Abstract
OBJECTIVE
Short sleep duration is associated with impaired glucose tolerance and an increased risk of diabetes. The effects of sleep restriction on insulin sensitivity have not been established. This study tests the hypothesis that decreasing nighttime sleep duration reduces insulin sensitivity and assesses the effects of a drug, modafinil, that increases alertness during wakefulness.
RESEARCH DESIGN AND METHODS
This 12-day inpatient General Clinical Research Center study included 20 healthy men (age 20–35 years and BMI 20–30 kg/m2). Subjects spent 10 h/night in bed for ≥8 nights including three inpatient nights (sleep-replete condition), followed by 5 h/night in bed for 7 nights (sleep-restricted condition). Subjects received 300 mg/day modafinil or placebo during sleep restriction. Diet and activity were controlled. On the last 2 days of each condition, we assessed glucose metabolism by intravenous glucose tolerance test (IVGTT) and euglycemic-hyperinsulinemic clamp. Salivary cortisol, 24-h urinary catecholamines, and neurobehavioral performance were measured.
RESULTS
IVGTT-derived insulin sensitivity was reduced by (means ± SD) 20 ± 24% after sleep restriction (P = 0.001), without significant alterations in the insulin secretory response. Similarly, insulin sensitivity assessed by clamp was reduced by 11 ± 5.5% (P < 0.04) after sleep restriction. Glucose tolerance and the disposition index were reduced by sleep restriction. These outcomes were not affected by modafinil treatment. Changes in insulin sensitivity did not correlate with changes in salivary cortisol (increase of 51 ± 8% with sleep restriction, P < 0.02), urinary catecholamines, or slow wave sleep.
CONCLUSIONS
Sleep restriction (5 h/night) for 1 week significantly reduces insulin sensitivity, raising concerns about effects of chronic insufficient sleep on disease processes associated with insulin resistance.
The average sleep duration in the U.S. has fallen below 7 h per night, a drop of ∼2 h per night over the last century and >1 h per night over the last 40 years (1,2). Cross-sectional and longitudinal studies have demonstrated a link between short sleep duration or poor sleep quality and increased risk of obesity (3–7), diabetes (7–11), hypertension (12), cardiovascular disease (13,14), the metabolic syndrome (15), and early mortality (14,16–21). Short-term sleep restriction (4 h/night for 1 week in a laboratory setting) impaired glucose tolerance during a frequently sampled intravenous glucose tolerance test (IVGTT) in healthy subjects (22).
In healthy subjects, the mechanisms leading to impaired glucose tolerance with short-term reductions in nightly sleep duration are unclear. Decreases in insulin secretion have been implicated, and sleep restriction increases cortisol levels, which could influence glucose tolerance (22). Further, insulin resistance has been reported in two very different models of disrupted sleep: sleep apnea (23) and experimental disruption of slow-wave sleep (24). In the latter model, the extent of slow-wave sleep disruption predicted reductions in insulin sensitivity (24).
Our primary goal was to test the hypothesis that sleep restriction in healthy subjects reduces insulin sensitivity as assessed by the hyperinsulinemic-euglycemic clamp. Insulin secretion was assessed using IVGTTs. To identify possible mechanisms by which sleep restriction may affect insulin sensitivity, we assessed the relationships between changes in insulin sensitivity and changes in cortisol, catecholamines, and slow-wave sleep. Further, we tested the ability of modafinil to ameliorate the adverse effects of sleep restriction on insulin sensitivity. Modafanil activates central, wake-promoting dopaminergic and noradrenergic mechanisms (25,26) and ameliorates the adverse effects of sleep deprivation on alertness and performance (27–29)—impairments that have been attributed to reduced brain glucose utilization (30). Thus, we performed hyperinsulinemic-euglycemic clamps and intravenous glucose tolerance twice: at baseline in sleep-replete individuals and after 7 nights of sleep restriction (5 h in bed) in healthy individuals randomized to daily treatment with placebo or modafinil.
RESEARCH DESIGN AND METHODS
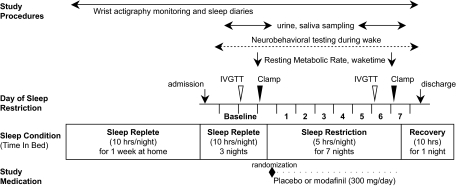
A schematic of this double-blind, placebo-controlled, randomized, clinical study is presented in Fig. 1. Procedures were approved by the Human Research Committee of the Brigham and Women's Hospital and conducted according to the principles expressed in the Declaration of Helsinki. All subjects provided written informed consent.
FIG. 1.
Protocol schema. (See research design and methods for a detailed description.)
Subject recruitment and screening.
Healthy male subjects were recruited using newspaper ads, flyers, and website postings. Subjects were screened for sleep patterns and medical and psychological history, underwent a physical examination by a licensed physician, and provided blood and urine samples to ensure that hematology and serum chemistry, including metabolic and thyroid panels, were within normal limits. All subjects passed a urine toxicology screen.
Prestudy conditions.
Before the experiment, subjects slept at home for at least 5 days (mean 8.9 days [range 5–21]) with 10 h per night of time in bed (TIB) from 10 p.m. to 8 a.m. (±1 h) in order to enter the experimental portion of the protocol in a nearly sleep-replete state, i.e., with similar and presumably minimal amounts of sleep debt (31). Subjects called into a time-stamped phone-answering system, wore wrist activity monitors (Minimitter, Bend, OR) and completed a sleep diary to assure compliance with this schedule.
Inpatient study conditions.
Subjects were admitted to the General Clinical Research Center at Brigham and Women's Hospital for a 12-day inpatient visit. The study began with a 3-day baseline period of 10/h night TIB (continued from the pretest period) and baseline metabolic assessments, after which subjects were scheduled for sleep restriction (5 h/night TIB) for the following 7 nights, with the sleep periods centered at the same clock time of 0300 h. During the periods of wakefulness, subjects were allowed to perform activities such as writing, reading, computer work, board or card games, movie viewing, arts and crafts, listening to or playing music, and light stretching. Subjects were observed by research technicians throughout the protocol either through direct interaction or remotely via video. Light levels during sleep periods were essentially complete darkness (<1 lux) and <90 lux during wakefulness, which simulations suggest would lead to a <9 min mean difference of circadian phase between sleep conditions (32). Subjects were randomized to modafinil (100 mg per tablet) or placebo (two tablets at 0600 h and one tablet at 1300 h) during the 7 days of sleep restriction. Post–sleep restriction metabolic assessments were performed, and subjects were discharged on day 12 after a recovery night of 10 h TIB (Fig. 1). Metabolic assessments are described below and consisted of an IVGTT, euglycemic-hyperinsulinemic clamp, and collection of saliva and urine for hormone measurements. During each sleep period, scalp surface electrodes (Beckman Instrument Company, Schiller Park, IL) were applied to specific locations on the subject's face and scalp at least 2 h prior to the scheduled sleep period for recordings of central (C3 and C4) and occipital (O1 and O2) electroencephalogram, electrooculogram, electromyogram, and electrocardiogram. Data were collected using Vitaport Three digital sleep recorders (TEMEC) and scored visually in 30-s epochs by registered polysomnographic technologists (33).
Controlled diet.
Throughout the inpatient portion of the study, subjects received an isocaloric, controlled-nutrient diet containing 58–60% carbohydrates, 15–17% protein, 25–27 ± 1% fat, 800–1,000 mg calcium, 100 ±2 mEq potassium, and 200 ±2 mEq sodium. Subjects were required to consume all food provided. An identical menu was provided on the day before and the day of each IVGTT and each euglycemic-hyperinsulinemic clamp procedure.
Subjective measures of sleepiness and alertness.
Every 3 hours during wake periods, subjects completed a short test battery including the Karolinska Sleepiness Scale (34) and the Psychomotor Vigilance Task (PVT) (3,24). The Psychomotor Vigilance Task involved a 10-min visual reaction time performance test in which the subject was instructed to maintain the fastest possible reaction time to a simple visual stimulus. Lapses of attention refer to the number of times the subject failed to respond to the signal within 500 ms.
Frequently-sampled IVGTT (insulin modified).
IVGTT tests were performed after an overnight fast on the mornings of days 3 and 10, e.g., following the penultimate night of 10 h TIB and the penultimate (sixth) night of 5 h TIB, respectively. Blood samples were drawn via an intravenous catheter every 5 min for 20 min starting at T = −20 min. At time 0 (9 a.m.), 0.3 g/kg glucose was infused over 1 min via an intravenous catheter in the nonsampling arm. Blood samples were then taken at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16 19, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min. At time 20 min, 0.02 units/kg human insulin (Novolin R U-100; Novo Nordisk, Princeton, NJ) was administered intravenously in <30 s, including saline flush. Minimal model analyses (Minmod Millennium 2000; R. Bergman, University of Southern California, Los Angeles, CA) were performed to determine first-phase area under the insulin curve from 0 to 10 min, glucose effectiveness and insulin sensitivity (SI). Glucose tolerance was calculated as the slope of the natural log of glucose values from minute 5 to minute 19.
Euglycemic-hyperinsulinemic clamp procedure.
Insulin sensitivity was measured using the insulin clamp procedure as previously described (35). Studies were performed on the mornings of days 4 and 11, following the final night of 10 h TIB and the final (seventh) night of 5 h TIB, respectively. Following an overnight fast, the subject remained in bed until the completion of the procedure. Intravenous lines were placed in each arm for infusion or blood draw. After a baseline sample was collected, subjects were infused with human insulin (Novolin R) with priming doses of 80 and 60 mU/m2 body surface area per min over the first two 5-min periods, respectively, followed by a constant infusion rate of 40 mU/m2 per min for 170 min. Blood samples were collected every 5 min from T = 0 to 180 min from a catheter placed retrograde in a dorsal vein of the wrist; this hand was placed in a hand warmer thermostatically controlled at 140°F to arterialize venous blood. Serum glucose levels were determined immediately at the bedside, and serum insulin was measured from frozen samples. Dextrose solution (20%) was variably infused to maintain serum glucose levels at 90 mg/dl throughout the clamp procedure. The mean dextrose infusion rate over the last 60 min of the clamp (M), after correcting for changes in plasma glucose concentration [expressed as mg (glucose) · kg−1 body weight · min−1 of infusion] as described by Defronzo et al. (36), was determined and used as an indicator of insulin sensitivity.
Resting metabolic rate.
Raising metabolic rate (RMR) was estimated from expired gases using a validated and FDA-approved indirect calorimeter (Medgem 100; Healthetech) that estimates RMR in kilocalories per day (20,37). Assessments were made upon waking in the sleep-replete condition while subjects were still in bed (i.e., after a 12-h fast) after a void and at least 10 min of quiet bed rest. The timing was at the same clock time in both conditions, ∼0820 h, and the test duration was ∼12–14 min until steady state was attained.
Saliva and urine sampling.
Saliva samples for determination of free cortisol levels were collected from 1500 to 2100 h on the last 2 days of each condition. Twenty-four hour urine collections were obtained on the last 2 days of each condition.
Assays.
Serum glucose during the clamp studies was measured using the Beckman Glucose Analyzer 2 (Beckman Coulter, Chaska, MN) with sensitivity of <10 mg/dl and precision <5%. Serum glucose during the IVGTT was measured using the COBAS Integra 400 (Roche Diagnostics, Indianapolis, IN) with sensitivity of 0.59 mg/dl and precision <4.3% (37). Serum insulin was measured by chemiluminescence immunoassay (Access Immunoassay System, Beckman Coulter, Chaska, MN) with sensitivity 0.03 IU/ml, precision <5.6%. Salivary cortisol was measured using a solid-phase radioimmunoassay (Coat-A-Count; DPC, Los Angeles, CA), with sensitivity <0.02 μg/dl and precision 4–5%. Urinary norepinephrine and epinephrine was assayed using the LDN CAT RIA kit (Immuno Biological Laboratories, Minneapolis, MN). The sensitivity of this method is 1.5 ng/ml for norepinephrine and 0.3 ng/ml for epinephrine; the precision is <15% for both assays (38).
Statistical analyses.
Mixed-effects models were applied to study the effects of the number of nights of sleep restriction and the effects of drug treatment on subjective and objective measures of sleepiness, including self-reported sleepiness and lapses of attention, and on insulin secretion, insulin sensitivity, cortisol levels, urinary norepinephrine and epinephrine, and resting metabolic rate. Treatment and sleep restriction status were considered fixed effects, and random intercepts were added to account for individual variation from the group mean. Data are presented as means ± SEM unless otherwise indicated.
RESULTS
Subjects.
Twenty healthy men (mean ± SD: age 26.8 ± 5.2 years; BMI 23.3 ± 3.1 kg/m2) completed the study (11 placebo and 9 active drug). An additional three subjects were withdrawn from the study after initiation of drug treatment as a result of 1) transient EKG changes in one subject, 2) tachycardia (up to 126 bpm) and elevated blood pressure (systolic 145 mmHg and diastolic 91 mmHg) in another subject, and 3) tachycardia (up to 127 bpm), elevated systolic blood pressure (systolic 147 mmHg and diastolic 87 mmHg), and urinary frequency in a third subject. These subjects were otherwise asymptomatic. Unblinding revealed that all three of these subjects had been receiving modafinil. All signs and symptoms resolved within a day of stopping the medication.
Subjective and objective measures of sleepiness.
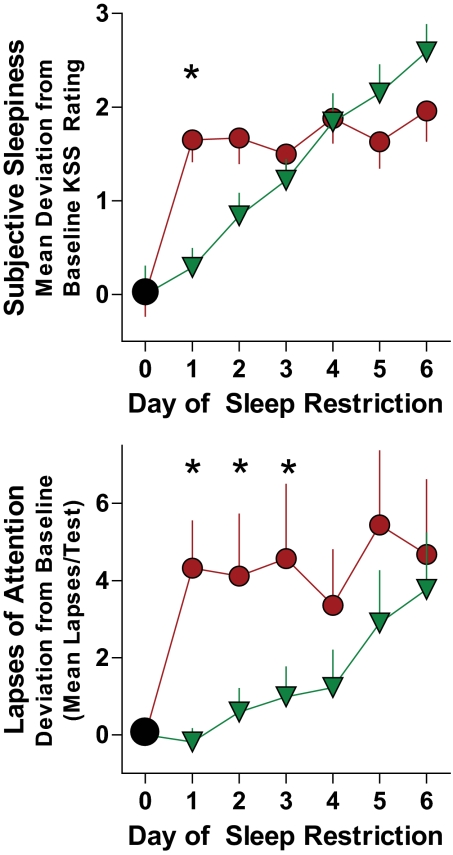
Sleep restriction increased self-reported sleepiness and objective measures of sleepiness compared with the sleep-replete baseline condition (Table 1 and Fig. 2); modafinil treatment significantly reduced the deleterious effects of sleep restriction on these measures of sleepiness.
TABLE 1.
| Sleep duration (TIB per study day) |
Baseline vs. sleep restriction condition (P) | Drug (P) | Interaction (P) | ||||
|---|---|---|---|---|---|---|---|
| Modafinil |
Placebo |
||||||
| 5 h | 10 h | 5 h | 10 h | ||||
| Sleepiness: KSS (1–9 scale) | 4.65 ± 0.12 | 3.23 ± 0.13 | 5.31 ± 0.11 | 3.61 ± 0.16 | <0.0001 | <0.0001 | <0.0001 |
| Performance: PVT lapses (lapses · test−1 · day−1) | 4.09 ± 0.45 | 2.48 ± 0.37 | 5.10 ± 0.73 | 0.92 ± 0.18 | <0.0001 | 0.008 | 0.002 |
| Sleep duration: total sleep time (min) | 268.2 ± 3.5 | 502.2 ± 8.9 | 274.0 ± 1.4 | 467.4 ± 10.8 | <0.0001 | 0.04 | 0.001 |
| Slow-wave sleep (min) | 78.6 ± 3.4 | 75.2 ± 5.9 | 75.4 ± 3.2 | 65.2 ± 7.4 | 0.12 | 0.50 | 0.57 |
| FSIVGTT | |||||||
| SI [(mU/l)−1 · min−1] | 4.61 ± 0.55 | 5.70 ± 0.88 | 4.81 ± 0.78 | 6.74 ± 0.94 | 0.001 | 0.62 | 0.24 |
| Acute insulin response (mU · l−1 · min) | 549.2 ± 127.2 | 514.0 ± 106.3 | 532.3 ± 94.1 | 566.7 ± 112.8 | 0.30 | 0.77 | 0.81 |
| Disposition index | 2,034.2 ± 231.6 | 2,500.6 ± 391.9 | 2,079.5 ± 254.1 | 2,993.7 ± 431.6 | 0.02 | 0.46 | 0.52 |
| Glucose tolerance (%/min) | −1.984 ± 0.137 | −2.295 ± 0.160 | −2.120 ± 0.162 | −2.465 ± 0.261 | 0.02 | 0.43 | 0.62 |
| Glucose effectiveness (min−1) | 0.017 ± 0.002 | 0.020 ± 0.002 | 0.021 ± 0.002 | 0.023 ± 0.003 | 0.21 | 0.15 | 0.76 |
| Clamp: M (mg · kg−1 · min−1) | 6.15 ± 0.66 | 7.11 ± 0.86 | 6.95 ± 0.81 | 7.39 ± 0.79 | 0.05 | 0.55 | 0.69 |
| Salivary cortisol (ng/ml) | 0.179 ± 0.017 | 0.112 ± 0.013 | 0.180 ± 0.015 | 0.125 ± 0.010 | <0.0001 | 0.70 | 0.48 |
| Catecholamines | |||||||
| Norepinephrine (μg/day) | 48.0 ± 4.5 | 29.7 ± 4.3 | 32.5 ± 4.4 | 27.5 ± 4.7 | <0.0001 | 0.14 | 0.02 |
| Epinephrine (μg/day) | 13.5 ± 1.2 | 7.0 ± 0.8 | 9.0 ± 2.1 | 7.4 ± 1.1 | 0.001 | 0.29 | 0.03 |
| Resting metabolic rate (kcal) | 1,931.4 ± 77.4 | 1,928.6 ± 79.1 | 1,851.0 ± 86.4 | 1,853.0 ± 116.6 | 0.99 | 0.56 | 0.96 |
Data are means ± SE unless otherwise indicated.
FIG. 2.
Subjective sleepiness and lapses of attention under sleep restriction conditions. Effects of sleep restriction on subjective sleepiness (top panel) and lapses of attention (bottom panel). Subjective sleepiness and objective lapses of attention in sleep-replete subjects were assessed after 10 h of TIB per night and after restricting sleep to 5 h/night over 1 week (days 1–6 averages) in subjects randomized to placebo (red circles) or modafinil administration (green triangles). Subjective sleepiness is defined as mean deviation from baseline Karolinska Sleepiness Scale (KSS). Lapses of attention are defined as reaction times >500 ms and are quantified as the absolute deviation from baseline (lapses/test per day). For self-reported sleepiness, we noted significant main effects of sleep restriction (P < 0.0001), treatment (P < 0.0001), and their interaction (P < 0.0001). With modafinil administration, self-reported sleepiness was significantly reduced compared with placebo after the first and second nights of sleep restriction only (P = 0.0276). For lapses of attention, there was a significant main effect of number of nights of sleep restriction (P = 0.0012) and a borderline effect for treatment (P = 0.0647), and their interaction was nonsignificant (P = 0.1488). With modafinil administration, lapses of attention were significantly reduced compared with placebo during the daytime testing after the first and second nights of sleep restriction only (P = 0.0141); after the second and third nights, the borderline P values were 0.0779 and 0.0770, respectively.
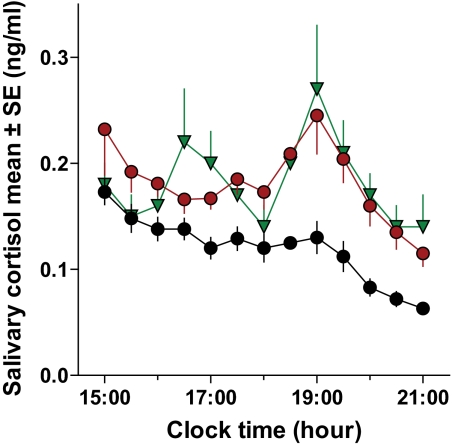
Cortisol and norepinephrine.
Salivary cortisol levels (assessed between 1500 and 2100 h) were elevated with sleep restriction compared with the baseline sleep-replete condition (Fig. 3). The mean increase in cortisol of 0.054 ± 0.01 ng/ml with sleep restriction and placebo was similar to the increase observed with sleep restriction and modafinil treatment (0.066 ± 0.01 ng/ml; P = 0.48). Compared with placebo, modafinil treatment significantly increased urinary epinephrine and norepinephrine with decreased sleep duration (Table 1). Changes in urinary catecholamines (sleep restricted − baseline sleep replete) were 4.98 ± 4.02 μg/day (placebo [t test P = 0.24]) and 18.29 ± 3.31 μg/day (modafinil [t test P = 0.0006]) for norepinephrine and 1.58 ± 1.72 μg/day (placebo [t test P = 0.39]) and 6.52 ± 0.94 μg/day (modafinil [t test P = 0.0001]) for epinephrine.
FIG. 3.
Salivary (free) cortisol levels with sleep restriction. Salivary cortisol levels were assessed hourly from 1500 to 2100 h under sleep-replete conditions (10 h/night TIB [black circles]) and under sleep-restricted conditions (5 h/night TIB for 1 week) in subjects receiving placebo (red circles) or modafinil (green triangles). Salivary cortisol levels (means ± SD) from 1500 to 2100 h were significantly affected by sleep duration only: 0.13 ± 0.03 ng/ml for the sleep-replete condition and 0.17 ± 0.04 ng/ml for the sleep-restricted condition (P < 0.0001 and Table 1). Identical (mixed composition) dinners were served just after the 1800-h saliva sample and finished before 1840 h.
Energy expenditure.
Fasted resting metabolic rate was unchanged from baseline (sleep replete) to sleep restriction (mean change 0 ± 44 kcal). There were no effects of drug treatment on the change in RMR (Table 1).
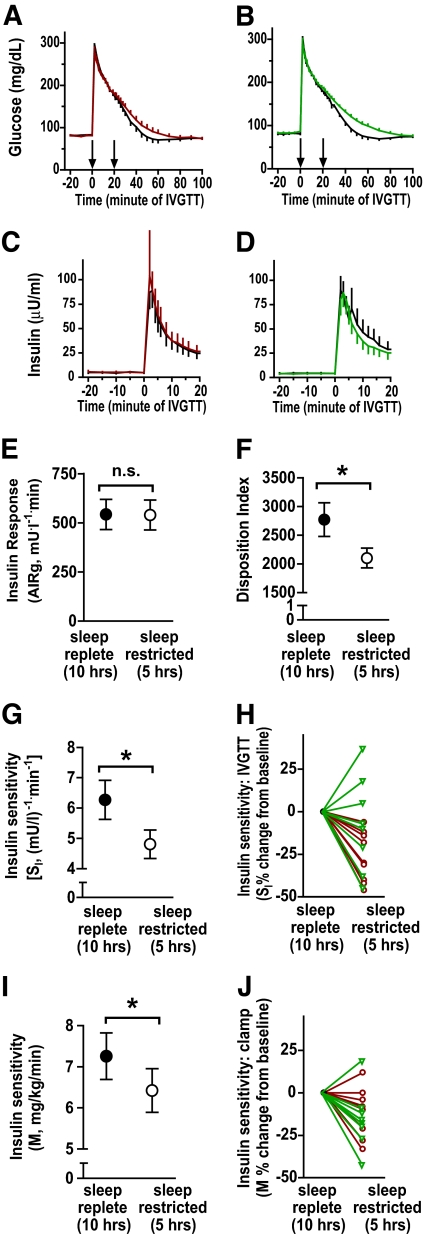
Insulin sensitivity and acute insulin response (IVGTT).
Insulin sensitivity assessed by minimal model analysis of IVGTT data was significantly reduced after sleep restriction compared with the sleep-replete baseline condition, with no significant effect of modafinil treatment (Table 1; Fig. 4E). Fifteen out of nineteen subjects had a decrease in SI with sleep restriction, with a mean decrease of 20 ± 24% (F1,18 = 15.18; P = 0.001) (Table 1 and Fig. 4E and F). The acute insulin response was not significantly affected by either sleep restriction or drug treatment (Table 1; Fig. 4C). With sleep restriction, the disposition index (the product of SI and acute insulin response), was significantly but slightly reduced (Table 1 and Fig. 4D) and glucose tolerance was significantly reduced (change of 0.31 ± 0.13% per min with modafinil and 0.17 ± 0.15% per min with placebo). There were no significant effects of sleep restriction or drug treatment on other minimal model parameters (Table 1).
FIG. 4.
Effects of sleep restriction on glucose metabolism. A and B: Mean glucose levels (± SE) from IVGTT during the baseline sleep-replete condition (10 h/night TIB [black line]) and following sleep restriction for 1 week (5 h/night TIB) in subjects receiving placebo (A) (red line) modafinil (B) (green line). Left arrow, glucose infusion at time = 0 min; right arrow, insulin infusion at time = 20 min. C and D: Mean insulin levels (± SE) from IVGTT. E–H: IVGTT parameters were calculated using Minmod Millennium software. Glucose and insulin data from insulin-modified IVGTT procedures under sleep-replete (filled symbols) and sleep-restricted conditions (open symbols) are shown. E: Acute insulin response (AIRg) (first phase). F: Disposition index. G: SI from IVGTT. H: relative changes in SI from IVGTT expressed as percent change from baseline sleep-replete condition in subjects randomized to placebo (red circles) or modafinil administration (green triangles). I: Insulin sensitivity (M) from euglycemic-hyperinsulinemic clamp procedure. J: Relative changes in insulin sensitivity (M) depicted as in F. There were no significant effects of drug administration on any metabolic parameters (Table 1).
Insulin sensitivity (euglycemic-hyperinsulinemic clamp).
Glucose and insulin levels at baseline and during euglycemic-hyperinsulinemic clamp protocols were similar between sleep-replete and sleep-restricted conditions and between modafinil and placebo treatments. Fasting insulin levels were 4.5 ± 0.4 μU/ml and increased to 57.8 ± 2.3 μU/ml during the insulin infusions. Serum glucose levels averaged 89.9 ± 0.3 mg/dl during the last 60 min of the clamp procedures. The dextrose infusion rate (M) needed to maintain euglycemia during the final hour of the clamp procedure was significantly reduced with sleep restriction compared with the baseline sleep-replete condition (Fig. 4G and H); there were no significant effects of drug treatment (Table 1). Ninety percent of subjects had a decrease in M with sleep restriction. The mean ± SE decrease for all subjects was 11 ± 5.5% (F1.18 = 4.64; P = 0.045) relative to the baseline sleep replete condition (Fig. 4). Importantly, changes in insulin sensitivity (M) assessed by the clamp procedure correlated with the change in insulin sensitivity (SI) assessed by the IVGTT procedure (r = 0.53; P = 0.02). Overall, there was a strong correlation of the absolute level of M with SI (r = 0.85; P < 0.0001).
Changes in slow-wave sleep.
Sleep restriction resulted in a significant decrease in total sleep time, but changes in the amount of slow-wave sleep (non–rapid eye movement stages 3 and 4), previously linked to changes in glucose metabolism (24), were not related to changes in insulin sensitivity (Table 1).
Predictors of changes in insulin sensitivity.
There was not a significant linear relationship between BMI and change in SI (F1,17 = 1.04; P = 0.32) or change in M (F1,17 = 1.23; P = 0.28), between change in cortisol and change in SI (F1,17 = 1.28; P = 0.27) or change in M (F1,17 = 0; P = 0.99), or between change in urinary catecholamine levels and change in either Si (F1,17 = 0.04, P = 0.85, for norepinephrine and F1,17 = 1.79, P = 0.20, for epinephrine) or M (F1,17 = 0.68, P = 0.42, for norepinephrine and F1,17 = 0.01, P = 0.91, for epinephrine). Similar results were obtained when the analysis was restricted to subjects receiving modafinil (data not shown).
DISCUSSION
Sleep restriction to 5 h/night (TIB) for 1 week in nonobese, healthy men significantly reduced insulin sensitivity as assessed by two techniques, the euglycemic-hyperinsulinemic clamp and the IVGTT, yet did not affect the acute insulin response to intravenous glucose administration. Sleep restriction led to elevations of afternoon and evening levels of free cortisol, but these increases were not linearly related to changes in insulin sensitivity. The effects of sleep restriction on measures of glucose metabolism and on salivary cortisol were not altered by administration of modafinil, though modafinil did improve subjective and objective measures of sleepiness. These changes in insulin sensitivity support the hypothesis that insufficient sleep duration leads to insulin resistance.
Our finding that sleep restriction leads to a decrease in insulin sensitivity is consistent with earlier studies showing impaired glucose metabolism with altered sleep duration. The earliest direct assessment of the relationship between sleep and glucose metabolism demonstrated that complete sleep deprivation for 3–4 days led to an elevation of glucose levels on an oral glucose tolerance test (39). Spiegel et al. (22) from the Van Cauter laboratory performed frequently sampled IVGTT (FSIVGTT) in healthy subjects during a sleep debt condition (4 h per night) and the sleep-replete condition (12 h/night). They found that the sleep debt condition led to impaired glucose metabolism characterized by 30–40% reductions in glucose tolerance, glucose effectiveness, and acute insulin response to glucose but a nonsignificant reduction in insulin sensitivity. We also demonstrated impairment in glucose metabolism with sleep restriction (to 5 h/night compared with a baseline sleep repletion of 10 h/night), but the impairment was attributable to a decrease in insulin sensitivity rather than to impairments in insulin secretion or glucose effectiveness. However, we did not observe a compensatory increase in insulin secretion despite the reduction in insulin sensitivity, so it is possible that more than one mechanism is contributing to impaired glucose metabolism with sleep restriction in our study. Our results are consistent with recent results in 11 overweight, middle-aged adults that sleep restriction to 5.5 h/night with an ad libitum diet reduces insulin sensitivity but does not change insulin secretion on an IVGTT (40). The current study extends from these findings with two techniques for assessing insulin sensitivity, the insulin-modified FSIVGTT and the gold standard euglycemic-hyperinsulinemic clamp, with concordant results. In further support of the hypothesis that alterations in sleep may affect insulin sensitivity, Van Cauter et al. (24) recently reported that the nearly total suppression of slow-wave sleep by acoustic disruption for 3 nights (without changing total sleep duration) reduces insulin sensitivity as well as acute insulin response.
Substantive differences in the current protocol compared with the results of Spiegel et al. may account for our different results. While both studies examined the effects of sleep restriction in healthy subjects, the baseline sleep-replete condition actually came after the sleep debt condition in the Spiegel protocol, so the sleep-replete condition may reflect more of a recovery process than the actual baseline for each individual. We believe that our sleep-replete baseline more accurately defines (in experimental and ecological terms) the changes in both sleep and metabolism from sleep-replete to sleep-restricted conditions. In addition, the current protocol carefully controlled food intake and activity, whereas the Spiegel protocol allowed subjects to leave the laboratory each day during sleep-restricted conditions. Also, the dose of sleep restriction could influence the results because Spiegel et al. restricted sleep to 4 h/night, whereas we used 5 h/night (to apply to a greater proportion of the adult population). Finally, the specific procedures to assess glucose metabolism differed between the two studies. The Spiegel study (24) used the tolbutamide-assisted FSIVGTT, and this procedure may not have had sufficient sensitivity to detect a significant increase in insulin resistance with sleep restriction.
Previous studies have shown that sleep restriction increases self-reported hunger and appetite for carbohydrate food (41,42). Therefore, to control for potential variations in diet, subjects in the current study consumed a consistent diet throughout the protocol. In addition, subjects maintained a sedentary (but not bed rest) level of activity. Thus, the current study did not allow behavioral changes in diet composition (43), caloric intake, or activity/exercise levels that may have contributed to the association of reduced habitual sleep duration and metabolic dysregulation in previous studies. We did not observe any changes in resting metabolic rate using indirect calorimetry, which is consistent with a prior report of a nonsignificant change in total energy expenditure with sleep restriction (42).
A further strength of the current study is that all subjects began the study in a similar sleep-replete state prior to the imposition of sleep restriction. Under our experimental conditions, there was a deterioration of subjective and objective measures of sleepiness with sleep restriction, consistent with the known effects of sleep restriction. Modafinil administration partially mitigated this effect. However, modafinil treatment had no discernable affect on glucose metabolism. Activation of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system are two key counterregulatory pathways for increasing glucose levels during hypoglycemia. Both these pathways also have been proposed as possible mediators of the impairments in glucose tolerance associated with sleep restriction (22,44–48). In the current study, sleep restriction led to a significant increase in salivary cortisol and urinary norepinephrine and epinephrine. Catecholamine increases with sleep restriction were amplified by modafinil treatment, consistent with prior reports of increased catecholamine levels with modafinil administration (49). However, we found no association, under our experimental conditions, between changes in SI and changes in hypothalamic-pituitary-adrenal axis function and sympathetic nervous system function, suggesting that these systems do not mediate the changes in SI with moderate sleep restriction.
In the present study, the relatively modest restriction of the sleep period to 5 h per night led to a small increase in slow-wave sleep amount by the third night, reflecting an increase in homeostatic sleep drive. A study that deliberately reduced slow-wave sleep through acoustic disruption without changing total sleep time led to a reduction in SI (24). In the current study, we observed reductions in SI due to reductions in total sleep time—not to reductions in slow-wave sleep. Our experimental design is much more closely related to the type of sleep restriction that occurs in healthy individuals who voluntarily restrict sleep.
The limitations of this study include the small sample size that is limited to healthy nonobese men and the lack of a control group that continued the sleep-replete condition of 10 h/night TIB from baseline through to the end of the study. Future studies are needed to determine the effects of sleep restriction on insulin sensitivity in other populations, including women, obese patients, and individuals with insulin resistance or diabetes. It is unlikely that the protocol alone (in the absence of sleep restriction) would lead to decreased insulin sensitivity because, in other published studies, repeating these intensive glucose metabolism test has not led to worsening of metabolic function. Other authors, in validating different types of metabolic challenge tests, have demonstrated the reproducibility of the test results, especially for SI (50). Furthermore, the careful control of diet and exercise allowed us to focus on effects of sleep restriction. However, sleep restriction increases appetite and increases the desire for high- carbohydrate/high-fat foods, so the control of food intake may have dampened the full effects of sleep restriction on glucose metabolism. In addition, we did not measure circadian phase changes directly. However, using a validated, data-based mathematical model, we estimate a <9 min variation in circadian phase under the experimental conditions and light levels employed in this study (32). Thus, circadian phase changes are unlikely to be responsible for the differences we found in insulin sensitivity.
Insufficient sleep duration (quantity) has been associated with an increased risk of obesity (3–6,51), type 2 diabetes (7–11), hypertension (12), cardiovascular disease (13,14), metabolic syndrome (a combination of cardiovascular and metabolic dysfunction) (15), and early mortality (14,16,17,19–21,52). Our finding that reducing sleep increases insulin resistance provides one possible mechanism for these associations. In prospective studies, decreases in the disposition index, the product of insulin secretion and SI, are a strong predictor of diabetes onset and worsening of metabolic function pre- and postdiagnosis (53). Our finding that sleep restriction reduces the disposition index further supports the hypothesis that sleep restriction contributes to the development of metabolic dysregulation resulting in elevated risk for diabetes. Future studies are needed to determine whether chronic short sleep has detrimental effects on insulin resistance and glucose metabolism and whether short sleep is a risk factor for disease processes associated with insulin resistance.
ACKNOWLEDGMENTS
This study was in part supported by NIH/NCRR Grant M01-RR02635.
This study was also supported by Cephalon Inc. No other potential conflicts of interest relevant to this article were reported.
O.B. designed the study, collected data, analyzed data, and wrote the manuscript. M.P. assisted with the study design, collected data, and reviewed and edited the manuscript. E.R. collected data and reviewed and edited the manuscript. W.W. analyzed data, performed statistical analyses, and reviewed and edited the manuscript. D.S. assisted with the study design, assisted with data analysis, and reviewed and edited the manuscript. G.A. assisted with the study design, collected data, assisted with data analysis, and reviewed and edited the manuscript.
We would like to thank the research volunteers for their participation, Brigham and Women's Hospital (BWH) General Clinical Research Center staff for their assistance with data collection, and the BWH Pharmacy for assistance with medications and randomization. For assistance with subject recruitment and data collection, we thank Megan Kunz and Jessica Jones. For assistance with data collection, we thank Keith Malarick, Andrea Muirhead, and Shawn O'Connor.
Footnotes
Clinical trial reg. no. NCT00895570, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hale L, Peppard PE, Young T. Does the demography of sleep contribute to health disparities? In Sleep Disorders, Their Impact on Public Health. Leger D, Pandi-Perumal SR. Eds. London, Informa Healthcare, 2007, p. 1–17 [Google Scholar]
- 2.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 2007;11:163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep 2005;28:1217–1220 [DOI] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289–1296 [DOI] [PubMed] [Google Scholar]
- 5.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 2004;27:661–666 [DOI] [PubMed] [Google Scholar]
- 6.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med 2005;165:25–30 [DOI] [PubMed] [Google Scholar]
- 7.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003;26:380–384 [DOI] [PubMed] [Google Scholar]
- 8.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007;30:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165:863–867 [DOI] [PubMed] [Google Scholar]
- 10.Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005;48:235–241 [DOI] [PubMed] [Google Scholar]
- 11.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006;29:657–661 [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 2006;29:1009–1014 [DOI] [PubMed] [Google Scholar]
- 13.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation 1995;92:3178–3182 [DOI] [PubMed] [Google Scholar]
- 14.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Int Med 2002;251:207–216 [DOI] [PubMed] [Google Scholar]
- 15.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep 2007;30:219–223, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep 2007;30:1659–1666, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond EC. Some preliminary findings of physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health 1964;54:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep 2007;30:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler M. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 2002;59:131–136 [DOI] [PubMed] [Google Scholar]
- 20.Meisinger C, Heier M, Löwel H, Schneider A, Döring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg Cohort study. Sleep 2007;30:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep 2004;27:440–444 [DOI] [PubMed] [Google Scholar]
- 22.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–1439, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530 [DOI] [PubMed] [Google Scholar]
- 24.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008;105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scammell T: Modafinil: mechanisms of action. In Narcolepsy and Hypersomnia. Bassetti C, Mignot E, Billiard M. Eds. New York, Informa Healthcare, 2007, p. 537–549 [Google Scholar]
- 26.Mitchell HA, Bogenpohl JW, Liles LC, Epstein MP, Bozyczko-Coyne D, Williams M, Weinshenker D. Behavioral responses of dopamine beta-hydroxylase knockout mice to modafinil suggest a dual noradrenergic-dopaminergic mechanism of action. Pharmacol Biochem Behav 2008;91:217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belenky G, Wesenstein NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 2003;12:1–12 [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–126 [DOI] [PubMed] [Google Scholar]
- 29.Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Jr, Kingsbury L, Arora S, Schwartz JRL, Niebler GE, Dinges DF. Modafinil for excessive sleepiness associated with shift work sleep disorder. N Engl J Med 2005;353:476–486 [DOI] [PubMed] [Google Scholar]
- 30.Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 1999;10:3745–3748 [DOI] [PubMed] [Google Scholar]
- 31.Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol 1993;265:R846–R857 [DOI] [PubMed] [Google Scholar]
- 32.St. Hilaire MA, Klerman EB, Khalsa SBS, Wright KP, Jr, Czeisler CA, Kronauer RE. Addition of a non-photic component to a light-based mathematical model of the human circadian pacemaker. J Theoretical Biol 2007;247:583–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, D.C., U.S. Government Printing Office, 1968 [Google Scholar]
- 34.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep 1994;17:236–241 [DOI] [PubMed] [Google Scholar]
- 35.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab 2001;86:5366–5371 [DOI] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 37.Caraway WT, Watts NB. Carbohydrates. In Fundamentals of Clin Chem. 3rd ed.Tietz NW. Ed. Philadelphia, WB Saunders, 1987, p. 422–447 [Google Scholar]
- 38.Pomares FJ, Cañas R, Rodriguez JM, Hernandez AM, Parrilla P, Tebar FJ. Differences between sporadic and multiple endocrine neoplasia type 2A pheochomocytoma. Clin Endocrinol (Oxf) 1998;48:195–200 [DOI] [PubMed] [Google Scholar]
- 39.Kuhn E, Brodan V, Brodanova M, Rysanek K. Metabolic reflection of sleep deprivation. Act Nerv Super (Praha) 1969;11:165–174 [PubMed] [Google Scholar]
- 40.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–850 [DOI] [PubMed] [Google Scholar]
- 42.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD: Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxton OM, Quintiliani LM, Yang MH, Ebbeling CB, Stoddard AM, Pereira LK, Sorensen G: Association of sleep adequacy with more healthful food choices and positive workplace experiences among motor freight workers. Am J Public Health 2009;99(Suppl. 3):636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997;20:865–870 [PubMed] [Google Scholar]
- 45.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991;88:934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 2000;85:3597–3603 [DOI] [PubMed] [Google Scholar]
- 47.Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–5771 [DOI] [PubMed] [Google Scholar]
- 48.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 49.Taneja I, Diedrich A, Black BK, Byrne DW, Paranjape SY, Robertson D. Modafinil elicits sympathomedullary activation. Hypertens 2005;45:612–618 [DOI] [PubMed] [Google Scholar]
- 50.Tripathy D, Wessman Y, Gullstrom M, Tuomi T, Groop L. Importance of obtaining independent measures of insulin secretion and insulin sensitivity during the same test: results with the Botnia clamp. Diabetes Care 2003;26:1395–1401 [DOI] [PubMed] [Google Scholar]
- 51.Patel SR, Redline S. Two epidemics: are we getting fatter as we sleep less? Sleep 2004;27:602–603 [PubMed] [Google Scholar]
- 52.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, Strax P, Pasternack BS. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst 1995;87:190–197 [DOI] [PubMed] [Google Scholar]
- 53.Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008;359:2220–2232 [DOI] [PubMed] [Google Scholar]