Abstract
OBJECTIVE
Chronic low-grade inflammation is a feature of obesity and is postulated to be causal in the development of insulin resistance and type 2 diabetes. The aim of this study was to assess whether overfeeding induces peripheral insulin resistance in lean and overweight humans, and, if so, whether it is associated with increased systemic and adipose tissue inflammation.
RESEARCH DESIGN AND METHODS
Thirty-six healthy individuals undertook 28 days of overfeeding by +1,250 kcal/day (45% fat). Weight, body composition, insulin sensitivity (hyperinsulinemic-euglycemic clamp), serum and gene expression of inflammation markers, immune cell activation, fat cell size, macrophage and T-cell numbers in abdominal subcutaneous adipose tissue (flow cytometry and immunohistochemistry) were assessed at baseline and after 28 days.
RESULTS
Subjects gained 2.7 ± 1.6 kg (P < 0.001) and increased fat mass by 1.1 ± 1.6% (P < 0.001). Insulin sensitivity decreased by 11% from 54.6 ± 18.7 to 48.9 ± 15.7 μmol/(kg of FFM)/min (P = 0.01). There was a significant increase in circulating C-reactive protein (P = 0.002) and monocyte chemoattractant protein-1 (P = 0.01), but no change in interleukin-6 and intercellular adhesion molecule-1. There were no changes in fat cell size, the number of adipose tissue macrophages or T-cells, or inflammatory gene expression and no change in circulating immune cell number or expression of their surface activation markers after overfeeding.
CONCLUSIONS
Weight gain-induced insulin resistance was observed in the absence of a significant inflammatory state, suggesting that inflammation in subcutaneous adipose tissue occurs subsequent to peripheral insulin resistance in humans.
Chronic low-level inflammation may be a pivotal link between obesity, insulin resistance, and type 2 diabetes (1). Studies performed in mouse models of obesity and in humans have shown increases in circulating proinflammatory mediators including C-reactive protein (CRP), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6) (2–4). Moreover, obesity is associated with increased macrophage accumulation in adipose tissue with the majority of macrophages being located in crownlike structures, where aggregates of macrophages surround dead adipocytes (5). Importantly, cytokines derived from adipose tissue macrophages inhibit the insulin signaling cascade and can contribute to systemic insulin resistance in humans (6–8). However, whether adipose tissue macrophage accumulation causes insulin resistance in obese humans remains unclear.
Obesity is associated not only with an increase in the total number of macrophages in adipose tissue (9) but also with a change in their activation state, from the alternative M2 to classical M1 phenotype. M1 macrophages are typically proinflammatory and release tumor necrosis factor, IL-6, and MCP-1, whereas M2 macrophages express an anti-inflammatory gene profile, characterized by higher expression of CD206 and IL-10 (10). Recent data has shown that M1 phenotype macrophages are >4-fold increased in subcutaneous adipose tissue from morbidly obese individuals as compared with lean individuals, with surgery-induced weight loss resulting in a twofold decrease in the M1/M2 ratio due to a concomitant decrease in M1 and increase in M2 macrophages (11). Importantly, both the number of M1 macrophages and the M1/M2 ratio have been shown to correlate with whole-body insulin sensitivity (12). There have been no studies examining the effects of moderate weight gain on M1/M2 phenotype in humans.
T-cells, with their activation and hyperpolarization into a proinflammatory T-helper 1 phenotype, may play an important role in initiating and perpetuating adipose tissue inflammation (13–16). In mouse models of obesity, T-cell infiltration in adipose tissue is observed after 5 weeks of high-fat diet (HFD) in conjunction with the induction of insulin resistance (14). Despite evidence linking T-cell infiltration to adipose tissue in animal models of obesity, there have been no studies examining T-cells in adipose tissue during overfeeding in humans. Furthermore, there is no data to clarify whether circulating immune cell activation is an early change contributing to chronic inflammation and development of insulin resistance or whether insulin resistance precedes immune activation.
The aims of this study were to investigate 1) whether short-term high-fat overfeeding and modest weight gain affects insulin sensitivity and systemic low-grade inflammation, immune cell counts and activation, and inflammatory gene expression and 2) the relationships between these variables. Our data suggest that modest weight gain and peripheral insulin resistance induced by overfeeding occurs prior to subcutaneous adipose tissue macrophage infiltration and induction of a significant inflammatory state in humans.
RESEARCH DESIGN AND METHODS
Subjects and study design.
The study design and protocol is described in detail elsewhere (17). Briefly, healthy normolipedemic, nondiabetic individuals completed 28 days of an overfeeding protocol outlined below. The cohort consisted of 19 females (5 postmenopausal) and 17 males, mean age 37 years (21–59 years); 16 subjects reported a family history of type 2 diabetes and 20 had no diabetic relatives. Subjects were not taking any medications. All subjects signed informed consent prior to starting the study. This study was approved by the Human Research and Ethics Committee from St. Vincent's Hospital and is registered as a clinical trial at clinicaltrials.gov, registration number NCT00562393.
Complete 3-day food intakes were provided before metabolic testing visits at baseline and study end. At baseline, this was at calculated energy requirements (17), with a nutrient composition of 30% fat, 15% protein, and 55% carbohydrate. During overfeeding, this was at 1,250 kcal/day above baseline energy requirements with a nutrient composition of 45% fat, 15% protein, and 40% carbohydrate. During the rest of the overfeeding phase (days 3–25), subjects consumed their regular diets and were provided with high-fat snacks (+1,250 kcal) (17). Average reported energy intake during this phase increased from 2,250 ± 480 to 3,250 ± 480 kcal/day, and the contribution of fat from 35 ± 7 at baseline to 46 ± 4%. Weight gain and compliance were monitored weekly by the research nurse and dietitian.
Metabolic testing at baseline and after 28 days of overfeeding.
All metabolic tests were conducted at the Clinical Research Facility at the Garvan Institute of Medical Research after a 12-h overnight fast. Weight was measured in a hospital gown after voiding. Height, blood pressure, and waist and hip circumference were also measured. Fasting blood samples were drawn and insulin sensitivity was assessed by a 2-h hyperinsulinemic-euglycemic clamp (60 mU/m2 body surface · min−1), as previously described (18). Steady-state glucose infusion rate (GIR) between 90 and 120 min was averaged and normalized for the fat free mass (FFM). Body composition was measured by dual-energy X-ray absorptiometry (Lunar DPX GELunar, Lunar Corp., Madison, WI). Liver fat content was measured by computed tomography as previously described (17). A needle biopsy of periumbilical subcutaneous adipose tissue was performed immediately prior to the clamp (19,20), to obtain ∼300 mg of tissue which was processed as described below. The same procedures were repeated on day 28. An additional blood sample for CRP was taken at 3 days of overfeeding.
Immunohistochemistry and fat cell size.
The biopsies were processed as previously described (9,21). We used antibodies targeted to macrophages (HAM56; Dako Cytomation, Trappes, France), M1 (CD40; R&D Systems, Minneapolis, MN) and M2 phenotype macrophages (CD206; R&D Systems, Minneapolis, MN), and T-lymphocytes (CD3; Neomarker Microm, Francheville France) (n = 33). Immunohistochemical detection was performed with the avidin–biotin–peroxidase method, and slides were counterstained with Mayer's hematoxylin. Positively labeled cells were identified by careful visual examination of the slides with increasing grades of magnification and were counted in ten randomly chosen areas at ×40 magnification. The number of positively labeled cells was expressed as a percentage per 100 adipocytes (i.e., number of positive stained cells/number of adipocytes × 100). In addition, adipocyte diameter measurement was performed blindly and for at least two fields of view. The mean diameter was calculated from an average of 400 cells per sample (17).
Immune cell preparation and flow cytometry analysis.
Using standard protocol, fresh whole blood was stained with fluorochrome-conjugated antibodies to various cell surface markers purchased from BD Biosciences (San Diego, CA) in a subset of 24 individuals pre- and post-intervention. All analyses were performed on a BD FACSCaliburTM (BD Biosciences, San Diego, CA) with an excitation laser line argon (488 nm) and red diode (635 nm) and running CellQuest software (version 3.3 from BD Biosciences). Data analysis software FlowJo version 7 from TreeStar Inc. was used. For comparative quantification of surface activation marker expression, the mean fluorescence intensity (MFI) of the marker was divided by the MFI of the unstained control to give relative MFI (rMFI). For quantification of Th1/Th2 cells, we used intracellular cytokine staining for the key cytokines interferon-γ (for Th1) and IL-4 (for Th2) (BD Bioscience Pharmingen, San Diego, CA). Briefly, peripheral blood mononuclear cells were activated with phorbol myristic acid (160 ng/ml) and Ionomycin (1,000 ng/ml) for 4 h at 37°C in the presence of GolgiPlug (BD Bioscience, San Diego, CA), allowing the identification of T-helper cell subsets. After surface staining for CD4+ and CD8+, cells were permeabilized using BD Cytofix/Cytoperm reagents (BD Bioscience Pharmingen, San Diego, CA), stained for intracellular cytokines, and analyzed immediately by flow cytometry.
Flow cytometry of adipose tissue stromovascular fraction.
Fresh periumbilical subcutaneous adipose tissue (∼150 mg) was placed in a prewarmed buffer (10 mmol/l CaCl2, 6 mmol/l Na2HPO4,125 mmol/l HEPES, 12 mmol/l MgSO4, 4 mmol/l NaH2PO4, 1.2M NaCl, 60 mmol/l KCl, 3 g BSA, and 0.09 g d-glucose in 100 ml H2O, pH 7.4, 37°C) and digested with collagenase type IV at 37°C. Stromovascular fraction was separated from mature adipocytes by centrifugation (1,200 rpm), washed twice with ice-cold PBS, and stained to quantify cell subsets and activation markers (22) by flow cytometry. Preadipocytes/CD34+ progenitor cells were identified by staining for stem cell marker CD34 and by excluding CD31+/endothelial cells. Macrophages were detected by CD14+ staining. Results are expressed as a percentage of all viable cells, and CD11b expression on macrophages by rMFI.
RNA extraction and quantitative real-time PCR.
Total RNA was extracted from 100–150 mg adipose tissue using TRIzol reagent (Invitrogen, Carlsbad, CA). The integrity and concentration of RNA was assessed by spectrophotometry (Nanodrop, 2000, Thermoline). cDNA was synthesized using Omniscript RT kit (Qiagen, GmbH, Germany) and Recombinant RNAsin RNase inhibitor (Promega, Madison, WI) according to kit instructions. For RT-PCR analyses, we chose a range of macrophage inflammation related genes and used gene-specific primer probes from Taqman (CD68, CD11c, CD206, MCP-1, IL-10, CD40, and Arginase 1) and Taqman universal PCR master mix (Applied Biosystems, Darmstadt, Germany). The samples were run in duplicate on an ABI Prism 7,900 system (Applied Biosystems, Darmstadt, Germany) with internal negative controls and a standard curve. The CT value for each sample was normalized to the CT value of β-actin, which was not different between days 0 and 28. CD40 and Arginase 1 expression were below detection.
Biochemical variables.
Plasma glucose was determined by the glucose oxidase method (Glucose analyzer 2,300 STAT PLUS 230V, YSI, Inc., Yellow Springs, OH). Serum insulin was measured by radioimmunoassay (Linco, St. Charles, MO). Serum total cholesterol, HDL cholesterol, and triglycerides were determined spectrophotometrically at 490 nm by enzymatic colorimetry (Roche, Basel, Switzerland). High-sensitivity CRP was measured using a Beckman Coulter Synchron LX system Chemistry Analyser, with reagents and calibrators supplied by Beckman Coulter Inc. (Sydney, Australia). CV was 3.7% at a level of 4.9 mg/l, and the sensitivity was 0.2 mg/l. IL-6, MCP-1, and soluble intercellular adhesion molecule (sICAM)-1 were measured using commercial high-sensitivity ELISAs (R&D Systems, Minneapolis MN). The observed CVs were 7%, 10%, and 10%, respectively.
Statistical analysis.
Analyses were performed using Statistical Package for Social Sciences, version 15.0. Because no differences were detected for any markers of inflammation at baseline or in response to overfeeding between individuals with and without a family history of diabetes, these groups were combined for all analyses. Comparisons between time points were performed using the paired t test (for normally distributed data) and the nonparametric Sign test (for skewed data). Correlations between variables were expressed as Pearson or Spearman correlation coefficients. P < 0.05 was considered significant, and data are presented as mean ± SD unless otherwise stated.
RESULTS
Clinical measures.
Anthropometric and metabolic measures of the 36 participants studied at baseline and after 28 days of overfeeding are summarized in Table 1. Weight gain was significant, with an increase of 2.7 ± 1.6 kg (+3.6%, P < 0.001). Similarly, BMI, waist circumference, % body fat, and liver fat were significantly increased (all P < 0.001). Fat cell size did not change. Fasting insulin and glucose increased (P < 0.01), and insulin sensitivity decreased by 11% (P = 0.01).
TABLE 1.
Anthropometric and metabolic measures of 36 subjects at baseline and after 28 days of overfeeding
| Variable | Day 0 | Day 28 | P |
|---|---|---|---|
| Weight (kg) | 75.9 ± 12.3 | 78.6 ± 12.7 | <0.001 |
| BMI (kg/m2) | 26.0 ± 3.6 | 26.9 ± 3.7 | <0.001 |
| Waist (cm) | 88.9 ± 10.8 | 91.4 ± 11.6 | <0.001 |
| WHR | 0.87 ± 0.08 | 0.87 ± 0.09 | 0.34 |
| Fat mass (%) | 35.8 ± 8.8 | 36.9 ± 8.5 | <0.001 |
| Fat cell size (μM) | 59.2 ± 6.6 | 58.3 ± 6.5 | 0.53 |
| Liver fat (Hu) | 54.9 ± 11.6 | 52.6 ± 11.5 | 0.001 |
| Abdominal fat (kg) | 2.0 ± 0.7 | 2.2 ± 0.7 | <0.001 |
| Glucose (mmol/l) | 4.5 ± 0.4 | 4.6 ± 0.3 | 0.01 |
| Fasting insulin (mU/ml) | 9.9 ± 3.3 | 11.4 ± 2.9 | 0.01 |
| GIR/FFM (μmol/kg/min) | 54.9 ± 18.7 | 48.9 ± 15.7 | 0.01 |
| Total cholesterol (mmol/l) | 4.7 ± 1.0 | 4.9 ± 1.2 | 0.01 |
| HDL cholesterol (mmol/l) | 1.3 ± 0.3 | 1.4 ± 0.5 | <0.001 |
| LDL cholesterol (mmol/l) | 2.8 ± 0.9 | 2.8 ± 0.9 | 0.75 |
| Triglycerides (mmol/l) | 1.1 ± 0.4 | 1.1 ± 0.6 | 0.89 |
Data are presented as means ± SD. P < 0.05 was considered statistically significant. WHR, waist-to-hip ratio.
Serum inflammation markers.
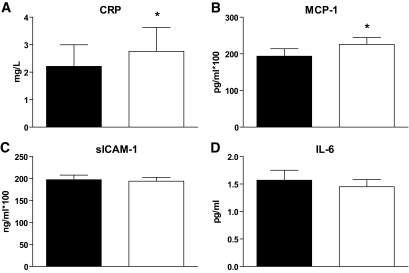
In response to 28 days of overfeeding, we observed significant increases in CRP (P = 0.003) and MCP-1 (P = 0.005), but no change in IL-6 or sICAM-1 (Fig. 1). CRP levels showed a similar increase after 3 days (P = 0.008, data not shown). At baseline, CRP correlated with BMI, % fat, and fat cell size (all r > 0.6, P < 0.001) and IL-6 correlated positively with % fat mass and increased liver fat (both r = 0.4, P = 0.04), but neither inflammatory marker correlated with baseline GIR/FFM. No associations were seen between levels of MCP-1 or sICAM-1 and any measure of adiposity, although MCP-1 levels were negatively correlated with GIR/FFM (r = −0.38, P = 0.03). At baseline, MCP-1 levels were correlated with the number of HAM56 labeled cells (r = 0.38, P = 0.04) and IL-6 levels were associated with increased liver fat (r = −0.41, P = 0.02). There were no relationships between changes in markers of inflammation and adiposity or insulin sensitivity in response to overfeeding.
FIG. 1.
Serum levels of inflammatory markers. (A) CRP, (B) MCP-1, (C) sICAM-1, and (D) IL-6 levels at baseline (black bars) and 28 days after overfeeding (open bars). Data are presented as means ± SEM. *P < 0.05.
Subcutaneous adipose tissue gene expression.
Macrophage and inflammation related genes, CD68, CD40, CD206, CD11c, MCP-1, and IL-10, were examined. We found no significant differences between baseline and 28 days after overfeeding for any genes measured (data not shown). Furthermore, there were no baseline or change from baseline associations between gene expression and measures of adiposity and insulin sensitivity.
Immunohistochemistry.
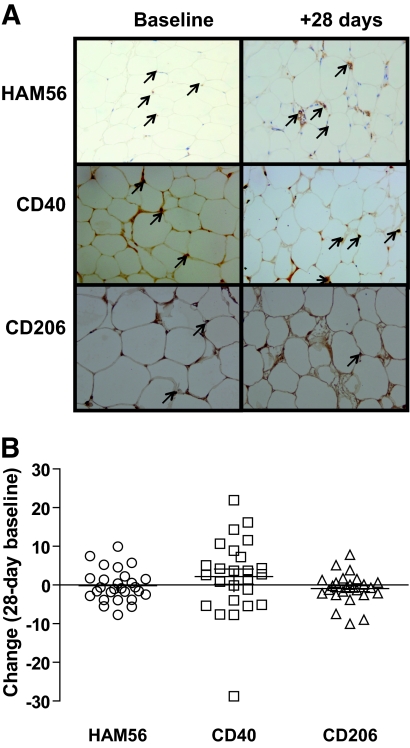
HAM56 labeled macrophages were mostly dispersed in the parenchyma and few crownlike structures were seen at baseline or 28 days after overfeeding (Fig. 2). As in a previous study (11), CD40 and CD206 were used as markers of M1 and M2 macrophages, respectively. We found a trend toward a higher M1/M2 ratio at 28 days of overfeeding (P = 0.05), although there were no statistical differences in the absolute numbers of CD40 (P = 0.36) and CD206 (P = 0.42) labeled cells (Fig. 2). There was little evidence of CD3 labeled T-cells at baseline or 28 days after overfeeding, with CD3+ cells only observed in 2/66 slides. At baseline, CD40 was related to liver fat (r = 0.4, P = 0.03) but no other markers of adiposity. There were no other associations between macrophage markers and adiposity or insulin resistance at baseline or in response to overfeeding (data not shown).
FIG. 2.
Immunohistochemistry staining of (A) macrophage-specific marker (HAM56), M1 marker (CD40), and M2 marker (CD206) phenotype macrophages in subcutaneous adipose tissue. Pictures are taken from paired representative slides, and positive cells are marked with a black arrow. B: Change in HAM56, CD40, CD206 positive cells from baseline in response to 28 days of overfeeding. (A high-quality digital representation of this figure is available in the online issue.)
Stromovascular fraction of adipose tissue.
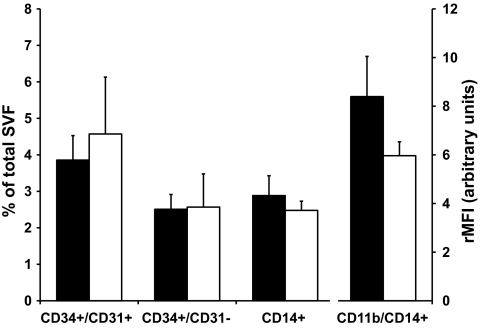
The cellular content of the stromovascular fraction of periumbilical subcutaneous adipose tissue was investigated by flow cytometry. The number of macrophages, preadipocytes, and endothelial cells were unchanged after 28 days of overfeeding (Fig. 3). Similarly, CD11b (MAC-1) expression on macrophages did not change. At baseline, the number of macrophages in subcutaneous adipose tissue was significantly correlated with central fat mass (r = 0.36, P = 0.02), liver fat (r = 0.53, P = 0.002), and insulin resistance (r = −0.37, P < 0.001). Also, CD11b expression on macrophages correlated positively with MCP-1 levels at baseline (r = 0.48, P = 0.01).
FIG. 3.
Analysis of stromovascular fraction of subcutaneous adipose tissue. Relative numbers of endothelial cells (CD34+/CD31+), preadipocytes/CD34+ precursor cells (CD34+/CD31−), macrophages (CD14+) and expression of activation marker CD11b on macrophages, shown as rMFI at baseline (black bars) and after 28 days of overfeeding (open bars). Data are presented as means ± SEM. SVF, stromovascular fraction.
Systemic changes in immune cell activation and T-cell polarization.
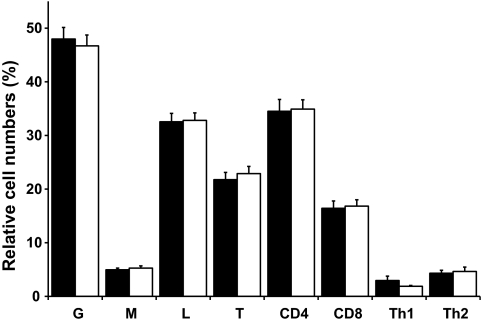
Circulating immune cells were also analyzed by flow cytometry at both visits, with a focus on cell numbers of major immune cell subsets and on expression of cell surface activation markers on different subsets. There was no change in relative cell number of neutrophils, monocytes, and lymphocytes after 28 days of overfeeding (Fig. 4). Similarly, numbers of CD4+ T-helper cells, including the specific phenotypes type 1 and type 2, as well as CD8+ cytotoxic T-cells, did not change over the 28-day overfeeding period. Furthermore, expression of activation markers CD66b, CD62L, and CD11b on neutrophils and monocytes, as well as CD69, CD62L, and CD25 on T-cells were not changed by overfeeding (Table 2).
FIG. 4.
Circulating immune cell numbers. Relative cell numbers of circulating immune cell subsets, expressed as a percentage of total white blood cells [granulocytes (G), monocytes (M), lymphocytes (L), or T-cells (T)] or as a percentage of T-lymphocytes (CD4, CD8) or as a percentage of CD4+ T-cells [T-helper cells type 1 (Th1) and type 2 (Th2)] at baseline (black bars) and after 28 days of overfeeding (open bars). Data are presented as means ± SEM.
TABLE 2.
Cell surface expression of activation markers on distinct immune cell subsets at baseline and after 28 days of overfeeding in a subset of 24 subjects
| Activation marker (relative mean fluorescence intensity) | Day 0 | Day 28 | P |
|---|---|---|---|
| Neutrophils CD66b | 7.5 ± 0.7 | 7.9 ± 0.8 | 0.68 |
| Neutrophils CD62L | 41.4 ± 5.6 | 42.3 ± 6.6 | 0.21 |
| Neutrophils CD11b | 1.8 ± 0.2 | 2.1 ± 0.5 | 0.40 |
| Monocytes CD66b | 0.9 ± 0.0 | 1.0 ± 0.0 | 0.21 |
| Monocytes CD62L | 55.2 ± 11.9 | 61.9 ± 10.4 | 0.99 |
| Monocytes CD11b | 6.9 ± 0.9 | 6.0 ± 1.4 | 0.10 |
| T-cells CD69 | 1.1 ± 0.1 | 1.0 ± 0.0 | 0.68 |
| T-cells CD62L | 30.0 ± 5.7 | 35.5 ± 6.2 | 0.99 |
| T-cells CD25 | 1.5 ± 0.3 | 1.2 ± 0.1 | 0.83 |
Data are presented as means ± SE. Degranulation marker CD66b, adhesion molecule CD62L, adhesion molecule CD11b, interleukin-2 receptor (CD25), activation marker CD69, and adhesion molecule CD62L.
DISCUSSION
Obesity and type 2 diabetes are characterized by the coexistence of insulin resistance and chronic low-grade inflammation (1). However, it is controversial whether insulin resistance is a cause or consequence of such “inflammation.” Our study demonstrates that overfeeding for 28 days in healthy humans results in moderate weight gain, with significant increases in total and abdominal fat mass and liver fat deposition. In parallel, we observed increases in fasting insulin and an 11% decrease in peripheral insulin sensitivity by hyperinsulinemic clamp. Importantly, this study is the first to show that peripheral insulin resistance occurred without substantial changes in inflammatory markers in the circulation or within subcutaneous adipose tissue in humans as demonstrated using flow cytometry, immunohistochemistry, and gene expression. Our data therefore suggest that low-grade systemic and subcutaneous adipose tissue inflammation, macrophage infiltration, and immune activation occur secondary to weight gain and peripheral insulin resistance in humans. Interpretation of these findings is limited to subcutaneous adipose tissue. The dynamics of the inflammatory response in other fat depots, particularly visceral adipose tissue, cannot be addressed in this study.
Inflammation has been implicated as a primary factor in the pathogenesis of insulin resistance and type 2 diabetes (3,8). This is supported by studies showing associations between elevated inflammatory markers and indexes of insulin resistance in obese animals and humans (23–25) and in vitro studies showing that addition of inflammatory cytokines inhibits the insulin signaling cascade (26). Moreover, studies in rodents have shown that there is a rapid induction of inflammatory gene expression in adipose tissue after HFD (27). Interestingly, Xu et al. also observed significant elevations in MCP-1 gene expression after 3 weeks of HFD, but did not detect changes in macrophage-specific markers (F4/80 or CD68) until 16 weeks of HFD (28). Strissel et al. also performed an elegant temporal investigation of macrophage infiltration and adipocyte death at 1, 4, 8, 16, and 20 weeks. They observed that adipocyte hypertrophy preceded adipocyte death and macrophage infiltration, which was significantly elevated 8 weeks after initiation of HFD as assessed by inflammatory gene expression and F4/80 staining. This increase coincided with impaired responsiveness to insulin, suggesting a link between macrophage infiltration of adipose tissue and induction of peripheral insulin resistance. However, the gold standard measure of insulin sensitivity, the hyperinsulinemic clamp, was not performed in this study and insulin resistance measured by clamp or by 2-deoxyglucose uptake is impaired as early as 3 weeks after initiation of HFD in rodents (29,30).
Few studies have prospectively examined the effects of overfeeding on adipose tissue macrophage infiltration in humans. In the current study, we detected no changes in subcutaneous adipose tissue macrophages by gene expression or by histology after 28 days of overfeeding with the exception of increased CD40/CD206 ratio, but without significant changes in the absolute numbers of CD40 and CD206 labeled cells and no change in circulating immune cells. It is possible that the single time point chosen here was inappropriate to detect substantial changes in macrophage infiltration. Regardless, 28 days of overfeeding induced peripheral insulin resistance, supporting the dissociation between macrophage infiltration and induction of insulin resistance in humans. Interestingly, mouse studies have shown that prevention of inflammation by macrophage-specific knockout of JNK or IKKβ only provides partial protection from diet-induced insulin resistance; that is, the knockouts are more insulin sensitive as compared with wild-types on HFD, but they are still more insulin resistant as compared with chow-fed animals (31,32). In humans, future studies to definitively answer whether insulin resistance precedes macrophage infiltration could involve more sampling time points or be performed with anti-inflammatory agents to determine whether dampening of the inflammatory cascade prevents insulin resistance during overfeeding in humans.
CRP, a validated inflammatory marker secreted by the liver, is highly correlated with adiposity and rapidly reduced by weight loss (33). We observed significantly increased serum CRP at 3 and 28 days of overfeeding and speculate that this could be due to the increase in liver fat after overfeeding. Associations have previously been reported between CRP and non-alcoholic fatty liver disease (NAFLD) (34–36), although we did not observe a correlation between change in CRP and change in liver fat. Interestingly, serum MCP-1 was also increased after 28 days of overfeeding, despite no detectable changes in subcutaneous adipose tissue MCP-1 expression, or HAM56+ macrophages, suggesting another source of MCP-1 (37,38). MCP-1 is involved in the recruitment of macrophages into adipose tissue (39) and transgenic mice overexpressing MCP-1 exhibit insulin resistance (39). CCR2 knockout mice have improved insulin sensitivity, reduced macrophage content, and reduced inflammatory profile in adipose tissue while on a HFD (40). Previous studies have shown that adipose MCP-1 mRNA levels increased within 2–7 days of HFD in mice, with an elevation in plasma MCP-1 detected after 4 weeks (27). Thus, we postulate that the early increase in circulating MCP-1 in this study may play a role in later recruitment of macrophages into adipose tissue.
Recent studies have suggested that adipose tissue infiltration by T-lymphocytes is a primary event leading to subsequent recruitment of macrophages (13–15). Kintscher et al. demonstrated development of glucose intolerance and T-cell infiltration after 3 weeks of high-fat feeding but did not observe any changes in adipose tissue macrophage infiltration until 8 weeks of HFD, where there was significant weight gain in mice. Together with other animal studies (13,15), these results suggest that glucose intolerance and T-cell activation may be primary events preceding macrophage infiltration and that macrophages only contribute later to insulin resistance by increased secretion of cytokines. In the current study, we saw little evidence of T-cell accumulation in subcutaneous adipose tissue. Future overfeeding studies in overweight to morbidly obese cohorts will be important for elucidating immune cell dynamics in adipose tissue in humans.
In conclusion, moderate weight gain after 28 days of overfeeding in healthy humans resulted in significantly increased body fatness, peripheral insulin resistance, and increased circulating CRP and MCP-1, without changes in subcutaneous adipose tissue immune cell histology or inflammatory gene expression. We hypothesized that adipose tissue infiltration by macrophages is dynamic and should occur at the time of fat deposition, if they are causative in insulin resistance. However, we detected no increase in the proportion of subcutaneous adipose tissue macrophages despite significant fat deposition and insulin resistance. These combined findings support the hypothesis that weight gain-induced insulin resistance from short-term overfeeding is an early metabolic defect preceding immune activation and macrophage recruitment into subcutaneous adipose tissue in humans.
ACKNOWLEDGMENTS
This study was funded by grants from the National Health & Medical Research Council (NHMRC #427639), Diabetes Australia Research Trust, and the Commission of the European Communities (Collaborative Project ADAPT, contract number HEALTH-F2-2008-201100). C.S.T. is funded by NHMRC/National Heart Foundation of Australia Postgraduate Biomedical Scholarship (#457224) and NHMRC Travelling Award for Research Training (#571180); A.V. is funded by GSK Don Chisholm Diabetes Research Fellowship; J.R.G. is funded by NHMRC Neil Hamilton Fairley Fellowship; and L.K.H. is funded by NHMRC Career Development Award (#481354).
No potential conflicts of interest relevant to this article were reported.
C.T., A.V., and L.H. wrote the manuscript. C.T., A.V., D.S., K.C., J.T., J.G., and K.T. designed/performed experiments and provided critical comments. L.H. and L.C. designed the study.
Our thanks go to the staff of the Garvan Clinical Research Facility and in particular the study coordinator, Lynne Schofield, and all the volunteers who participated in the study.
We also thank Pr. Daniele Hugol, Patricia Bonjour, and Beatrice from the Anathomopathology Department at Hotel-Dieu Hospital for technical assistance with the CD3 staining.
Footnotes
Clinical trial registry no.: NCT00562393; clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998;83:847–850 [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 4.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 6.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005;54:2939–2945 [DOI] [PubMed] [Google Scholar]
- 7.Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M, Krogh-Madsen R, Erikstrup C, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Associations between insulin resistance and TNF-alpha in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 2007;50:2562–2571 [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665–668 [DOI] [PubMed] [Google Scholar]
- 9.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005;54:2277–2286 [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clément K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 2009;94:4619–4623 [DOI] [PubMed] [Google Scholar]
- 12.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009;58:2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffaut C, Galitzky J, Lafontan M, Bouloumié A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 2009;384:482–485 [DOI] [PubMed] [Google Scholar]
- 14.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304–1310 [DOI] [PubMed] [Google Scholar]
- 15.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 16.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. Eur J Endocrinol 2006;154:691–697 [DOI] [PubMed] [Google Scholar]
- 17.Samocha-Bonet D, Campbell LV, Viardot A, Freund J, Tam CS, Greenfield JR, Heilbronn LK. A family history of type 2 diabetes increases risk factors associated with overfeeding. Diabetologia 2010;53:1700–1708 [DOI] [PubMed] [Google Scholar]
- 18.Kriketos AD, Greenfield JR, Peake PW, Furler SM, Denyer GS, Charlesworth JA, Campbell LV. Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care 2004;27:2033–2040 [DOI] [PubMed] [Google Scholar]
- 19.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, Smith SR. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab 2004;89:1844–1848 [DOI] [PubMed] [Google Scholar]
- 20.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clément K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006;55:1554–1561 [DOI] [PubMed] [Google Scholar]
- 22.Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol 2005;205:114–122 [DOI] [PubMed] [Google Scholar]
- 23.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O'Reilly DS, Packard CJ, Sattar NWest of Scotland Coronary Prevention Study C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002;51:1596–1600 [DOI] [PubMed] [Google Scholar]
- 24.Thorand B, Löwel H, Schneider A, Kolb H, Meisinger C, Fröhlich M, Koenig W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med 2003;163:93–99 [DOI] [PubMed] [Google Scholar]
- 25.Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology 2010;151:971–979 [DOI] [PubMed] [Google Scholar]
- 26.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Görgün CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 2006;4:465–474 [DOI] [PubMed] [Google Scholar]
- 27.Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, Mudgett J, Chen H, MacNeil DJ, Reitman ML, Qian S. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res 2005;13:1311–1320 [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009;58:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005;54:3530–3540 [DOI] [PubMed] [Google Scholar]
- 31.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 32.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 2007;6:386–397 [DOI] [PubMed] [Google Scholar]
- 33.Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol 2001;21:968–970 [DOI] [PubMed] [Google Scholar]
- 34.Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-sensitivity C-reactive protein as a serum predictor of nonalcoholic fatty liver disease based on the Akaike Information Criterion scoring system in the general Japanese population. J Gastroenterol 2009;44:313–321 [DOI] [PubMed] [Google Scholar]
- 35.Oruc N, Ozutemiz O, Yuce G, Akarca US, Ersoz G, Gunsar F, Batur Y. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol 2009;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 2007;42:573–582 [DOI] [PubMed] [Google Scholar]
- 37.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005;25:2594–2599 [DOI] [PubMed] [Google Scholar]
- 38.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009;117:95–109 [DOI] [PubMed] [Google Scholar]
- 39.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006;281:26602–26614 [DOI] [PubMed] [Google Scholar]
- 40.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]