studies, the subjects stayed in a respiration chamber throughout the whole period.
The reduction in insulin-stimulated glucose uptake observed in the present study confirms previous observations showing that prolonged fasting reduced glucose Rd, which was accounted for by a reduction in both insulin-stimulated glucose oxidation and nonoxidative glucose disposal (40). In agreement with previous reports (40), we also detected a decreased metabolic flexibility (i.e., the ability to switch from predominantly fat oxidation to glucose oxidation upon insulin stimulation) upon prolonged fasting (Fig. 3). However, not all studies show this effect (41).
As anticipated, whole-body fat oxidation increased significantly upon prolonged fasting. Therefore, the decrease in mitochondrial capacity in skeletal muscle is counterintuitive, especially since this decrease was substrate-independent and also apparent upon a lipid substrate.
These results indicate that the reduced mitochondrial capacity is secondary to the fatty acid surplus associated with the insulin-resistant state. It is important to note however, that the reduction in muscle mitochondrial capacity does not (yet) affect the capability of the body to enhance fat oxidation. This is an important finding since it has generally been assumed that a reduction in muscle mitochondrial function will result in reductions in whole-body fat oxidative capacity (3–5,42,43). Here we show that this extrapolation may not be justified, although we cannot exclude that the fasting-induced reduced mitochondrial function in muscle may decrease muscle-specific fat oxidation compensated by increased fat oxidation in other organs, or may have an impact on the capacity to switch from carbohydrate to fat oxidation (metabolic flexibility).
In conclusion, 60 h of fasting in humans lowered insulin-stimulated glucose uptake down to ∼50% along with drastically elevated plasma FFA and IMTG levels. This was accompanied by an overall reduction in intrinsic mitochondrial functional capacity in skeletal muscle, despite a pronounced increase in whole-body fat oxidation. Since prolonged fasting is a physiologic condition in which increased fat oxidation becomes very important, a reduced mitochondrial function seems unbeneficial from a physiologic point of view. Our findings suggest that the elevated plasma FFA and/or intramuscular lipid levels associated with the insulin-resistant state are responsible for the secondary negative effects on mitochondrial function.
ACKNOWLEDGMENTS
This study was funded by Top Institute Food and Nutrition. J.H. was supported by a grant from the Netherlands Organization for Scientific Research. M.K.C.H. is supported by a VIDI Research Grant for innovative research from the Netherlands Organization for Scientific Research (Grant 917.66.359). P.S. is supported by a VICI Research Grant for innovative research from the Netherlands Organization for Scientific Research (Grant 918.96.618).
No potential conflicts of interest relevant to this article were reported.
J.H. wrote the manuscript and researched data. N.A.v.H. researched data and reviewed/edited the manuscript. M.M. contributed to discussion and reviewed/edited the manuscript. E.M.-K. and D.v.B. were involved in analysis of material. M.K.C.H. reviewed/edited the manuscript. P.S. edited the manuscript and contributed to discussion.
The authors thank Esther Phielix and Gert Schaart for practical help and technical support.
Footnotes
The study has been registered at www.trialregister.nl with registration number NTR 2042.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 4.Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 2008;57:2943–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007;56:1592–1599 [DOI] [PubMed] [Google Scholar]
- 6.Pagel-Langenickel I, Bao J, Joseph JJ, Schwartz DR, Mantell BS, Xu X, Raghavachari N, Sack MN. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem 2008;283:22464–22472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006;55:3309–3319 [DOI] [PubMed] [Google Scholar]
- 8.Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, Nowotny P, Wolzt M, Waldhausl W, Roden M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeks J, Hesselink MK, Russell AP, Mensink M, Saris WH, Mensink RP, Schrauwen P. Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia 2006;49:2419–2426 [DOI] [PubMed] [Google Scholar]
- 10.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006;55:136–140 [PubMed] [Google Scholar]
- 11.Johnson NA, Stannard SR, Mehalski K, Trenell MI, Sachinwalla T, Thompson CH, Thompson MW. Intramyocellular triacylglycerol in prolonged cycling with high- and low-carbohydrate availability. J Appl Physiol 2003;94:1365–1372 [DOI] [PubMed] [Google Scholar]
- 12.Stannard SR, Thompson MW, Fairbairn K, Huard B, Sachinwalla T, Thompson CH. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am J Physiol Endocrinol Metab 2002;283:E1185–1191 [DOI] [PubMed] [Google Scholar]
- 13.Rabol R, Hojberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 2009;94:1372–1378 [DOI] [PubMed] [Google Scholar]
- 14.Hoeks J, van Baak MA, Hesselink MK, Hul GB, Vidal H, Saris WH, Schrauwen P. Effect of beta1- and beta2-adrenergic stimulation on energy expenditure, substrate oxidation, and UCP3 expression in humans. Am J Physiol Endocrinol Metab 2003;285:E775–782 [DOI] [PubMed] [Google Scholar]
- 15.Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med 1985;6:197–201 [DOI] [PubMed] [Google Scholar]
- 16.Schoffelen PF, Westerterp KR, Saris WH, Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol 1997;83:2064–2072 [DOI] [PubMed] [Google Scholar]
- 17.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr 1997;66:276–282 [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand 1967;71:140–150 [DOI] [PubMed] [Google Scholar]
- 19.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 20.Roorda BD, Hesselink MK, Schaart G, Moonen-Kornips E, Martinez-Martinez P, Losen M, De Baets MH, Mensink RP, Schrauwen P. DGAT1 overexpression in muscle by in vivo DNA electroporation increases intramyocellular lipid content. J Lipid Res 2005;46:230–236 [DOI] [PubMed] [Google Scholar]
- 21.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol 2001;116:63–68 [DOI] [PubMed] [Google Scholar]
- 22.Shepherd D, PB G: Citrate synthase from rat liver. In Methods in Enzymology. Vol XIIINew York, Academic Press, 1969, p. 11–16 [Google Scholar]
- 23.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007;50:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrauwen P, Mensink M, Schaart G, Moonen-Kornips E, Sels JP, Blaak EE, Russell AP, Hesselink MK. Reduced skeletal muscle uncoupling protein-3 content in prediabetic subjects and type 2 diabetic patients: restoration by rosiglitazone treatment. J Clin Endocrinol Metab 2006;91:1520–1525 [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 26.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A 2003;100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Khalili L, Forsgren M, Kannisto K, Zierath JR, Lonnqvist F, Krook A. Enhanced insulin-stimulated glycogen synthesis in response to insulin, metformin or rosiglitazone is associated with increased mRNA expression of GLUT4 and peroxisomal proliferator activator receptor gamma co-activator 1. Diabetologia 2005;48:1173–1179 [DOI] [PubMed] [Google Scholar]
- 28.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 29.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 2006;103:2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 2008;295:E678–685 [DOI] [PubMed] [Google Scholar]
- 31.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez AO, Kamath S, Jani R, Sharma LK, Monroy A, Abdul-Ghani MA, Centonze VE, Sathyanarayana P, Coletta DK, Jenkinson CP, Bai Y, Folli F, Defronzo RA, Tripathy D. Effect of short-term free fatty acids elevation on mitochondrial function in skeletal muscle of healthy individuals. J Clin Endocrinol Metab 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci U S A 2007;104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeks J, Briede JJ, de Vogel J, Schaart G, Nabben M, Moonen-Kornips E, Hesselink MK, Schrauwen P. Mitochondrial function, content and ROS production in rat skeletal muscle: effect of high-fat feeding. FEBS Lett 2008;582:510–516 [DOI] [PubMed] [Google Scholar]
- 35.Schrauwen P, Hoeks J, Hesselink MK. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism? Prog Lipid Res 2006;45:17–41 [DOI] [PubMed] [Google Scholar]
- 36.Moreno M, Lombardi A, De Lange P, Silvestri E, Ragni M, Lanni A, Goglia F. Fasting, lipid metabolism, and triiodothyronine in rat gastrocnemius muscle: interrelated roles of uncoupling protein 3, mitochondrial thioesterase, and coenzyme Q. FASEB J 2003;17:1112–1114 [DOI] [PubMed] [Google Scholar]
- 37.Cadenas S, Buckingham JA, Samec S, Seydoux J, Din N, Dulloo AG, Brand MD. UCP2 and UCP3 rise in starved rat skeletal muscle but mitochondrial proton conductance is unchanged. FEBS Lett 1999;462:257–260 [DOI] [PubMed] [Google Scholar]
- 38.Tunstall RJ, Mehan KA, Hargreaves M, Spriet LL, Cameron-Smith D. Fasting activates the gene expression of UCP3 independent of genes necessary for lipid transport and oxidation in skeletal muscle. Biochem Biophys Res Commun 2002;294:301–308 [DOI] [PubMed] [Google Scholar]
- 39.Johnson NA, Stannard SR, Rowlands DS, Chapman PG, Thompson CH, O'Connor H, Sachinwalla T, Thompson MW. Effect of short-term starvation versus high-fat diet on intramyocellular triglyceride accumulation and insulin resistance in physically fit men. Exp Physiol 2006;91:693–703 [DOI] [PubMed] [Google Scholar]
- 40.Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab 2007;293:E1103–1111 [DOI] [PubMed] [Google Scholar]
- 41.Soeters MR, Sauerwein HP, Dubbelhuis PF, Groener JE, Ackermans MT, Fliers E, Aerts JM, Serlie MJ. Muscle adaptation to short-term fasting in healthy lean humans. J Clin Endocrinol Metab 2008;93:2900–2903 [DOI] [PubMed] [Google Scholar]
- 42.Ortenblad N, Mogensen M, Petersen I, Hojlund K, Levin K, Sahlin K, Beck-Nielsen H, Gaster M. Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 2005;1741:206–214 [DOI] [PubMed] [Google Scholar]
- 43.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54:8–14 [DOI] [PubMed] [Google Scholar]