Abstract
OBJECTIVE
The redox enzyme p66Shc produces hydrogen peroxide and triggers proapoptotic signals. Genetic deletion of p66Shc prolongs life span and protects against oxidative stress. In the present study, we evaluated the role of p66Shc in an animal model of diabetic wound healing.
RESEARCH DESIGN AND METHODS
Skin wounds were created in wild-type (WT) and p66Shc−/− control and streptozotocin-induced diabetic mice with or without hind limb ischemia. Wounds were assessed for collagen content, thickness and vascularity of granulation tissue, apoptosis, reepithelialization, and expression of c-myc and β-catenin. Response to hind limb ischemia was also evaluated.
RESULTS
Diabetes delayed wound healing in WT mice with reduced granulation tissue thickness and vascularity, increased apoptosis, epithelial expression of c-myc, and nuclear localization of β-catenin. These nonhealing features were worsened by hind limb ischemia. Diabetes induced p66Shc expression and activation; wound healing was significantly faster in p66Shc−/− than in WT diabetic mice, with or without hind limb ischemia, at 1 and 3 months of diabetes duration and in both SV129 and C57BL/6 genetic backgrounds. Deletion of p66Shc reversed nonhealing features, with increased collagen content and granulation tissue thickness, and reduced apoptosis and expression of c-myc and β-catenin. p66Shc deletion improved response to hind limb ischemia in diabetic mice in terms of tissue damage, capillary density, and perfusion. Migration of p66Shc−/− dermal fibroblasts in vitro was significantly faster than WT fibroblasts under both high glucose and hypoxia.
CONCLUSIONS
p66Shc is involved in the delayed wound-healing process in the setting of diabetes and ischemia. Thus, p66Shc may represent a potential therapeutic target against this disabling diabetes complication.
Impaired cutaneous wound healing is a major source of morbidity and mortality in the diabetic population (1,2). It is estimated that foot ulcers affect about 15% of diabetic patients with an annual amputation rate of about 1% (3). Importantly, mortality after a major amputation is very high (4). Chronic nonhealing ulcers also lead to disability and have a profound negative impact on patients' quality of life (5). The pathophysiology of chronic diabetic ulcers is complex and still incompletely understood, the most important predisposing factors being diabetic neuropathy and vasculopathy. Both micro- and macroangiopathy strongly contribute to development and delayed healing of diabetic wounds, through an impaired tissue feeding and response to ischemia. Wound healing is a complex physiological process that integrates the cooperation of multiple cell types, including the epithelium, endothelium, and fibroblasts (6). The biochemical basis of delayed wound healing in diabetes resembles the pathogenesis of other chronic complications, and includes stimulation of the polyol and glucosamine pathways, overactivity of protein kinase C and mitogen-activated protein kinase, and formation of advanced glycation end products. Oxidative stress is recognized as the common precursor mechanism of all these biochemical alterations (7). In diabetes, excessive reactive oxygen species (ROS) are produced either in the cytoplasm through the NADPH oxidase (8) or in mitochondria, through respiratory chain overflow. One source of mitochondrial ROS is the 66-kDa isoform of the mammal ShcA gene, p66Shc (9). Once believed to act as a docking adaptor protein for mitogenic signals, p66Shc has been characterized as a redox enzyme that, upon phosphorylation on serine 36, translocates into the mitochondrial intermembrane space and catalyzes the production of hydrogen peroxide (H2O2). This eventually causes mitochondrial permeability transition and leads the way to apoptotic cell death.
We have previously shown that the expression of p66Shc is increased in diabetic patients in relation to glucose control and markers of oxidative stress (10). This observation formed the basis to explore the role of p66Shc in diabetes complications. Genetic deletion of p66Shc in mice confers resistance to oxidative damage and prolongs life span (11). Interestingly, some studies show that p66Shc−/− mice are protected from the development of certain diabetes complications, such as nephropathy, endothelial dysfunction, and cardiomyopathy (12–14).
In the present study, we tested the hypothesis that p66Shc is involved in the delayed wound healing induced by diabetes and ischemia, and that p66Shc knockout improves features of wound healing in these settings. We show that deletion of p66Shc reverses many features of delayed wound healing typical of diabetes, such as impaired development and vascularization of the granulation tissue, response to ischemia, and reepithelialization.
RESEARCH DESIGN AND METHODS
An expanded description of materials and methods can be found in the online supplement at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1727/DC1. All the procedures involving animals and their care were conducted in accordance with international guidelines, laws, and policies, and with the National Institutes of Health Principles of Laboratory Animal Care (National Institutes of Health publication no. 85–23, revised 1985). Most experiments were conducted with wild-type (WT) and knockout (KO) mice on an SV129 genetic background. Some results were confirmed in C57BL/6 mice. The protocol was authorized by our local institutions. Diabetes was induced through a single intraperitoneal injection of 150 mg/kg STZ, and experiments were performed 4 or 12 weeks after confirmation of hyperglycemia (blood glucose level >300 mg/dl). Hind limb ischemia was induced by femoral artery ligation and excision, and experiments were performed 2 weeks later. Full-thickness skin wounds, 4 mm in diameter, were created on the dorsal surface of the left hind limbs with a biopsy punch, and closure was monitored with a digital camera. A block including the wound area with surrounding tissue was collected for histological analyses at mid-closure time. Tissue sections were stained with hematoxylin and eosin, Masson trichrome, and B4-isolectin immunofluorescence. Apoptosis was quantified with the ApopTag Plus assay kit. C-myc expression and nuclear localization of β-catenin in the epithelial layer were assessed with quantitative confocal immunofluorescence. Skin concentrations of A1C and pentosidine were determined by HPLC, and α-nitrotyrosines were measured with a commercial ELISA kit. Dermal fibroblasts were isolated from WT and p66Shc−/− mice and cultured in normal (5 mmol/l) and high (25 mmol/l) glucose in normoxia or hypoxia. Migration was assessed after growth in monolayer using a commercially available device.
Adductor muscle samples were collected for analysis of necrosis and inflammation (hematoxylin and eosin), apoptosis (ApopTag Plus), and capillary density (B4 isolectin/laminin double immunofluorescence). Perfusion was assessed in vivo before death with laser doppler perfusion imager. Circulating endothelial progenitor cells (EPCs) were determined by flow cytometry on the basis of the expression of CD34 (a stem cell marker) and Flk-1 (type 2 vascular endothelial growth factor receptor) on blood cells. Comparison between two groups was performed with unpaired Student t test, and statistical significance was accepted at P < 0.05.
RESULTS
Diabetes and high glucose induce p66Shc expression and activation.
p66Shc protein expression was determined in lysates of skin samples from control and diabetic WT mice. We found that experimental diabetes increased p66Shc protein content. Serine 36–phosphorylated p66Shc was increased in diabetic compared with control mice as well (supplemental Fig. IA), indicating activation of p66Shc. p66Shc protein expression was also determined in dermal fibroblasts exposed to normoxia or hypoxia in 5 or 25 mmol/l glucose; we found that high glucose increased p66Shc expression, especially in the presence of hypoxia (supplemental Fig. IB). Finally, p66Shc gene expression was also significantly increased in the heart, kidney, and skeletal muscle of diabetic versus control WT mice (supplemental Fig. 1C).
p66Shc knockout accelerates healing of diabetic and ischemic wounds.
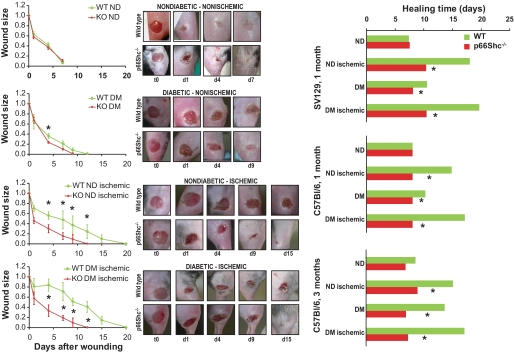
Wounds 4 mm in diameter were created on the dorsal surface of the right hind limb in WT and p66Shc−/− SV129 mice, in the presence of either 4-week STZ-induced diabetes, 2-week hind limb ischemia, or both. In separated experiments, wounds and ischemia were induced in WT and p66Shc−/− C57BL/6 mice after 4 and 12 weeks of diabetes and in coeval nondiabetic C57BL/6 mice. Wound area was monitored with a digital camera till complete macroscopic closure. As shown in Fig. 1, with both the SV129 and C57BL/6 backgrounds, in nondiabetic nonischemic animals, wound healing was not different between WT and p66Shc−/− mice. Four-week diabetes delayed wound healing by about 70% in WT SV129 animals and 30% in p66Shc−/− animals, resulting in a significantly faster healing in p66Shc−/− than in WT diabetic nonischemic mice (wound area at day 4: 23.6 ± 2.2 vs. 35.9 ± 5.0%; P = 0.028). Hind limb ischemia delayed wound healing by about 185% in WT and 70% in p66Shc−/− mice, resulting in a faster healing in p66Shc−/− versus WT nondiabetic ischemic mice (P < 0.05 from day 1 to 11). The coexistence of both diabetes and hind limb ischemia further exacerbated the differences between WT and p66Shc−/− mice. Similarly, p66Shc deletion in C57BL/6 mice prevented the delay in wound healing induced by 4-week diabetes and hind limb ischemia. In ischemic and nonischemic C57BL/6 mice 12 weeks after diabetes induction, absence of p66Shc was associated with significantly faster wound healing compared with the WT micce (Fig. 1, right panels). With this diabetes duration and genetic background, induction of ischemia was often followed by hind limb auto-amputation in WT mice (30 and 50% in nondiabetic and diabetic animals, respectively), while no amputation was seen in p66Shc−/− mice (supplementary Fig. II).
FIG. 1.
Wound healing. Healing of a 4-mm–diameter cutaneous wound was monitored using digital photography in wild-type (WT) and p66Shc-knockout (KO) diabetic (DM) and nondiabetic (ND) mice with or without hind limb ischemia. Representative photos are shown. Wound size is reported as fraction of the initial wound area. On the right, healing time is shown for WT and p66Shc−/− mice with or without diabetes and limb ischemia on the SV129 or the C57BLC57BL/6 background at 4 or 12 weeks of diabetes duration. n ≥ 3 mice for each group. *P < 0.05 in KO vs. WT. (A high-quality digital representation of this figure is available in the online issue.)
p66Shc knockout rescues the impaired granulation tissue in diabetic wounds.
A well-developed and vascularized granulation tissue is essential for wound healing. We show that diabetes, especially in combination with ischemia, impairs development and maturation of the granulation tissue in WT mice. After 4 weeks of diabetes in SV129 mice, collagen-stained area (18.7 ± 4.0 vs. 36.0 ± 2.0%; P = 0.002) and thickness of the granulation tissue (105.5 ± 18.4 vs. 323.0 ± 35.0 μm2; P < 0.0001) were significantly reduced in diabetic ischemic wounds compared with control wounds (Fig. 2). Within the granulation tissue of diabetic wounds, capillary density was reduced (nonischemic 91.2 ± 12.7 vs. 159.6 ± 11.2 hpf; P = 0.002; ischemic 42.8 ± 6.6 vs. 135.7 ± 18.9 hpf; P = 0.002), while apoptosis was increased (nonischemic 89.9 ± 22.9 vs. 19.2 ± 6.0 hpf; P = 0.009) compared with nondiabetic wounds. In parallel, closure of the epithelial gap was delayed in diabetic wounds (nonischemic 11.3 ± 3.6 vs. 26.6 ± 4.6%; P = 0.015; ischemic 20.6 ± 5.9% vs. 29.4 ± 6.8%; P = 0.33). All these alterations were reversed in p66Shc−/− mice: collagen area and tissue thickness were increased in diabetic wounds, while apoptosis was reduced and epithelial re-covering was significantly improved. Interestingly, capillary density within the granulation tissue was significantly lower in nondiabetic nonischemic p66Shc−/− mice compared withWT mice (50.8 ± 3.1 vs. 159.6 ± 11.2 hpf; P < 0.0001), but this difference was abrogated in the presence of either diabetes or ischemia, and was inverted in the presence of both diabetes and ischemia (92.8 ± 18.7 vs. 42.8 ± 6.6 hpf; P = 0.002). Results were confirmed in mice with the C57BL/6 genetic background after 1 month of diabetes (supplementary Fig. III). Collectively, these data using two different mouse strains strongly indicate that deletion of p66Shc reverts the negative effect of diabetes on development and ischemia-induced vascularization of the granulation tissue.
FIG. 2.
Characteristics of the granulation tissue in SV129 mice. Several morphological characteristics of the granulation tissue were evaluated in WT and p66Shc knockout (KO), diabetic (DM) (4-week duration), and nondiabetic (ND) mice with or without hind limb ischemia. Masson trichrome staining allowed determination of collagen area and granulation tissue thickness. Reepithelialization was quantified in histological sections by measuring the percentage of re-covered epithelial gap. Capillary density within the granulation tissue was quantified by B4 isolectin immunofluorescence. Apoptosis was assessed with the ApopTag kit in the granulation tissue and at wound edges, a critical site for active wound healing. Representative images are shown for control (nondiabetic nonischemic) and diabetic ischemic WT and KO mice. n ≥ 3 mice for each group; n ≥ 5 sections for tissue samples. *P < 0.05 in KO vs. corresponding WT group. †P < 0.05 in diabetic vs. corresponding nondiabetic group. (A high-quality digital representation of this figure is available in the online issue.)
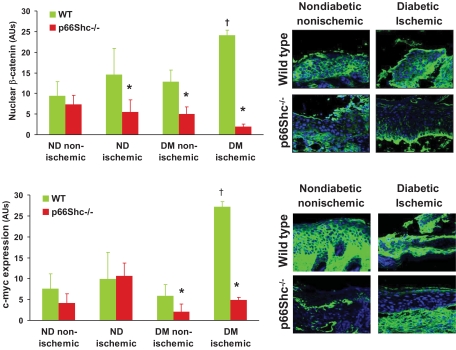
p66Shc knockout abrogates nonhealing features of diabetic wounds.
Nonhealing wounds display some typical cellular and molecular footprints. For example, high expression of c-myc and nuclear localization of β-catenin have been shown to mark the presence of ulcerogenic cells at the wound edges, associated with delayed healing (15). We show in WT mice that diabetic ischemic wounds had increased expression of c-myc (27.3 ± 7.3 vs. 7.6 ± 3.8 arbitrary units; P = 0.03) and nuclear localization of β-catenin (24.1 ± 1.2 vs. 9.4 ± 3.5 arbitrary units; P = 0.02) at their edges compared with control. In p66Shc−/− mice, development of this nonhealing feature was inhibited, as diabetic ischemic and nonischemic wounds displayed significantly lower expression of c-myc and lower nuclear β-catenin (Fig. 3).
FIG. 3.
Biomarkers of epithelial healing. In the epidermis, nuclear localization of β-catenin and high expression of c-myc, as features on nonhealing wounds, were quantified by immunofluorescence in wild-type (WT) and p66Shc -knockout (KO) diabetic (DM) and nondiabetic (ND) mice with or without hind limb ischemia. Representative images are shown for control (nondiabetic nonischemic) and diabetic ischemic WT and KO mice. n ≥ 3 mice for each group; n ≥ 5 sections for tissue samples. *P < 0.05 in KO vs. corresponding WT group. †P < 0.05 in diabetic vs. corresponding nondiabetic group. (A high-quality digital representation of this figure is available in the online issue.)
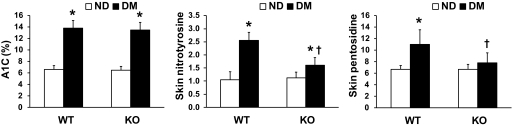
p66Shc knockout reduces nitrotyrosines and pentosidine in diabetic skin.
Fasting glucose levels were not significantly different between WT and p66Shc−/− diabetic mice (mean ± SEM 388 ± 28 vs. 401 ± 34 mg/dl; P = 0.77). Consistently, glycated hemoglobin was equally increased in WT and KO diabetic animals compared with nondiabetic animals (13.8 ± 1.4% vs. 6.6 ± 0.7% and 13.5 ± 1.2% vs. 6.5 ± 0.6%). The skin concentration of nitrotyrosines, a marker of tissue oxidative stress, was significantly increased by 2.5-fold in WT diabetic versus nondiabetic mice, while it was not significantly different in diabetic versus nondiabetic p66Shc−/− mice. Similarly, skin pentosidine concentration, an indicator of advanced glycation, was significantly increased by about 75% in WT diabetic versus nondiabetic mice, while it was not significantly different in diabetic versus nondiabetic p66Shc−/− mice (Fig. 4).
FIG. 4.
Biomarkers of glyco-oxidation. Blood A1C and skin concentration of nitrotyrosines (a marker of tissue oxidative stress) and pentosidine (a marker of advanced glycation) were determined by high-performance chromatography in wild-type (WT) and p66Shc knockout (KO) diabetic (DM) and nondiabetic (ND) mice. n = 3 mice for each group. *P < 0.05 in DM vs. ND; †P < 0.05 in KO vs. WT.
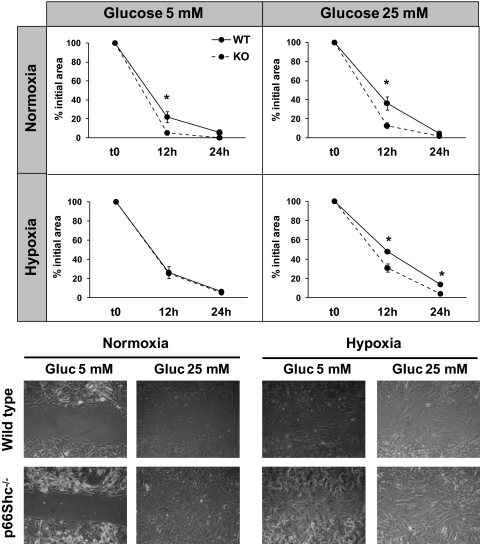
Improved migration of dermal fibroblasts in p66Shc knockout mice.
It is well known that deletion of p66Shc protects from oxidative damage (11). We first confirmed that p66Shc−/− dermal fibroblasts are resistant to hydrogen peroxide in vitro, while about 90% WT cells died (supplemental Fig. IV). We then developed an in vitro wound model assay using dermal fibroblasts isolated from WT and p66Shc−/− nondiabetic nonischemic mice. p66Shc−/− cells migrated significantly faster than WT fibroblasts in normoxic 5 mmol/l glucose (% of initial area at 12 h 5.3 ± 2.2 vs. 22.1 ± 5.9; P = 0.037) and when exposed to high glucose (25 mmol/l) in both normoxia (12.8 ± 3.2% vs. 36.1 ± 11.0%; P = 0.01) and hypoxia (30.9 ± 4.1% vs. 47.9 ± 2.2%; P = 0.026) (Fig. 5). These data indicate that p66Shc partly mediates the detrimental effects of hypoxia and high glucose on the migratory activity of dermal fibroblasts.
FIG. 5.
Migration of dermal fibroblasts. Fibroblasts were isolated from dermis of wild-type (WT) and p66Shc knockout (KO) mice. Migration of dermal fibroblasts were assessed in vitro under normal (5 mmol/l) or high (25 mmol/l) glucose in normoxia or hypoxia. Closure of the monolayer gap was monitored at 12 and 24 h. Migration is expressed as percentage closure of the gap. n = 3 experiments for each condition. *P < 0.05 in KO vs. WT.
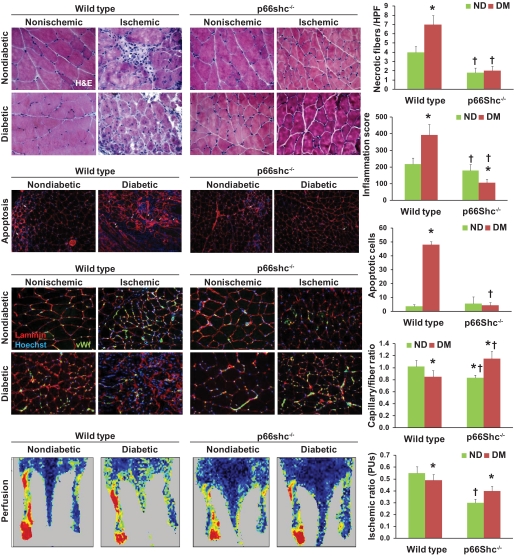
Reduced skeletal muscle ischemic damage in p66Shc knockout mice.
Ischemia is a strong inhibitor of wound healing and a risk factor of wound development, especially in the setting of diabetes. Thus, we examined the ischemic responses in WT and p66Shc−/− diabetic and nondiabetic muscles. In addition to reducing blood flow, ischemia induced inflammation, apoptosis, and necrosis in skeletal muscle, all of which were worsened by diabetes in WT animals: compared with nondiabetic WT mice, diabetic WT mice showed increased apoptotic (48.1 ± 2.2 vs. 2.6 ± 0.9 hpf; P < 0.0001) and necrotic (7.0 ± 1.0 vs. 3.9 ± 0.7 hpf; P = 0.002) cell death and inflammatory score (385 ± 72 vs. 210 ± 51 arbitrary units; P = 0.023). Postischemic capillary density and muscle perfusion were lowered by diabetes in WT mice (capillaries 0.83 ± 0.07 vs. 1.05 ± 0.07 fiber; P = 0.047; ischemic-to-nonischemic perfusion ratio 0.48 ± 0.06 vs. 0.54 ± 0.05; P = 0.05). In p66Shc−/− diabetic mice, necrosis, apoptosis, and inflammation did not increase after ischemia compared with nondiabetic p66Shc−/− mice. Interestingly, in nondiabetic animals, p66Shc knockout reduced postischemic perfusion recovery compared with WT animals (capillaries 0.81 ± 0.03 vs. 1.05 ± 0.07 fiber; P = 0.05; ischemic-to-nonischemic perfusion ratio 0.30 ± 0.03 vs. 0.54 ± 0.05; P = 0.02). However, p66Shc knockout improved perfusion recovery in diabetic compared with nondiabetic mice (capillaries 1.15 ± 0.11 vs. 0.81 ± 0.03/fiber; P = 0.04; ischemic/nonischemic perfusion ratio 0.40 ± 0.05 vs. 0.30 ± 0.03; P = 0.04), suggesting that p66Shc deletion counteracts the negative impact of diabetes on blood flow recovery after ischemia (Fig. 6).
FIG. 6.
Skeletal muscle damage and repair. Several morphological characteristics of skeletal muscle were evaluated in wild-type (WT) and p66Shc knockout (KO) diabetic (DM) and nondiabetic (ND) mice before and after hind limb ischemia. Hematoxylin and eosin staining was used to evaluate necrosis and to quantify inflammatory infiltrate. Apoptosis was quantified using the ApopTag assay kit while the capillary network was stained and quantified using double immunofluorescence for basement membrane (anti–α-laminin) and endothelium (anti-GSL I-isolectin B4). Limb perfusion was monitored 2 weeks after ischemia using a laser-doppler imager. Representative figures from the different groups of animals are shown. Histograms report ischemic versus nonischemic rations. n ≥ 3 experiments for each condition; n = 5 sections for each tissue sample. *P < 0.05 in DM vs. ND; †P < 0.05 in KO vs. WT. (A high-quality digital representation of this figure is available in the online issue.)
To understand the differential angiogenic response to ischemia, we assessed circulating endothelial progenitor cells (EPCs), which normally increase after tissue ischemia in control but not in diabetic animals (16,17). In both diabetic and nondiabetic p66Shc−/− mice, there was no EPC mobilization after ischemia and baseline levels were not significantly different from WT mice (not shown), suggesting that the effect of p66Shc on ischemic response is not mediated by EPCs.
DISCUSSION
We show for the first time that deletion of the life span–determinant redox enzyme p66Shc accelerates healing of diabetic and ischemic wounds, through induction of a histologic pattern consistent with reduced tissue damage, enhanced tissue repair, and modulation of the ischemic response. Herein, we also confirm our previous observation that diabetes increases p66Shc gene expression in humans (10), by showing that diabetes and high glucose increase p66Shc expression and activation in a variety of tissues including skin and skeletal muscle. These data collectively indicate that p66Shc is involved in the negative effects of diabetes and ischemia on wound healing.
Delayed wound healing represents one of the most disabling diabetes complications (1–5). It is attributable to a number of cellular and molecular mechanisms, most of which are stimulated by oxidative stress (7). p66Shc generates H2O2, mediates proapoptotic signals, and is linked to mitochondrial and nonmitochondrial oxidative stress (9,18). In compliance with the oxidative theory of aging, deletion of p66Shc prolongs life span by about 30% in mice (19). It is also involved in diseases prominently mediated by oxidative stress, including diabetes complications (20). p66Shc−/− mice are protected against experimental diabetic glomerulopathy (12), diabetic cardiomyopathy (14), and hyperglycemia-induced endothelial dysfunction (13), indicating that p66Shc is part of the signal transduction pathway of hyperglycemic damage. We now present data linking p66Shc to delayed wound healing. Typical features of nonhealing diabetic wounds include impaired deposition of the granulation tissue, which is thinner and devoid of vascular support (21). This is associated with defective migration of connective tissue cells within the wound area, delayed apposition of wound edges, and incomplete reendothelialization (22). In the present study, we document all these morphological aspects of delayed wound healing in a mouse model of STZ-induced diabetes. Some of these features, such as defect of granulation tissue and capillary network, were worsened by the simultaneous presence of diabetes and limb ischemia. It has been previously shown in chronic nonhealing lesions that keratinocytes at wound edges have canonical Wnt-induced β-catenin activation; this inhibits keratinocyte migration by activating c-myc, blocking the effects of epidermal growth factor, and causing cytoskeletal rearrangement (15). We found that the β-catenin/ c-myc pathway was overactivated in diabetic ischemic wounds, consistently with a reduced reendothelialization and wound closure.
The most important finding of the present study is that p66Shc−/− mice showed significantly improved healing of diabetic wounds, in the presence or absence of ischemia. This was confirmed at 1 and 3 months of diabetes duration and in both outbred SV129 and inbred C57BL/6 mice. As shown by nitrotyrosine and pentosidine levels, p66Shc deletion prevented diabetes-induced oxidative stress and advanced glycation in the skin. In addition, p66Shc−/− mice had reduced wound edge cell apoptosis and improved migration of connective tissue cells in high glucose with or without hypoxia. As a result, histologic aspects of healing were improved in diabetic wounds and ischemic diabetic wounds of p66Shc−/− mice compared with WT mice. Furthermore, activation of the β-catenin/c-myc pathway, which prevents epithelial healing, was strongly inhibited by p66Shc deletion in diabetic wounds and ischemic diabetic wounds. This suggests that oxidative stress and p66Shc signaling are involved in the deranged epithelial cell differentiation and migration that mark nonhealing wounds in diabetes. However, the exact biochemical pathway that links p66Shc, oxidative stress, and β-catenin/ c-myc remains to be defined.
In addition to these local and biochemical events, diabetes delays wound healing because it is often complicated by ischemia (23). Diabetic macroangiopathy is aggressive and often not susceptible to revascularization (24), resulting in a steady shortage of oxygen and nutrients to the tissue. Moreover, the local angiogenic response to ischemia is impaired in diabetes (25). Herein, we confirm in WT mice that capillary network of diabetic ischemic wounds is significantly reduced compared with nondiabetic wounds. In hind limb muscles, ischemia caused more severe damage and inflammation in diabetic than in nondiabetic mice, which was accompanied by a defective postischemic recovery of vascular network and perfusion. We found that diabetes induced p66Shc expression in skeletal muscle, and the excess muscle ischemic damage associated with diabetes was strongly inhibited by p66Shc deletion. While capillary density and perfusion were lower in p66Shc−/− mice at baseline, the postischemic recovery of skeletal muscle vascularization was significantly improved in diabetic versus nondiabetic p66Shc−/− mice. Similarly, the vascular support of granulation tissue in diabetic ischemic wounds was doubled by p66Shc deletion, even if SV129 and C57BL/6 mice differed slightly in the regulation of granulation tissue vascularity. With longer diabetes duration (12 weeks) on the C57BL/6 background, which is more susceptible to ischemic damage, we also found that p66Shc−/− mice are completely protected from amputation, which occurred in 30–50% of WT mice. These data indicate that blocking p66Shc prevents the diabetes-related inhibition of postischemic angiogenesis and support a role for p66Shc in regulating angiogenesis and mediating the negative effects of diabetes on ischemic responses. Despite the fact that p66Shc has been shown to reverse the defect of diabetic EPCs in vitro (26), we found no evidence that this is responsible for the regulation of angiogenesis by p66Shc in vivo, likely because prevention of the ischemic damage in p66Shc−/− mice reduced the signal for EPC mobilization. The regulation of local angiogenic signals by p66Shc and oxidative stress deserves further investigation.
Conclusions.
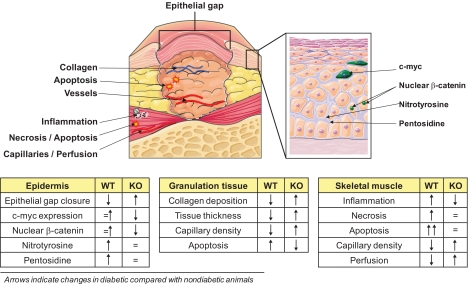
In conclusion, we show data supporting that p66Shc is part of the signaling network that delays wound healing in diabetes. Genetic deletion of p66Shc accelerated diabetic wound healing by reducing advanced glycation, oxidative stress, and tissue damage; promoting development and vascularization of the granulation tissue; inhibiting the antihealing β-catenin/c-myc epithelial pathway; and modulating vascular response to ischemia. p66Shc knockout also significantly protected from diabetes-related ischemic damage in limb muscles, a known factor contributing to diabetic foot disease (Fig. 7). Although the exact biochemical pathways are not yet fully elucidated, p66Shc is revealed as a potential therapeutic target against this disabling diabetic complication.
FIG. 7.
Wound healing. The many differences in wound healing features between wild-type (WT) and p66Shc knockout (KO) mice are shown. The figure identifies single features, and the arrows in the tables indicate changes in diabetic compared with nondiabetic mice. (A high-quality digital representation of this figure is available in the online issue.)
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a European Foundation for the Study of Diabetes (EFSD) Grant to A.A., and by the Integrated Project “HeartRepair” LSHM-CT-2005-018630, Heart Failure, and Cardiac Repair (www.heartrepair.eu), and partly supported by an EFSD grant to G.P.F.
No potential conflicts of interest relevant to this article were reported.
G.P.F. designed the study and experiments, performed animal experiments, analyzed data, and wrote the manuscript. M.A. performed animal experiments and tissue analysis and analyzed and interpreted data. L.M. performed culture experiments and analyzed data. E.B. performed and analyzed flow cytometry experiments. E.P. and E.I. performed and interpreted WB and PCR experiments. C.C. and A.L. performed, analyzed, and interpreted glycol-oxidation experiments. V.P. provided support for performing and interpreting ischemia experiments. C.A. contributed to the discussion and wrote the manuscript. P.G.P., M.G., M.S., and M.A. performed breeding, provided animals, interpreted data, and wrote the manuscript. A.A. supervised the project, analyzed and interpreted data, contributed to the discussion, and wrote the manuscript.
Parts of this study were presented in abstract form at the 45th Annual Meeting of the European Association for the Study of Diabetes (EASD), Vienna, Austria, 29 September–2 October 2009, and published in Diabetologia (2009) 52:S442.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–387 [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 3.Bartus CL, Margolis DJ. Reducing the incidence of foot ulceration and amputation in diabetes. Curr Diab Rep 2004;4:413–418 [DOI] [PubMed] [Google Scholar]
- 4.Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Quarantiello A, Lupattelli T, Luppattelli T, Morabito A. Long-term prognosis of diabetic patients with critical limb ischemia: a population-based cohort study. Diabetes Care 2009;32:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48:1906–1910 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol 2001;28:521–534 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. The pathobiology of diabetes complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 8.Avogaro A, Pagnin E, Calò L. Monocyte NADPH oxidase subunit p22(phox) and inducible hemeoxygenase-1 gene expressions are increased in type II diabetic patients: relationship with oxidative stress. J Clin Endocrinol Metab 2003;88:1753–1759 [DOI] [PubMed] [Google Scholar]
- 9.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005;122:221–233 [DOI] [PubMed] [Google Scholar]
- 10.Pagnin E, Fadini G, de Toni R, Tiengo A, Calò L, Avogaro A. Diabetes induces p66Shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab 2005;90:1130–1136 [DOI] [PubMed] [Google Scholar]
- 11.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309–313 [DOI] [PubMed] [Google Scholar]
- 12.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 2006;55:1642–1650 [DOI] [PubMed] [Google Scholar]
- 13.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Lüscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A 2007;104:5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Lüscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66Shc gene. Circ Res 2006;99:42–52 [DOI] [PubMed] [Google Scholar]
- 15.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 2005;167:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 2007;30:1305–1313 [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006;49:3075–3084 [DOI] [PubMed] [Google Scholar]
- 18.Tomilov AA, Bicocca V, Schoenfeld RA, Giorgio M, Migliaccio E, Ramsey JJ, Hagopian K, Pelicci PG, Cortopassi GA. Decreased superoxide production in macrophages of long-lived p66Shc knockout mice. J Biol Chem 2010;285:1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinton P, Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle 2008;7:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellegrini M, Baldari CT. Apoptosis and oxidative stress-related diseases: the p66Shc connection. Curr Mol Med 2009;9:392–398 [DOI] [PubMed] [Google Scholar]
- 21.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608 [DOI] [PubMed] [Google Scholar]
- 22.Hehenberger K, Heilborn JD, Brismar K, Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen 1998;6:135–141 [DOI] [PubMed] [Google Scholar]
- 23.Gershater MA, Löndahl M, Nyberg P, Larsson J, Thörne J, Eneroth M, Apelqvist J. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia 2009;52:398–407 [DOI] [PubMed] [Google Scholar]
- 24.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 25.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A 2009;106:13505–13510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stefano V, Cencioni C, Zaccagnini G, Magenta A, Capogrossi MC, Martelli F. p66ShcA modulates oxidative stress and survival of endothelial progenitor cells in response to high glucose. Cardiovasc Res 2009;82:421–429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.