Abstract
OBJECTIVE
To test whether induction of chimerism lowers the amount of donor islets required for reversal of diabetes and renders the pancreas a suitable site for islet grafts in autoimmune diabetic mice.
RESEARCH DESIGN AND METHODS
The required donor islet dose for reversal of diabetes in late-stage diabetic NOD mice after transplantation into the liver or pancreas was compared under immunosuppression or after induction of chimerism. Recipient mice were monitored for blood glucose levels and measured for insulin-secretion capacity. Islet grafts were evaluated for β-cell proliferation, β-cell functional gene expression, and revascularization.
RESULTS
With immunosuppression, transplantation of 1,000, but not 600, donor islets was able to reverse diabetes when transplanted into the liver, but transplantation of 1,000 islets was not able to reverse diabetes when transplanted into the pancreas. In contrast, after induction of chimerism, transplantation of as few as 100 donor islets was able to reverse diabetes when transplanted into either the liver or pancreas. Interestingly, when lower doses (50 or 25) of islets were transplanted, donor islets in the pancreas were much more effective in reversal of diabetes than in the liver, which was associated with higher β-cell replication rate, better β-cell functional gene expression, and higher vascular density of graft islets in the pancreas.
CONCLUSIONS
Induction of chimerism not only provides immune tolerance to donor islets, but also markedly reduces the required amount of donor islets for reversal of diabetes. In addition, this process renders the pancreas a more superior site than the liver for donor islets in autoimmune mice.
Type 1 diabetes results from autoimmune destruction of insulin-secreting pancreatic β-cells (1). A cure for type 1 diabetes will require a reversal of autoimmunity as well as regeneration or replacement of islet β-cells (2,3). Currently, islet transplantation is the only therapy with the potential to reverse refractory late-stage type 1 diabetes in animal models and patients (4), although hundreds of regimens have been reported to prevent type 1 diabetes (3), and anti-CD3 therapies have been shown to reverse or ameliorate new-onset type 1 diabetes in mouse models or patients (5–8). However, islet transplantation under the current Edmonton protocol of administration of immunosuppressants, including rapamycin, tacrolimus, and anti-IL-2 mAb, only provides insulin independence for 1 to 2 years in most recipients (9).
The obstacles that prevent islet transplantation as a curative therapy for refractory late-stage type 1 diabetes include: 1) chronic rejection of islet grafts mediated by allo- and autoimmunity (10,11); 2) chronic toxicity of immunosuppressants and their impairment of islet graft function and β-cell regeneration (12–14); 3) limited availability of donor islets (e.g., under the Edmonton protocol, more than two cadaveric donors are usually required for each recipient, which limits the widespread application of islet transplantation); and 4) lack of an ideal graft site. For instance, in current clinical islet transplantation, donor islets are usually injected via the portal vein into the liver. Unfortunately, this procedure has shortcomings. First, portal vein injection of islets can cause bleeding, thrombosis, and portal system hypertension. Second, studies with syngeneic islet transplantation in animal models have shown that islet grafts in the liver, but not in the pancreas, demonstrate significant dysfunction 1 month after transplantation (15,16). Third, glucagon secretion has been found to be defective in response to insulin-induced hypoglycemia in some patients with islet grafts in the liver, although islet transplantation has impressively decreased the number and severity of episodes of hypoglycemia (17,18). As one alternative, the pancreas itself could provide a site for donor islets; however, it is still unknown whether the pancreas of an autoimmune individual can be a graft site for donor islets. Taken together, a regimen that can overcome each of these obstacles could promote islet transplantation as a curative therapy for refractory type 1 diabetes.
Induction of mixed chimerism is one of the most effective approaches for induction of organ transplantation tolerance (19), and this concept has been recently demonstrated in humans (20–22). In addition, the induction of chimerism is also effective in reversal of autoimmunity in autoimmune NOD mice (2,23–26). Furthermore, we recently reported that induction of chimerism under a radiation-free and graft versus host disease (GVHD) preventive anti-CD3-based conditioning regimen led to reversal of autoimmunity and elimination of insulitis (2,27). Beyond this, it has been reported that adult β-cells can replicate to reverse hyperglycemia in nonautoimmune mice (12). In addition, we have observed that after elimination of insulitis by induction of chimerism, residual islet β-cells in the pancreas proliferated to reverse diabetes in new-onset, although not in late-stage, diabetic NOD mice (2).
In the current study, we tested whether induction of chimerism under the radiation-free anti-CD3-based conditioning regimen lowers the amount of donor islets required for reversal of diabetes and whether the pancreas would provide a suitable site for islet grafts in late-stage diabetic NOD mice. Herein, we have demonstrated that, after induction of chimerism, the required amount of donor islets for reversal of diabetes was markedly reduced when transplanted into the liver or pancreas. In addition, our studies suggest that after induction of chimerism, the pancreas may provide a superior site for donor islets in comparison with the liver, as assessed by enhanced β-cell replication, better long-term β-cell function, and improved revascularization of the graft islets in the pancreas.
RESEARCH DESIGN AND METHODS
Female NOD/LtJ (H-2g7) and FVB/N (H-2q) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The luciferase transgenic (Luc+) FVB/N line was generated as previously described (28). All animals were maintained in a pathogen-free room at City of Hope Research Animal Facilities (Duarte, CA). The animal-use procedures were approved by the Institutional Committee of City of Hope.
Immunosuppressant therapy.
Late-stage (>3 weeks after onset) diabetic NOD mice were given Edmonton protocol of chemotherapy before islet transplantation, including administration of rapamycin (i.p. 2 mg/kg daily, LC Laboratories, MA), tacrolimus (i.p. 0.6 mg/Kg daily, LC Laboratories, Woburn, MA), and anti-mouse-IL-2 mAb (i.p. 0.5 mg/mouse weekly, S4B6–1).
Bone marrow transplantation.
These procedures were described in our previous publications (2,29) and in the supplementary materials and methods, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0450/DC1.
Isolation of islets, islet transplantation, in vivo and ex vivo bioluminescent imaging, insulin level, and glucose tolerance test.
These procedures have been described in publications of ours and others (2,29,30) and in the supplementary materials and methods.
Histopathology of pancreatic islets, bromodeoxyuridine labeling and sequential chlorodeoxyuridine (CLDU) and iododeoxyuridine (IDU) labeling of proliferating β-cells and immunofluoresent staining.
These procedures have been described in publications of ours and others (2,27,31) and in the supplementary materials and methods.
Measurement of gene expression levels of the retrieved islet grafts.
Retrieving graft islets from the liver and pancreas were performed as described previously (32,33). The primers used for real-time PCR measurement of gene expression levels of luciferace, insulin, pancreatic duodenal homeobox-1 (PDX-1), GLUT2, glucokinase (GCK), and glucagon were described by others (34,35), and real-time PCR was performed as described in our previous publications (36) and in the supplementary materials and methods.
Evaluation of vascular density.
Sections were stained for lectin from Bandeiraea simplicifolia (Sigma) followed by Texas Red conjugated Streptavidin (Jackson ImmunoResearch). Vascular density was identified and evaluated as previously described (37,38) and in the supplementary materials and methods.
Amylase test.
Amylase activity was measured by EnzChek Ultra Amylase Assay Kit (E33651; Molecular Probes) following the manufacturer's protocol.
Statistical Analysis.
Comparison of changes of serum blood glucose levels after hematopoietic cell transplantation (HCT) was evaluated by the log-rank test (Prism, version 4.0, GraphPad, San Diego, CA). Comparison of kinetic blood glucose change in glucose tolerance test (GTT) test was evaluated with two-way ANOVA. Comparison of means among multiple groups was evaluated with one-way ANOVA; comparison of two means was performed with two-tailed Student t test.
RESULTS
A large amount of donor islets that could reverse diabetes when implanted in the liver did not reverse diabetes when implanted in the pancreas in late-stage diabetic NOD mice under immunosuppressant therapy.
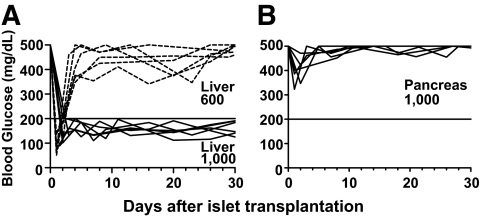
It was reported that islet grafts functioned better in the pancreas than in the liver in nonautoimmune recipients (15,16). However, it is not yet clear whether the pancreas of the autoimmune recipients can be used as a site of donor islets. Under immunosuppressant therapy, 600 or 1,000 donor islets were required to reverse diabetes in diabetic NOD mice (39,40). Therefore, we transplanted 600 or 1,000 donor islets into the liver or pancreas of the late-stage diabetic NOD mice, while immunosuppressant therapy of Edmonton protocol was used to prevent graft rejection. Harvesting 1,000 islets required ∼5 donors because the average yield in our studies was 209 ± 8 per donor (mean ± SE, N = 20), which was similar to a previous report (41). We observed that although 1,000 donor islets implanted in the liver were able to reverse diabetes in all (6 of 6) of the recipients, the same amount of donor islets implanted in the pancreas did not reverse diabetes (0 of 6) (P < 0.001, Fig. 1A and B). Lowering the donor islet dose to 600 in the liver also resulted in an inability to stably reverse diabetes (Fig. 1A). These results indicate that under this form of immunosuppressant therapy, the pancreas of the autoimmune NOD mice is not a suitable site for donor islets.
FIG. 1.
Transplantation of a large number of donor islets was needed to reverse diabetes in immunosuppressant-treated late-stage diabetic NOD mice. Late-stage diabetic NOD mice were transplanted with 1,000 or 600 islets from FVB/N mice into the liver (A) or the pancreas (B) after the mice were given immunosuppressant therapy. The recipients were monitored twice weekly for blood glucose for 30 days after transplantation. For recipients with 600 donor islets in the liver or 1,000 donor islets in the pancreas, exogenous insulin was used to ensure the survival of the recipients after transplantation. If a recipient's blood glucose was >500 mg/dl, the mouse was injected with insulin (1 unit daily) for the subsequent 5 days, and injections were stopped 2 days before blood glucose measuring. There were 6 recipients in each group.
Induction of chimerism not only markedly reduced the required donor islet dose for reversal of late-stage diabetes, but also rendered the pancreas of the autoimmune NOD mice a suitable site for islet grafts.
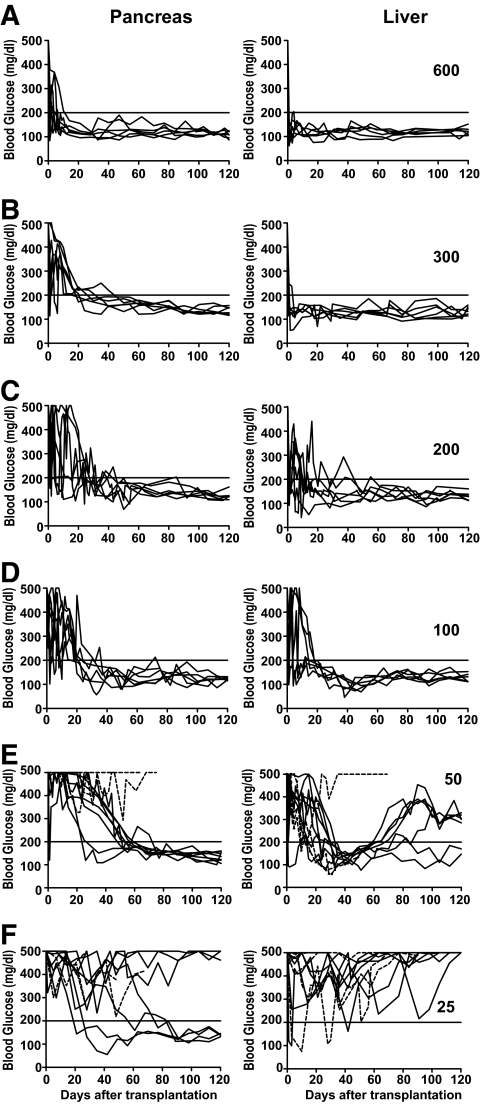
We previously reported that induction of chimerism resulted in reversal of autoimmunity, elimination of insulitis, and immune tolerance to donor islets when implanted under the kidney capsule (2). Therefore, we tested whether the pancreas of the chimeric diabetic NOD recipients could be a suitable site for islet grafts. Accordingly, late-stage diabetic NOD mice were induced to develop chimerism by conditioning with anti-CD3/CD8 along with one injection of donor CD4+ T-depleted spleen cells and bone marrow cells (50 × 106 each), as described in research design and methods and in our previous publication (2). Next, 600 donor islets, which did not reverse diabetes in the late-stage diabetic NOD mice under the above mentioned immunosuppressant therapy, were transplanted into the pancreas or liver of the chimeric recipients on the next day after injection of donor bone marrow cells. We found that 100% (6 of 6) of the chimeric recipients with 600 donor islets in the pancreas or liver showed normal glycemia for >120 days, although they all had hyperglycemia (>500 mg/dl) before islet transplantation (Fig. 2A). The chimeric late-stage diabetic NOD mice without islet transplantation continued to have hyperglycemia, and the chimeric recipients with islet grafts under kidney capsule showed hyperglycemia again after nephrectomy (supplementary Fig. 1). Next, we titrated down the amount of donor islets and transplanted 300, 200, and 100 donor islets into the pancreas or liver of the chimeric recipients. We found that transplantation of 100 or more donor islets was able to reverse hyperglycemia in all the recipients (Fig. 2A–D).
FIG. 2.
A small number of donor islets was needed to reverse diabetes in chimeric late-stage diabetic NOD mice. Late-stage diabetic NOD mice were injected with BM and CD4+ T-depleted spleen cells from FVB/N donors to induce chimerism after conditioning with anti-CD3 and anti-CD8. The next day after HCT, titrated numbers (600–25) of islets from FVB/N donors were transplanted into the pancreas or liver of the chimeric recipients (A–F). The recipients were checked weekly for blood glucose for 120 days after islet transplantation. For recipients with 25 donor islets, exogenous insulin was used soon after transplantation to ensure the survival of the recipients. If a recipient's blood glucose was >500 mg/dl, the mouse was injected with insulin (1 unit daily) for the subsequent 5 days, and injections were stopped 2 days before blood glucose measuring. There were 6–10 recipients in each group.
In addition, we found that transplantation of 100 islets from syngeneic NOD mice was also able to reverse late-stage diabetes in the chimeric NOD mice in which pre-existing insulitis cleared up after transplantation. Furthermore, the chimeric recipients rapidly rejected the islets from third-party donors when implanted in the pancreas (supplementary Fig. 2). These results indicate that the chimeric recipients are tolerant to both donor- and host-type graft islets in the pancreas. Taken together, induction of chimerism not only reverses autoimmunity and provides immune tolerance to donor islets, but also markedly reduces the required amount of donor islets for reversal of diabetes and renders the pancreas a suitable site for islet grafts.
Small amounts of donor islets implanted in the pancreas were more effective in reversing diabetes than in the liver after induction of chimerism.
Next, we further titrated down the dose of donor islets to 50 and 25 islets per chimeric recipient. We observed that although 50 islets implanted in the pancreas resulted in long-term reversal of diabetes in 70% (7 of 10) chimeric recipients, 50 islets implanted in the liver resulted in long-term reversal in only 20% (2 of 10) of the chimeric recipients (P < 0.01, Fig. 2E). We should point out that some of the chimeric recipients with 50 donor islets in the liver showed transient normal glycemia and relapse of hyperglycemia. This relapse of hyperglycemia was not caused by graft rejection, because there was still strong bioluminescent imaging (BLI) signaling of the islet grafts, and no infiltration observed in the grafts (supplementary Fig. 3). In addition, 40% (4 of 10) of the chimeric recipients with 25 donor islets in the pancreas reached normal glycemia, although it took >2 months for some recipients. In contrast, none (0 of 10) of the chimeric recipients with 25 donor islets in the liver reached normal glycemia (P < 0.01, Fig. 2F). These results indicate that when small amounts of donor islets are transplanted, the pancreas is a better site than the liver for islet grafts.
Graft islet β-cells in the pancreas had better long-term function than those in the liver.
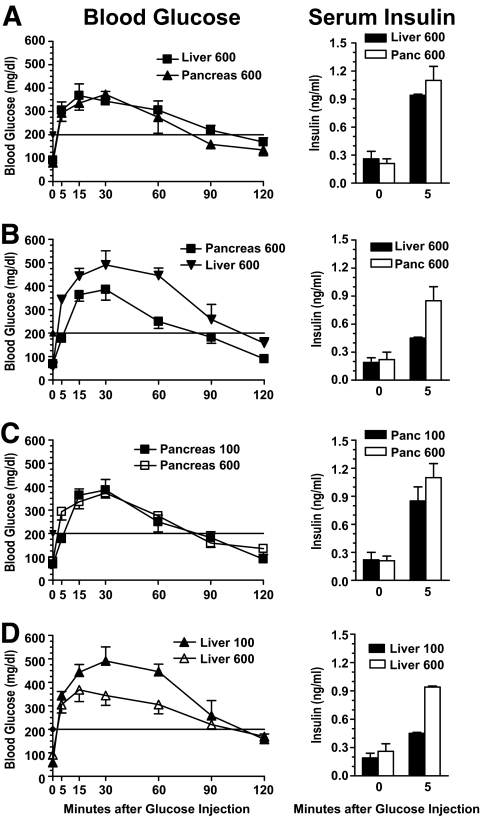
It has been reported that, in a syngeneic transplantation, islet grafts in the liver showed reduced capacity of insulin production compared with grafts in the pancreas (15). Thus, 120 days after islet transplantation, we compared the insulin secretion capacity of islet grafts in the pancreas and liver of the long-term chimeric recipients by GTT. We found that there was no significant difference in changes of serum blood glucose or insulin levels in long-term recipients transplanted with 600 donor islets in the pancreas or liver during GTT (Fig. 3A). However, the recovery rate of serum blood glucose in recipients with 100 graft islets in the liver was significantly slower than that of recipients with 100 islets in the pancreas, especially at 15–90 min after glucose injection (P < 0.01, Fig. 3B). Similarly, the serum insulin levels of recipients with 100 graft islets in the liver was twofold lower than that of recipients with 100 graft islets in the pancreas 5 min after glucose injection (P < 0.01, Fig. 3B). In addition, although there was no significant difference in the blood glucose recovery rate or serum insulin levels between recipients with 600 and 100 graft islets in the pancreas (Fig. 3C), there was a significant difference between recipients having 600 or 100 graft islets in the liver (P < 0.01, Fig. 3D). These results indicate that 1) islet grafts in the pancreas may maintain better long-term function than those in the liver; and 2) islet grafts in the pancreas may expand better than those in the liver when small amounts of donor islets are transplanted.
FIG. 3.
Comparison of insulin secretion capacity of long-term islet grafts in the liver and pancreas of chimeric recipients by GTT test. At 120 days after islet transplantation, a GTT test was performed with chimeric recipients with 100 or 600 graft islets in the liver and pancreas. Blood glucose recovery curve and serum insulin levels before and 5 min after glucose injection are shown. Mean ± SE of 6 mice in each group is shown.
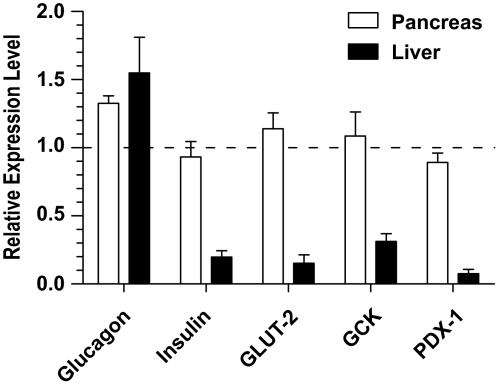
To determine whether long-term islet grafts in the pancreas maintained superior function to those in the liver, we retrieved islets from the pancreas and liver as previously described (33), and identified the donor islets with ex vivo BLI (supplementary Fig. 4). We compared the expression levels of metabolic genes of islet grafts using donor islet-specific luciferase mRNA as an internal control to avoid the influence of differences in the purity of the retrieved islets. We found that although the expression levels of insulin, PDX-1, and GLUT2 by islet grafts from the pancreas were similar to those before transplantation (P > 0.1; Fig. 4), the expression levels of those genes by islet grafts from the liver was reduced by ∼5- to ∼10-fold (P < 0.01; Fig. 4). The expression levels of glucagon by islet grafts from the liver were not different from islet grafts from the pancreas or from those before transplantation control (P > 0.1; Fig. 4). These results indicate that long-term graft islet β-cells in the liver, but not in the pancreas, become defective in functional gene expression.
FIG. 4.
Long-term islet grafts in the pancreas maintained better function than those in the liver. Islet grafts were retrieved from the pancreas and liver, and the donor-type islets were identified with ex vivo BLI. The expression levels of metabolic genes of β-cells were compared using donor islet-specific luciferase mRNA as an internal control to avoid the influence of differences in the purity of the retrieved islets. The relative expression levels of insulin, PDX-1, GLUT2, GCK, and glucagon were calculated by the expression levels of retrieved grafts versus islets before transplantation. Mean ± SE of four replicate experiments is shown.
Graft islet β-cells replicated better in the pancreas than in the liver.
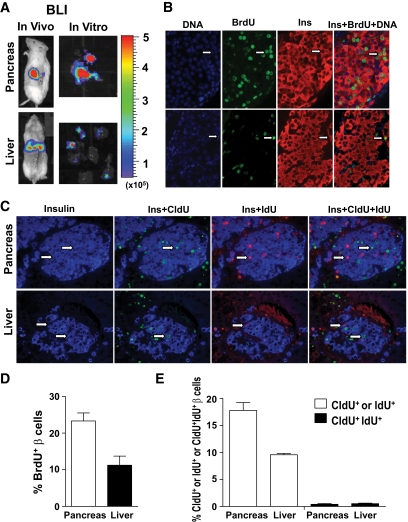
We previously reported that residual islets in new-onset diabetic NOD mice could proliferate to reverse diabetes after induction of chimerism (2). We tested whether graft islet β-cells could also proliferate in the pancreas of the chimeric recipients. Accordingly, the chimeric recipients with donor islet grafts in the pancreas or liver were injected intraperitoneally with bromodeoxyuridine (BrdU) (50 μg/g body weight) for 2 weeks immediately after islet transplantation. The islet grafts were identified by in vivo and ex vivo BLI (Fig. 5A). We found that β-cells in grafts from both pancreas and liver proliferated, as determined by incorporation of BrdU, but the graft islet β-cell proliferation in pancreas was twofold more vigorous in the pancreas than in the liver, as judged by the percentage of BrdU+Insulin+ β-cells in the graft (P < 0.01, Fig. 5B and D). Furthermore, we used sequential CldU and IdU labeling to test whether the proliferation of β-cells were from β-cell replication or neogenesis as described by Teta et al. (31). We found that the proliferating β-cells in islet grafts were either CldU+ or IdU+, and few were CldU+IdU+ (Fig. 5C and E). The percentage of CldU+ or IdU+ insulin-secreting β-cells in islet grafts from the pancreas was nearly twofold higher than those from the liver (P < 0.01, Fig. 5C and E). These results indicate that the proliferating β-cells in islet grafts are from β-cell replication, but not neogenesis, and donor islet β-cells replicate better in the pancreas than in the liver.
FIG. 5.
Islet graft β-cell proliferation/replication in the liver and pancreas as judged by BrdU-labeling and sequential CldU and IdU labeling. We transplanted 100 donor islets from luc+ FVB/N donors into the liver or pancreas of chimeric recipients. The day after islet transplantation, the recipients were given BrdU labeling or sequential CldU and IdU labeling. Islet grafts were harvested under the guidance of ex vivo BLI. Formalin-fixed graft tissues were stained for DNA, BrdU, and insulin; or CldU, IdU, and insulin. The proliferating β-cells were identified as BrdU+Insulin+. The replicating β-cells were identified with CldU+Insulin+ or IdU+Insulin+. β-cells from neogenesis were identified as CldU+IdU+Insulin+. Representatives of proliferating or replicating β-cells are indicated by arrows. A: In vivo and in vitro BLI of islet grafts. B: A representative staining pattern of BrdU-labeling of proliferating β-cells. C: A representative staining pattern of CldU and IdU-labeling of replicating β-cells. D: Mean ± SE of percentage of proliferating β-cells of 6 islet grafts in each group. E: Mean ± SE of percentage of β-cells from replication or neogenesis of 4 islet grafts in each group. (A high-quality digital representation of this figure is available in the online issue.)
Graft islets in the pancreas had higher vascular density than in the liver.
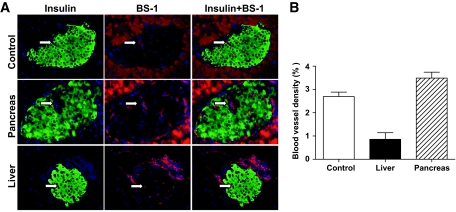
It has been reported that revascularization of islets was important for graft islet β-cell proliferation and function (42–44). It was also reported that revascularization of graft islets was initiated within 2–4 days and was completed by 14 days after islet transplantation (45,46). Therefore, we compared the revascularization of graft islets in the pancreas and in the liver 3 weeks after transplantation. Accordingly, 3 weeks after islet transplantation, graft islets were identified by in vivo and ex vivo BLI and harvested as described in Fig. 5. The sections of the selected pancreas tissues were stained with the lectin Bandeiraea simplicifolia (BS-1) to visualize blood vessels inside the graft islets as described previously (37,38). We found that vascular density in the graft islets from the pancreas was similar to the donor islets before transplantation and was approximately fourfold higher than in the graft islets from the liver (P < 0.01, Fig. 6). These results indicate that the better proliferation and function of the graft islets in the pancreas than in the liver may result from better intraislet revascularization of graft islets in the pancreas.
FIG. 6.
Graft islet revascularization in the liver and pancreas as judged by BS-1 staining. One hundred donor islets from luc+ FVB/N donors were transplanted into the liver or pancreas of chimeric recipients. Three weeks later, islet grafts were harvested under the guidance of ex vivo BLI. Formalin-fixed graft tissues were stained for DNA (blue), insulin (green), and BS-1 (red). Vascularization was identified with BS-1 staining (red) inside of insulin staining (green). A and B: A representative BS-1 staining pattern of control islets and islet grafts in the liver and pancreas (A); mean ± SE of percentage of vascular density of 4 islet grafts in each group (B). (A high-quality digital representation of this figure is available in the online issue.)
No pancreatitis is induced by islet transplantation into the pancreas.
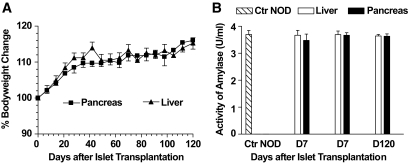
One major concern regarding implantation of islets in the pancreas is induction of pancreatitis. Therefore, we carefully compared the body weight and serum amylase levels of the recipients with islet grafts in the pancreas and liver. We found that all the recipients with 300–600 islets in the pancreas or liver showed healthy appearance, similar body weight, and little amylase in their serum over an observation period of 120 days (Fig. 7A and B).
FIG. 7.
No increase of serum amylase levels in the recipients with islet grafts in pancreas. Chimeric late-stage diabetic NOD mice were given islet transplantation (300 or 600) into the liver or pancreas. The recipients were monitored weekly for body weight (A). Serum levels of amylase were measured before and 7 and 120 days after islet transplantation (B). There were 12 mice in each group. Mean ± SE of bodyweight change or serum amylase units of 12 mice in each group is shown. Ctr NOD, control NOD.
DISCUSSION
We have demonstrated that compared with immunosuppressant therapy, induction of chimerism under the anti-CD3-based conditioning regimen not only provided immune tolerance to donor islets, but also markedly reduced the required amount of donor islets for reversal of diabetes. Furthermore, induction of chimerism rendered the pancreas of autoimmune diabetic recipients a better site than the liver for donor islets, especially when small amounts of donor islets were transplanted, which was associated with better replication and long-term function of donor islet β-cells, as well as better revascularization of the graft islets in the pancreas.
We observed that induction of chimerism markedly reduced the required amount of donor islets for reversal of severe late-stage type 1 diabetes. We found that with immunosuppressants of the Edmonton protocol for prevention of islet graft rejection, we needed to transplant >600 donor islets (>3 donors) in the liver to reverse diabetes. In contrast, as few as 100 donor islets implanted in the liver (∼½ islets from a donor) were able to reverse the disease after induction of chimerism. This is more than a sixfold reduction. This required amount of donor islets for reversal of diabetes in chimeric recipients is equivalent to the required amount in syngeneic islet transplantation in the liver as reported by others (47,48). In fact, transplantation of donor islets into the recipients with donor BM chimerism is similar to a syngeneic islet transplantation. Thus, the marked reduction of required donor islet amount for reversal of diabetes in the chimeric recipients is associated with avoidance of the immune rejection mediated by allo- and autoimmunity, as well as the avoidance of chemotoxicity to donor islets.
We also observed that induction of chimerism rendered the pancreas of an autoimmune diabetic recipient a suitable site for donor islet grafts. It was shown that the pancreas appears to be a better site than the liver for donor islets in the syngeneic islet transplantation (15,16). However, we found that under immunosuppressant therapy for prevention of rejection, a large dose (1,000) of donor islets that could reverse diabetes when implanted in the liver failed to reverse diabetes when implanted in the pancreas of the late-stage diabetic NOD mice. In contrast, after induction of chimerism, a transplantation of as few as 25 islets into the pancreas was able to reverse the disease in 40% of the recipients. This is a more than a 40-fold reduction in the required amount of donor islets for reversal of diabetes, as compared with immunosuppressant therapy for prevention of rejection. One important factor for induction of chimerism to render the pancreas a suitable site for donor islets is the elimination of pre-existing insulitis. Our previous studies have shown that induction of chimerism not only prevented the recurrence of autoimmunity, but also eliminated insulitis in the host pancreas (2,27). This graft versus autoimmunity (GVA) effect was mediated by donor CD8+ T-cells in the bone marrow transplant, and this GVA effect was not associated with GVHD in the recipients conditioned with anti-CD3–based regimen as shown in our previous reports (27,29).
We observed that when small numbers of islets (i.e., less than 100) were transplanted, islet grafts in the pancreas were much more effective in reversing diabetes than in the liver of the chimeric recipients. Furthermore, this difference resulted from better replication of graft islet β-cells early after transplantation and better long-term β-cell function in the pancreas than in the liver, which was associated with improved revascularization of the graft islets in the pancreas. It is not yet clear how graft islets in the pancreas can have better revascularization and proliferation than in the liver. It was reported that during pregnancy in adult rats, insulin from islet β-cells augmented islet vascular endothelial cell proliferation, and in turn, endothelial growth factor from the vascular endothelial cells augmented β-cell proliferation (44). Therefore, it is possible that the existence of an endothelial-endocrine axis in the pancreas favors β-cell proliferation and islet revascularization. In addition, the defective expression of β-cell functional genes of the graft islets in the liver of long-term recipients may be caused by graft islets in the liver (but not in the pancreas) that are chronically exposed to high levels of glucose absorbed from the intestine and produced by the hepatocytes that are toxic to islet β-cells (49).
There have been concerns that the differences in gene expression profiles of islet grafts from the liver and pancreas could be caused by the difference in purity of the retrieved islets, as noted in a previous publication (15). In the current study, this concern has been markedly reduced, if not eliminated. We used luciferase transgenic donor islets for transplantation and we used luciferase mRNA as baseline to avoid the impact of purity difference in retrieved islets when we compared the gene expression levels of the retrieved graft islets.
We are aware that it is still a concern that injection of donor islets into the pancreas could potentially induce pancreatitis in humans. However, we did not observe any clinical signs of pancreatitis after injection of donor islets into the mouse pancreas, because the bodyweight changes and serum levels of amylase in the mouse recipients with islet grafts in the pancreas or liver were similar. Reports by others also showed that intrapancreatic injection of donor islets in both rats and dogs did not induce pancreatitis (16), and intrapancreatic injection of marrow stem cells in humans did not induce pancreatitis either (50). Therefore, it is possible that intrapancreatic injection of donor islets in humans will not cause pancreatitis, and a nonhuman primate trial is warranted to further test the feasibility.
In summary, we have demonstrated that induction of chimerism under the radiation-free anti-CD3-based conditioning regimen markedly reduces the required amount of donor islets for reversal of late-stage diabetes and also renders the pancreas of autoimmune diabetic recipients a more suitable site for graft islets than the liver. Thus, induction of chimerism under a nontoxic conditioning regimen (i.e., anti-CD3-based conditioning) and implantation of donor islets into the pancreas of the chimeric recipient may be a curative therapy in the future for refractory late-stage type 1 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health (R21 DK-71007 to D.Z.) and Juvenile Diabetes Research Foundation (Research Grant 1-2006-136 to D.Z.).
No potential conflicts of interest relevant to this article were reported.
C.Z., M.W., and D.Z. researched data and wrote the manuscript. J.J.R., H.L., C.-L.L., I.N., J.L., Y.-A.C., and I.T. researched data. M.A. researched data and discussed, reviewed, and edited the manuscript.
The authors thank Dr. Arthur Riggs for his continuous encouragement and support of this research; Lucy Brown at City of Hope Flow Cytometry Facility; Sofia Loera at City of Hope Anatomic Pathology Laboratory; Alina Avakian-Mansoorian at the Department of Diabetes The Beckman Research Institute of City of Hope; and Clive Wasserfall at University of Florida for their excellent technical assistance. The authors also thank Dr. Richard Ermel and his staff at City of Hope Research Animal Facility for providing excellent animal care.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 2007;7:988–994 [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Todorov I, Lin CL, Atkinson M, Kandeel F, Forman S, Zeng D. Elimination of insulitis and augmentation of islet beta cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci U S A 2007;104:2337–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson MA. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can't we prevent or reverse this disease? Diabetes 2005;54:1253–1263 [DOI] [PubMed] [Google Scholar]
- 4.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant 2008;8:1990–1997 [DOI] [PubMed] [Google Scholar]
- 5.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 8.Bresson D, von Herrath M. Limitations in immunotherapy with CD3 antibodies: comment on the article by Drs. Chatenoud and Bach. Rev Diabet Stud 2005;2:187–189; discussion 190–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 10.Eisenbarth GS, Stegall M. Islet and pancreatic transplantation–autoimmunity and alloimmunity. N Engl J Med 1996;335:888–890 [DOI] [PubMed] [Google Scholar]
- 11.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes 2009;58:2175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimodahira M, Fujimoto S, Mukai E, Nakamura Y, Nishi Y, Sasaki M, Sato Y, Sato H, Hosokawa M, Nagashima K, Seino Y, Inagaki N. Rapamycin impairs metabolism-secretion coupling in rat pancreatic islets by suppressing carbohydrate metabolism. J Endocrinol 2010;204:37–46 [DOI] [PubMed] [Google Scholar]
- 14.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM, Thompson DM, Verchere CB, Warnock GL. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant 2009;18:833–845 [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, Jansson L, Carlsson PO. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes 2007;56:1544–1550 [DOI] [PubMed] [Google Scholar]
- 16.Stagner JI, Rilo HL, White KK. The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. Jop 2007;8:628–636 [PubMed] [Google Scholar]
- 17.Kendall DM, Teuscher AU, Robertson RP. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes 1997;46:23–27 [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Zhang T, Bogdani M, Oseid E, Parazzoli S, Vantyghem MC, Harmon J, Slucca M, Robertson RP. Intrahepatic glucose flux as a mechanism for defective intrahepatic islet alpha-cell response to hypoglycemia. Diabetes 2008;57:1567–1574 [DOI] [PubMed] [Google Scholar]
- 19.Sykes M. Mixed chimerism and transplant tolerance. Immunity 2001;14:417–424 [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008;358:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 2008;358:362–368 [DOI] [PubMed] [Google Scholar]
- 22.Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, Smith A, Webster B, Shaw PJ, Lammi A, Stormon MO. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med 2008;358:369–374 [DOI] [PubMed] [Google Scholar]
- 23.Li H, Kaufman CL, Boggs SS, Johnson PC, Patrene KD, Ildstad ST. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in nonobese diabetic (NOD) mice. J Immunol 1996;156:380–388 [PubMed] [Google Scholar]
- 24.Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA, Greiner DL. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood 2000;95:2175–2182 [PubMed] [Google Scholar]
- 25.Beilhack GF, Scheffold YC, Weissman IL, Taylor C, Jerabek L, Burge MJ, Masek MA, Shizuru JA. Purified allogeneic hematopoietic stem cell transplantation blocks diabetes pathogenesis in NOD mice. Diabetes 2003;52:59–68 [DOI] [PubMed] [Google Scholar]
- 26.Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes 2004;53:376–383 [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Huang T, Zhang C, Todorov I, Atkinson M, Kandeel F, Forman S, Zeng D. Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb. Blood 2005;105:2180–2188 [DOI] [PubMed] [Google Scholar]
- 28.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A 2004;101:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, Contag CH, Kandeel F, Forman S, Zeng D. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol 2007;178:838–850 [DOI] [PubMed] [Google Scholar]
- 30.Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, Reeves R, Taylor-Edwards C, Schulz S, Doyle TC, Fathman CG, Robbins RC, Herzenberg LA, Negrin RS, Contag CH. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation 2005;80:134–139 [DOI] [PubMed] [Google Scholar]
- 31.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 32.Laybutt DR, Hawkins YC, Lock J, Lebet J, Sharma A, Bonner-Weir S, Weir GC. Influence of diabetes on the loss of beta cell differentiation after islet transplantation in rats. Diabetologia 2007;50:2117–2125 [DOI] [PubMed] [Google Scholar]
- 33.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes 2004;53:948–954 [DOI] [PubMed] [Google Scholar]
- 34.Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 2005;54:2755–2763 [DOI] [PubMed] [Google Scholar]
- 35.Ritz-Laser B, Gauthier BR, Estreicher A, Mamin A, Brun T, Ris F, Salmon P, Halban PA, Trono D, Philippe J. Ectopic expression of the beta-cell specific transcription factor Pdx1 inhibits glucagon gene transcription. Diabetologia 2003;46:810–821 [DOI] [PubMed] [Google Scholar]
- 36.Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood 2008;112:2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau J, Kampf C, Mattsson G, Nyqvist D, Kohler M, Berggren PO, Carlsson PO. Beneficial role of pancreatic microenvironment for angiogenesis in transplanted pancreatic islets. Cell Transplant 2009;18:23–30 [DOI] [PubMed] [Google Scholar]
- 38.Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J 1987;19:225–234 [DOI] [PubMed] [Google Scholar]
- 39.Molano RD, Pileggi A, Berney T, Poggioli R, Zahr E, Oliver R, Malek TR, Ricordi C, Inverardi L. Long-term islet allograft survival in nonobese diabetic mice treated with tacrolimus, rapamycin, and anti-interleukin-2 antibody. Transplantation 2003;75:1812–1819 [DOI] [PubMed] [Google Scholar]
- 40.Ansari MJ, Fiorina P, Dada S, Guleria I, Ueno T, Yuan X, Trikudanathan S, Smith RN, Freeman G, Sayegh MH. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin Immunol 2008;126:140–147 [DOI] [PubMed] [Google Scholar]
- 41.Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, Anzai K, Ono J, Taniguchi M, Ikeda S. Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice. Transplantation 2007;83:1085–1092 [DOI] [PubMed] [Google Scholar]
- 42.Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008;118:3790–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes 2008;57:1870–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 2006;147:2315–2324 [DOI] [PubMed] [Google Scholar]
- 45.Sandberg JO, Margulis B, Jansson L, Karlsten R, Korsgren O. Transplantation of fetal porcine pancreas to diabetic or normoglycemic nude mice. Evidence of a rapid engraftment process demonstrated by blood flow and heat shock protein 70 measurements. Transplantation 1995;59:1665–1669 [DOI] [PubMed] [Google Scholar]
- 46.Mendola J, Corominola H, Gonzalez-Clemente JM, Esmatjes E, Saenz A, Fernandez-Cruz L, Gomis R. Follow-up study of the revascularization process of cryopreserved islets of Langerhans. Cryobiology 1996;33:530–543 [DOI] [PubMed] [Google Scholar]
- 47.Toyofuku A, Yasunami Y, Nabeyama K, Nakano M, Satoh M, Matsuoka N, Ono J, Nakayama T, Taniguchi M, Tanaka M, Ikeda S. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes 2006;55:34–39 [PubMed] [Google Scholar]
- 48.Yasunami Y, Kojo S, Kitamura H, Toyofuku A, Satoh M, Nakano M, Nabeyama K, Nakamura Y, Matsuoka N, Ikeda S, Tanaka M, Ono J, Nagata N, Ohara O, Taniguchi M. Valpha14 NK T cell-triggered IFN-gamma production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med 2005;202:913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makhlouf L, Duvivier-Kali VF, Bonner-Weir S, Dieperink H, Weir GC, Sayegh MH. Importance of hyperglycemia on the primary function of allogeneic islet transplants. Transplantation 2003;76:657–664 [DOI] [PubMed] [Google Scholar]
- 50.Fernandez Vina JFL RJ, Kraft D, Saslavsky J, Camozzi L, Fernandez Vina R, Vrsalovick F, Andrin O. Report case of using marrowminer system for cell therapy in diabetes type1 patient. In ISCT Annual Meeting; Miami, Florida, 2008, p. 07-A-170-ISCT [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.