Abstract
OBJECTIVE
To create an immunodeficient mouse model that spontaneously develops hyperglycemia to serve as a diabetic host for human islets and stem cell–derived β-cells in the absence or presence of a functional human immune system.
RESEARCH DESIGN AND METHODS
We backcrossed the Ins2Akita mutation onto the NOD-Rag1null IL2rγnull strain and determined 1) the spontaneous development of hyperglycemia, 2) the ability of human islets, mouse islets, and dissociated mouse islet cells to restore euglycemia, 3) the generation of a human immune system following engraftment of human hematopoietic stem cells, and 4) the ability of the humanized mice to reject human islet allografts.
RESULTS
We confirmed the defects in innate and adaptive immunity and the spontaneous development of hyperglycemia conferred by the IL2rγnull, Rag1null, and Ins2Akita genes in NOD-Rag1null IL2rγnull Ins2Akita (NRG-Akita) mice. Mouse and human islets restored NRG-Akita mice to normoglycemia. Insulin-positive cells in dissociated mouse islets, required to restore euglycemia in chemically diabetic NOD-scid IL2rγnull and spontaneously diabetic NRG-Akita mice, were quantified following transplantation via the intrapancreatic and subrenal routes. Engraftment of human hematopoietic stem cells in newborn NRG-Akita and NRG mice resulted in equivalent human immune system development in a normoglycemic or chronically hyperglycemic environment, with >50% of engrafted NRG-Akita mice capable of rejecting human islet allografts.
CONCLUSIONS
NRG-Akita mice provide a model system for validation of the function of human islets and human adult stem cell, embryonic stem cell, or induced pluripotent stem cell–derived β-cells in the absence or presence of an alloreactive human immune system.
Investigators are working to develop functional human β-cells from adult or embryonic stem cells (ESCs) or from induced pluripotent stem (iPS) cells (1–4), and human ESC-derived β-cells can restore euglycemia in streptozotocin (STZ) diabetic immunodeficient mice (5). However, STZ can have detrimental effects on many organs in addition to β-cells (6,7), and there is sometimes recovery of the host β-cell function (8,9). In addition, it will be important to transplant human β-cells in the presence of an allogeneic human immune system.
We have developed an immunodeficient mouse model of spontaneous hyperglycemia based on NOD-Rag1null Prf1null mice bearing the Ins2Akita mutation (10). Mice heterozygous for Ins2Akita develop spontaneous hyperglycemia at 3–6 weeks of age (11,12), and transplantation of human islets restores euglycemia (10). However, human immune system engraftment is relatively inefficient in this model (13). Immunodeficient scid and Rag1null NOD mice bearing a mutation in the interleukin (IL)-2 receptor common γ chain (IL2rγnull) gene are now available (14,15) and can be engrafted with functional human immune systems (14–18).
We now describe the development of the NOD-Rag1null IL2rγnull Ins2+/Akita (NRG-Akita) strain. These mice develop spontaneous hyperglycemia, and euglycemia can be restored following transplantation with mouse or human islets or with dissociated mouse islet cells. A human immune system develops following engraftment with human hematopoietic stem cells (HSCs), and in some mice, this immune system is capable of rejecting human islet allografts.
RESEARCH DESIGN AND METHODS
NOD-Rag1null IL2rγnull (NRG) (14), NOD-Rag1null Prf1null Ins2Akita (10), and NOD-scid IL2rγnull (NSG) (15) mice have previously been described. To generate NRG-Akita mice, NOD.Cg-Rag1tm1Mom Prf1null Ins2Akita mice were crossed with the NOD-Rag1null IL2rγnull strain. Additional crosses fixed the wild-type Prf1 allele and the IL2rγnull mutation to homozygosity while maintaining the Ins2Akita mutation in the heterozygous state. Mice were housed in a specific pathogen-free facility in microisolator cages (15). All animal use was in accordance with the guidelines of the IACUCs of the University of Massachusetts and The Jackson Laboratory.
Antibodies and flow cytometry.
The phenotypes of murine cells were determined using four color analyses (15). Anti-human FoxP3 (eBioscience, San Diego, CA), anti-human CD123 and BDCA2 (Miltenyi Biotec, Bergisch Gladbach, Germany), anti-human CD11c (Biolegend, San Diego, CA), and anti-human CD235a (Coulter Immunotech, Miami, FL) were obtained as indicated. All other antibodies were purchased from BD Biosciences (San Jose, CA).
Bone marrow, spleen, and thymus cell suspensions and heparinized blood were incubated with anti-mouse FcR11b to block Fc binding (14,19). Cells were prepared for flow cytometry, and at least 50,000 events were acquired on BD Biosciences LSRII or FACSCalibur instruments (BD Biosciences) (14,15,20). For rare populations, we acquired at least 500 specific events. Data analysis was performed with FlowJo (Tree Star, Ashland, OR) software.
Mouse and human islet transplantation.
Handpicked BALB/c islets (21) were also dissociated into single-cell suspensions (22). Dissociated islet cell suspensions were treated with Cytofix/Cytoperm (BD Biosciences) for intracellular staining using a biotinylated anti-insulin antibody and developed with Streptavidin-allophycocyanin (SA-APC). Dissociated preparations contained a mean ± SD of 62 ± 19% (n = 10) insulin-positive cells. Islets or dissociated cells were transplanted intrapancreatically or subrenally into STZ diabetic NSG mice (150 mg/kg) or unmanipulated diabetic NRG-Akita recipients.
Human islets were obtained from Juvenile Diabetes Research Foundation Islet Isolation Centers or the National Institutes of Health Integrated Islet Distribution Program. Human islets (IEQs) (4,000) were transplanted subrenally into diabetic NSG or NRG-Akita mice. Nonfasting blood glucose levels were determined twice weekly. Loss of graft function was determined by two consecutive blood glucose values >250 mg/dl.
Engraftment of mice with human HSCs.
NRG mice or progeny of NRG × NRG-Akita matings (∼50% heterozygous for Ins2Akita) 1–3 days old were irradiated with 400 cGy and engrafted with umbilical cord blood. HSCs were provided by the University of Massachusetts Memorial Umbilical Cord Blood Donation Program under insitutional review board approval (14,20). Mice were analyzed phenotypically 12–16 weeks later and/or transplanted with human islet allografts.
Histology.
Pancreata and islet transplants were stained with hemotoxlin-eosin (H-E) and for insulin, glucagon, and human CD45 (16). Unilateral nephrectomy of the graft-bearing kidney was performed on selected cohorts to confirm recurrent hyperglycemia. In some cases, islet allograft survival was inferred with histological study.
Statistical analyses.
Most data are presented as means ± SD, and flow cytometry data are presented as means ± SEM. Parametric data were compared by one-way ANOVA with Bonferroni posttests to compare individual pairwise groupings. Nonparametric data were compared by a Kruskal-Wallis with Dunns posttest to compare individual pairwise groupings. Significant differences were assumed for P values <0.05. All statistical analyses were performed using GraphPad Prism software (version 4.0c; GraphPad, San Diego, CA).
RESULTS
Phenotype of the hematopoietic system of NRG-Akita mice.
To develop an immunodeficient strain of mice bearing the Ins2Akita mutation that will support the development of a human immune system following engraftment with HSC, we generated NRG-Akita mice. Because mice homozygous for Ins2Akita develop a rapid hyperglycemia and die shortly after weaning (10), all studies were performed using mice heterozygous for the Ins2Akita allele.
Flow cytometry analysis of NRG-Akita splenocytes confirmed the lack of mature T, B, and natural killer (NK) cells (supplemental Table 1, available in the online appendix [http://diabetes.diabetesjournals.org/cgi/content/full/db10-0323/DC1]). Although cells with an NK phenotype (DX5+/CD122+) were observed, these cells are not mature NK cells (15). Populations of granulocytes and macrophages in NRG and NRG-Akita mice were similar (supplemental Table 1), documenting that NRG-Akita mice are deficient in adaptive immunity and NK cells.
Spontaneous hyperglycemia in NRG-Akita mice and reversal by islet transplantation.
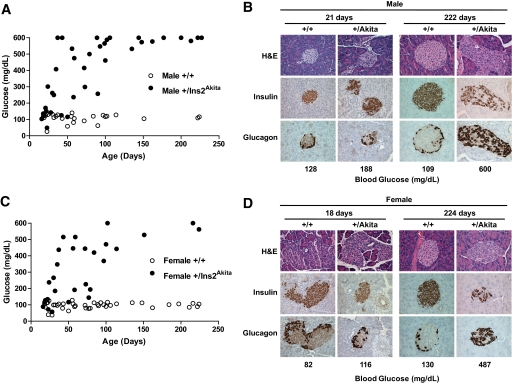
All male and female NRG-Akita mice developed hyperglycemia between 3 and 5 weeks of age and remained diabetic, viable, and healthy in the absence of exogenous insulin (Fig. 1). In contrast, NRG (Ins2+/+) littermates remained euglycemic throughout their life span.
FIG. 1.
Onset of hyperglycemia and islet morphology in male and female NRG-Akita mice. Male (A) and female (C) NRG-Akita and NOD-Rag1null IL2r γnull littermate control mice were followed for >200 days with blood glucose monitored at the days of age indicated as described in research design and methods. Data points shown are of individual animals. Pancreata from male (B) and female (D) NRG-Akita and NOD-Rag1null IL2rγnull littermate control mice at the indicated ages were stained with H-E and immunohistochemically for insulin and glucagon. Young and older control NRG (+/+) mice and young NRG-Akita (+/Akita) mice displayed normal architecture and histochemical structure with insulin-positive cells throughout the islets and a peripheral rim of glucagon-positive cells. Older NRG-Akita mice displayed collapsed islet structure with fewer insulin-positive β-cells and numerous glucagon positive cells scattered throughout the islets. Blood glucose levels of the mice at the time of recovery of the pancreas are shown at the bottom of each panel. Magnification ×400. (A high-quality digital representation of this figure is available in the online issue.)
Normal islet architecture and insulin and glucagon staining were observed in the pancreata of NRG mice at all ages (Fig. 1). At ∼3 weeks of age, the pancreata of NRG-Akita mice were only slightly disorganized and strong insulin staining was observed. By ∼32 weeks of age in hyperglycemic NRG-Akita mice, islet architecture was disorganized and condensed. Some insulin-positive cells were present but scattered throughout the islet. No inflammatory infiltrates were observed.
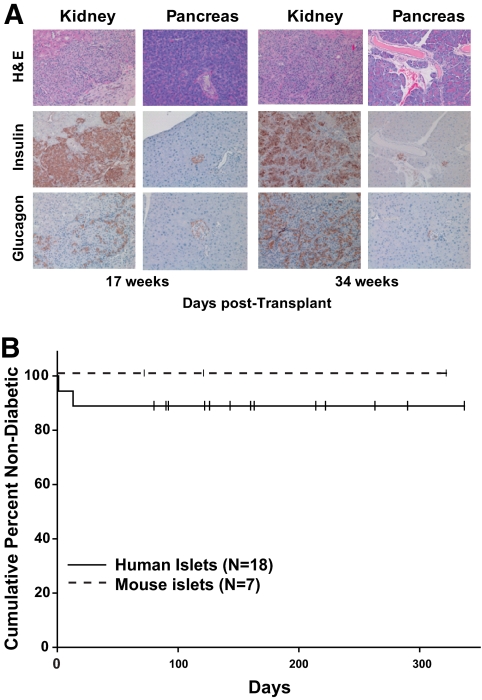
Subrenal transplantation of 20 mouse islets/g body wt into hyperglycemic NRG-Akita mice restored normoglycemia (Fig. 2). Islet graft recipients remained normoglycemic to the end of observation periods or, in selected cases, reverted to hyperglycemia following removal of the graft-bearing kidney (data not shown).
FIG. 2.
Transplantation of mouse and human islets into diabetic NRG-Akita mice. Diabetic NRG-Akita mice were transplanted in the renal subcapsular space with 4,000 IEQ human islets or with 20 islets/g body wt mouse islets as described in research design and methods. Blood glucose levels were determined, and the kidney bearing the islet transplant and the host pancreas were recovered at the end of the experiment for histological and immunohistological analyses. A: H-E, insulin, and glucagon staining of transplanted mouse (left panel) and human (right panel) islets and host pancreas of the NRG-Akita transplant recipient at the times indicated after islet transplantation. In the renal subcapsular space, there was robust engraftment of mouse and human islets. Magnification ×200. B: Frequency of diabetes in mouse or human islet recipients. No significant differences were observed between recipients of mouse or human islets. Small vertical bars indicate censored data, i.e., mice that were found dead or were removed from the study for other analyses. (A high-quality digital representation of this figure is available in the online issue.)
Transplantation of 4,000 IEQ human islets into the subrenal capsular space routinely restored euglycemia in NRG-Akita mice (Fig. 2). These islets remained functional throughout the observation periods—in some cases over 300 days. Graft survival was confirmed by histology or by removal of the graft-bearing kidney and reversion to hyperglycemia (data not shown).
Restoration of normoglycemia by transplantation of mouse islets or dissociated islet cells into diabetic NSG or NRG-Akita mice.
Intrapancreatic transplantation of 20 islets/g body wt into STZ diabetic NSG mice failed to restore normoglycemia, whereas 40 islets/g body wt restored normoglycemia in two of seven recipients (supplemental Fig. 1). Using dissociated islet cells, more than 5 × 105 insulin-positive β-cells was required for restoration of normoglycemia following subrenal transplantation in diabetic NSG mice (supplemental Fig. 1).
In diabetic NRG-Akita mice, intrapancreatic transplantation of 40 mouse islets/g body wt restored normoglycemia in three of four NRG-Akita mice, whereas 20 islets/g body wt restored normoglycemia in only one of three NRG-Akita recipients (supplemental Fig. 2). This is in contrast to restoration of normoglycemia in diabetic NRG-Akita recipients following subrenal transplantation of 20 mouse islets/g body wt (Fig. 2).
Intrapancreatic transplantation of dissociated mouse islet cells containing 1–4 × 106 insulin-positive β-cells restored euglycemia in only one of five diabetic NRG-Akita recipients (supplemental Fig. 2). Only two of six hyperglycemic NRG-Akita mice became euglycemic after subrenal transplantation with 1–4 × 106 dissociated mouse islet cells (supplemental Fig. 2). These data document 1) that dissociated mouse islets can restore normoglycemia in diabetic NSG and NRG-Akita mice and 2) that fewer insulin-positive cells are required for restoration of normoglycemia following subrenal compared with intrapancreatic transplantation.
Engraftment of human HSCs in NRG-Akita mice.
We have shown that NRG mice engraft with human HSC at levels similar to those observed in NSG mice (14), but the effect of chronic hyperglycemia on the development of a human immune system is unknown.
NRG-Akita and NRG littermates were injected with human HSCs (14,20). Twelve weeks later, hyperglycemia was confirmed in NRG-Akita mice (data not shown). Similar percentages of splenic human CD45+ cells and all human cell subsets were observed in hyperglycemic NRG-Akita acompared with euglycemic NRG mice (Table 1). Comparable levels of human CD45+ and human cell subsets were also observed in the bone marrow, blood, and thymus of hyperglycemic NRG-Akita and euglycemic NRG mice (supplemental Tables 2–4). These data document that the human immune systems that develop in hyperglycemic NRG-Akita mice appear phenotypically comparable with those developing in normoglycemic NRG mice.
TABLE 1.
Human HSC engraftment in the spleen of NRG and NRG-Akita mice
| NRG | NRG-Akita | |
|---|---|---|
| n | 16 | 12 |
| Total splenocyte number (×106) | 17.4 ± 4.3 | 23.1 ± 3.5 |
| Total human leukocytes (%) | ||
| CD45+ | 58.8 ± 6.1 | 50.98 ± 4.9 |
| B lineage (% of CD45) | ||
| CD20+ | 69.4 ± 2.9 | 71.2 ± 2.8 |
| T lineage (% of CD45) | ||
| CD3+ | 5.2 ± 1.6 | 8.6 ± 3.9 |
| CD4+ CD45RA+ | 5.7 ± 2.6 | 4.2 ± 1.2 |
| CD4+ CD45RO+ | 3.3 ± 1.1 | 4.5 ± 1.4 |
| CD4+ FoxP3+ | 0.7 ± 0.2 | 0.8 ± 0.2 |
| CD8+ CD45RA+ | 4.7 ± 1.7 | 6.3 ± 2.0 |
| CD8+ CD45RO+ | 1.2 ± 0.4 | 1.6 ± 0.6 |
| Dendritic subsets (from murine CD45− cells) | ||
| (Monocytic) CD11c+ BDCA2− | 2.5 ± 0.3 | 3.6 ± 0.4 |
| (Plasmacytoid) CD123+ BDCA2+ | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Monocyte/macrophage (% of CD45) | ||
| CD14+ | 1.3 ± 0.3 | 2.0 ± 0.4 |
Newborn NRG and NRG-Akita mice were irradiated with 400 cGY and injected with T cell–depleted Umbilical cord blood (UCB) containing 3 × 104 human CD34+ HSCs by intracardiac injection as described in research design and methods. The percent of various human cell populations in the spleen was determined by flow cytometry 12 weeks later. No significant differences were observed.
Transplantation of human islet allografts in HSC-engrafted NRG-Akita mice.
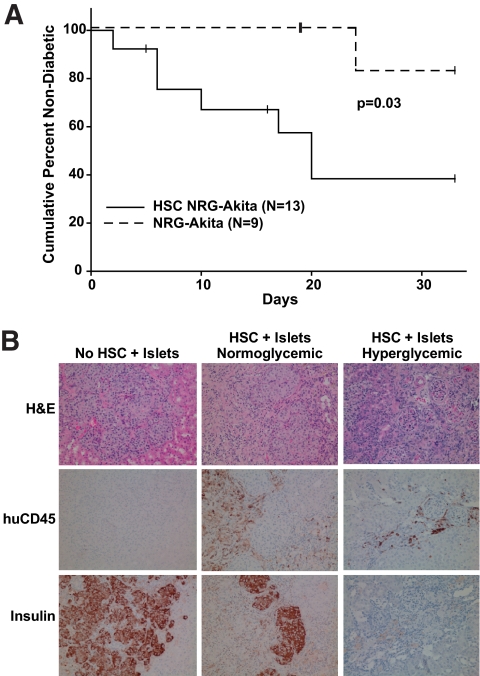
Eight of nine human islet recipients of 4,000 IEQ restored normoglycemia (<250 mg/dl) (Fig. 3) in non–HSC-engrafted diabetic NRG-Akita mice. In contrast, eight of 13 human islet allografts were rejected in HSC-engrafted NRG-Akita mice (Fig. 3). Insulin staining in the absence of mononuclear cell infiltration was detected in human islet allografts in non–HSC-engrafted NRG-Akita mice (Fig. 3). In the eight HSC-engrafted NRG-Akita human islet recipients that reverted to hyperglycemia, human CD45+ cell infiltration into the graft site and loss of β-cells were observed. In the five HSC-engrafted NRG-Akita mice that did not revert to hyperglycemia, insulin-positive cells and human CD45+ cell islet infiltration were observed (Fig. 3).
FIG. 3.
Transplantation of human islet allografts into diabetic NRG-Akita mice engrafted with human HSC. Diabetic NRG-Akita and HSC-engrafted NRG-Akita mice were transplanted subrenally with 4,000 human IEQ as described in research design and methods. A: Frequency of diabetes in islet allograft recipients. NRG-Akita vs. HSC-engrafted NRG-Akita, P = 0.03. B: Representative histology and immunochemical staining patterns are shown. Note the abundance of insulin-positive cells and the absence of human CD45-positive cells in non–HSC engrafted NRG-Akita mice (left panel). Note the presence of fewer insulin-positive cells and islet graft infiltration by human CD45+ cells in HSC-engrafted NRG-Akita mice that were normoglycemic at the end of the experiment (middle panel). Note the scarcity of insulin-positive cells and moderate numbers of human CD45+ cells in HSC-engrafted NRG Akita mice that were hyperglycemic at the end of the experiment and had rejected their human islet allografts (right panel). Magnification ×200. huCD45, human CD45 staining; Insulin, insulin staining. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
We have previously described the development of spontaneously diabetic NOD-Rag1null Prf1null Ins2Akita mice (10), but NOD-Rag1null Prf1null mice engraft poorly with human immune systems (23). To address this limitation, we generated NRG-Akita mice that spontaneously develop hyperglycemia. Euglycemia can be restored following transplantation with mouse or human islets or with dissociated mouse islet cells. Even in the presence of a state of chronic hyperglycemia, a human immune system develops following engraftment with human HSCs that is capable of rejecting human islet allografts in some of the transplanted mice.
Unmanipulated NRG-Akita mice maintain a phenotype consistent with a severely immunodeficient mouse bearing IL2rγ and Ins2Akita mutated genes. Both male and female mice develop hyperglycemia between 3 and 5 weeks of age, and islets progressively lose insulin-positive cells with age. However, small numbers of insulin-positive cells remain throughout life, perhaps explaining why administration of exogenous insulin is not required for long-term survival.
We confirmed that transplantation of mouse or human islets restores euglycemia in diabetic NRG-Akita and NSG mice. Higher numbers of islets were required following intrapancreatic implantation than were needed following subrenal transplantation to restore euglycemia. Our data also suggest that single-cell suspensions of insulin-producing cells may be less functional than comparable numbers contained within an islet structure.
We confirmed that a human immune system develops in diabetic NRG-Akita mice engrafted with human HSCs, and this immune system is phenotypically comparable with that generated in euglycemic NRG mice. However, only ∼60% of human immune system–engrafted NRG-Akita mice rejected human islet allografts. In separate studies, HSC-engrafted NSG mice readily reject human skin allografts (M.A.B., unpublished observations), but it is known that skin allografts prompt a more robust alloimmune response than do islet allografts (24,25). Alternatively, the human immune system may be impaired as a result of the hyperglycemic environment—or we may not have allowed sufficient time for graft rejection.
In summary, we have developed a new model of spontaneous hyperglycemia based on immunodeficient NOD mice bearing mutations in the IL2rγ gene and the Ins2Akita gene. NRG-Akita mice can be engrafted with a human immune system, providing a recipient environment similar to that which will be encountered when transplanting allogeneic β-cells in the clinic. These mice provide a novel model system suitable for validation of the function of stem cell–derived human β-cells in the absence or presence of an alloreactive human immune system.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants (AI46629, DK53006, HL077642, DK66636, DK69603, DK68854, and DK72473), the VA Research Service, an institutional Diabetes Endocrinology Research Center (DERC) grant (DK32520), Cancer Center Core Grant CA34196, the Vanderbilt Diabetes Research and Training Center (DK20593), the Beta Cell Biology Consortium (DK72473), an institutional Center for AIDS Research grant (AI042845), and grants from the Juvenile Diabetes Foundation, International, and the Helmsley Foundation. D.L.G. is a member of the UMass DERC (DK32520). Human islets were obtained from the Juvenile Diabetes Research Foundation Islet Isolation Centers or the Islet Cell Resource Consortium (now termed the Integrated Islet Distribution Program).
No potential conflicts of interest relevant to this article were reported.
M.A.B. researched data, wrote the manuscript, and reviewed and edited the manuscript. R.B. reviewed and edited the manuscript. P.D. researched data, contributed to discussion, and reviewed and edited the manuscript. J.L. researched data and contributed to discussion. J.L. reviewed and edited manuscript. A.C. researched data. C.Y. reviewed and edited the manuscript. M.H. reviewed and edited the manuscript. L.B. researched data and wrote the manuscript. B.G. researched data and wrote the manuscript. O.F. researched data. A.C.P. contributed to discussion and wrote the manuscript. D.L.G. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. L.D.S. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited manuscript.
We thank Linda Paquin, University of Massachusetts Medical School, Allison Ingalls, The Jackson Laboratory, and Greg Poffenberger, Vanderbilt University Medical Center, for their technical assistance, and Anoop Kavirayani, The Jackson Laboratory, for critical reading of the manuscript. We also thank the medical staff of the University of Massachusetts Memorial Umbilical Cord Blood Donation Program.
Footnotes
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science 2008;322:1811–1815 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Melton DA. Pathways to new beta cells. Cold Spring Harb Symp Quant Biol 2008;73:175–181 [DOI] [PubMed] [Google Scholar]
- 3.Fellous TG, Guppy NJ, Brittan M, Alison MR. Cellular pathways to beta-cell replacement. Diabete Metab Res Rev 2007;23:87–99 [DOI] [PubMed] [Google Scholar]
- 4.Sordi V, Bertuzzi F, Piemonti L. Diabetes mellitus: an opportunity for therapy with stem cells? Regen Med 2008;3:377–397 [DOI] [PubMed] [Google Scholar]
- 5.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 6.Levine BS, Henry MC, Port CD, Rosen E. Toxicologic evaluation of streptozotocin (NSC 85998) in mice, dogs and monkeys. Drug Chem Toxicol 1980;3:201–212 [DOI] [PubMed] [Google Scholar]
- 7.Manikandan R, Sundaram R, Thiagarajan R, Sivakumar MR, Meiyalagan V, Arumugam M. Effect of black tea on histological and immunohistochemical changes in pancreatic tissues of normal and streptozotocin-induced diabetic mice (Mus musculus). Microsc Res Tech 2009;72:723–726 [DOI] [PubMed] [Google Scholar]
- 8.Guz Y, Nasir I, Teitelman G. Regeneration of pancreatic beta cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology 2001;142:4956–4968 [DOI] [PubMed] [Google Scholar]
- 9.Guz Y, Torres A, Teitelman G. Detrimental effect of protracted hyperglycaemia on beta-cell neogenesis in a mouse murine model of diabetes. Diabetologia 2002;45:1689–1696 [DOI] [PubMed] [Google Scholar]
- 10.Pearson T, Shultz LD, Lief J, Burzenski L, Gott B, Chase T, Foreman O, Rossini AA, Bottino R, Trucco M, Greiner DL. A new immunodeficient hyperglycaemic mouse model based on the Ins2Akita mutation for analyses of human islet and beta stem and progenitor cell function. Diabetologia 2008;51:1449–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 12.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002;73:1333–1336 [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Banuelos S, Lyons B, Samuels R, Burzenski L, Gott B, Lang P, Leif J, Appel M, Rossini A, Greiner DL. NOD/LtSz-Rag1null Prf1null mice: a new model system with increased levels of human peripheral leukocyte and hematmopoietic stem cell engraftment. Transplantation 2003;76:1036–1042 [DOI] [PubMed] [Google Scholar]
- 14.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, Cuthbert A, Burzenski L, Gott B, Lyons B, Foreman O, Rossini AA, Greiner DL. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol 2008;154:270–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rγnull mice engrafted with mobilized human hematopoietic stem cell. J Immunol 2005;174:6477–6489 [DOI] [PubMed] [Google Scholar]
- 16.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, Atkinson MA, Wasserfall C, Herold KC, Woodland RT, Schmidt MR, Woda BA, thompson mj, Rossini AA, Greiner DL. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol 2008;126:303–314 [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS one 2009;4:e7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, Cohen JI, Munz C. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med 2009;206:1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Burzenski L, Gott B, Foreman O, Kavirayani A, Herlihy M, Rossini AA, Shultz LD, Greiner DL. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol 2010;135:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol 2008;Chapter 15:Unit 15.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markees TG, Serreze DV, Phillips NE, Sorli CH, Noelle RJ, Woda BA, Greiner DL, Mordes JP, Rossini AA. NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes 1999;48:967–974 [DOI] [PubMed] [Google Scholar]
- 22.Pipeleers DG, Pipeleers-Marichal MA. A method for the purification of single A, B and D cells and for the isolation of coupled cells from isolated rat islets. Diabetologia 1981;20:654–663 [DOI] [PubMed] [Google Scholar]
- 23.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007;7:118–130 [DOI] [PubMed] [Google Scholar]
- 24.Pearson T, Markees TG, Wicker LS, Serreze DV, Peterson LB, Mordes JP, Rossini AA, Greiner DL. NOD congenic mice genetically protected from autoimmune diabetes remain resistant to transplantation tolerance induction. Diabetes 2003;52:321–326 [DOI] [PubMed] [Google Scholar]
- 25.Phillips NE, Markees TG, Mordes JP, Greiner DL, Rossini AA. Blockade of CD40-mediated signaling is sufficient for inducing islet but not skin transplantation tolerance. J Immunol 2003;170:3015–3023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.