Abstract
OBJECTIVE
Dietary factors influence diabetes development in the NOD mouse. Diet affects the composition of microbiota in the distal intestine, which may subsequently influence intestinal immune homeostasis. However, the specific effects of antidiabetogenic diets on gut immunity and the explicit associations between intestinal immune disruption and type 1 diabetes onset remain unclear.
RESEARCH DESIGN AND METHODS
Gut microbiota of NOD mice fed a conventional diet or ProSobee formula were compared using gas chromatography. Colonic lamina propria immune cells were characterized in terms of activation markers, cytokine mRNA and Th17 and Foxp3+ T-cell numbers, using real-time PCR and flow cytometry. Activation of diabetogenic CD4 T-cells by purified B-cells was assessed in both groups. Immune tolerance to autologous commensal bacteria was evaluated in vitro using thymidine-incorporation tests.
RESULTS
Young NOD mice showed a disturbed tolerance to autologous commensal bacteria. Increased numbers of activated CD4 T-cells and (CD11b+CD11c+) dendritic cells and elevated levels of Th17 cells and IL23 mRNA were moreover observed in colon lamina propria. These phenomena were abolished when mice were fed an antidiabetogenic diet. The antidiabetogenic diet also altered the expression levels of costimulatory molecules and the capacity of peritoneal B-cells to induce insulin-specific CD4 T-cell proliferation.
CONCLUSIONS
Young NOD mice show signs of subclinical colitis, but the symptoms are alleviated by a diet change to an antidiabetogenic diet. Disrupted immune tolerance in the distal intestine may influence peritoneal cell pools and B-cell–mediated activation of diabetogenic T-cells.
Dietary and microbial factors may be partly responsible for the increase in type 1 diabetes incidence. The intestinal mucosa is constantly exposed to these factors, and it is therefore important to thoroughly understand how these factors affect the intestinal immune system.
Evidence suggesting that gut immune disruptions may trigger type 1 diabetes originated from studies that showed correlations between a high prevalence of cow-milk antibodies, brief breastfeeding in infancy, and an increased risk of type 1 diabetes (1,2). This hypothesis gained further support from the discovery that lymphocytes accumulating in the islets share homing characteristics with gut-associated lymphocytes (3–5). Research in both humans and animals has thereafter lead to an understanding that type 1 diabetes is associated with increased permeability and enteropathy in the small intestine (6–9). The impaired barrier functions of the small intestine may subsequently cause alterations in antigen responses and thus disrupt the immunological homeostasis of the intestine. This in turn could cause intestinal inflammation and induce immune responses that lead to autoimmunity (7,10,11).
Considerably less attention has been paid to the potential role of the large intestinal immune system in type 1 diabetes development. The large intestine differs immunologically in several aspects from the small intestine: The disruptions in small intestinal immunity is linked foremost to ingested antigens or allergens such as insulin from cow milk, cereal-based allergens, and other food derivatives. The most immediate sources of immune disruption in the large intestine, however, are the vast quantities of bacteria residing therein. Moreover, large intestinal lamina propria lymphocytes differ markedly in terms of population dynamics and cytokine expression from the lamina propria lymphocytes of the small intestine (12).
There is some evidence that directly associates immune responses in the large intestine with the pancreas; lymph from the transverse colon has been reported to drain specifically to the pancreatic lymph nodes (13). This could allow innate immune stimuli to interfere with induction of peripheral immune tolerance to antigens. Accordingly, studies in BDC2.5/NOD mice have indicated that dextran sodium sulfate, which disrupts the barrier functions of the colonic epithelium, enhances the activation of islet reactive T-cells in pancreatic lymph nodes of NOD mice (14). It is therefore of interest to further investigate the role of the colonic immune system in type 1 diabetes.
Dendritic cells as well as macrophages play a major part in large intestinal mucosal immune counterbalance. Macrophages and CD11b− dendritic cells have been reported to secrete anti-inflammatory cytokines, such as IL-10, while CD11b+ dendritic cells elicit the production of proinflammatory IL-17 (15,16). Macrophages, which are capable of suppressing dendritic cell-induced IL-17 secretion (17), are reduced in numbers in the lamina propria of mice suffering from colitis, concomitant with a substantial increase of lamina propria CD11b+ dendritic cells (15). Intestinal macrophages in humans have moreover been described as inflammatorily anergic, producing only low levels of proinflammatory cytokines (18).
The cytokine IL-23 has importance in several inflammatory disorders (19). IL-23 is capable of promoting both Th17 and Th1 responses in the intestinal lamina propria, because its absence decreases both Th1- and Th17-type cytokines in the intestine (20). The exact effects of IFN-γ and IL-17 on type 1 diabetes are nevertheless unclear. Though increased production of IFN-γ has been associated with type 1 diabetes, NOD mice lacking IFN-γ or IFN-γ receptor develop type 1 diabetes to a degree equal to wild-type NOD mice (21,22). IL-17 promotes pancreatic inflammation (23) and is upregulated in diabetic mice (24,25). Furthermore, treatment with IL-25, which inhibits the Th17 cell population, and with IL-17 neutralizing antibody, prevent diabetes in NOD mice (26). Treatment with IFN-γ can likewise prevent diabetes in NOD mice, probably by decreasing the production of IL-17 in the spleen and pancreas (27). Th17 cells can moreover transform to Th1-like cells under the influence of IL-12, demonstrating a considerable degree of plasticity between the Th17 and the Th1 cell lineages (28).The results presented in this study indicate that newly weaned NOD mice suffer from a mild level of colitis, which alters the colonic immune cell standing toward a proinflammatory status. This is moreover associated with a disruption of immune tolerance toward autologous intestinal microbiota. As recently reported, NOD peritoneal B cells show a significantly higher efficiency in activating insulin-specific T-cell reactivity than spleen-derived conventional B-cells (29). Remarkably, most of the abnormalities in the colon and peritoneal B-cell antigen-presenting activity of young NOD mice can be abrogated when NOD mice are fed an antidiabetogenic diet from the time of weaning. The substantial effects of the antidiabetogenic diet on the colonic and peritoneal immune system call attention to the importance of colon immune homeostasis in the development of type 1 diabetes in NOD mice.
RESEARCH DESIGN AND METHODS
NOD mice were either on a regular diet (CRM-E, SDS, Tapvei) (hereafter referred to as “NOD” mice) or ProSobee infant formula (Mead Johnson Nutritionals) (referred to as “PNOD” mice). The ProSobee formula was given to the mothers from 2 weeks after giving birth and to the offspring immediately after weaning, and continued throughout the study period. The average weight of 4.5 week old NOD and PNOD mice was not significantly different; NOD, 21.0 g (±1.1 g), and PNOD, 19.6 g (±1.2 g). The diet modifications thus did not impede growth in mice.
Diabetes incidence was assessed through weekly blood tests, and mice were considered diabetic when blood glucose levels exceeded 14 mmol/l on two consecutive measurements. NOD and BALB/c mice originating from commercial breeders have been housed and bred for more than two decades in the central animal laboratory of Turku University. All animal experiments were approved by the National Laboratory Animal Care & Use Committee in Finland and conformed to the legal acts, regulations, and requirements set by the European Union concerning protection of animals used for research.
Cell proliferation in response to commensal bacteria.
Fresh fecal pellets were collected from individual BALB/c and NOD mice, incubated in PBS (one pellet/0.1 ml) for 30 min at 37°C, then vortexed and centrifuged to remove undissolved fibrous pieces. The suspension was further incubated for 2 h at 60°C to inactivate the bacteria and sonicated to produce a suspension of dead bacterial components. The bacterial density was adjusted using absorbance measurements relying on a standard curve created on the basis of titration of colony-forming unit values for different absorbance values. Bacterial sonicate was added to cell culture plates containing mesenteric lymph node (MLN) cells (200,000 cells/well) from the same mouse from which the pellets were collected (bacterial sonicate from autologous intestine) or from a littermate (bacterial sonicate from heterologous intestine). The cells were incubated at 37°C for 72 h with the addition of [H3] thymidine (0.4 μCi/ml) during the last 6 h of incubation. Finally, cells were collected using an automatic cell harvester (Tomtec Harvest 96), and the radioactivity was counted using a beta counter (Wallac). Each experimental condition was performed in triplicate.
Assessment of intestinal histology.
For histological studies of the colon, mice were killed at the age of 4.5, 6, or 10 weeks. Colons were excised, washed with PBS, and fixed in 10% buffered formalin. After rehydration, 4–5-μm thick paraffin-embedded sections were stained with hematoxylin and eosin, and hyperplasia was assessed by measuring the thickness of the epithelial crypts using light microscopy (Olympus).
Gas chromatographic analysis of cellular fatty acid profiles of gut bacteria.
The intestinal flora of NOD and PNOD mice were assessed for overall differences using gas–liquid chromatographic (GLC) techniques. This method allowed computerized profiling of cellular fatty acids of bacteria in NOD and PNOD stool samples. Differing fatty acid profiles correlate to differences in bacterial species because the fatty acid composition is species-specific (30).
To assess the differences in gut flora, stool samples were collected from NOD and PNOD mice and stored at −70°C until processing. Before proceeding to GLC analysis, bacterial mass was separated from other fatty acids present in the feces as described in ref. (31) using sedimentation and centrifugation steps. The bacterial mass was further saponified and methylated, and GLC was run as described in ref. (30).
Isolation of lamina propria lymphocytes and myeloid cells.
Colons were excised, washed, and cut into pieces. The pieces were incubated for 3 × 20 min at 37°C in Hanks' balanced salt solution supplemented with 2% fetal calf serum (FCS) (Life Technologies) and 2 mmol/l EDTA to remove the epithelial layer and intraepithelial lymphocytes.
The colon pieces were then washed with RPMI-1640 (Life Technologies) and digested with Collagenase A (Roche) (0.5 mg/ml) for 1 h at 37°C in RPMI-1640 supplemented with 10% FCS. Undigested pieces were minced and filtered through a nylon mesh. Leukocytes were purified from the resulting cell suspension using Lympholyte-M (Cedarlane) gradient centrifugation (1250 g, room temperature) and thereafter washed twice in culture medium before further use.
Flow cytometry.
Anti-CD4 and anti-CD8 conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to detect T-cell populations. Cell populations were further stained using PE-conjugated anti-α4 or PE-conjugated anti-CD86 (BD Pharmingen), or FITC-conjugated anti-CD44, PE-conjugated anti-CD69 or FITC-conjugated anti-CD62L (Immunotools).
Peritoneal washout cells were stained with FITC-conjugated anti-CD11b (Immunotools) and allophycocyanin conjugated anti-CD45R (Caltag Laboratories) to identify B1-cells. PE-conjugated anti-CD40 or anti-CD86 (Immunotools) was used for peritoneal B-cell activation marker detection.
Subsets of myeloid antigen-presenting cells were characterized as follows: CD11b+ F4/80+ macrophages, F4/80−CD11b+CD11c+ myeloid dendritic cells, and F4/80−CD11b− CD11c+ lymphoid/plasmacytoid DC (all antibodies for this characterization were either FITC or PE conjugated and purchased from Immunotools). The samples were run with FACSCalibur and analyzed using cellQuest software (Becton Dickinson).
Quantitation of colon cytokine gene expression using real-time PCR.
Mouse colon samples were cut into pieces and stored in RNA Later (Qiagen). Total RNA was purified with RNeasy Mini Kit (Qiagen). RNA purity and quantity was determined using a Nanodrop spectrophotometer (Nanodrop Technologies). cDNA was synthesized with DyNAmo cDNA Synthesis Kit (Finnzymes), using oligo-dT primers provided with the kit. Levels of cytokine expression in colons of individual mice were evaluated with real-time quantitative PCR using Maxima SYBR Green qPCR Master Mix (Fermentas) and RotoGene cycler (Corbett Research). Ct-values were normalized to the endogenous housekeeping gene GAPDH and are expressed as copy numbers relative to the GADPH copy numbers. Primer sequences are given in supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0147/DC1.
Analysis of Th17, Th1, and Foxp3 cells in colon lamina propria.
Purified colonic lamina propria lymphocytes (LPLs) were incubated in complete RPMI 1,640 (supplemented with 10% FCS, 2 mmol/l L-glutamine, 100 units/ml penicillin, and streptomycin) containing 0.1 μmol/l PMA, 1 μmol/l ionomycin, and 10 μg/ml Brefeldin A (Sigma-Aldrich) for 4 h at 37°C.
Stimulated cells were surface-stained using FITC-conjugated anti-CD4 and allophycocyanin-conjugated anti-CD25. The cells were then fixed with 2% paraformaldehyde and permeabilized with 0.5% saponin. Fc block was used to block nonspecific binding. PE-conjugated anti-IFN-γ, PE-conjugated anti-Foxp3, or PE-conjugated anti-IL-17 and appropriate isotype controls (all reagents from eBiosciences) were used for the intracellular staining.
B-cell antigen presentation capacity.
The antigen presentation assay was performed using the same experimental settings as in ref. (29); NOD mice were immunized with 50 μg insulin peptide (Insulin B 9-23, Anaspec) subcutaneously on the hind flank. Ten days later, spleens were collected from these animals and the splenic CD4+ T-cells were purified using CD4 MicroBeads (Miltenyi Biotec). These purified T-cells were cocultured (175,000 cells/well) with either NOD or PNOD peritoneal- or splenic B-cells (150,000 cells/well) purified with B220 MicroBeads (Miltenyi Biotech). Purified B-cells (>93% B220+) were irradiated with 3 Gy before adding to cell culture. Additionally, either insulin peptide (4 μmol/l; Insulin B 9-23; Anaspec) or intact insulin (20 μg/ml; σ-Aldrich) was added to the wells in triplicate. The cells were incubated in 37°C for 72 h with the addition of [H3] thymidine (0.4 μCi/ml) during the last 16 h of incubation. The cells were harvested and analyzed as described above.
RESULTS
Lack of tolerance to commensal bacteria in NOD mice.
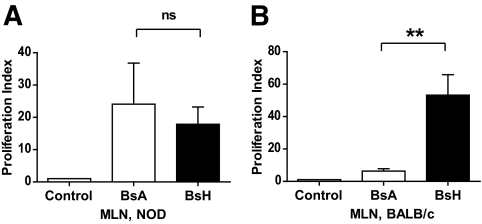
MLN cells from NOD mice proliferated vigorously in response to bacterial sonicate from a littermate (heterologous sonicate). Proliferation was, however, at an equal level regardless of whether cells were stimulated with autologous or heterologous bacterial sonicate (Fig. 1A). Contrarily, in BALB/c mice, high-level proliferation was observed when the commensal bacteria originated from a heterologous source (littermate), whereas autologous bacterial sonicate failed to induce any significant response (Fig. 1B). These results are indicative of a state of inflammation in the NOD colon, because loss of tolerance to autologous commensal bacteria is associated with inflammation and colitis (32,33).
FIG. 1.
Lack of tolerance to autologous bacterial flora in NOD. BsA, bacterial sonicate from autologous intestine, BsH, bacterial sonicate from heterologous intestine. A: MLN cells from NOD mice; n = 10. B: MLN cells from BALB/c mice; n = 3. **P < 0.01 as calculated using one-way ANOVA and Bonferroni post hoc test. ns, no significant difference.
Villous hyperplasia in the colons of NOD mice.
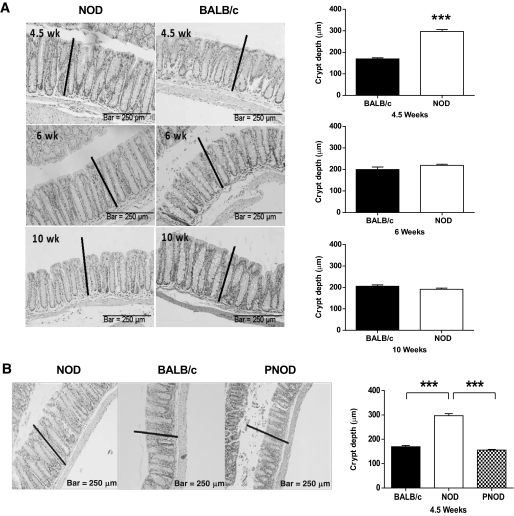
A histological analysis of the colon revealed hyperplasia in the epithelial crypts of NOD mice at 4.5 weeks of age. The crypts of NOD colons were thicker than the crypts of BALB/c colons at 4.5 weeks of age. However, the epithelial layer of the NOD colon was thinner at 6 and 9 weeks of age compared with 4.5 weeks. This was in contrast to the development of BALB/c colons, where a gradual age-dependent thickening was observed (Fig. 2A). There were no other signs of frank colitis, such as leukocyte infiltration, goblet cell loss, or crypt abscesses in the NOD colons. When NOD mice were kept on ProSobee diet, hyperplasia was not observed at 4.5 weeks of age, indicating that the occurrence of hyperplasia in newly weaned NOD mice is diet related (Fig. 2B).
FIG. 2.
Colon epithelial layer hyperplasia in young NOD mice. A (Left): Representative images of longitudinal sections of colons from NOD (left) and BALB/c (right) mice at 4.5 (top), 6 (middle), and 10 weeks (bottom), stained with hematoxylin and eosin. The black line represents the thickness of the NOD colon at 4.5 weeks. (Right): Average crypt depth ± SEM for 4.5-, 6-, and 10-week-old BALB/c and NOD mice. n = 4 per group. B: Longitudinal sections of colons from NOD, BALB/c, and PNOD mice at 4.5 weeks of age. The black lines represent the thickness of the NOD colon at this age. The bar graph to the right represents average crypt depths ± SEM for NOD, BALB/c, and PNOD at 4.5 weeks. n = 4 per group. ***P < 0.001 using Student t test (A) or one-way ANOVA and Bonferroni post hoc test (B).
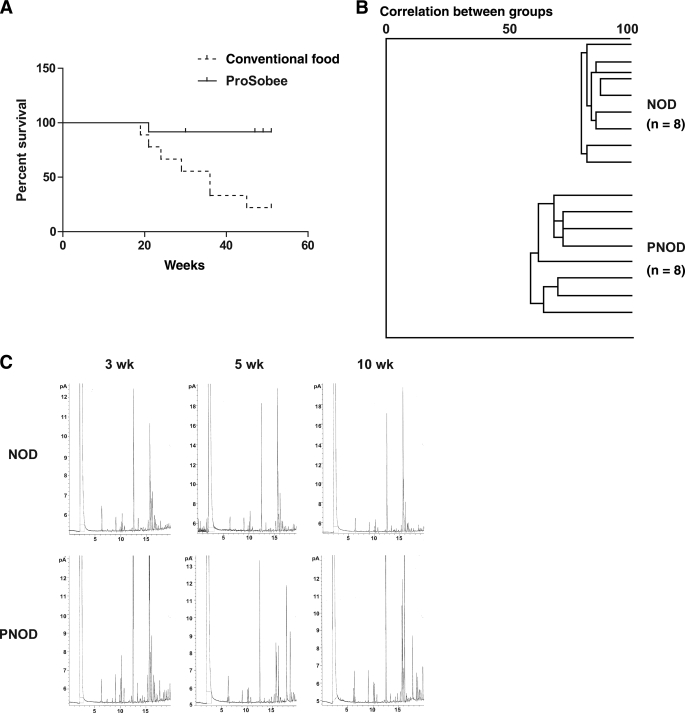
Differences in NOD and PNOD diabetes incidence and gut flora.
Diabetes incidence was significantly lower in NOD mice that had been fed ProSobee instead of conventional food (Fig. 3A). The fatty acid profile of NOD and PNOD did not differ at 3 weeks of age (preweaning). However, at 5 and 10 weeks of age, GLC analysis revealed profound differences in the bacterial fatty acid profiles of NOD and PNOD gut bacteria (Fig. 3B and C).
FIG. 3.
Diabetes incidence and bacterial fatty acid composition for NOD and PNOD mice. A: Diabetes incidence for NOD mice that have been raised on conventional murine food (broken line) and on ProSobee (continuous line). n = 18 per group. B: Example of a cluster analysis of fatty acid profiles from the stool samples. All 16 samples are compared with each other and clustered accordingly. An index of 100 indicates complete similarity with the same fatty acids (peaks in the chromatogram) found in the same concentrations in the samples compared; an index of 0 indicates complete dissimilarity. C: GLC analysis of fecal bacterial fatty acids from NOD and PNOD mice. Each peak in the graph represents an individual fatty acid. Graphs are representative of NOD (top row) and PNOD (bottom row) at 3 weeks (left column), 5 weeks (middle column), and 10 weeks (right column). n = 13–17 mice per group.
Mice within the same age and diet group exhibited consistently similar fatty acid profiles. Moreover, no significant differences between 5- and 10-week-old NOD mice were observed. PNOD mice, contrarily, showed differing fatty acid profiles at 5 and 10 weeks of age. A summary of P values based on these comparisons is provided in Table 1. This data demonstrates that diet has a substantial effect on bacterial species prevalence in NOD mice.
TABLE 1.
Bacterial fatty acid composition comparisons between NOD and PNOD mice at 3, 5, and 10 weeks
| Mice, n | NOD |
PNOD |
|||
|---|---|---|---|---|---|
| 5 weeks | 10 weeks | 3 weeks | 5 weeks | 10 weeks | |
| NOD | |||||
| 3 weeks | 68.59 ± 16.88*** | 74.47 ± 14.03*** | 85.41 ± 17.75, ns | 47.73 ± 12.96*** | 50.21 ± 9.18*** |
| 5 weeks | 80.89 ± 16.27, ns | 34.69 ± 11.62*** | 49.97 ± 13.38*** | 52.90 ± 9.95*** | |
| 10 weeks | 39.21 ± 11.87*** | 56.01 ± 12.21*** | 56.74 ± 11.10*** | ||
| PNOD | |||||
| 3 weeks | 53.43 ± 17.10*** | 69.46 ± 20.42*** | |||
| 5 weeks | 62.01 ± 17.54** | ||||
Data are expressed as a similarity index of the fatty acid profile of the grouped samples when compared with the fatty acid profile of the grouped samples of the other group ± SD of individual samples within the group. An index of 100 indicates complete similarity with the same fatty acids (peaks in the chromatogram) found in the same concentrations in the samples compared; an index of 0 indicates complete dissimilarity. n = 13–17 mice per group.
**P < 0.01;
***P < 0.001; ns, not significantly different.
Inflammatory lymphocytes and dendritic cells in NOD colon lamina propria.
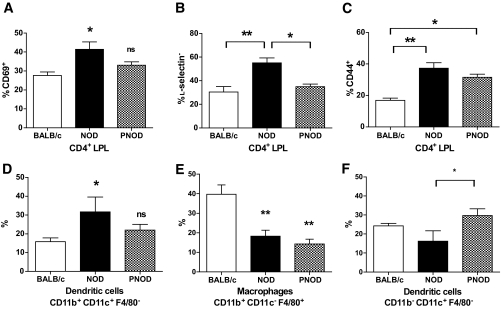
Compared with BALB/c mice, NOD mice had a higher proportion of CD4+ T-cells expressing CD44 and CD69 in colon lamina propria. Furthermore, l-selectin was downregulated on the majority of NOD colonic CD4+ T-cells. CD4+ lamina propria cells from NOD mice on ProSobee, in contrast, showed the same level of CD69 and l-selectin expression as BALB/c mice, and intermediary levels of CD44 expression (Fig. 4A–C). The colons of NOD mice, moreover, contained an increased fraction of CD11b+CD11c+ (myeloid, inflammatory) dendritic cells and a decreased percentage of macrophages and CD11b−CD11c+ dendritic cells compared with BALB/c mice (Fig. 4D–F). PNOD lamina propria contained both less macrophages and CD11b+CD11c+ dendritic cells, but more dendritic cells with the phenotype CD11b−CD11c+. The increase of CD11b+CD11c+ dendritic cells in NOD lamina propria may be indicative of colonic inflammation because these cells have been reported to increase in mice with colitis (15).
FIG. 4.
Colon lamina propria CD4+ T-cell activation markers and lamina propria dendritic cell/macrophage populations in BALB/c (white bars) and (black bars) NOD or PNOD (hatched bars) LPLs. A: Percent CD69 positive CD4 LPL. B: Percent l-selectin negative CD4 LPL. C: Percent CD44 positive CD4 LPL. D: Percent CD11b+CD11c+ dendritic cells. E: Percent CD11b+CD11c−F4/80+ macrophages. F: Percent CD11b−CD11c+ dendritic cells. Bars represent means ± SEM. n = 4–8 mice per group. *P < 0.05 and **P < 0.01 as calculated using one-way ANOVA and Bonferroni post hoc test.
Real-time PCR measurement of colonic cytokine expression.
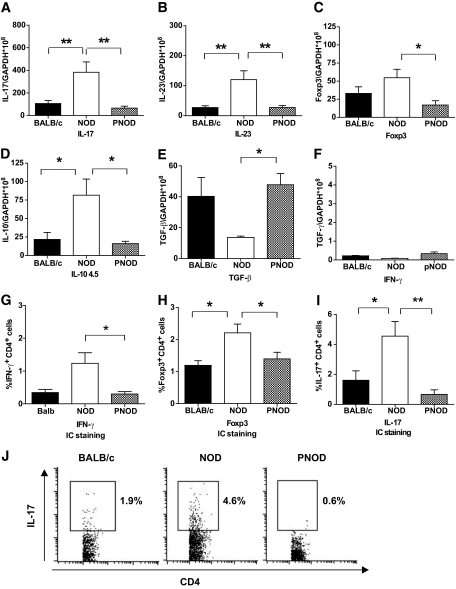
To further elucidate the inflammatory nature of NOD colon immune cells, real-time quantitative PCR was used to measure the expression of different cytokines in BALB/c, NOD, and PNOD colons. Colons from NOD mice showed elevated expression levels of IL-17, IL-23, and IL-10 and decreased expression of TGF-β. IFN-γ expression was also assessed, but all mouse groups only expressed barely detectable levels of it. Foxp3 was also upregulated in NOD colonic cells at 4.5 weeks. This may be a counterbalancing phenomenon to the proinflammatory occurrences. All of the differences observed between NOD and BALB/c mouse cytokine expression leveled out with dietary manipulation (ProSobee diet); PNOD cytokine expression for all of the above listed cytokines was similar to that of BALB/c. (Fig. 5A–F).
FIG. 5.
Colonic cytokine and Foxp3 mRNA and IFNg, Foxp3, and IL-17 protein expression measured with real-time PCR and intracellular staining methods. Colon samples derive from 4.5-week-old BALB/c (black bars) and NOD (white bars) or PNOD (hatched bars). A–F: mRNA expression, normalized to GADPH copy numbers, of IL-17, IL-23, Foxp3, IL-10, TGF-β, and IFN-γ. G–I: Percent IFN-γ+, Foxp3+, and IL-17+ CD4+ cells in colonic lamina propria measured with intracellular (IC) staining of BALB/c, NOD, and PNOD samples. Bars represent mean values ± SEM. n = 4–8 per group. *P = 0.05 and **P < 0.01 as calculated with one-way ANOVA and Bonferroni post hoc test. J: Representative dot plots of intracellular staining for IL-17.
Intracellular staining of colonic lamina propria CD4+ lymphocytes.
Intracellular staining was performed to confirm that the differences in IL-17 mRNA expression correlated with increased IL-17 production in CD4+ T-cells. The results were similar to RT-PCR; a higher percentage of IL-17 producing CD4 T-cells were present in the colon of 4.5-week-old NOD than in that of BALB/c or PNOD mice (Fig. 5I and J). The results for IFN-γ and Foxp3 intracellular staining (Fig. 5G and H, respectively) likewise coincided with the RT-PCR analysis. For IFN-γ intracellular stainings, the percentages of CD4+ IFN-γ+ cells were higher in NOD than in PNOD lamina propria cells. The levels, however, were low (Fig. 5H).
Peritoneal B-cell activation markers and antigen-presenting capacity.
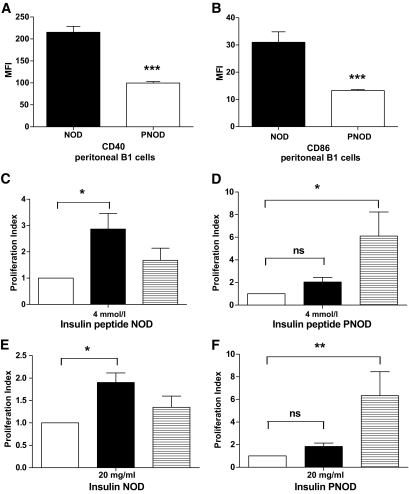
NOD peritoneal B1 cells express abnormally high levels of CD40 and CD86, are more effective than splenic B-cells at presenting antigen to diabetogenic T-cells, and migrate at an enhanced rate to the pancreatic lymph nodes (29). The expression of costimulatory molecules CD40 and CD80 is significantly decreased on PNOD peritoneal B1-cells compared with NOD B1-cells (Fig. 6A and B). Peritoneal and splenic B-cells from NOD and PNOD were next tested in parallel for their antigen-presenting efficiency in presenting insulin or insulin peptide (insulin B9-23) to insulin B9-23 primed NOD CD4 T-cells (Fig. 6C–F). In contrast to NOD mice, peritoneal B-cells from PNOD mice were less efficient than splenic B-cells at presenting antigen (Fig. 6D and F). It is suggested that the decreased expression of CD40 and CD86 and the lessened antigen-presenting capacity in PNOD peritoneal cells is a consequence of lower activation status in the peritoneum, which in turn is a result of the lower inflammation level in the colon of PNOD mice.
FIG. 6.
Peritoneal B-cells' activation markers and antigen-presenting cell (APC) efficiency. A: Mean fluorescence intensity (MFI) of CD40 on peritoneal B1-cells from NOD (black bar) or PNOD (white bar). B: MFI of CD86 on peritoneal B1 cells from NOD (black bar) and PNOD (white bar). Data represent means ± SEM. ***P < 0.001, Student t test. n = 3–5 per group. C–F: Antigen-presenting cell capacity of peritoneal B-cells from NOD and PNOD to 4 μmol/l insulin peptide (C, D) and 20 μg/ml intact insulin (E and F). The graphs show B-cell induced T-cell proliferation using purified NOD (C and E) or PNOD (D and F) peritoneal B-cells (black bars) or splenic B-cells (striped bars). White bars indicate control values (relative baseline proliferation of T-cells + B-cells in the absence of insulin/insulin peptide). Baseline counts per min ranged between 50 and 300 cpm. Data present mean values ± SEM. n = 3–4 per group. *P < 0.05, **P < 0.01 as calculated with one-way ANOVA and Dunnet post hoc test. Peritoneal cells were pooled for each experiment from four mice to yield sufficient numbers of purified B-cells. Splenic B-cells were pooled and purified from the same donors.
DISCUSSION
The evidence presented herein indicates that young NOD mice suffer from a mild level of colitis, which disrupts the immune homeostasis of the large intestine. Intolerance to autologous microbiota, colonic hyperplasia, increased numbers of dendritic cells, and increased levels of IL-17 and IL-23 in the NOD colon are all indicative of colonic inflammatory activity. Remarkably, this condition is alleviated if the standard mouse diet is changed to an antidiabetogenic diet (ProSobee) from the time of weaning. With respect to human type 1 diabetic patients, an increased risk of type 1 diabetes in the offspring of mothers diagnosed with ulcerative colitis has been observed (34). Moreover, mucosal inflammation in the small intestine has been associated with type 1 diabetes in humans (35,36). However, a mild and perhaps transient colonic inflammation, like that observed in NOD mice, would easily escape diagnosis in human type 1 diabetic patients.
The increased levels of IL-17 and IL-23 in the colons of 4.5-week-old NOD mice are a clear indication of an inflammatory response. Increased levels of Foxp3 and IL-10, which were also observed, may demonstrate a countereffect to the ongoing inflammation in the colon. A significant increase in IL-10 in inflamed mucosa of patients with ulcerative colitis has in fact already been reported (37). Similarly, the accumulation of naturally occurring T-regulatory cells has been detected in inflamed pancreatic lymph nodes and in the pancreas (38). Increased numbers of Foxp3 T cells have also been detected in the small intestine of children with both celiac disease and type 1 diabetes (39).
Though antibiotics alleviate intestinal inflammation (40), depleting commensal bacteria by antibiotic treatment has been shown to render the colon more susceptible to chemically induced epithelial injury in mice (41). Proper immune recognition of bacteria, rather than just commensal bacteria per se, is thus considered a critical element in the immune regulation of the colon (41,42). The immune cells in healthy individuals are hyporesponsive to resident bacterial flora, but this immune tolerance is broken in patients suffering from inflammatory bowel disease (32,33). Moreover, disruption of the balance between potentially pathogenic and potentially beneficial commensal bacteria may also underlie inflammatory bowel disorders (43,44). BB diabetes-prone and diabetes-resistant rats differ in the composition of microbial species present in the gut (45). Moreover, it has been reported that Bacteroides fragilis has the ability to suppress IL-17 production in a model of H. hepaticus induced colitis (44). Recent studies indicate that intestinal Th17 cells are controlled by the specific composition of intestinal microbiota and that the segmented filamentous bacteria with the candidate name Artromitus are particularly potent inducers of Th17 cells (46). Removal of the MyD88 protein moreover protects against diabetes by modulating the composition of gut microbiota (42). It is thus becoming ever more evident that the composition of the bacterial species in the gut profoundly affects the immune homeostasis of the intestinal immune system.
A wheat-free diet reduces the number of microbes in the intestine (47), and delayed introduction of wheat into the diet has positive long-term effects on diabetes prevention in mice (48). It is thought that the intestinal immune system of newly weaned individuals may be particularly sensitive to immune disruption due to the yet immature immune system, higher permeability of the intestinal wall, and lower numbers of IgA positive B-cells in infancy (49). The findings presented herein indicate that a diet change from standard murine pellet food to ProSobee infant formula dramatically alters microbial species prevalence in the intestine. The ProSobee diet moreover brings about a decline in colonic proinflammatory cytokine levels and eases the hyperplasia observed in NOD colons. At 6 weeks of age, differences between BALB/c, NOD, and PNOD had largely leveled out (results not shown), indicating that the changes seen in NOD at 4.5 weeks may be transient. This correlates with the idea that the weaning period is particularly critical for the development of gut immunity, because the mice were weaned at ∼3 weeks of age.
It has been proposed that the pancreatic lymph nodes are the primary draining sites for the transverse colon (13). Furthermore, dextran sodium sulfate, which causes colitis, has been reported to promote T-cell activation in the pancreatic lymph nodes (14). Thus, it is possible that a direct connection exists between NOD colonic immune interruptions and the onset of autoimmune events in the pancreatic lymph nodes, which ultimately lead to type 1 diabetes. However, it is also possible that events occurring in the colonic lamina propria may cause a response in the peritoneal immune cell pool. Indomethacine, which disrupts the epithelial barrier mainly in the small intestine, causes rapid changes in the composition of cells in the peritoneum (50). The peritoneal cavity B-cells in turn preferentially migrate to the pancreatic lymph nodes (29) and, hence, may provide the link between gut immune system disruption and type 1 diabetes onset. The elevated expression of activation markers CD40 and CD86 and the enhanced efficiency of NOD peritoneal B-cells to present antigen to diabetogenic T-cells decline when NOD mice are fed ProSobee instead of the conventional diet. The events in the peritoneum of NOD mice thus may be interlinked with intestinal immune regulation.
The evidence brought forward in this study emphasizes the importance of the colonic immune system and the role of microbial prevalence in the development of type 1 diabetes in the NOD model. It is suggested herein that the antidiabetogenic effects of the ProSobee diet derive, at least in part, from its capacity to restore colonic immune homeostasis in NOD, where a proinflammatory bias otherwise prevails. The anti-inflammatory effects of the ProSobee diet also have implications outside of the gastrointestinal immune system, because it changes the properties of the peritoneal B-cells. It can be proposed that the colonic immune imbalance in NOD mice reflects on the peritoneal immune cells, which subsequently aid in initiating an autoimmune response in the pancreatic lymph nodes, triggering type 1 diabetes development.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by The Academy of Finland, The Päivikki and Sakari Sohlberg Foundation, Finland and The Finnish Diabetes Research Foundation. No potential conflicts of interest relevant to this article were reported.
C.A. and A.H. researched the data and wrote the manuscript. S.V. and V.P. researched the data. J.J. contributed to discussion. E.E. researched the data and contributed to discussion.
We also thank Jani Jaakkola (Turku University of Applied Sciences) and Seija Lindqvist (Central Animal Laboratory of Turku University) for skillful technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Vaarala O, Knip M, Paronen J, Hämäläinen AM, Muona P, Väätäinen M, Ilonen J, Simell O, Akerblom HK. Cow's milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes 1999;48:1389–1394 [DOI] [PubMed] [Google Scholar]
- 2.Virtanen SM, Saukkonen T, Savilahti E, Ylönen K, Räsänen L, Aro A, Knip M, Tuomilehto J, Akerblom HK. Diet, cow's milk protein antibodies and the risk of IDDM in Finnish children. Childhood Diabetes in Finland Study Group. Diabetologia 1994;37:381–387 [DOI] [PubMed] [Google Scholar]
- 3.Hänninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes 1996;45:1173–1180 [DOI] [PubMed] [Google Scholar]
- 4.Hänninen A, Jaakkola I, Jalkanen S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J Immunol 1998;160:6018–6025 [PubMed] [Google Scholar]
- 5.Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes 1997;46:1542–1547 [DOI] [PubMed] [Google Scholar]
- 6.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006;49:2824–2827 [DOI] [PubMed] [Google Scholar]
- 7.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AM. Enteropathy precedes type 1 diabetes in the BB rat. Gut 2004;53:1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurano F, Mazzarella G, Luongo D, Stefanile R, D'Arienzo R, Rossi M, Auricchio S, Troncone R. Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia 2005;48:931–937 [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre DE, Powell KL, Strom A, Scott FW. Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu Rev Nutr 2006;26:175–202 [DOI] [PubMed] [Google Scholar]
- 10.Vaarala O. Leaking gut in type 1 diabetes. Curr Opin Gastroenterol 2008;24:701–706 [DOI] [PubMed] [Google Scholar]
- 11.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008;57:2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reséndiz-Albor AA, Esquivel R, López-Revilla R, Verdín L, Moreno-Fierros L. Striking phenotypic and functional differences in lamina propria lymphocytes from the large and small intestine of mice. Life Sci 2005;76:2783–2803 [DOI] [PubMed] [Google Scholar]
- 13.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med 1974;139:1189–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 2005;102:17729–17733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruickshank SM, English NR, Felsburg PJ, Carding SR. Characterization of colonic dendritic cells in normal and colitic mice. World J Gastroenterol 2005;11:6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rescigno M, Lopatin U, Chieppa M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr Opin Immunol 2008;20:669–675 [DOI] [PubMed] [Google Scholar]
- 17.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 2007;8:1086–1094 [DOI] [PubMed] [Google Scholar]
- 18.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005;115:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, Hibi T. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut 2008;57:1682–1689 [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res 2010;35:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes 1996;45:812–817 [DOI] [PubMed] [Google Scholar]
- 22.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes 2000;49:2007–2011 [DOI] [PubMed] [Google Scholar]
- 23.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 2009;39:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan S, Bolick DT, Lukashev D, Lappas C, Sitkovsky M, Lynch KR, Hedrick CC. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes 2008;57:484–493 [DOI] [PubMed] [Google Scholar]
- 25.Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, Aronow BJ, Katz JD. Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics 2005;21:201–211 [DOI] [PubMed] [Google Scholar]
- 26.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 2009;58:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 2008;205:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 2009, 2February (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam C, Valkonen S, Ohls S, Törnqvist K, Hänninen A. Enhanced trafficking to the pancreatic lymph nodes and auto-antigen presentation capacity distinguishes peritoneal B lymphocytes in non-obese diabetic mice. Diabetologia 2010;53:346–355 [DOI] [PubMed] [Google Scholar]
- 30.Eerola E, Lehtonen OP. Optimal data processing procedure for automatic bacterial identification by gas–liquid chromatography of cellular fatty acids. J Clin Microbiol 1988;26:1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toivanen P, Vaahtovuo J, Eerola E. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 2001;69:2372–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duchmann R, Schmitt E, Knolle P, Meyer zum Büschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol 1996;26:934–938 [DOI] [PubMed] [Google Scholar]
- 34.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia 2009;52:1820–1828 [DOI] [PubMed] [Google Scholar]
- 35.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 2003;52:2287–2295 [DOI] [PubMed] [Google Scholar]
- 36.Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004;53:1680–1683 [DOI] [PubMed] [Google Scholar]
- 37.Matsuda R, Koide T, Tokoro C, Yamamoto T, Godai T, Morohashi T, Fujita Y, Takahashi D, Kawana I, Suzuki S, Umemura S. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naive ulcerative colitis during active and quiescent disease. Inflamm Bowel Dis 2009;15:328–334 [DOI] [PubMed] [Google Scholar]
- 38.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes 2008;57:113–123 [DOI] [PubMed] [Google Scholar]
- 39.Vorobjova T, Uibo O, Heilman K, Rägo T, Honkanen J, Vaarala O, Tillmann V, Ojakivi I, Uibo R. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand J Gastroenterol 2009;44:422–430 [DOI] [PubMed] [Google Scholar]
- 40.Videla S, Vilaseca J, Guarner F, Salas A, Treserra F, Crespo E, Antolín M, Malagelada JR. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut 1994;35:1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 2008;205:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008;453:620–625 [DOI] [PubMed] [Google Scholar]
- 45.Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, Li N, Mai V, Wasserfall CH, Schatz D, Atkinson MA, Neu J, Triplett EW. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J 2009;3:536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen AK, Ling F, Kaas A, Funda DP, Farlov H, Buschard K. Diabetes preventive gluten-free diet decreases the number of caecal bacteria in non-obese diabetic mice. Diabetes Metab Res Rev 2006;22:220–225 [DOI] [PubMed] [Google Scholar]
- 48.Flohé SB, Wasmuth HE, Kerad JB, Beales PE, Pozzilli P, Elliott RB, Hill JP, Scott FW, Kolb H. A wheat-based, diabetes-promoting diet induces a Th1-type cytokine bias in the gut of NOD mice. Cytokine 2003;21:149–154 [DOI] [PubMed] [Google Scholar]
- 49.Vaarala O. Is it dietary insulin? Ann N Y Acad Sci 2006;1079:350–359 [DOI] [PubMed] [Google Scholar]
- 50.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 2006;203:2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.