Abstract
OBJECTIVE
To investigate age-period-cohort effects on the temporal trend of type 1 diabetes in children age 0–14 years in Italian registries.
RESEARCH DESIGN AND METHODS
This report is based on 5,180 incident cases in the period 1990–2003 from the Registry for Type 1 Diabetes Mellitus in Italy (RIDI). Multilevel (random intercept) Poisson regression models were used to model the effects of sex, age, calendar time, and birth cohorts on temporal trends, taking into account the registry-level variance component.
RESULTS
The incidence rate was 12.26 per 100,000 person-years and significantly higher in boys (13.13 [95% CI 12.66–13.62]) than in girls (11.35 [10.90–11.82]). Large geographical variations in incidence within Italy were evident; incidence was highest in Sardinia, intermediate in Central-Southern Italy, and high in Northern Italy, particularly in the Trento Province, where the incidence rate was 18.67 per 100,000 person-years. An increasing temporal trend was evident (2.94% per year [95% CI 2.22–3.67]). With respect to the calendar period 1990–1992, the incidence rates increased linearly by 15, 27, 35, and 40% in the following time periods (P for trend < 0.001). With respect to the 1987–1993 birth cohort, the incidence rate ratio increased approximately linearly from 0.63 (95% CI 0.54–0.73) in the 1975–1981 cohort to 1.38 (1.06–1.80) in the 1999–2003 cohort. The best model, however, included sex, age, and a linear time trend (drift).
CONCLUSIONS
Large geographical variations and an increasing temporal trend in diabetes incidence are evident among type 1 diabetic children in Italy. Age-period-cohort analysis shows that the variation over time has a linear component that cannot be ascribed to either the calendar period or the birth cohort.
The incidence of type 1 diabetes among children is increasing worldwide with great geographical heterogeneity (1–6). Recently, the EURODIAB Study showed that within Europe the most striking changes during the 1989–2003 period were observed in central and eastern European countries (7). The etiology of the disease is unclear, although a genetic component is evident, and other nongenetic factors, such as infections, nutritional components, and toxins, have been suggested (8). Investigating the spatial and temporal variations of diabetes incidence might provide insights into the etiology of the disease (9–15). Moreover, if associations between environmental factors and the disease exist, it is relevant to establish whether increasing temporal trends are occurring cross-sectionally, affecting all age-groups at the same point in time (period effects), or longitudinally, affecting all age-groups from the same birth cohort (cohort effects) (16). Whereas a nonlinear period increase would suggest an abrupt exposure to an environmental determinant, a nonlinear cohort increase would be consistent with the effect of an epidemic of congenital infections. The linear dependence among age, period, and cohort makes it necessary to apply specific modeling strategies for this task (16). Whereas national diabetes temporal trends (17) and regional age-period-cohort modeling have been published previously (15,18), the age-period-cohort analysis of the Registry of Type 1 Diabetes Mellitus in Italy (RIDI) for the combined national data has never been conducted. The aim of this report is to describe the incidence time trends of childhood-onset type 1 diabetes in Italy in the 1990–2003 period using an age-period-cohort approach.
RESEARCH DESIGN AND METHODS
RIDI was set up in 1997 to coordinate registries for the incidence of type 1 diabetes in Italy (17). All registries reported newly diagnosed cases of childhood type 1 diabetes using a special form, which includes each patient's personal identification number, date of birth, sex, date of diagnosis (defined as the date when the first insulin injection was given), and municipality of residence. A diagnosis of type 1 diabetes was based on permanent insulin treatment within 6 months of diagnosis, fasting C-peptide levels ≤0.20 nmol/l, or positivity for insulinoma-associated protein 2, islet cell antibody, or GAD antibody. Case subjects diagnosed as having type 2 diabetes or other specific types were excluded. Each registry used at least two independent data sources for case ascertainment (17), and the completeness of ascertainment was estimated by the capture-recapture method. In a previous report (17), we presented incidence rates based on 3,602 incident case subjects aged 0–14 years, who had been registered during 1990–1999 by nine registries. To date, 12 registries regularly update incidence rates as part of RIDI: seven regional registries (Liguria, Marche, Umbria, Lazio, Abruzzo, Campania, and Sardinia) and five province registries (Trento, Torino, Pavia, Modena, and Firenze-Prato), covering an at-risk population of 3,321,459 children (39.7% of the whole Italian population aged 0–14 years (a geographical distrubution map is available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0151/DC1).
This report is based on 5,180 incident cases registered in the 1990–2003 period. Most registries contributed data over the whole study period. The registries of Modena and Trento contributed cases in periods 1996–2003 and 1998–2003, respectively, whereas registries of Abruzzo, Lazio, and Umbria contributed data in periods 1990–1995, 1990–1999, and 1990–2001, respectively. Data on at-risk residents in the geographical area covered by each registry for each year of the study period were obtained from the National Institute for Statistics.
Statistical analysis.
The presence of extra-Poisson variability has been evaluated assuming a γ distribution of the true incidence rates through the likelihood ratio test. Multilevel random intercept Poisson regression models have been used to estimate the effects of sex, age (five 3-year age-groups: 0–2, 3–5, 6–8, 9–11, and 12–14 years), calendar time (four 3-year periods: 1990–1992, 1993–1995, 1996–1998, 1999–2001; and one 2-year period: 2002–2003), and birth cohorts (nine 6-year birth cohorts: 1978, 1981, 1984, 1987, 1990, 1993, 1996, 1999, 2001 mid-years), taking into account the registry-level variance component. The models assume a γ distribution of the registry-level random intercept, accounting also for within-registry dependence. Six models were fitted to data: sex; sex, age; sex, age, linear time trend (drift); sex, age, cohort; sex, age, period; and sex, age, period, cohort. The term “drift” denotes a temporal variation of rates that does not distinguish between the influences of two of the three temporal variables involved in the analysis. The hierarchically ordered models were compared by likelihood ratio test. The χ2 test for trend in incidence rates used is the Mantel extension of the Armitage-Cochran trend test (19). The trend test is adjusted by sex. Poisson regression analyses were performed using xtpoisson in STATA, version 9.0. Age-period-cohort models were fitted using apc.fit in R (http://www.R-project.org) (16).
RESULTS
In the 1990–2003 period, 5,180 incident cases of type 1 diabetes were identified among children aged 0–14 years. All registries provided high estimated completeness of ascertainment (Table 1). Incidence rates by sex, age-group, calendar period, birth cohort, Italian macro-area (North, Center-South, Island [Sardinia]), and registry are shown in Table 1. The incidence rate was 12.26 per 100,000 person-years (95% CI 11.93–12.60), with significantly lower risk in girls (11.35 [95% CI 10.90–11.82]) than in boys (13.13 [12.66–13.62]). In peninsular Italy, the incidence rates were 9.53 (95% CI 9.22–9.84) overall, 9.97 (9.54–10.42) in boys, and 9.06 (8.64–9.50) in girls.
TABLE 1.
Incidence rates of type 1 diabetes among Italian children 0–14 years old in the years 1990–2003 by sex, age-group, calendar period, and geographical area of residence
| Incident cases (n) | Person-years at risk (n) | Incidence rates per 100,000 person-years (95% CI) | % Estimated completeness of ascertainment (range) | |
|---|---|---|---|---|
| All | 5,180 | 42,246,144 | 12.26 (11.93–12.60) | |
| Boys | 2,840 | 21,629,264 | 13.13 (12.66–13.62) | |
| Girls | 2,340 | 20,616,878 | 11.35 (10.90–11.82) | |
| Age-group (years) | ||||
| 0–2 | 479 | 7,984,265 | 6.00 (5.49–6.56) | |
| 3–5 | 961 | 8,002,496 | 12.01 (11.27–12.79) | |
| 6–8 | 1,118 | 8,259,614 | 13.54 (12.77–14.35) | |
| 9–11 | 1,366 | 8,672,116 | 15.75 (14.94–16.61) | |
| 12–14 | 1,256 | 9,327,652 | 13.47 (12.74–14.23) | |
| Calendar period | ||||
| 1990–1992 | 1,089 | 10,659,853 | 10.22 (9.63–10.84) | |
| 1993–1995 | 1,139 | 9,871,152 | 11.54 (10.89–12.23) | |
| 1996–1998 | 1,184 | 9,297,165 | 12.74 (12.03–13.48) | |
| 1999–2001 | 1,091 | 7,836,490 | 13.92 (13.12–14.77) | |
| 2002–2003 | 677 | 4,581,483 | 14.78 (13.7–15.93) | |
| Birth cohort | ||||
| 1975–1981 | 273 | 2,536,714 | 10.76 (9.56–12.12) | |
| 1978–1984 | 560 | 4,440,171 | 12.61 (11.61–13.70) | |
| 1981–1987 | 819 | 6,100,235 | 13.43 (12.54–14.38) | |
| 1984–1990 | 1,012 | 7,278,051 | 13.90 (13.07–14.79) | |
| 1987–1993 | 1,047 | 8,137,636 | 12.87 (12.11–13.67) | |
| 1990–1996 | 715 | 6,285,553 | 11.38 (10.57–12.24) | |
| 1993–1999 | 457 | 4,186,785 | 10.92 (9.96–11.96) | |
| 1996–2002 | 226 | 2,385,021 | 9.48 (8.32–10.80) | |
| 1999–2003 | 71 | 895,977 | 7.92 (6.28–10.00) | |
| Northern Italy | 945 | 8,006,808 | 11.80 (11.07–12.58) | |
| Turin | 419 | 3,823,910 | 10.96 (9.96–12.06) | 99.0 (98.2–99.4) |
| Liguria | 280 | 2,377,687 | 11.78 (10.47–13.24) | 98.2 (96.7–99.0) |
| Pavia | 93 | 768,584 | 12.10 (9.87–14.83) | 99.0 (97.8–99.8) |
| Modena | 74 | 613,452 | 12.06 (9.61–15.15) | 99.3 (98.1–100) |
| Trento | 79 | 423,175 | 18.67 (14.97–23.27) | 99.4 (98.7–100) |
| Central-Southern Italy | 2,728 | 30,550,760 | 8.93 (8.6–9.27) | |
| Firenze-Prato | 214 | 1,923,090 | 11.13 (9.73–12.72) | 98.8 (98.0–100) |
| Marche | 284 | 2,696,075 | 10.53 (9.38–11.83) | 99.0 (97.8–99.9) |
| Lazio | 678 | 7,522,247 | 9.01 (8.36–9.72) | 97.0 (95.3–98.8) |
| Umbria | 145 | 1,255,832 | 11.55 (9.81–13.59) | 99.0 (98.0–100) |
| Abruzzo | 115 | 1,196,101 | 9.61 (8.01–11.54) | 98.1 (97.2–100) |
| Campania | 1,292 | 15,957,414 | 8.10 (7.67–8.55) | 96.2 (94.2–98.5) |
| Island | ||||
| Sardinia | 1,507 | 3,688,576 | 40.86 (38.84–42.97) | 91.0 (89.0–96.0) |
The likelihood ratio test showed substantial and statistically significant presence of overdispersion (P < 0.001). To account for this heterogeneity, multilevel random-intercept Poisson regression models were fitted to data accounting for the registry-level variance component. In the regression analysis, after controlling for age, the rate ratio (RR) for girls with respect to boys was 0.87 (95% CI 0.82–0.92); corresponding values in peninsular Italy and in Sardinia were 0.91 (0.85–0.97) and 0.77 (0.70–0.85), respectively. The incidence steeply increased from the age-group 0–2 to the age-group 3–5 years (Table 1), was quite similar in age-groups 6–8 and 12–14, and peaked at 9–11 years.
Large geographical variations in type 1 diabetes incidence were evident (Table 1), with the highest rate in Sardinia, an intermediate rate in Central-Southern Italy, and a high rate in Northern Italy, particularly in the Trento Province (18.67 per 100,000 person-years [95% CI 14.97–23.27]). The lowest incidence rate was recorded in the Campania Region (8.10 [7.67–8.55]).
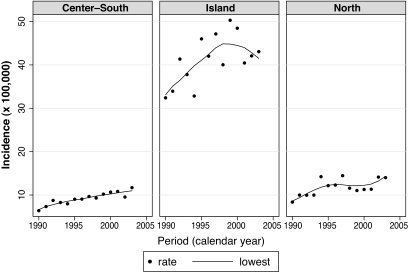
An increasing temporal trend was evident both examining the whole Italian area and the three Italian macro-areas separately; in Sardinia we found a tendency toward lower risk in more recent years (Fig. 1). Overall, the rates increased from 10.22 per 100,000 person-years in 1990–1992 to 14.78 per 100,000 in 2002–2003 (Table 1). Controlling for age and sex, the annual increase was 2.94% (95% CI 2.22–3.67).
FIG. 1.
Incidence rates of type 1 diabetes among Italian children 0–14 years old in the years 1990–2003 in the three Italian macro-areas (Center-South, Island [Sardinia], and North).
Table 2 shows age-specific incidence rates by calendar period (diagonals) and birth cohorts. An increasing temporal trend across calendar period was evident (P < 0.0001): with respect to the calendar period 1990–1992, the RR was 1.15 (95% CI 1.06–1.25) in the period 1993–1995, 1.27 (1.17–1.39) in the period 1996–1998, 1.35 (1.24–1.47) in the period 1999–2001, and 1.40 (1.27–1.55) in the period 2002–2003. In all periods (diagonals), the highest incidence rates were found in the age-group 9–11 years; in the last period (2002–2003) only, after a rapid increase from the first to the second age-group, rates remained similarly high from 6 years old onwards.
TABLE 2.
Age-specific incidence rates (per 100,000 person-years) of type 1 diabetes among Italian children 0–14 years old in the years 1990–2003, by birth cohorts 1978–2001, and by calendar periods (diagonals) 1990–1992, …, 2002–2003
| Birth cohorts |
Yearly increase (%) | P | α* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1978 | 1981 | 1984 | 1987 | 1990 | 1993 | 1996 | 1999 | 2001 | ||||
| Age-group (years) | ||||||||||||
| 0–2 | 5.0 (95) | 4.9 (92) | 6.4 (114) | 7.1 (107) | 7.9 (71) | 3.9 | 0.001 | 0.3 | ||||
| 3–5 | 10.3 (199) | 12.4 (229) | 12.1 (223) | 12.6 (191) | 13.5 (119) | 1.6 | 0.056 | 0.2 | ||||
| 6–8 | 10.9 (225) | 13.1 (246) | 14.4 (263) | 14.6 (232) | 17.0 (152) | 3.3 | <0.001 | 0.2 | ||||
| 9–11 | 13.4 (297) | 15.2 (309) | 16.2 (299) | 18.2 (293) | 17.6 (168) | 2.4 | 0.001 | 0.2 | ||||
| 12–14 | 10.8 (273) | 11.9 (263) | 14.3 (285) | 16.5 (268) | 17.4 (167) | 4.0 | <0.001 | 0.3 | ||||
| RR (95% CI) | 0.63 (0.54–0.73) | 0.72 (0.64–0.80) | 0.82 (0.74–0.90) | 0.91 (0.83–0.99) | Ref. | 0.99 (0.89–1.09) | 1.12 (1.00–1.26) | 1.18 (1.01–1.38) | 1.38 (1.06–1.80) | |||
Number of cases are in parentheses. Percent yearly increases, test for age-specific trends adjusted by sex over calendar periods, and estimated variance of the registry-level random intercept (α) are shown in the last column.
*Likelihood ratio test of α = 0; P < 0.001 in all age-groups. RR, rate ratio for each birth cohort taking as reference those born in 1987–1993 (1990 mid-year).
With respect to birth cohort 1987–1993 (1990 mid-year), the incidence rates increased approximately linearly. As shown in Table 2, the RR increased from 0.63 (95% CI 0.54–0.73) in birth cohort 1975–1981 (1978 mid-year) to 1.38 (1.06–1.80) in birth cohort 1999–2003 (2001 mid-year).
Multilevel Poisson regression analysis was also used to assess whether the observed temporal increase in incidence rates could be due to period or cohort effects. Table 3 shows the main results of fitting different models to the data. The best model (model 3) included sex, age, and a linear time trend (drift). Therefore, the variation over time has a linear component that cannot be ascribed to either the calendar period or the cohort. Indeed, the sex, age, and cohort model (model 4) and the sex, age, and period model (model 6) were not significantly better than the sex, age, and drift model (model 3). The estimated variance of the registry-level random intercept showed statistically significant heterogeneity between registries (P value of the likelihood ratio test <0.001 in all models, Table 3).
TABLE 3.
Comparison of different age-period-cohort models fitted to incidence rates of type 1 diabetes among Italian children 0–14 years old in the years 1990–2003
| Covariates included in the model | DF | Likelihood ratio χ2 | P | α* |
|---|---|---|---|---|
| 1. Sex | ||||
| 2. Sex + age | 4 | 385.00 | <0.0001 | 0.21 |
| 3. Sex + age + drift | 1 | 65.33 | <0.0001 | 0.20 |
| 4. Sex + age + cohort | 7 | 8.51 | 0.29 | 0.20 |
| 5. Sex + age + period + cohort | 3 | 2.23 | 0.53 | 0.20 |
| 6. Sex + age + period | −7 | −6.51 | 0.48 | 0.20 |
| 3. Sex + age + drift | −3 | −4.24 | 0.24 | 0.20 |
Each model is compared with the one above through the likelihood ratio test.
*Likelihood-ratio test of α = 0; P < 0.001 in all models. DF, difference in the number of degrees of freedom.
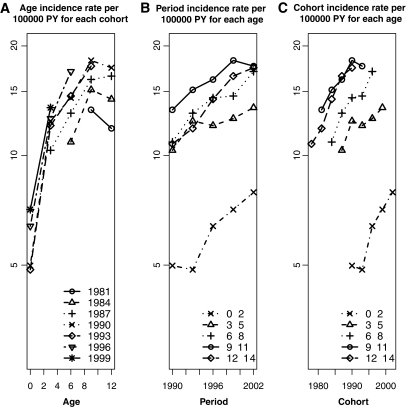
Figure 2 shows incidence rates (on a logarithmic scale) over age-groups by birth cohorts, over calendar period by age-groups, and over birth cohorts by age-groups. Results were similar when data were analyzed by geographical macro-areas (data not shown).
FIG. 2.
Incidence rates of type 1 diabetes among Italian children 0–14 years old in the years 1990–2003 over age-groups by birth cohorts (mid-year of birth cohorts) (A), over calendar period by age-groups (B), and over birth cohorts by age-groups (C).
DISCUSSION
Results of the present analyses of the RIDI study provide evidence of 1) large geographical variations in risk of childhood type 1 diabetes within Italy, with high-risk areas in both Sardinia and Trento, in the North-East of Italy; 2) a significantly lower risk in girls than in boys, which is more pronounced in Sardinia (−23%) than in peninsular Italy (−9%); and 3) a linear increasing temporal trend, with an annual increment of 2.94%, affecting all age-groups and both sexes. In our dataset, however, it was impossible to ascribe this increase definitively to either a birth cohort or a calendar period effect.
Our findings are based on a national registry using standardized methods of ascertainment of incident cases, covering 40% of the Italian population at risk; moreover, the long time period on which this report is based allows us to add to current knowledge on epidemiology of type 1 diabetes. Heterogeneity of type 1 diabetes risk has been reported worldwide, but Italy is one of the countries with greater variation within it. Indeed, incidence in peninsular Italy is fourfold lower than in Sardinia. In the latter, rates appeared to decline in more recent years, suggesting the possibility that an upper limit has been reached. The novel finding of the updated report of the RIDI Study is that a new high-risk area has been identified in the North-East of Italy, in Trento. The lower boundary of the CI of the incidence rate for Trento is higher than the upper value of the CI of all other peninsular areas (with the exception of Modena), and is similar to risk of children living in neighboring European areas, Germany, and Austria (4–5). Whether this heterogeneity is due to either genetic or environmental factors is difficult to establish on the basis of available data and advocates further studies. Moreover, the extension of the RIDI project to other Italian areas not covered by registries would provide a more accurate estimate of risk at national level.
Sex differences in risk of both type 1 and type 2 diabetes, with an almost two-fold higher risk in males than in females, have been previously reported in young Italian adults (20–21). The large number of cases on which this report is based allows us to provide evidence that the male excess in risk of type 1 diabetes is evident even in Italian children, consistent with studies performed in high-risk areas (22). This finding could suggest a role for sex-linked differences in β-cell function or in insulin sensitivity, with a higher rate of β-cell exhaustion in male than in female subjects.
Our analyses show that the increasing temporal trend involved all age-groups similarly. This finding is consistent with a recent report from the registry of Turin, showing a similar increase in incidence of childhood and adulthood diabetes since 1984, suggesting that widespread environmental determinants are involved in this phenomenon (18). This finding, however, is in contrast with studies suggesting that the incidence increase could be explained by a shift to a younger age at onset (7,11–12,14,23–24). In our study, we carried out an age-period-cohort analysis that included evaluation of both the drift (the linear variation of the incidence in time due to either a calendar period or a cohort effect) and the nonlinear components of the effects of the two temporal variables. We did not find evidence of nonlinear effects because there were no improvements in the models when the nonlinear components of calendar period or birth cohort were introduced. Because the incidence increase was linear, it was not possible to distinguish between the effects of calendar period and birth cohort, and our data do not support the hypothesis of a shift to a younger age at onset.
Previously published age-period-cohort models in childhood diabetes temporal trend have shown inconclusive results. A period effect was found in Poland but not in a U.S. county in the years 1970–1985 (25). In 1978–1987 in Sweden (13), in 1965–1984 in Finland (14), and in 1973–2000 in Norway (9) linear and nonlinear effects of the calendar period were evident, but no statistically significant cohort effect was found; the Finnish study (14) found only some evidence of younger age at diabetes onset. A later Swedish study (24) found no evidence of an increase in incidence rates, whereas it strongly supported a cohort effect because of a steeper increase in younger age-groups. In 1970–2000 in Denmark, a modest drift effect and a significant cohort effect were found, with increased risk for children born after 1985 (12). Similarly, in 1978–2000 in Yorkshire, U.K., increased incidence rates were associated with late birth cohorts (in particular 1985 and 1995) and with calendar periods (12). Finally, the most recent EURODIAB study (7) showed a statistically significant yearly increase in all age-groups (+5.4% in 0–4, +4.3% in 5–9, and +2.9% in 10–14 years old) and a statistically significant interaction between calendar year and age-group, with the highest increase in the youngest age-groups. An analysis of the Registry of Turin in the period 1984–2003 for the age-group 0–29 years found a linear effect that could not be ascribed to either the calendar period or the birth cohort (18). The RIDI study does not support the interpretation of the observed steady increase as a cohort or a calendar period effect, and reasoning on causal and biological plausibility of different hypotheses becomes crucial. Because prevalence of childhood obesity is increasing over time, the “accelerated hypothesis” has been suggested as a possible explanation of the increasing temporal trend of type 1 diabetes (26). Other studies have proposed that the decreasing early life exposure to infectious diseases, which typically occurred over the past three decades in developed countries, could be involved in this trend (27). Evidence supporting both hypotheses, however, is still inconclusive.
In conclusion, the incidence of type 1 diabetes among Italian children increased during the study period. Such an increase cannot be ascribed to either the calendar period or the birth cohort.
Supplementary Material
ACKNOWLEDGMENTS
This study was partially supported by the Italian Association for Cancer Research (AIRC), the Compagnia San Paolo/Fondazione Internazionale in Medicina Sperimentale (FIRMS), and Regione Piemonte (Ricerca Sanitaria Finalizzata 2008).
No potential conflicts of interest relevant to this article were reported.
G.B. researched data and wrote the manuscript. M.M. researched data and wrote the manuscript. F.M. researched data and reviewed and edited the manuscript. G.N. researched data. A.F., A.I., L.I., E.A., G.d'A., S.P., P.P., D.I., M.S., F.R., and S.T. researched data and contributed to the discussion. F.C. and V.C. researched data and reviewed and edited the manuscript.
APPENDIX
Members of the RIDI Study Group: F. Cerutti (Department of Pediatrics, University of Turin, Turin, Italy); G. Novelli (Department of Internal Medicine, University of Turin, Turin, Italy); S. Franchini, L. Bianchi (Unit of Observational Epidemiology, Trento, Italy); R. Lorini and N. Minuto (Department Of Pediatrics, University of Genova, Genova, Italy); S. Sacco and F. Ramondetti (Department of Preventive Medicine, University of Pavia, Pavia, Italy); B. Predieri (Department of Pediatrics, University of Modena, Modena, Italy); S. Reali and A. Medici (Department of Pediatrics, University of Florence, Florence, Italy); M. Biagioni (Polytechnic University of Marche, Marche, Italy); R. Gesuita (Department of Epidemiology, Biostatistics and Medical Information Technology, Polytechnic University of Marche, Ancona, Italy); F. Santeusanio (Department of Internal Medicine, University of Perugia, Perugia, Italy); G. De Giorgi (Pediatrics Clinic, University Hospital, Perugia, Italy); N. Visalli (Pertini Hospital, Rome, Italy); C. Bizzarri (Bambino Gesù Hospital, Rome, Italy); F. Chiarelli and S. Tumini (Department of Epidemiology, University of L'Aquila, L'Aquila, Italy); F. Prisco and S. Confetto (Department of Pediatrics, II University of Naples, Naples, Italy); and P. Frongia and A. Marinaro (Diabetes Clinic, San Michele Hospital, Cagliari, Italy).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Myers M, Zimmet P. Halting the accelerating epidemic of type 1 diabetes. Lancet 2008;371:1730–1731 [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371(9626):1777–1782 [DOI] [PubMed] [Google Scholar]
- 3.Barat P, Valade A, Brosselin P, Alberti C, Maurice-Tison S, Lévy-Marchal C. The growing incidence of type 1 diabetes in children: the 17-year French experience in Aquitaine. Diabete Metab 2008;34:601–605 [DOI] [PubMed] [Google Scholar]
- 4.Ehehalt S, Blumenstock G, Willasch AM, Hub R, Ranke MB, Neu ADIARY-study Group Baden-Württemberg Continuous rise in incidence of childhood Type 1 diabetes in Germany. Diabet Med 2008;25:755–757 [DOI] [PubMed] [Google Scholar]
- 5.Schober E, Rami B, Waldhoer TAustrian Diabetes Incidence Study Group Steep increase of incidence of childhood diabetes since 1999 in Austria. Time trend analysis 1979–2005. A nationwide study. Eur J Pediatr 2008;167:293–297 [DOI] [PubMed] [Google Scholar]
- 6.Svensson J, Lyngaae-Jørgensen A, Carstensen B, Simonsen LB, Mortensen HBDanish Childhood Diabetes Registry Long-term trends in the incidence of type 1 diabetes in Denmark: the seasonal variation changes over time. Pediatr Diabetes 2009;10:248–254 [DOI] [PubMed] [Google Scholar]
- 7.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész GEURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multiCenter prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 8.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005;54:(Suppl. 2):S125–136 [DOI] [PubMed] [Google Scholar]
- 9.Aamodt G, Stene LC, Njølstad PR, Søvik O, Joner GNorwegian Childhood Diabetes Study Group Spatiotemporal trends and age-period-cohort modeling of the incidence of type 1 diabetes among children aged <15 years in Norway 1973–1982 and 1989–2003. Diabetes Care 2007;30:884–889 [DOI] [PubMed] [Google Scholar]
- 10.McNally RJ, Feltbower RG, Parker L, Bodansky HJ, Campbell F, McKinney PA. Space-time clustering analyses of type 1 diabetes among 0- to 29-year-olds in Yorkshire, U.K. Diabetologia 2006;49:900–904 [DOI] [PubMed] [Google Scholar]
- 11.Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, U.K.: time trends in 0–14 and 15–29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437–441 [DOI] [PubMed] [Google Scholar]
- 12.Svensson J, Carstensen B, Mølbak A, Christau B, Mortensen HB, Nerup J, Borch-Johnsen K. Increased risk of childhood type 1 diabetes in children born after 1985. Diabetes Care 2002;25:2197–2201 [DOI] [PubMed] [Google Scholar]
- 13.Nyström L, Dahlquist G, Rewers M, Wall S. The Swedish childhood diabetes study. An analysis of the temporal variation in diabetes incidence 1978–1987. Int J Epidemiol 1990;19:141–146 [DOI] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Rewers M, Reunanen A, Lounamaa P, Lounamaa R, Tuomilehto-Wolf E, Akerblom HK. Increasing trend in type 1 (insulin-dependent) diabetes mellitus in childhood in Finland. Analysis of age, calendar time and birth cohort effects during 1965 to 1984. Diabetologia 1991;34:282–287 [DOI] [PubMed] [Google Scholar]
- 15.Bruno G, Merletti F, Biggeri A, Cerutti F, Grosso N, De Salvia A, Vitali E, Pagano GPiedmont Study Group for Diabetes Epidemiology Increasing trend of type I diabetes in children and young adults in the province of Turin (Italy). Analysis of age, period and birth cohort effects from 1984 to 1996. Diabetologia 2001;44:22–25 [DOI] [PubMed] [Google Scholar]
- 16.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med 2007;26:3018–3045 [DOI] [PubMed] [Google Scholar]
- 17.Carle F, Gesuita R, Bruno G, Coppa GV, Falorni A, Lorini R, Martinucci ME, Pozzilli P, Prisco F, Songini M, Tenconi MT, Cherubini VRIDI Study Group Diabetes incidence in 0- to 14-year age-group in Italy: a 10-year prospective study. Diabetes Care 2004;27:2790–2796 [DOI] [PubMed] [Google Scholar]
- 18.Bruno G, Novelli G, Panero F, Perotto M, Monasterolo F, Bona G, Perino A, Rabbone I, Cavallo-Perin P, Cerutti FPiedmont Study Group for Diabetes Epidemiology The incidence of type 1 diabetes is increasing in both children and young adults in Northern Italy: 1984–2004 temporal trends. Diabetologia 2009;52:2531–2535 [DOI] [PubMed] [Google Scholar]
- 19.Clayton D, Hills M. Statistical Models in Epidemiology. 1st ed.Oxford, U.K., Oxford University Press, 1993 [Google Scholar]
- 20.Bruno G, Merletti F, Vuolo A, Pisu E, Giorio M, Pagano G. Sex differences in the incidence of insulin-dependent diabetes (IDDM) in the age group 15–29: higher risk in males in the Province of Turin (Italy). Diabetes Care 1993;16:133–136 [DOI] [PubMed] [Google Scholar]
- 21.Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, Novelli G, Trovati M, Cerutti F, Pagano GPiedmont Study Group for Diabetes Epidemiology Incidence of type 1 and type 2 diabetes in adults aged 30–49 years: the population-based registry in the province of Turin, Italy. Diabetes Care 2005;28:2613–2619 [DOI] [PubMed] [Google Scholar]
- 22.Joner G, Stene LC, Søvik ONorwegian Childhood Diabetes Study Group Nationwide, prospective registration of type 1 diabetes in children aged <15 years in norway 1989–1998: no increase but significant regional variation in incidence. Diabetes Care 2004;27:1618–1622 [DOI] [PubMed] [Google Scholar]
- 23.Dahlquist G, Mustonen L. Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Swedish Childhood Diabetes Study Group. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 24.Pundziute-Lyckå A, Dahlquist G, Nyström L, Arnqvist H, Björk E, Blohmé G, Bolinder J, Eriksson JW, Sundkvist G, Ostman JSwedish Childhood Diabetes Study Group The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0–34 years group in Sweden 1983–1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 25.Rewers M, Stone RA, LaPorte RE, Drash AL, Becker DJ, Walczak M, Kuller LH. Poisson regression modeling of temporal variation in incidence of childhood insulin-dependent diabetes mellitus in Allegheny County, Pennsylvania, and Wielkopolska, Poland, 1970–1985. Am J Epidemiol 1989;129:569–581 [DOI] [PubMed] [Google Scholar]
- 26.Gale EA. To boldly go–or to go too boldly? The accelerator hypothesis revisited. Diabetologia 2007;50:1571–1575 [DOI] [PubMed] [Google Scholar]
- 27.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.