Abstract
OBJECTIVE
Mitochondrial reactive oxygen species (ROS) plays a key role in diabetic retinopathy (DR) pathogenesis. However, whether simvastatin decreases diabetes-induced mitochondrial ROS production remains uncertain. The aim of this study was to clarify the beneficial effects and mechanism of action of simvastatin against diabetes-induced retinal vascular damage.
RESEARCH DESIGN AND METHODS
Diabetic rats and control animals were randomly assigned to receive simvastatin or vehicle for 24 weeks, and bovine retinal capillary endothelial cells (BRECs) were incubated with normal or high glucose with or without simvastatin. Vascular endothelial growth factor (VEGF) and peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) in the rat retinas or BRECs were examined by Western blotting and real-time RT-PCR, and poly (ADP-ribose) polymerase (PARP), and p38 MAPK were examined by Western blotting. Mitochondrial membrane potential (Δψm) and ROS production were assayed using the potentiometric dye 5,5′,6,6′- Tetrachloro1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1) or CM-H2DCFDA fluorescent probes.
RESULTS
Simvastatin significantly upregulated PGC-1α (P < 0.01), subsequently decreased Δψm (P < 0.05) and ROS generation (P < 0.01), inhibited PARP activation (P < 0.01), and further reduced VEGF expression (P < 0.01) and p38 MAPK activity (P < 0.01). Those changes were associated with the decrease of retinal vascular permeability, retinal capillary cells apoptosis, and formation of acellular capillaries.
CONCLUSIONS
Simvastatin decreases diabetes-induced mitochondrial ROS production and exerts protective effects against early retinal vascular damage in diabetic rats in association with the inhibition of mitochondrial ROS/PARP pathway mediated by PGC-1α. The understanding of the mechanisms of action of statins has important implications in the prevention and treatment of mitochondrial oxidative stress-related illness such as DR.
The 3-hydroxy-3-methylglutaryl-CoA(HMG-CoA) reductase inhibitors, or statins, which are extensively used in the treatment of hypercholesterolemia, are potent inhibitors of cholesterol biosynthesis (1). By inhibiting l-mevalonic acid synthesis, statins may also decrease mitogenic responses and antiangiogenic actions that are independent of the cholesterol-lowering effect (2). Previous studies revealed that treatment with simvastatin-attenuated leukocyte-endothelial cell interactions and the subsequent breakdown of the blood-retinal barrier via the suppression of vascular endothelial growth factor (VEGF) induced intercellular adhesion molecule (ICAM)-1 expression in db/db mice retinas (2,3). Moreover, this effect is associated with the inhibition of NADPH oxidase-mediated reactive oxygen species (ROS) production by statins (4,5).
Progression of vascular abnormalities, in addition to blood-retinal barrier breakdown, including the selective loss of pericytes and formation of acellular capillaries, characterizes early diabetic retinopathy (DR). The increased VEGF expression, on one hand, leads to increased vascular permeability whereby statins decrease retinal vascular permeability via VEGF downregulation (3,6). On the other hand, it is paradoxically associated with the selective loss of pericytes and formation of acellular capillaries as a potent survival factor for endothelial cells in vivo (7). Therefore, there is a need to explore the mechanisms underlying the apoptosis of vascular cells in diabetic rats and the possible role of statins in this process.
It has been reported that hyperglycemia-induced ROS production is primarily associated with mitochondria and NADPH oxidase (8), and an increase in oxidative stress in vascular cells plays a key role in DR pathogenesis (9–11). A strong in vivo correlation exists between ROS production and the expression of neovascularization and growth factors in diabetic retinas (12,13). Recently, Brownlee (16) suggested a unifying hypothesis integrating various mechanisms discussed in past years that an overproduction of mitochondrial ROS is an initiating cause in the pathogenesis of diabetic complications. This concept proposes that high glucose levels (HG) in the endothelial cells lead to overproduction of mitochondrial ROS, which then inactivates glyceraldehyde-3-phosphate dehydrogenase by poly (ADP-ribose) polymerase (PARP) activation and subsequent ADP ribosylation (14–16). However, there is no evidence as to whether there is an effect of statins on mitochondria-derived ROS under HG conditions.
Thus, the aim of this study was to investigate the potential effect and molecular mechanisms of statins on mitochondria-derived ROS and retinal vascular cell apoptosis under HG. Our results showed that simvastatin significantly inhibited the increase of mitochondria-derived ROS and prevented the apoptosis of retinal vascular cells mediated by p38 MAPK through PARP under HG, in addition to attenuating retinal vascular permeability via downregulation of VEGF. The effect was associated with the upregulation of peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α).
RESEARCH DESIGN AND METHODS
All experiments in this study comply with the requirements of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All chemicals were reagent grade quality and were purchased from Sigma Chemicals (St. Louis, MO), unless stated otherwise.
Eight-week-old male Sprague-Dawley rats weighing ∼200 g (Shanghai Laboratory Animal Center, Chinese Academy of Sciences) were randomly assigned into groups receiving either 60 mg/kg streptozotocin (STZ) intraperitoneally or citrate buffer alone. Rats were categorized as diabetic when the blood glucose exceeded 16.7 mmol/l at 48 h after STZ administration. Diabetic rats were randomly assigned into groups receiving either 10 mg/kg/day simvastatin administered via drinking water for 24 weeks or no treatment at all. Age-matched rats that had not received STZ served as controls. All rats were provided free access to standard rat food and drinking water. Diabetic rats received subcutaneous insulin (Humulin-N, Eli Lilly, Indianapolis, IN) twice weekly to maintain body weight and maximize survival rate. Every week the plasma glucose concentrations were monitored using the glucose oxidase technique. Plasma triglycerides and cholesterol levels were determined with a commercially available enzyme kit (Leadman Biotech, Beijing, China). At the end of the experiments, the eyes were resected from deeply anesthetized animals. The right eyes were assigned for preparation of retinal digests, and the left eyes for the extraction procedures.
Retinal digest procedures.
The eyes were immediately placed in 4% buffered paraformaldehyde for 24 h. Retinal trypsin digestion was performed according to the method of Cogan et al. (17). Preparations of retinal vascular networks were set onto polylysine-coated glass slides in distilled water and then dried. The preparations were stored at −20°C until used for periodic acid-Schiff (PAS) and hematoxylin staining. The capillary network was evaluated to identify the number of pericytes and acellular capillaries by using previously described quantitative methods (11).
Measurement of retinal blood vessel leakage using Evans blue dye.
Retinal blood vessel leakage was quantitated using Evans blue dye, which noncovalently binds to plasma albumin in the blood stream (18). With the rats under deep anesthesia, Evans blue dye was injected through the tail vein at a dosage of 30 mg/kg. After the dye had circulated for 60 min, the chest cavity was opened and the left heart ventricle was cannulated. After each mouse was perfused, the eyes were enucleated, and the retinas were carefully dissected away under an operating microscope. The weight of each retina was measured after thorough drying in a Speed-Vac. Albumin leakage into the retinal tissue was estimated via the measurement of extravasated Evans blue dye. Evans blue was extracted by incubating each retina in 60 μl formamide for 18 h at 70°C. The extract was ultracentrifuged at a speed of 70,000 rpm for 45 min at a temperature of 4°C. The absorbance of the supernatant was measured with a spectrophotometer at 620 nm, the absorption maximum for Evans blue in formamide. The concentration of dye in the extracts was calculated from a standard curve of Evans blue in formamide and normalized to the dry retinal weight.
Cell culture.
The primary culture of bovine retinal capillary endothelial cells (BRECs) was obtained as described in our previous study (11). The endothelial cells generated after four passages were used in later experiments. The cells were incubated with either normal glucose (5 mmol/l glucose), normal glucose plus 25 mmol/l mannitol, HG (30 mmol/l glucose), normal d-glucose(5 mmol/l) plus H2O2 (500 μmol/l), HG plus PJ-34 (3 μmol/l; a potent-specific PARP inhibitor), or HG plus simvastatin (5 μmol/l; Merck and Co., China) in the absence or presence of either mevalonate (100 μmol/l dissolved in ethanol), the general ROS scavenger N-acetylcysteine (NAC) (10 mmol/l), or NAC and GW9662 (an irreversible peroxisome proliferator-activated receptor γ [PPARγ] inhibitor; 20 μmol/l). The cells were washed with PBS after 72 h for subsequent experiments.
For knockdown studies, BRECs were infected with either a control adenovirus vector expressing green fluorescent protein, or 50 nmol/l PGC-1α siRNA, as indicated, for 48 h. Adenoviruses for PGC-1α siRNA were generated as described (19).
Mitochondrial membrane potential (Δψm).
The potentiometric dye 5,5′,6,6′- Tetrachloro1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1; Molecular Probes) exhibits membrane potential-dependent loss as J-aggregates (polarized mitochondria) are converted to JC-1 monomers (depolarized mitochondria), as indicated by the fluorescence emission shift from red to green (20). Hence, mitochondrial depolarization was determined by an increase in the green/red fluorescence intensity ratio. Mitochondrial membrane potential (Δψm) measurements were performed by flow cytometry (Coulter Epics XL, Beckman-Coulter) as described in our previous study (11).
Real-time RT-PCR.
Total RNA was extracted from rat retinal tissue and BRECs by using the TRIzol reagent (Invitrogen Life Technologies, Gaithersburg, MD) and stored at −80°C. The DyNAmo Flash SYBR Green qPCR Kit (Finnzymes Oy, Espoo, Finland) was used according to the manufacturer's instructions. The primer sequences (sense/antisense) used were as follows: VEGF, 5′-GCGGGCTGCTGCAATG-3′/5′-TGCAACGCGAGTCTGTGTTT-3′; PGC-1α, 5′-CACCAAACCCACAGAGAACA-3′/5′-GGGTCATTTGGTGACTCTGG-3′; β-actin, 5′-GCACCGCAAATGCTTCTA-3′/5′-GGTCTTTACGGATGTCAACG-3′. The specificity of the amplification product was determined by performing a melting curve analysis. Standard curves were generated for expression of each gene by using serial dilutions of known quantities of the corresponding cDNA gene template. Relative quantification of the signals was performed by normalizing the signals of different genes with the β-actin signal.
Western blotting.
The protein concentration in the supernatant was measured using the Bio-Rad DC protein assay. Fifty micrograms of protein obtained from each sample (retinas or BRECs) were subjected to SDS-PAGE in a Bio-Rad miniature slab gel apparatus and electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked in 5% nonfat dried milk solution and incubated overnight with partially purified mouse anti-VEGF monoclonal antibody (mAb; 1:500; Chemicon, Temecula, CA), rabbit anti-PGC-1α mAb (1:500) (Cell Signaling Technology), rabbit anti-phospho-p38 MAP kinase polyclonal antibody (polyAb; 1:500; Cell Signaling Technology), or rabbit anti-PPARγ polyAb (1:500) (Upstate, Millipore). Detection of β-actin expression with a mAb (1:1,000; Sigma Chemical) was used as an internal control to confirm equivalent total protein loading. Signal intensities in the control lanes were arbitrarily assigned a value of 1.0. Western blots were repeated 3 to 5 times and qualitatively similar results were obtained.
Statistical analysis.
The experimental data are expressed as means ± SD. Group means were compared by one-way ANOVA using the GraphPad Prism 4.0 software system (GraphPad, San Diego, CA) and the statistical software program SPSS 13.0 for Windows (Chicago, IL). Pearson correlation tests were also performed. P values < 0.05 were considered significant in all cases.
RESULTS
Animal data.
Nonfasting blood glucose, 24-h urine volume, triglycerides, and cholesterol levels were strikingly increased in the diabetic group as compared with those in the normal control group (P < 0.01). Administration of simvastatin did not ameliorate the severity of hyperglycemia in diabetic rats. The values obtained for nonfasting blood glucose and 24-h urine levels determined throughout the duration of the experiment were comparable between the two diabetic groups (diabetes and diabetes plus simvastatin), but were significantly different (P < 0.01) from those of the normal control group (Table 1). Compared with the diabetic group, treatment with simvastatin significantly reduced the plasma triglycerides and cholesterol levels in the diabetic rats (triglycerides, P < 0.05; cholesterol, P < 0.01). Diabetic rats were treated with insulin to prevent weight loss. Body weight in both diabetic rat groups remained significantly lower than those of the nondiabetic control rats (P < 0.05). Long-term simvastatin administration did not have adverse effects on the health or lifespan of the diabetic rats.
TABLE 1.
Metabolic and physical parameters of the experimental groups (24 weeks)
| Control | DM | DM + S | |
|---|---|---|---|
| Body weight (g) | 581 ± 53 | 388 ± 31* | 409 ± 45* |
| Nonfasting blood glucose (mmol/l) | 4.6 ± 0.3 | 26.2 ± 5.3** | 27.1 ± 3.3** |
| 24-hour urine volume (ml) | 21 ± 5.2 | 203 ± 56** | 189 ± 63** |
| Triglycerides (mg/dl) | 103.6 ± 9.3 | 146.8 ± 11.6** | 119 ± 8.9# |
| Cholesterol (mg/dl) | 89.6 ± 5.2 | 154.5 ± 9.8** | 112.6 ± 8.2## |
**P ≤ 0.01 for the difference between the diabetic and nondiabetic groups or between the nondiabetic and diabetic + simvastatin groups;
#P ≤ 0.05 for the difference between the diabetic and diabetic + simvastatin groups;
##P ≤ 0.01 for the difference between the diabetic and diabetic + simvastatin groups. Control, control rats; DM, diabetic rats; DM + S, diabetic rats treated with simvastatin.
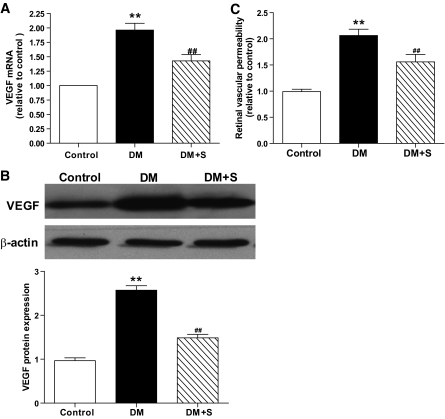
Alleviation of retinal vascular permeability in diabetic rats by simvastatin is associated with downregulation of VEGF.
Compared with the control, the VEGF mRNA and protein were upregulated significantly in the retinas of diabetic rats with a concomitant increase in retinal vascular permeability, whereas simvastatin significantly inhibited these changes (Fig. 1A–C). Pearson correlation tests showed significant positive correlations between the changes in retinal vascular permeability and the changes in VEGF expression in diabetic rats with or without treatment with simvastatin (data not shown).
FIG. 1.
Changes of retinal VEGF levels in the control rats (control), diabetic rats (DM), and diabetic rats treated with simvastatin (DM + S). A: Real-time RT-PCR determination of VEGF mRNAs relative to the control values. B: Western blot analysis of VEGF protein expression in the three groups. Equal protein loading was confirmed by detection of β-actin. C: Retinal vascular permeability in the three groups. Bars indicate SD. A representative experiment of the three is shown (**P < 0.01 vs. control; ##P < 0.01 vs. DM; n = 8).
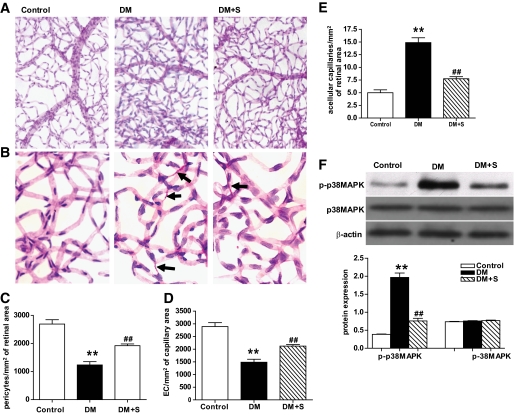
Simvastatin-mediated decrease of apoptosis of retinal capillary cells in diabetic rats is associated with reduced activation of p38 MAPK.
Accelerated death of capillary cells is believed to be the major cause of acellular capillary formation (21). Compared with the control rats, the number of pericytes and endothelial cells significantly decreased (P < 0.01), and that of acellular vessels significantly increased (P < 0.01) in diabetic rat retinas. In addition, there was a significant increase and reduction, respectively, in the diabetic eyes treated with simvastatin compared with untreated diabetic rats (P < 0.01) (Fig. 2A–E). Concomitantly, in diabetic rat retinas, the activity of p38 MAPK significantly increased as compared with those in the nondiabetic animals, and the changes were significantly inhibited by simvastatin (Fig. 2F). By immunohistochemical analysis of trypsin-digested retinal blood vessels, it was found that simvastatin significantly decreased the activation of p38MAPK induced by diabetes (Fig. 2G and supplementary data in the online appendix available at http://diabetes.diabetesjournals.org/content/early/2010/06/15/db10-0638/suppl/DC1). Pearson correlation tests showed significant positive correlations between the changes in apoptosis of retinal capillary cells and acellular vessel formation and the changes in activity of p38 MAPK in diabetic rats with or without simvastatin treatment, respectively (data not shown).
FIG. 2.
Observation of the number of retinal capillary cells and activity of p38 MAPK in the control, DM, and DM + S groups. A and B: Low- and high-magnification photomicrographs (×50 and ×400, respectively) of trypsin-digested retinal blood vessels obtained from the three groups. All preparations were stained with PAS and hematoxylin; arrowheads indicate acellular capillary. C–E: Determination of pericyte/mm2 of capillary area (C), endothelial cell (EC)/mm2 of capillary area (D), and acellular capillary segment/mm2 in the retinal vessels (E) in the three groups. F: Western blot analysis of activity of p38 MAPK (phosphorylation of p38 MAPK, p-p38 MAPK) in the three groups. Bars indicate SD. A representative experiment of the three is shown (**P < 0.01 vs. control; ##P < 0.01 vs. DM; n = 8). (A high-quality digital representation of this figure is available in the online issue.)
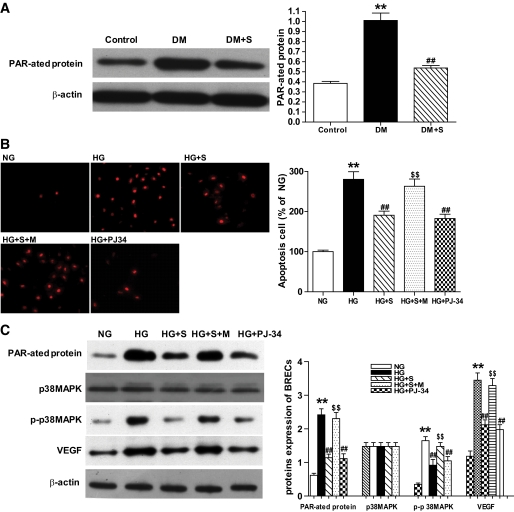
Protective effect of simvastatin on retinopathy in diabetic rats is associated with the inhibition of PARP activity.
PARP activity was demonstrated using a mAb to detect poly(ADP-ribosyl)ated proteins, which is the product of the enzyme. As illustrated in Fig. 3A, a marked increase was observed in poly(ADP-ribosyl)ation of the proteins obtained from the retinal extract of 6-month diabetic rats as compared with the nondiabetic controls, and it was significantly inhibited by simvastatin, which was accompanied by upregulation of VEGF (Fig. 1A and B) and activation of p38MAPK (Fig. 2F). Moreover, the effects were similar to that with PJ-34 (data not shown). In vitro, PJ-34 also significantly inhibited VEGF expression and activation of p38 MAPK and prevented the apoptosis of BRECs incubated in HG, as did simvastatin (Fig. 3B and C). In addition, we found that PARP antisense oligonucleotides downregulate VEGF expression (data not shown) and inhibited the activation of p38 MAPK in BRECs (22). These results suggest that the effect of simvastatin on retinopathy in diabetic rats may be associated with the inhibition of PARP activity.
FIG. 3.
Protein expression and apoptosis of cell analysis in retinas and BRECs. A: Representative Western blot analysis of poly(ADP-ribosyl)ated protein expression in retinas in control, DM, and DM + S. Equal protein loading was confirmed by detection of β-actin. Quantification of signal intensity by poly(ADP-ribosyl)ated protein densitometry in the retinas obtained from all three groups. B: Apoptosis cell in normal glucose (NG), high glucose (HG), HG + S, HG + S + mevalonate (HG + S + M), and HG + PJ-34. C: Representative Western blot analysis of poly(ADP-ribosyl)ated, VEGF, p38 MAPK, and p-p38 MAPK protein expression in BRECs in the five groups. Bars indicate SD. A representative experiment of the three is shown (**P < 0.01 vs. control; ##P < 0.01 vs. DM; n = 8; **P < 0.01 vs. NG; ##P < 0.01 vs. HG; $$P < 0.01 vs. HG + S; n = 9). (A high-quality digital representation of this figure is available in the online issue.)
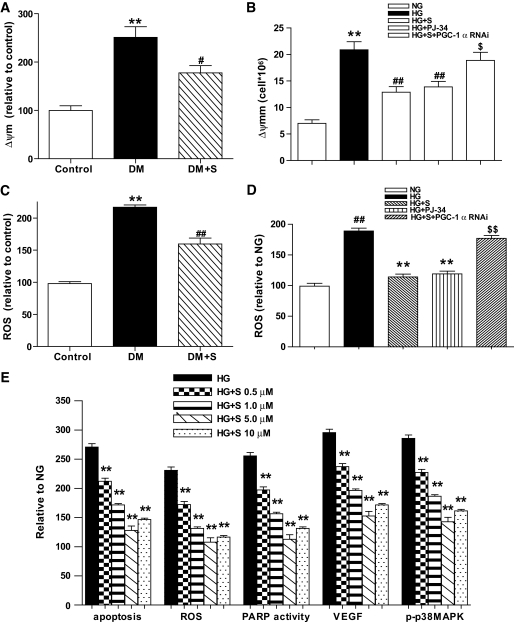
PARP activation is mediated by mitochondrial ROS in vivo and in vitro.
Our study revealed that the production of Δψm and ROS was significantly increased in the retinas of diabetic rats compared with nondiabetic rats (P < 0.01), and simvastatin treatment reduced these effects (Fig. 4A and C). Similarly, we found that in vitro exposure of BRECs to HG increased Δψm, ROS synthesis, and PARP activity, upregulated VEGF, increased p38 MAPK activity, and concomitantly induced the apoptosis of the cells; however, these changes were significantly inhibited by simvastatin (Figs. 3B and C, Fig. 4B and D). Moreover, we observed dosage-dependent effects of simvastatin on cell apoptosis, ROS generation, PARP activity, VEGF, and p-p38MAPK in BRECs in serum-free DMEM containing HG (Fig. 4E). As described in our previous study (23), localization of ROS production was investigated in cultured cells double-labeled with CM-H2DCFDA to detect ROS production and MitoTracker Red CM-H2XRos (MTR; Molecular Probes) to visualize mitochondria. The results of this study indicated that mitochondria are the major source of ROS production after exposure to HG (data not shown).
FIG. 4.
Mitochondrial membrane potential (Δψm) and ROS production (the mitochondria suspensions were diluted to 1 mg protein/ml) in retina or BRECs. A and B: Δ ψm in retina in the control, DM, and DM + S groups (A) or in BRECs in NG, HG, HG + S, HG + PJ-34, and HG + S+ PGC-1α RNAi (B) was examined with the molecular probe JC-1. C and D: ROS production in the retinas of the three groups (C) or in BRECs of the five groups was identified with the fluorescent probe CM-H2DCFDA in BRECs in serum-free DMEM containing HG (D). E: Effects of simvastatin (0, 0.5, 1.0, 5.0, 10.0 μmol/l) on cell apoptosis, ROS, poly(ADP-ribosyl)ated protein, VEGF, and p-p38MAPK in BRECs in serum-free DMEM containing HG. Bars indicate SD. A representative experiment of the three is shown (**P < 0.01 vs. control, #P < 0.05 vs. DM, ##P < 0.01 vs. DM, n = 8; **P < 0.01 vs. NG, ##P < 0.01 vs. HG, $P < 0.05 vs. HG + S, $$P < 0.01 vs. HG + S, n = 9).
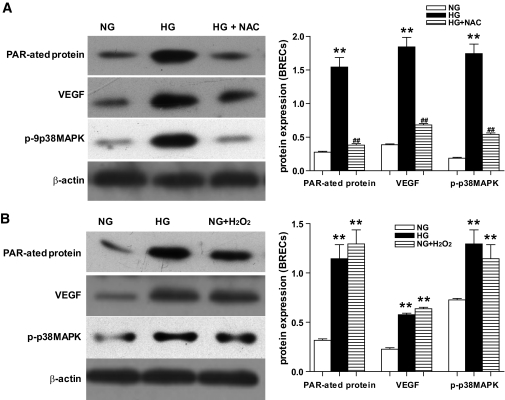
To test our hypothesis that ROS is an upstream molecule of PARP/VEGF/p38MAPK pathway activation under high glucose condition, we employed two approaches: one with ROS scavengers NAC, and the other with exogenous hydrogen peroxide (H2O2) (commonly used on behalf of ROS). First, our results showed that NAC, a ROS scavenger, prevented the activation of PARP, arrested the elevation of VEGF, and inhibited the activation of p38 MAPK induced by hyperglycemia (Fig. 5A). Second, our results indicated that incubation with H2O2 induced the activation of PARP, upregulated VEGF expression, and increased p38 MAPK activity. In addition, the effects were similar to that by HG (Fig. 5B). Our results also demonstrated that the effect of H2O2 on them was both time- and dosage-dependent (data not shown). To rule out the influence of osmolarity on ROS generation, mannitol was used to treat the cells, but we found that mannitol had no effect (data not shown).
FIG. 5.
Representative Western blot analysis of poly(ADP-ribosyl)ated protein expression, VEGF protein expression, and activity of p38 MAPK in BRECs. A and B: poly(ADP-ribosyl)ated protein expression, VEGF protein expression, and activity of p38 MAPK in BRECs in NG, HG, HG + NAC (A), or in NG, HG, and NG + H2O2 (B). Equal protein loading was confirmed by detection of β-actin (**P < 0.01 vs. NG; ##P < 0.01 vs. HG; n = 9).
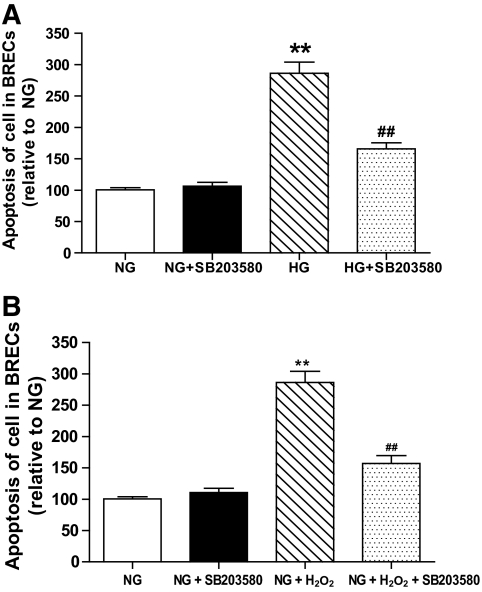
Inhibition of p38 MAPK restores cell survival.
To confirm the role of p38 MAPK activation in the proapoptotic effects of HG or exogenous H2O2, we treated the cells with the specific p38 MAPK inhibitor SB203580 (25 μmol/l) and assessed apoptosis. The p38 MAPK inhibitor did not affect cell death in the normal glucose control, but significantly reduced the apoptosis induced in HG-maintained or exogenous H2O2 cultures (Fig. 6A and B).
FIG. 6.
Observation of cellular apoptosis. A and B: Apoptosis of BRECs in NG, HG, NG + SB203580, and HG + SB203580 (A), and in NG, NG + H2O2, NG + SB203580, and NG + H2O2+ SB203580 (B). (**P < 0.01 vs. NG; #P < 0.05 vs. HG; n = 9).
Simvastatin reduces ROS production and inhibits the activation of its downstream pathway through the PGC-1α pathway.
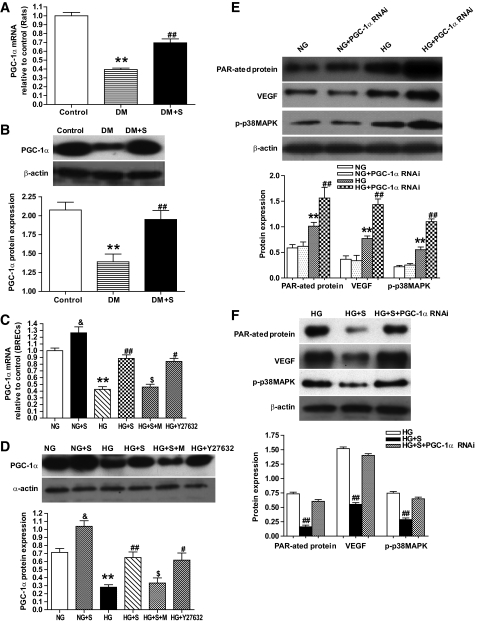
A previous study revealed that PGC-1α is a broad and powerful regulator of ROS metabolism (24). To explore the mechanism underlying the inhibition of mitochondrial ROS by simvastatin, PGC-1α was assayed. In the present study, we first found that PGC-1α mRNA and protein expressions were significantly decreased in the retinas of diabetic rats compared with nondiabetic rats (P < 0.01), and simvastatin treatment inhibited this effect (Fig. 7A and B). In vitro, simvastatin treatment not only inhibited the downregulation of PGC-1α mRNA and protein expression in BRECs induced by HG, but also directly upregulated the expression of PGC-1α mRNA and protein under NG conditions (Fig. 7C and D). Moreover, the effects of simvastatin on PGC-1α expression in BRECs incubated by HG were offset by the addition of mevalonate; however, Y27632, an inhibitor of Rho kinase, inhibited the HG-induced PGC-1α downregulation, and the action was similar to that with simvastatin (Fig. 7C and D).
FIG. 7.
PGC-1α mRNA and protein expression in retina or BRECs. A: Real-time RT-PCR determination of PGC-1α mRNA in control, DM, and DM + S. B: Western blot analysis of PGC-1α protein expression in the three groups. C: Real-time RT-PCR determination of PGC-1α mRNA in BRECs in NG, NG + S, HG, HG + S, HG + S + M, and HG + Y27632. D: Western blot analysis of PGC-1α protein expression in the six groups. E: Western blot analysis of PAR-ated protein, VEGF and p-p38MAPK expression in NG, NG + PGC-1α RNAi, HG, and HG + PGC-1α RNAi. F: Western blot analysis of PAR-ated protein, VEGF, and p-p38MAPK expression in HG, HG + S, and HG + S + PGC-1α RNAi. Bars indicate SD. A representative experiment of the three is shown (**P < 0.01 vs. control; ##P < 0.01 vs. DM; n = 8; &P < 0.05 vs. NG; **P < 0.01 vs. NG; #P < 0.05 vs. HG; ##P < 0.01 vs. HG; $P < 0.01 vs. HG + S, n = 9).
We also found that the effect of simvastatin on Δψm and ROS production in BRECs incubated by hyperglycemia were lost in cells infected with viruses expressing RNAi directed against PGC-1α (Fig. 4B and D). Furthermore, the PGC-1α null cells displayed a higher activation of PARP and p38 MAPK, and upregulation of VEGF induced HG; in contrast, under NG conditions, PGC-1α null cells showed no definitive activation of PARP, p38 MAPK, and VEGF (Fig. 7E and F). These data indicate that PGC-1α null BRECs have a higher activation level of the mitochondrial ROS pathway and a greater sensitivity to oxidative stress induced by HG. Therefore, the inhibition of mitochondrial ROS by simvastatin may be mediated by the PGC-1α pathway.
DISCUSSION
This study demonstrates, for the first time, that simvastatin can decrease the mitochondria-derived ROS generation through the PGC-1α pathway, subsequently downregulating VEGF expression and blocking the activation of p38 MAPK through the inhibition of PARP activity in retinas of diabetic rats and in BRECs incubated with HG. Concomitantly, this leads to the alleviation in retinal vascular permeability and reduction of apoptosis of retinal capillary cells in diabetic rats.
Recent trials revealed lower coronary risk, despite comparable cholesterol levels, in patients treated with statins compared with those in the placebo group (25). Statins inhibit the synthesis of mevalonic acid, a precursor of many nonsteroidal isoprenoid compounds such as farnesylpyrophosphate and geranylgeranylpyrophosphate (26). In endothelial cells, farnesylation and geranylgeranylation are responsible for the translocation of Ras and Rho from the cytoplasm to the membrane, that is, the site of action of these proteins (27). In the present study, we found that simvastatin upregulated PGC-1α expression, subsequently resulting in the decrease of Δψm and ROS production in retina in diabetic rats and in BRECs incubated by hyperglycemia. This action may be executed through the inhibition of the synthesis of mevalonic acid since it was offset by the addition of mevalonate. These results imply that the effect of statins on PGC-1α expression is mediated by the inhibition of the pathway from isoprenoid to small G proteins. This is supported by the observation that Y27632 could mimic the effects of the statins.
The transcriptional coactivator PGC-1α was identified through its functional interaction with the nuclear receptor PPARγ in brown adipose tissue (BAT), a mitochondria-rich tissue specialized for thermogenesis (28). A PPARγ-responsive element is located in the distal region of the PGC-1α gene promoter that binds PPARγ/retinoid X receptor heterodimers (29). In addition to BAT, PGC-1α is strongly expressed in heart, skeletal muscle, kidney, and brain, all of which are highly oxidative tissues (30–32). PGC-1α is highly versatile and has the ability to interact with many different transcriptional factors, including nuclear respiratory factors (NRF-1 and NRF-2), estrogen-related receptor-α, Gabpa/b, PPARs, and the thyroid hormone receptor. These transcriptional factors support mitochondrial biogenesis through the expression of genes encoding proteins involved in oxidative phosphorylation, fatty acid oxidation, heme biosynthesis, and mitochondrial protein import (33).Through binding to and coactivating different transcriptional factors, PGC-1α activates distinct biologic processes in different tissues. For example, when ectopically expressed in fat or muscle cells, PGC-1α strongly promotes increases in mitochondrial DNA as well as expression of a large set of nuclear and mitochondrial-encoded genes (30). Thus, PGC-1α initiates the elevation of mitochondrial biogenesis and oxidative metabolism, participates in the regulation of the downstream steps of this biologic program, and is also involved in pathogenic conditions such as obesity, diabetes, neurodegeneration, and cardiomyopathy (34). In this study, we first demonstrated that PGC-1α was expressed in rat retina and in BRECs, that diabetes and HG downregulate PGC-1α, and furthermore, that simvastatin inhibited these changes. Upregulation of PGC-1α by simvastatin suppressed the production of Δψm and ROS, and the effects of simvastatin on ROS were blocked by PGC-1α RNAi. In addition, PGC-1α null BRECs displayed a greater sensitivity to oxidative stress induced by HG. With respect to the mechanisms underlying the action of PGC-1α on ROS, a previous study demonstrated that PGC-1a induces the classic ROS-scavenging enzymes by an oxidative stressor, and importantly, the induction of anti-ROS genes is not limited to those of the mitochondria; GPx1, catalase, and SOD1 are all present substantially or totally in the nonmitochondrial cytoplasm and peroxisomes (24). Also key is the fact that PGC-1a can regulate the expression of UCP2 and UCP3, both of which are now understood to be important regulators of ROS formation, as we and others have demonstrated (11,24,35).
We also investigated the mechanism underlying the effect of simvastatin on PGC-1a. BRECs were coincubated with mevalonate or Y27632 (an inhibitor of Rho kinase) in the presence of simvastatin. Mevalonate is a precursor for cholesterol and isoprenoid intermediates such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate. The isoprenoids are important lipid moieties added during post-translational modification of a variety of proteins, including G-proteins and G-protein subunits, Ras, and Ras-like proteins such as Rho, Rab, Rae, Ral, or Ra. Statins inhibit the biosynthesis of mevalonate. In this present study, we found that mevalonate prevented the effects of simvastatin, suggesting that the observed effects of HMG-CoA reductase inhibition on ROS are primarily dependent on the absence of mevalonate, but may not be entirely related to cholesterol reduction. We also found that Y27632 inhibits the HG-induced PGC-1α downregulation, similarly to that with simvastatin, indicating that its effect may be through the inhibition of Rho kinase.
As stated in the introduction, HG in the endothelial cells leads to overproduction of mitochondrial ROS, which inactivates glyceraldehyde-3-phosphate dehydrogenase by PARP activation and subsequent ADP ribosylation (14,15). PARP is a family of eukaryotic nuclear enzymes and plays an important role in regulating DNA repair, gene transcription, cell cycle progression, chromatin function, genomic stability, and cell death. However, excessive activation of PARP represents a fundamental step in the pathogenesis of numerous diseases and in diabetic complications (14,15,36). A previous study revealed that PARP activation is involved in diabetes- and hypoxia-induced VEGF production, which is located downstream from sorbitol pathway activation and oxidative stress (37). Recent evidence from our previous study and a study of PARP knockout mice suggests that PARP function is involved in signal transduction, important in enhancing the p38/MAPK signaling in response to HG or inflammatory stimulation (22,38). In this study, our results revealed that PARP activity was increased in the retinal tissues of diabetic rats and simvastatin treatment inhibited PARP activation, downregulated VEGF expression, and reduced the activation of p38 MAPK; the latter finding is consistent with the report indicating that simvastatin attenuates blood retinal barrier breakdown via VEGF suppression (3,6). Moreover, it was found that p38 MAPK activity decreased concomitantly with the retinal vascular cell apoptosis.
In the present study, first, we found that ROS is an upstream molecule of PARP, and this is in agreement with previous studies (14,15) in which NAC, a ROS scavenger, could inhibit hyperglycemia-induced activation of PARP, whereas H2O2 increased PARP and p38 MAPK activity, upregulated VEGF expression, and induced the apoptosis of BRECs. Additionally, we found that VEGF expression and p38 MAPK activity were mediated by PARP activity, as the PARP inhibitor PJ-34 or PARP antisense oligonucleotides downregulate VEGF expression in BRECs (data not shown), and inhibited the activation of p38 MAPK and HUVECs apoptosis (22). Finally, we found that the effects of simvastatin on VEGF expression, p38 MAPK activity, and cell apoptosis were associated with the suppression of PARP activity since the action of simvastatin was similar to that by PJ-34 or PARP antisense oligonucleotides.
VEGF, an angiogenic cytokine secreted by a variety of cells, is a potent survival factor for endothelial cells in vivo and in vitro (7). However, diabetes or HG treatment blocks the prosurvival effect of VEGF and causes accelerated endothelial cell apoptosis via the action of peroxynitrite in causing tyrosine nitration of PI 3-kinase, inhibiting the activity of Akt-1 kinase, and increasing the activity of p38 MAPK (39). In the present study, we found similar results in that HG or H2O2 upregulated VEGF, increased the activity of p38 MAPK, and induced the apoptosis of the endothelial cell (data not shown). Furthermore, the action of simvastatin in the apoptosis of BRECs was similar to that by the specific p38 MAPK inhibitor SB203580. Therefore, the upregulation of VEGF induced by hyperglycemia leads to not only increased vascular permeability, but also to the selective loss of pericytes and formation of acellular capillaries by increasing the activity of p38 MAPK.
In conclusion, simvastatin alleviated retinal vascular permeability and inhibited the apoptosis of retinal capillary cells and formation of acellular capillaries in diabetic rats through the inhibition of VEGF expression and p38 MAPK activity mediated by the PGC-1α/mitochondrial ROS/PARP pathway. These results suggest that statins have potential clinical applications in the prevention and treatment of mitochondrial oxidative stress-related illness such as DR.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Research Fund for the National Nature Science Funding of China (numbers 30871204 and 30872828), National Key Basic Research Program (2010CB535006), and the Funding of Shanghai Key Laboratory for Retinal Disease (number 0904).
No potential conflicts of interest relevant to this article were reported.
Z.Z. and H.C. researched data, contributed to the discussion, and wrote and reviewed/edited the manuscript. H.W., B.K., B.Z., Q.L., P.L., and L.S. researched data and contributed to the discussion. Q.G. researched data. X.X. researched data, contributed to the discussion, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation 2000;101:207–213 [DOI] [PubMed] [Google Scholar]
- 2.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation 2002;105:739–745 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res 2009;89:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev 2005;1:59–63 [DOI] [PubMed] [Google Scholar]
- 5.Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci 2008;49:3231–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol 2004;164:1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998;273:13313–13316 [DOI] [PubMed] [Google Scholar]
- 8.Coughlan MT, Cooper ME, Forbes JM. Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxid Redox Signal 2007;9:331–342 [DOI] [PubMed] [Google Scholar]
- 9.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 2007;27:1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008;57:1446–1454 [DOI] [PubMed] [Google Scholar]
- 11.Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, Gu Q, Xu X, Ho PC. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes 2009;58:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001;50:1938–1942 [DOI] [PubMed] [Google Scholar]
- 13.Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB. Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med 2000;28:91–101 [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 15.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003;112:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 17.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 1961;66:366–378 [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 2001;42:789–794 [PubMed] [Google Scholar]
- 19.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003;300:1574–1577 [DOI] [PubMed] [Google Scholar]
- 20.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett 1997;411:77–82 [DOI] [PubMed] [Google Scholar]
- 21.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Jia W, Xu X, Fan Y, Zhu D, Wu H, Xie Z, Zheng Z. Upregulation of PEDF expression by PARP inhibition contributes to the decrease in hyperglycemia-induced apoptosis in HUVECs. Biochem Biophys Res Commun 2008;369:718–724 [DOI] [PubMed] [Google Scholar]
- 23.Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi R, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 2006;83:807–816 [DOI] [PubMed] [Google Scholar]
- 24.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006;127:397–408 [DOI] [PubMed] [Google Scholar]
- 25.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990;343:425–430 [DOI] [PubMed] [Google Scholar]
- 27.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997;389:990–994 [DOI] [PubMed] [Google Scholar]
- 28.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–839 [DOI] [PubMed] [Google Scholar]
- 29.Hondares E, Mora O, Yubero P, Rodriguez de la Concepcion M, Iglesias R, Giralt M, Villarroya F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology 2006;147:2829–2838 [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115–124 [DOI] [PubMed] [Google Scholar]
- 31.Esterbauer H, Oberkofler H, Krempler F, Patsch W. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 1999;62:98–102 [DOI] [PubMed] [Google Scholar]
- 32.Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol 2000;20:2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A 2009;106:2188–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005;1:361–370 [DOI] [PubMed] [Google Scholar]
- 35.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007;56:2457–2466 [DOI] [PubMed] [Google Scholar]
- 36.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes 2004;53:2960–2967 [DOI] [PubMed] [Google Scholar]
- 37.Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med 2004;14:55–64 [PubMed] [Google Scholar]
- 38.Ha HC. Defective transcription factor activation for proinflammatory gene expression in poly(ADP-ribose) polymerase 1-deficient glia. Proc Natl Acad Sci U S A 2004;101:5087–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.el-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci 2005;118:243–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.