Abstract
OBJECTIVE
Vascular endothelial growth factor (VEGF-A or VEGF) is a major pathogenic factor and therapeutic target for diabetic retinopathy (DR). Since VEGF has been proposed as a survival factor for retinal neurons, defining the cellular origin of pathogenic VEGF is necessary for the effectiveness and safety of long-term anti-VEGF therapies for DR. To determine the significance of Müller cell-derived VEGF in DR, we disrupted VEGF in Müller cells with an inducible Cre/lox system and examined diabetes-induced retinal inflammation and vascular leakage in these conditional VEGF knockout (KO) mice.
RESEARCH DESIGN AND METHODS
Leukostasis was determined by counting the number of fluorescently labeled leukocytes inside retinal vasculature. Expression of biomarkers for retinal inflammation was assessed by immunoblotting of TNF-α, ICAM-1, and NF-κB. Vascular leakage was measured by immunoblotting of retinal albumin and fluorescent microscopic analysis of extravascular albumin. Diabetes-induced vascular alterations were examined by immunoblotting and immunohistochemistry for tight junctions, and by trypsin digestion assays for acellular capillaries. Retinal integrity was analyzed with morphologic and morphometric analyses.
RESULTS
Diabetic conditional VEGF KO mice exhibited significantly reduced leukostasis, expression of inflammatory biomarkers, depletion of tight junction proteins, numbers of acellular capillaries, and vascular leakage compared to diabetic control mice.
CONCLUSIONS
Müller cell-derived VEGF plays an essential and causative role in retinal inflammation, vascular lesions, and vascular leakage in DR. Therefore, Müller cells are a primary cellular target for proinflammatory signals that mediates retinal inflammation and vascular leakage in DR.
Diabetic retinopathy (DR) is a microvascular complication of diabetes and a leading cause of vision loss in working-age adults in developed countries. During diabetes, hyperglycemia and oxidative stress upregulates a major angiogenic factor, vascular endothelial growth factor (VEGF-A or VEGF), which induces retinal neovascularization, vascular leakage, and perhaps macular edema (1,2). DR is also known as a chronic inflammatory disorder. During the early stage of DR, proinflammatory proteins, such as intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor-α (TNF-α), are upregulated, and increased leukostasis is observed (3–5). These early pathologic changes are associated with upregulation of VEGF (6–8).
Clinical observations that elevated VEGF levels are associated with DR have led to intensive studies on VEGF action over the past decade, and have resulted in anti-VEGF treatments as a major therapeutic strategy for DR. Surprisingly the role of VEGF in the pathogenesis of DR has not been well investigated at the cellular level. Since VEGF may be required for the maintenance and survival of retinal neurons (9–12), revealing cellular mechanism of VEGF actions becomes necessary to the safety and effectiveness of anti-VEGF therapies.
In the retina, VEGF is mainly expressed in Müller cells (13), endothelial cells (14), astrocytes (15), retinal pigment epithelium (RPE) (16), and ganglion cells (17). At present, the in vivo function of VEGF produced by these retinal cell-types remains largely uninvestigated. Although the role of retinal Müller cell-produced VEGF in DR was probed a decade ago (13), its function in the disease is unclear. An in vitro study demonstrated that Müller cells produce a large amount of VEGF in response to hypoxia (14). This has led to speculation that Müller cells are a major source of VEGF in DR and a major cellular target for the treatment of the disease. To determine the role of Müller cell-derived VEGF and dissect the cellular mechanism of DR, we generated conditional VEGF knockout (KO) mice by mating floxed VEGF mice with transgenic mice expressing Cre recombinase in retinal Müller cells (18,19), with a Cre/lox-based conditional gene KO approach (20). Using these conditional VEGF KO mice, we recently demonstrated that Müller cell-derived VEGF contributed significantly to ischemia-induced retinal neovascularization (21). This report describes our investigation of the role of Müller cell-derived VEGF in retinal inflammation and vascular leakage that occurs as a consequence of diabetes.
RESEARCH DESIGN AND METHODS
Treatment of conditional VEGF knockout mice.
All animal experiments followed the guidelines established by the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committees. Conditional VEGF KO mice were generated by mating floxed VEGF mice with transgenic mice expressing Cre recombinase in retinal Müller cells, as described previously (18,19,21). Doxycycline was administered through the drinking water of pregnant mice at a concentration of 2 mg/ml in 5% sucrose from embryonic day 15 to postnatal day 1. To minimize the influence of genetic background, littermates were used as controls in this study. PCR analysis of tail DNA was performed for genotyping mice using primer pairs a (5′-CCT GGC CCT CAA GTA CAC CTT −3′) and b (5′-TCC GTA CGA CGC ATT TCT AG-3′) to detect a 108-bp product for the wild-type (WT) allele and a 148-bp product for the floxed VEGF allele (21). Primer pairs c (5′-AGG TGT AGA GAA GGC ACT TAG C-3′) and d (5′-CTA ATC GCC ATC TTC CAG CAG G-3′) were used to detect a 411-bp product for Cre (22).
Diabetes was induced in 6- to 10-week-old mice (weighing 18–22 g). After an 8-h fast, mice were injected intraperitoneally with freshly prepared streptozotocin (STZ; Sigma, St. Louis, MO) at a concentration of 55 mg/kg body weight in 10 mmol/l citrate buffer (pH 4.5), daily for 5 days. Age-matched controls received citrate buffer only. Mice with blood glucose levels >300 mg/dl 7 days after the first STZ-injection were deemed diabetic.
Immunoblotting and immunohistochemistry.
For immunoblotting, retinas were dissected and sonicated in a lysis buffer containing 50 mmol/l Tris (pH 7.6), 150 mmol/l NaCl, 5 mmol/l EDTA, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, and a protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN), which is similar to that used for previously successful extraction of occludin (23). The lysate was centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was collected. Monoclonal antibody against β-actin (1:5,000 dilution) was from Sigma (St. Louis, MO); polyclonal antibodies against VEGF, ICAM-1, occludin, hypoxia-inducible factor-1α (HIF-1α), nuclear factor-kappaB (NF-κB, p65), and phosphorylated NF-κB (p65) (1:500 dilution) were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibody against TNF-α (1:500 dilution) was from Abcam (Cambridge, MA). Polyclonal antibody against zonula occludens 1 (ZO-1, 1:500 dilution) was from Zymed (San Francisco, CA), and polyclonal antibody against albumin (1:1,000 dilution) was from Bethyl (Montgomery, TX). Horseradish peroxidase-linked anti-rabbit, mouse, or goat IgG (1:2,000 dilution, Santa Cruz Biotechnology) were used for secondary detection in immunoblotting. Immunoreactivity was visualized by enhanced chemiluminescence using Super Signal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL) and images were captured by a Chemi Genius Image Station (SynGene, Frederick, MD). Band intensities were quantified using the Gene Tools program (SynGene). Immunohistochemical staining was performed according to a previous method (21).
Quantification of retinal leukostasis.
Quantification of leukostasis was performed as described previously (24,25). The chest cavity of each deeply anesthetized mouse was carefully opened. The descending aorta was clamped and a perfusion needle was inserted into the left ventricle. The perfusate was drained by cutting the right atrium immediately before perfusion. Mice were perfused with 10 ml of PBS and heparin (0.1 mg/ml) to wash out nonadherent blood cells. FITC-conjugated concanavalin A (ConA) (20 μg/ml in PBS; pH 7.4; 5 mg/kg body weight; Vector Laboratories, Burlingame, CA) was then perfused to label adherent leukocytes and vascular endothelial cells. Unbound ConA was flushed by perfusion with 10 ml PBS. Eyes were removed and fixed in 4% paraformaldehyde for 1 h. Retinas were dissected and flat mounted. Images of retinas were observed by epifluorescence or confocal microscopy, and the total number of adherent leukocytes per retina was counted.
Visualization and quantification of retinal vascular leakage.
Anesthetized mice received femoral vein injections of fluorescein isothiocyanate-BSA (FITC-BSA, 100 mg/kg) (Sigma). After 20 min, mice were killed and eyes were removed and fixed in 4% paraformaldehyde for 30 min. Retinas were dissected, flat mounted, and imaged by fluorescent microscopy. A computer-assisted method was used to quantify leakage using Adobe Photoshop 7.0 software. In this procedure, the intensity of basal level of fluorescence in nonleakage areas was used as background fluorescence. After deduction of background signals, the total intensity of fluorescence contributed by the leaked FITC-labeled albumin was used to represent the leakage.
Quantification of retinal vascular histopathology.
Retinal vasculature was isolated by the trypsin digest method (26). Briefly, 10% buffered formalin-fixed retinas were dissected, washed, and incubated with 3% Difco crude trypsin (BD Biosciences, Sparks, MD) containing 0.2 mol/l NaF at 37°C for 1 h. Retinal tissues were brushed away, and the isolated vasculature was mounted, dried on glass slides, and stained with periodic acid-Schiff and hematoxylin. Acellular capillaries were counted in six field areas in the midretina (×400 magnification) and expressed as the total number/mm of retina area.
Retinal morphometry and transmission electron microscopy.
Transmission electron microscopy (TEM) was performed as described previously (27). Morphometric analysis of hematoxylin and eosin (H&E)-stained retinal sections was performed as described previously (21). The total number of ganglion nuclei in H&E-stained retinal sections was used to represent ganglion cell density.
Statistical analysis.
All results that required statistic analysis were expressed as the mean ± SEM. Statistical significance was evaluated by Student t test with P value < 0.05 considered significant.
RESULTS
Preparation of diabetic conditional VEGF knockout mice.
In an effort to generate human VMD2 promoter controlled tetracycline-inducible RPE-specific Cre mice (28), we identified a mouse line displaying Cre function predominantly localized to the retinal Müller cells (29). Further characterization demonstrated that this mouse line is feasible for conditional gene expression in Müller glia (19). The transgenic Cre mice (cre+) were mated with mice carrying a floxed VEGF (Vegfff) allele (18) to generate the conditional Müller glial VEGF KO mice. Using primary cultures derived from the conditional VEGF KO mice, we determined that there was a 66.5% reduction of VEGF expression in Müller cells (21). Immunoblotting assays demonstrated a significant decrease (43.2%) in VEGF expression in the retinas of conditional VEGF KO mice (cre+Vegfff) compared with WT (cre−Vegfff) controls (Fig. 1A), confirming that Müller cells are a major source of VEGF in the retina.
FIG. 1.
Analysis of VEGF and HIF-1α expression in normal and diabetic conditional VEGF KO mice. A and B: Immunoblotting analysis for VEGF (A) and HIF-1α (B) in retinas from conditional VEGF KO mice and WT controls 2 months after diabetes. C and D: Confocal microscopic analysis of immunostained retinas for VEGF expression (green) in conditional VEGF KO mice and WT controls subjected to a diabetic stress. Blue: nuclear staining (DAPI). Scale bar equals 40 μm. ONL, outer nuclear layer; INL, inner nuclear layer. Error bar: SEM. ***P < 0.001. ns, not significant. VEGF expression was significantly reduced in the retinas of conditional VEGF KO mice under normal or diabetic conditions. Although diabetes upregulated HIF-1α, no significant change in the levels of retinal HIF-1α was observed in diabetic conditional VEGF KO mice. (A high-quality digital representation of this figure is available in the online issue.)
Diabetes was induced by STZ-injection in conditional VEGF KO mice and WT controls. Blood glucose levels and average body weights of mice at the time of experiments are shown in Table 1. A significant elevation of blood glucose level and loss of body weight were observed in diabetic groups compared with nondiabetic controls (P < 0.001) 2 and 6 months after induction of diabetes. No significant changes in blood glucose levels or body weight were observed between conditional VEGF KO and WT mice under normal or diabetic conditions (Table 1), indicating that disruption of Müller cell-derived VEGF did not affect blood glucose levels or body weight. Under diabetic stress, expression of VEGF increased dramatically (2.16-fold) in WT mice (Fig. 1A). In contrast, disruption of Müller cell-derived VEGF resulted in a 51.4% reduction in VEGF (Fig. 1A). This result was supported by immunohistochemical analysis in conditional VEGF KO mice subjected to diabetic stress (Fig. 1C–D). As HIF-1α is upregulated in diabetic retinas and contributes to increased levels of VEGF (30), we verified the state of hypoxia in conditional VEGF KO mice by measuring the expression of HIF-1α in diabetic retinas. Our results demonstrated a greater than twofold upregulation of HIF-1α (Fig. 1B) in diabetic retinas of both WT and conditional VEGF KO mice, which is consistent with a previous observation (31). However, no significant difference was observed in HIF-1α levels between WT and conditional VEGF KO mice, suggesting that the loss of Müller cell-derived VEGF did not alter diabetes-induced HIF-1α accumulation.
TABLE 1.
Body weights and blood glucose levels of normal and diabetic mice
| Group | Body weight (g) |
Blood glucose (mg/dl) |
||
|---|---|---|---|---|
| 2 months | 6 months | 2 months | 6 months | |
| Diabetic WT | 21.46 ± 1.60* | 23.03 ± 2.35* | 461.43 ± 66.66* | 497.71 ± 6.05* |
| Diabetic KO | 20.86 ± 1.22* | 23.29 ± 0.63* | 432.86 ± 65.15* | 480.86 ± 50.65* |
| Nondiabetic WT | 28.17 ± 2.28 | 32.43 ± 1.66 | 178.14 ± 33.35 | 184.14 ± 22.44 |
| Nondiabetic KO | 28.04 ± 2.08 | 31.86 ± 2.53 | 199.29 ± 24.28 | 176.14 ± 12.23 |
Data are means ± SE, n = 20–30.
*P < 0.001 compared with nondiabetic WT or KO.
Retinal inflammatory responses.
Leukostasis, a major parameter for inflammation and early pathologic changes in DR, was quantified by FITC-conjugated ConA staining assay 2 months after inducing diabetes (3). Under normal conditions, the number of adherent leukocytes in the retinal microvasculature of WT and conditional VEGF KO mice was negligible (data not shown). The number of adherent leukocytes in the retinal microvasculature of diabetic WT mice was elevated significantly and increased threefold compared with diabetic conditional VEGF KO mice (Fig. 2A–C).
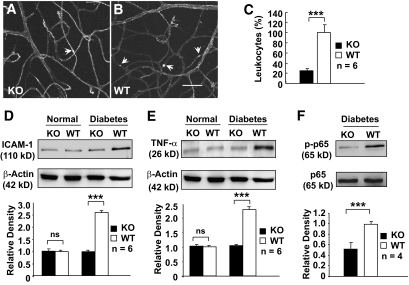
FIG. 2.
Analysis of retinal inflammation in conditional VEGF KO mice 2 months after inducing diabetes. A and B: FITC-conjugated ConA staining for adherent leukocytes (arrows) in retinal microvasculatures of diabetic conditional VEGF KO mice and WT controls. Scale bar represents 100 μm. C: Quantification of adherent leukocytes in retinal vasculatures of diabetic conditional VEGF KO mice and WT controls. D and E: Immunoblotting analysis of ICAM-1 (D) and TNF-α (E) expression in conditional VEGF KO mice. F: Immunoblotting analysis of NF-κB p65 phosphorylation in diabetic retinas of conditional VEGF KO mice. Error bar: SEM. ***P < 0.001. Loss of Müller cell-derived VEGF caused a significant reduction in the number of adherent leukocytes, expression of ICAM-1 and TNF-α, and phosphorylated NF-κB p65 in diabetic retinas. ns, not significant.
To verify whether disrupting Müller cell-derived VEGF significantly reduced retinal inflammation, we examined the expression of proinflammatory markers ICAM-1 and TNF-α by immunoblotting. Retinas from diabetic WT mice demonstrated a greater than twofold increase in the levels of both ICAM-1 and TNF-α compared with those from nondiabetic animals (Fig. 2D–E). No apparent increases in ICAM-1 and TNF-α were observed in diabetic conditional VEGF KO mice. These results suggest that the loss of Müller cell-derived VEGF significantly reduces inflammation in diabetic retinas.
The transcription factor NF-κB is activated in diabetes and plays a major role in diabetes-induced early pathologic changes in DR (32,33). To determine the relationship between Müller cell-derived VEGF and NF-κB in diabetic retinas, we investigated the level and phosphorylation state at serine 276 of NF-κB p65 subunit, as phosphorylation of this subunit increases transcriptional activity of NF-κB (34). Although there was no detectable difference in the overall level of NF-κB p65 subunit between diabetic conditional VEGF KO and control mice, loss of Müller cell-derived VEGF resulted in a twofold decrease in phosphorylated NF-κB p65 in the retinas 2 months after diabetes (Fig. 2F), suggesting that Müller cell-derived VEGF regulates NF-κB transcriptional activity in DR.
Retinal vascular histopathology and leakage.
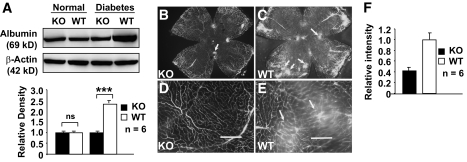
To determine the role of Müller cell-derived VEGF in vascular leakage, we performed immunoblotting for extravascular albumin content in the retinas and vitreous from PBS-perfused mice 6 months after inducing diabetes. The extravascular albumin content in diabetic WT mice was 2.5-fold higher than that in nondiabetic WT mice (Fig. 3A). However, there was a 58.9% reduction of extravascular albumin in the retinas and vitreous of diabetic conditional VEGF KO mice (Fig. 3A). Using FITC-labeled albumin, we visualized and quantified vascular leakage in conditional VEGF KO mice 6 months after inducing diabetes (Fig. 3B–F). Compared with diabetic WT mice, diabetic conditional VEGF KO mice had significantly fewer areas of leaked FITC-labeled albumin (arrows in Fig. 3C and E) in their retina. Computer-assisted quantitative analysis demonstrated a similar level of reduction (61.5%) of FITC-labeled albumin leakage in diabetic conditional VEGF KO mice compared with diabetic WT controls (Fig. 3F).
FIG. 3.
Analysis of retinal vascular leakage in conditional VEGF KO mice 6 months after the onset of diabetes. A: Immunoblotting analysis of retinal extravascular albumin in conditional VEGF KO mice. B and E: Microscopic images of retinal flat-mounts (B and C) and enlarged areas (d and E) showing fluorescently labeled albumin (arrows) in diabetic retinas of WT and conditional VEGF KO mice after injection with FITC-labeled albumin. Scale bars represent 100 μm. F: Computer-assisted quantitative analysis of FITC-labeled albumin in diabetic retinas of conditional VEGF KO mice. Error bar: SEM. ***P < 0.001. Loss of Müller cell-derived VEGF caused a significant reduction in diabetes-induced retinal vascular leakage.
To determine the mechanism of Müller cell-derived VEGF in diabetes-induced vascular leakage, we examined the expression of occludin and ZO-1, two important tight junction proteins. Although diabetes caused 39.2% and 58.1% decreases of retinal occludin and ZO-1 in WT mice, virtually no change in retinal occludin and ZO-1 contents were observed in diabetic conditional VEGF KO mice (Fig. 4A and B). Compared with WT controls, the diabetic conditional VEGF KO mice demonstrated 59.7% and 130.3% increases of occludin and ZO-1, respectively, in their retinas (Fig. 4A–B). This result was confirmed by immunohistochemical analysis of ZO-1 in retinal vessels (Fig. 4C–F). Our results suggest that the loss of Müller cell-derived VEGF significantly reduced diabetes-induced retinal vascular leakage by attenuating the depletion of occludin and ZO-1.
FIG. 4.
Analysis of tight junction protein expression in retinas of conditional VEGF KO mice 6 months after diabetes. A and B: Immunoblotting and quantification of tight junction protein occludin and ZO-1 in retinas of diabetic conditional VEGF KO mice. Error bar: SEM. ***P < 0.001. C–F: Confocal microscopic analysis of immunostained tight junction protein ZO-1 in retinal vasculature (C and D) from the diabetic retinas of conditional VEGF KO mice. Scale bars represent 50 μm. Loss of Müller cell-derived VEGF caused a significant attenuation of diabetes-induced depletion of tight junction proteins occludin and ZO-1. ns, not significant.
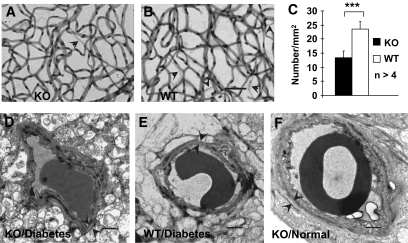
To assess retinal vascular lesions, we quantified the number of acellular capillaries in trypsin digestion assay and examined basement membrane thickness by TEM in conditional VEGF KO mice 6 months after inducing diabetes. The diabetic conditional VEGF KO mice had significantly fewer acellular capillaries than diabetic WT controls (Fig. 5A–C). However, we did not observe significant differences in basement membrane thickness between diabetic conditional VEGF KO and WT mice and between diabetic and nondiabetic mice (Fig. 5D–F).
FIG. 5.
Retinal vascular histopathology in the conditional VEGF KO mice 6 months after inducing diabetes. A–C: Analysis of acellular capillaries (arrowheads) with trypsin digestion in diabetic retinas of the conditional VEGF KO mice and WT controls. Error bar: SEM. ***P < 0.001. Scale bar in B represents 50 μm. Scale bars in D–F represent 1 μm. D–F: Representative TEM images for basement membrane thickness in retinal vascular capillaries. Although the number of acellular capillaries was significantly decreased in diabetic conditional VEGF KO mice, no apparent change in the thickness (arrowheads) of basement membrane was observed among all mice examined.
Morphometric and ultrastructural analyses.
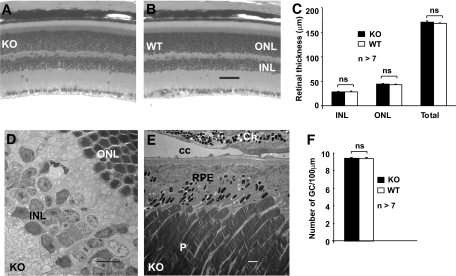
To determine if the loss of Müller cell-derived VEGF in the conditional VEGF KO mice affected retinal neuron survival, we examined retinal integrity in conditional VEGF KO mice 6 months after inducing diabetes. Retinal morphology was carefully examined and retinal thickness, a direct correlation of retinal neuronal number, was quantified by morphometry. No significant differences in outer nuclear layer, inner nuclear layer, or whole retina were observed between diabetic conditional VEGF KO mice and WT controls (Fig. 6A–C), indicating no loss in photoreceptors or inner retinal neurons. In addition, we counted the number of ganglion cells and observed no differences between diabetic conditional VEGF KO mice and WT controls (Fig. 6F). Furthermore, no morphologic changes indicative of neuronal apoptosis were observed at the ultrastructural level (Fig. 6D and E). These results suggest that disrupting VEGF from Müller glia does not result in enhanced neuronal loss in conditional VEGF KO mice 6 months after inducing diabetes.
FIG. 6.
Retinal morphology and morphometry in conditional VEGF KO mice 6 months after onset of diabetes. A and B: Morphology of H&E-stained retinal sections. C: Thickness of outer nuclear layer, inner nuclear layer, and total retina. D and E: Representative TEM images showing no detectable defects in diabetic retina of conditional VEGF KO mice. F: Average number of ganglion cells. Scale bar equals 50 μm in B and 1 μm in d and E. No detectable changes in retinal thickness, numbers of ganglion cells, and retinal ultrastructure were observed in diabetic conditional VEGF KO mice. Ch, choroid; CC, choriocapillaris; GC, ganglion cells; INL, inner nuclear layer; ns, not significant; ONL, outer nuclear layer; P, photoreceptors.
DISCUSSION
VEGF is produced by multiple cellular origins in the retina and has paracrine activity. To date, no in vivo study has convincingly demonstrated the cellular origin of VEGF induced in DR. Since VEGF has been suggested to play a role in retinal neuron survival and choriocapillaris fenestration (9–12) and anti-VEGF treatments have become a major therapeutic strategy for DR, revealing the cellular origins of pathogenic VEGF becomes paramount. Müller cells have long been suspected as a major contributor to DR (13). Because of the lack of appropriate animal models (35), in vivo function of Müller cell-derived VEGF in DR remained unaddressed until recently when we developed an inducible Cre/lox system to delete VEGF from Müller cells (19).
Increased VEGF levels in DR are responsible for blood-retina barrier (BRB) breakdown and lead to vascular leakage (36). However, the causative role of Müller cell-derived VEGF in diabetes-induced BRB breakdown has not been established. In this study, we demonstrate that the loss of Müller cell-derived VEGF significantly inhibits diabetes-induced vascular leakage (Fig. 3). Since the integrity of tight junction proteins in endothelial cells is crucial for BRB function, we measured retinal tight junction proteins occludin and ZO-1 (Fig. 4) to determine how increases in Müller cell-derived VEGF might result in diabetes-induced vascular leakage. Our result that diabetes-induced depletion of occludin and ZO-1 was attenuated in conditional VEGF KO mice supports a role for Müller cell-derived VEGF in diabetes-induced reorganization of tight junctions (37–39). Our results also suggest that reducing Müller cell-derived VEGF attenuates capillary acellularity (Fig. 5A–C), a relatively early vascular lesion in diabetic rodent retinas. Since VEGF is a survival factor for endothelial cells, one might expect that reducing VEGF levels might accelerate endothelial loss and the development of acellular capillaries in diabetic retinas, which is contrary to our observation. However, our result is consistent with that in a study demonstrating an increase in acellular capillaries in transgenic mice overexpressing VEGF165 from rod photoreceptors (40). As inflammation may play a central role in developing pathologic changes, including acellular capillaries in DR (41), we speculate that the diabetes-induced inflammatory responses may play a more prominent role in endothelial death and capillary acellularity and that the reduced inflammatory responses in diabetic conditional VEGF KO mice may attenuate the development of acellular capillaries. Furthermore, as we did not disrupt VEGF in endothelial cells, autocrine endothelium-derived VEGF signaling could play a role in promoting endothelial survival and in reducing capillary acellularity in diabetic conditional VEGF KO mice. Based on our results, we cannot conclude if the Müller cell-derived VEGF plays a role in the thickening of basement membranes, as we did not observe significant change in this process between diabetic mice and nondiabetic controls 6 month after STZ-injection (Fig. 5A–C). This result is in agreement with a previous observation in similar aged diabetic and nondiabetic mice (42).
Leukostasis, adhesion of leukocytes to the vascular wall, is increased in the diabetic retina and results in retinal vascular occlusion (5). The diabetes-induced leukostasis is mediated via upregulation of ICAM-1 and its ligand CD18 (3). Disruption of ICAM-1 and CD18 in mice results in decreases in both leukostasis and vascular permeability (41). It has been shown that VEGF increases expression of ICAM-1 in endothelial capillaries and intravitreal injection of VEGF upregulates the level of retinal ICAM-1 (8). TNF-α is an inflammatory cytokine which is upregulated in diabetic retinas. It has also been reported that an inhibitor of TNF-α is capable of reducing diabetes-induced leukostasis and BRB breakdown (41) and that anti-VEGF treatment inhibited the expression of TNF-α (43). In the current report, we demonstrate that disrupting Müller cell-derived VEGF inhibits diabetes-induced leukostasis and overexpression of ICAM-1 and TNF-α (Fig. 2), early pathologic changes in DR. We also demonstrated that disruption of Müller cell-derived VEGF caused a significant reduction of phosphorylated NF-κB p65 subunit (Fig. 2F), suggesting that reduced transcriptional activation affects downstream effectors for inflammatory responses and vascular complications in diabetic retinas (44,45).
Diabetic conditional VEGF KO mice and nondiabetic WT mice produced a comparable level of VEGF (Fig. 1A). This level of VEGF may not exceed the threshold of causing significant pathologic changes in DR, as observed throughout this study. Very low levels of retinal inflammation and vascular leakage and the inability for other retinal VEGF-producing cells to compensate the loss of VEGF in diabetic conditional VEGF KO mice only suggest that Müller cell-derived VEGF is a primary source of VEGF involved in the diabetes-induced retinal inflammation and vascular leakage. However, we cannot exclude the possibility that VEGF derived from other retinal cells contributes to diabetes-induced retinal inflammation and vascular leakage in the presence of Müller cell-derived VEGF, which may serve as a key to the “pathologic threshold.”
In this study, HIF-1α expression was upregulated in the retinas of diabetic conditional VEGF KO mice and WT controls, which is consistent with a previous finding that HIF-1α is increased in diabetic retina and that inducing HIF-1α contributes to the increased levels of VEGF (30). However, loss of Müller cell-derived VEGF did not altered the expression of HIF-1α in diabetic retina (Fig. 1B). This result indicates that the phenotypic changes observed in the conditional VEGF KO mice are not attributed to the altered expression of the upstream regulator HIF-1α. Therefore, the reduction of diabetes-induced retinal inflammation and vascular leakage in the conditional VEGF KO mice appears to be a direct consequence of reduced VEGF expression. This result may suggest that HIF-1α is a potential target for a more stringent therapeutic strategy to treat DR, which is supported by a recent finding that inhibition of HIF-1α has an effect on ischemia-induced vascular leakage (46).
We demonstrated that disrupting Müller cell-derived VEGF did not cause significant changes in retinal morphology/morphometry in diabetic conditional VEGF KO mice 6 months after STZ-injection (Fig. 6), suggesting that the reduction in VEGF level may not be sufficient to cause accelerated retinal neuron loss within the time frame. Therefore, the phenotypic changes described herein can be directly attributed to the disruption of Müller cell-derived VEGF, not the loss of retinal neurons. Since Cre expression in retinal ganglion cells is negligible (19), the reduced retinal inflammation and vascular leakage in our conditional VEGF KO mice is not likely caused by disruption of VEGF in ganglion cells. The inability for ganglion cells to compensate for the loss of Müller cell-produced VEGF in conditional VEGF KO mice may suggest that the major function of ganglion cell-derived VEGF is not pathogenic, and is perhaps important for neuronal survival under stress conditions (10,11). A significant decrease of VEGF produced by retinal endothelial cells under hyperglycemia suggests a minor role for endothelial-cell–derived VEGF in the pathogenesis of DR (47). The role of the RPE-derived VEGF in DR is intriguing. As the RPE barrier is tighter than the endothelial barrier, the significance of the RPE-derived VEGF/outer BRB breakdown in DR has not been focused sufficiently. Although the RPE-derived VEGF has been suggested to play a role in RPE-choriocapillaris interaction (9), RPE-derived soluble VEGF may regulate outer BRB function (37). The breakdown of the RPE barrier has been observed in diabetic patients (48). Some DR patients with no significant changes in the endothelial barrier have eventually demonstrated a severe outer BRB breakdown (49). However, the significance of the RPE-derived VEGF and RPE barrier dysfunction in overall pathologic changes in DR remains largely uninvestigated, which is caused by difficulties in distinguishing the leakage from each BRB. With the development of cell-specific gene expression systems, a better understanding of the outer BRB dysfunction in DR is likely to emerge shortly.
In summary, the results from the current investigation and a previous study demonstrating a significant role for Müller cell-derived VEGF in retinal neovascularization (21) suggest that the Müller cell-derived VEGF plays a causative role in major pathologic changes in DR, including retinal neovascularization, vascular leakage, vascular lesions, and inflammation. Therefore, Müller cells are a primary cellular source for signals that mediate pathologic changes in DR. As VEGF is a target for clinical trials to treat DR, this information is valuable to the design of the delivery mechanisms for anti-VEGF therapies.
ACKNOWLEDGMENTS
This study was supported by American Diabetes Association grants 1-06-RA-76 and 1-10-BS-94, American Health Assistance Foundation grant M2008-059, Foundation Fighting Blindness grant BR-CMM-0808-0453-UOK, National Institutes of Health (NIH) grants R01EY19494, R01EY20900, P20RR17703, P20RR024215, and P30EY12190, Beckman Initiative for Macular Research grant 1003; Oklahoma Center for Advancement of Science and Technology grants HR09-058 and HR09-028, and unrestricted research awards from Hope for Vision and Research to Prevent Blindness, Inc.
No potential conflicts of interest relevant to this article were reported.
The authors thank Dr. Y. Bai for technical assistance, Drs. J. Ma and B. Zhang for helpful suggestions, and J. Wilkerson and Image Core Facility at Oklahoma Medical Research Foundation for transmission electron microscopy. Characterization of the animal model in this study is a necessary experiment described in NIH R01EY20900 grant application.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 2.Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445–450 [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A 1999;96:10836–10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 2002;16:438–440 [DOI] [PubMed] [Google Scholar]
- 5.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 1991;139:81–100 [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 2000;156:1733–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 2002;160:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Perez VL, Ma N, Miyamoto K, Peng HB, Liao JK, Adamis AP. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci 1999;40:1808–1812 [PubMed] [Google Scholar]
- 9.Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 1999;155:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol 2007;171:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilic U, Kilic E, Jarve A, Guo Z, Spudich A, Bieber K, Barzena U, Bassetti CL, Marti HH, Hermann DM. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci 2006;26:12439–12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS ONE 2008;3:e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 1995;92:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 1995;113:1538–1544 [DOI] [PubMed] [Google Scholar]
- 15.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995;15:4738–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997;13:37–50 [DOI] [PubMed] [Google Scholar]
- 17.Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci 1996;37:290–299 [PubMed] [Google Scholar]
- 18.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149–1159 [DOI] [PubMed] [Google Scholar]
- 19.Ueki Y, Ash JD, Zhu M, Zheng L, Le Y. Expression of Cre recombinase in retinal Müller cells. Vision Research 2009;49:615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Y, Sauer B. Conditional gene knockout using Cre recombinase. Mol Bioltechnol 2001;17:269–275 [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Ma J, Guo J, Wang J, Zhu M, Chen Y, Le Y. Müller cell-derived VEGF is a significant contributor to ischemia-induced retinal neovascularization. J Pathol 2009;219:446–454 [DOI] [PubMed] [Google Scholar]
- 22.Le Y, Ash JD, Al-Ubaidi MR, Chen Y, Ma J, Anderson RE. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol Vis 2004;10:1011–1018 [PubMed] [Google Scholar]
- 23.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci 2006;47:5106–5115 [DOI] [PubMed] [Google Scholar]
- 24.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001;158:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci 2003;44:5410–5416 [DOI] [PubMed] [Google Scholar]
- 26.Kern TS, Engerman RL. Vascular lesions in diabetes are distributed non-uniformly within the retina. Experimental eye research 1995;60:545–549 [DOI] [PubMed] [Google Scholar]
- 27.Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochemi 2010;112:1584–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Y, Zheng W, Rao P, Zheng L, Anderson RE, Esumi N, Zack DJ, Zhu M. Inducible expression of Cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci 2008;49:1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu M, Ueki Y, Ash JD, Zheng L, Le YZ. Unexpected transcriptional activity of human VMD2 promoter. Adv Exp Med Biol 2010;664:211–216 [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem 2004;279:37997–38006 [DOI] [PubMed] [Google Scholar]
- 31.Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 2006;47:1126–1141 [DOI] [PubMed] [Google Scholar]
- 32.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res 2003;37:1169–1180 [DOI] [PubMed] [Google Scholar]
- 33.Kowluru RA, Engerman RL, Kern TS. Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr Eye Res 2000;21:814–819 [DOI] [PubMed] [Google Scholar]
- 34.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005;30:43–52 [DOI] [PubMed] [Google Scholar]
- 35.Koury CB. Genetic system offer option for studying the role of retinal Müller cells. Retina Today 2007;I:31 [Google Scholar]
- 36.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 1998;47:1953–1959 [DOI] [PubMed] [Google Scholar]
- 37.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res 2003;77:593–599 [DOI] [PubMed] [Google Scholar]
- 38.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci 2007;48:5257–5265 [DOI] [PubMed] [Google Scholar]
- 39.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999;274:23463–23467 [DOI] [PubMed] [Google Scholar]
- 40.Shen WY, Lai CM, Graham CE, Binz N, Lai YK, Eade J, Guidolin D, Ribatti D, Dunlop SA, Rakoczy PE. Long-term global retinal microvascular changes in a transgenic vascular endothelial growth factor mouse model. Diabetologia 2006;49:1690–1701 [DOI] [PubMed] [Google Scholar]
- 41.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B., Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed] [Google Scholar]
- 42.Miller-Lotan R, Miller B, Nakhoul F, Aronson D, Asaf R, Levy AP. Retinal capillary basement membrane thickness in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev 2007;23:152–156 [DOI] [PubMed] [Google Scholar]
- 43.Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol 2006;168:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 2007;56:337–345 [DOI] [PubMed] [Google Scholar]
- 45.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007;2007:95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang J, Xia XB, Xu HZ, Xiong Y, Song WT, Xiong SQ, Li Y. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1α and VEGF. J Cell Physiol 2009;218:66–74 [DOI] [PubMed] [Google Scholar]
- 47.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol 2008;295:C1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol 1989;134:231–235 [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberger D, Fink-Cohen S, Gaton DD, Priel E, Yassur Y. Non-retinovascular leakage in diabetic maculopathy. Br J Ophthalmol 1995;79:728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]