Abstract
Background
Vitamin E supplements may reduce the risk of developing rheumatoid arthritis (RA) through antioxidant effects. While previous observational studies have investigated this question, no randomized trial data are available.
Methods
The Women’s Health Study is a randomized, double-blind, placebo-controlled trial designed to evaluate the benefits and risks of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer among 39,876 female health professionals age 45 years and older throughout the US, conducted between 1992 and 2004. After excluding women with self-reported RA at baseline, 39,144 women were included in the present study. The primary endpoint, definite RA, was confirmed using a connective tissue disease screening questionnaire (CSQ), followed by medical record review for ACR criteria.
Results
During an average follow-up of 10 years, 106 cases of definite RA occurred, 50 in the vitamin E group and 56 in the placebo group. Sixty-four (60%) RA cases were rheumatoid factor positive; 42 (40%) rheumatoid factor negative. There was no significant association between vitamin E and risk of definite RA (relative risk [RR], 0.89; 95% confidence interval, 0.61–1.31). There also were no significant risk reductions for either seropositive (RR, 0.64 (0.39–1.06)) or seronegative RA (RR 1.47 (0.79–2.72)).
Conclusion
600 IU every other day of vitamin E supplements are not associated with a significant reduction in the risk of developing RA among women in a randomized, double-blind, placebo-controlled trial.
Keywords: rheumatoid arthritis, randomized controlled trial, vitamin E, antioxidants
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, affecting approximately 1% of the population.1, 2 Its etiology is unknown but it is presumed to be an immunologic disease with genetic3–7, hormonal8–10 and environmental risk factors such as cigarette smoking.11–13 Oxidative damage has been implicated in the pathogenesis of RA, and free radicals have been found within the rheumatoid synovium14–17 and in the plasma of patients with RA.18 Such molecules may induce endothelial cell damage and promote the production of pro-inflammatory cytokines and adhesion molecules, thereby contributing to the ongoing inflammatory response in RA.
Dietary antioxidant micronutrients act as scavengers of reactive oxygen radicals and may protect against free radical mediated tissue damage in an inflamed joint.19–21 Antioxidants may also protect against the development of RA.22 Vitamin E (α-tocopherol) is the major lipid soluble, chain-breaking antioxidant found in biological membranes. Lipids are important constituents of normal synovial fluid, and alterations in synovial lipids can occur in RA.23 Low levels of vitamin E may therefore have a detrimental effect in inflammatory arthritis. The mean synovial fluid concentrations of α-tocopherol in patients with inflammatory arthritis is significantly lower than that found in controls24–26 suggesting that α-tocopherol is consumed within the inflamed joint. Chronic inflammation can affect serum antioxidant vitamin levels in RA patients,27 with some studies showing that RA patients and children with chronic arthritis have lower serum vitamin E levels than healthy subjects.28–30 Observational case-control and cohort studies suggest an inverse relation between vitamin E and the risk of developing RA,31–37 but the data are inconsistent. No data from randomized trials are available; therefore, to provide further information, we examined the association of vitamin E supplementation and risk of developing RA in the Women’s Health Study randomized trial.
Methods
Study Design and Participants
The Women’s Health Study is a randomized, double-blind, placebo-controlled 2×2 factorial trial designed to evaluate the balance of benefits and risks of low-dose aspirin (100 mg every other day; Bayer Healthcare) and vitamin E (600 IU every other day; Natural Source Vitamin E Association) in the primary prevention of cardiovascular disease and cancer among 39,876 female health professionals (including nurses, physicians, and dentists), aged 45 years and older, throughout the United States and Puerto Rico.38–40 The trial initially contained a beta carotene component, which was terminated after a median treatment duration of 2.1 years after consultation with the Data and Safety Monitoring Board of the study, due to the null findings found in another clinical trial and a suggestion of harmful effects among individuals at high risk for lung cancer in two other trials.41 Written informed consent was obtained from all participants and the trial was approved by the institutional review board of Brigham and Women’s Hospital.
Detailed methods of the trial have been described elsewhere.42, 43 In brief, letters of invitation and baseline questionnaires were sent to 1.7 million female health care professionals between September 1992 and May 1995. A total of 453,787 women completed the questionnaire, and 65,169 women were willing and eligible to participate. Eligibility criteria included: age 45 years or older; no previous history of coronary heart disease, cerebrovascular disease, cancer (except non-melanoma skin cancer), or other major chronic illnesses; no history of side effects from aspirin; no use of aspirin or non-steroidal anti-inflammatory medications (NSAIDs) more than once a week, or willingness to forego their use; no use of anticoagulants or corticosteroids; no use of individual supplements of vitamin A, E, or beta-carotene more than once a week. Eligible women were enrolled into a 3-month run-in period with placebo medications. Following the run-in period, 39,876 women remained willing, eligible and compliant, and were randomly assigned to vitamin E or placebo.
For the present study, we further excluded 732 women who reported prevalent RA on their baseline questionnaires, or who reported RA during follow-up with a diagnosis date that preceded randomization, leaving 39,144 women.
Study Treatment and Follow-up
Every 6 months during the first year and annually thereafter, participants received follow-up questionnaires that asked about compliance with pill taking (including outside use of the study agents), potential adverse effects to the study agents, diagnoses of outcomes of interest including RA, and risk factors, such as cigarette smoking. Study medication and endpoint ascertainment was continued in a blinded fashion through the scheduled end of the trial (March 31, 2004). At the end of the trial, morbidity and mortality follow-up were 97.2% and 99.4% complete, respectively. Compliance, defined as taking at least two-thirds of the study capsules (reported on follow-up questionnaires), was 75.8% averaged throughout the trial, with no difference between active and placebo groups (p=0.64). Non-trial use of individual supplements of vitamin E on ≥4 days/month (”drop-ins”), averaged throughout the trial, was somewhat lower in the active (8.6%) than placebo group (8.9%) (p=0.07).
Confirmation of End Points
When women reported RA, in response to the question, “Have you EVER been diagnosed by a physician as having rheumatoid arthritis?”, we confirmed the diagnosis in a two-stage process. First, they were asked to complete a connective tissue disease screening questionnaire (CSQ)44, 45, that has sensitivity for detecting RA of 85% and specificity of 92%. On the request letter women were asked to check a box if they did not have a diagnosis of RA. Subjects who screened positive for possible RA on the CSQ – at least 3 RA symptoms, or a positive rheumatoid factor – were then asked for release of medical records. Medical records were independently reviewed by two board certified rheumatologists (EWK, NAS) blinded to the second reviewer’s result for American College of Rheumatology (ACR) classification criteria.46, evidence of RA-specific medication treatment, the treating physician’s diagnosis. The reviewers met to discuss and resolve discrepancies and determine a consensus diagnosis. The primary endpoint for the present analysis, definite RA, was defined as occurring in subjects who, on medical record review, met at least 4 ACR criteria for RA (95%), or had confirmed RA based on clinical symptoms, laboratory tests, medication treatment, and the expert reviewers’ consensus (5%). Secondary endpoints included seropositive RA, defined as definite RA with a positive rheumatoid factor test documented in the medical record; seronegative RA, defined as definite RA with a negative rheumatoid factor test; and inflammatory polyarthritis, defined as ≥ 2 ACR criteria for rheumatoid arthritis on medical record review. Additionally, we also examined two other secondary endpoints: RA defined using the CSQ (≥ 3 RA symptoms on the CSQ44, 45, and self-reported RA from the follow-up questionnaires that was not later denied.
Statistical Analysis
Analyses were performed using the SAS statistical software package (release 8.2; SAS Institute Inc, Cary, NC). We used Cox proportional hazards models to calculate relative risks and 95% confidence intervals, comparing event rates in the vitamin E and placebo groups. Models were adjusted for age and the other randomized treatment assignments (aspirin, β carotene). Statistical significance was set at p<0.05, using 2-sided tests.
Results
The mean age of women at randomization was 54.6 (±7) years. Table 1 presents the characteristics of the 39,144 women in the present analysis, according to vitamin E and placebo groups. There were no significant differences among groups with regard to age, smoking, body mass index, reproductive variables, or use of postmenopausal hormones.
Table 1.
Baseline Characteristics of Women by Group, Women’s Health Study
| Characteristic | ||||
|---|---|---|---|---|
| Vitamin E N=19576 | Placebo N=19568 | Total N=39144 | P-value | |
| Age, y | ||||
| Mean (SD) | 54.6 (7) | 54.6 (7) | 54.6 (7) | 0.85† |
| ≤ 54 | 10977 (56) | 10966(56) | 21943(56) | |
| 55–64 | 6629 (34) | 6619 (34) | 13248 (34) | |
| ≥ 65 | 1970 (10) | 1983 (10) | 3953 (10) | |
| Smoking status | ||||
| Never | 9973 (51) | 10017 (51) | 19990 (51) | 0.58‡ |
| Past | 7064 (36) | 6970 (36) | 14034 (36) | |
| Current | 2520 (13) | 2564 (13) | 5084 (13) | |
| Body mass index** | ||||
| Mean (SD) | 26.0 (5.0) | 25.9(5.0) | 26.0 (5.0) | 0.89† |
| < 25 | 9741 (51) | 9808 (51) | 19549(51) | |
| 25–29 | 5943 (31) | 5900 (31) | 11843 (31) | |
| ≥ 30 | 3477 (18) | 3472 (18) | 6949 (18) | |
| Age at menarche, y | ||||
| ≤ 10 | 1601 (8) | 1623 (8) | 3224 (8) | 0.39‡‡ |
| 11 | 3167 (16) | 3162 (16) | 6329(16) | |
| 12 | 5519 (28) | 5559 (28) | 11078 (28) | |
| 13 | 5607 (29) | 5659 (29) | 11266 (29) | |
| ≥ 14 | 3653 (19) | 3547 (18) | 7200(18) | |
| Parity | ||||
| Nulliparous | 2437 (13) | 2524 (13) | 4961 (13) | 0.20‡ |
| Parous | 17047 (87) | 16984 (87) | 34031 (87) | |
| Menopausal status and hormone therapy (HT) | ||||
| Premenopausal | 5406 (28) | 5460 (28) | 10866 (28) | 0.94‡ |
| Uncertain | 3500 (18) | 3511 (18) | 7011 (18) | |
| Postmenopausal – no HT | 3240 (17) | 3185(16) | 6425(16) | |
| Postmenopausal – past HT use | 1438 (7) | 1424(7) | 2862 (7) | |
| Postmenopausal – current HT use | 5943(30) | 5937 (30) | 11880 (30) |
Numbers do not always sum to group totals due to missing information for some variables
Weight in kilograms divided by the square of height in meters
Non-paired T- test
Chi-squared test
Cochran-Armitage trend test
Case Validation
During an average follow-up of 9.99 years (± 1.03 years), 1110 women reported a diagnosis of RA on their follow-up questionnaires. Of these women, 803 (72%) responded to requests for the CSQ. Non-responders had similar age, smoking status, and body mass index to responders. Non-responders were equally likely to have received Vitamin E as placebo (p=0.82). At this second contact, 456 women (41% of all self-reports) denied that they had a diagnosis of RA. Of the 347 responders who did not deny the diagnosis (“unrefuted self-reports”), 177 (51%) screened positive for RA based on the CSQ. Definite RA was confirmed on medical record review in 106 women, yielding an annual incidence rate of 27.1 cases per 100,000 person-years. Of the 106 women with definite RA, 64 (60%) had seropositive RA, and 42 (40%) seronegative RA.
Primary Endpoint
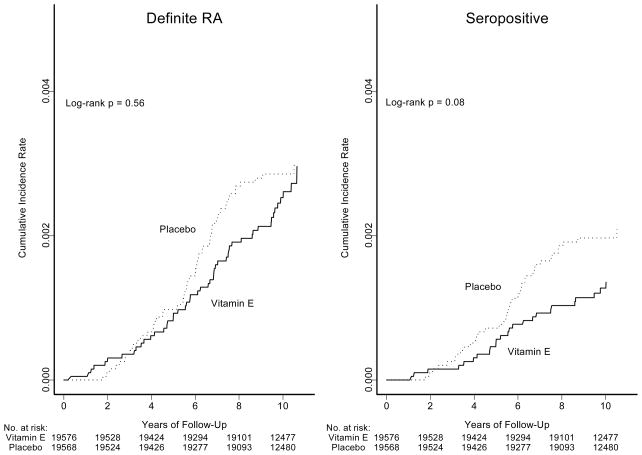
The primary endpoint of this study was definite RA, confirmed on medical record review. The average duration between randomization and the diagnosis of definite RA was 5.8 (SD, 2.4) years. Of the 106 cases of definite RA, 50 occurred in the vitamin E group and 56 in the placebo group (Table 2). This corresponded to a non-significant relative risk (RR) of 0.89 (95% confidence interval [CI], 0.61–1.31; p=0.56) associated with vitamin E. Cumulative incidence rates of definite RA and of seropositive RA over time among women in the two groups are shown in Figure 1. These curves were not significantly different, and the test for non-proportionality over time was not significant for definite RA (p=0.95) or for seropositive RA (p=0.99).
Table 2.
Relative Risks of Rheumatoid Arthritis (RA) by Group, Women’s Health Study
| No.of Events | |||||
|---|---|---|---|---|---|
| Outcome | No.of cases | Vitamin E | Placebo | Relative Risk** (95% Confidence Interval) | P- value |
| Definite RA* | 106 | 50 | 56 | 0.89 (0.61–1.31) | 0.56 |
| Seropositive RA* | 64 | 25 | 39 | 0.64 (0.39–1.06) | 0.08 |
| Seronegative RA | 42 | 17 | 25 | 1.47 (0.79–2.72) | 0.22 |
| Inflammatory polyarthritis† | 134 | 63 | 71 | 0.89 (0.63–1.25) | 0.49 |
| RA defined using CSQ ‡ | 177 | 89 | 88 | 1.01 (0.75–1.36) | 0.94 |
| Self-reported RA‡‡ | 654 | 321 | 333 | 0.96 (0.83–1.12) | 0.63 |
Confirmed on medical record review. Seropositive RA was defined as cases with a positive rheumatoid factor test documented in the medical record.
Cox proportional hazards models adjusted for age at randomization and randomized assignment to aspirin and beta carotene
Defined as ≥ 2 American College of Rheumatology criteria for rheumatoid arthritis46 on medical record review.
Defined as ≥ 4 RA symptoms on the Connective Tissue Disease Screening Questionnaire (CSQ)44
Includes self-reports of RA on the initial questionnaire that were not refuted at the second contact
Figure 1.
Cumulative Incidence Rates of Definite Rheumatoid Arthritis and Seropositive Rheumatoid Arthritis by Randomized Vitamin E Assignment
Secondary Endpoints
Five secondary endpoints were examined in this study: seropositive RA, seronegative RA, inflammatory polyarthritis, RA defined using the CSQ, and self-reported RA. Self-reported RA was defined as those women who reported RA on the yearly questionnaire AND did not refute the diagnosis at the second contact. Vitamin E was not significantly associated with lower risks of either seropositive or seronegative RA (RR 0.64, 0.39–1.06 and 1.47, 0.79–2.72) respectively (Table 2). While there was a marginally significant lower risk for seropositive RA, cumulative incidence curves over time of this endpoint did not differ significantly between the vitamin E and placebo groups (log-rank test, p = 0.08; Figure 1). There also were no significant associations for inflammatory polyarthritis (RR = 0.89 (0.63–1.25)), for RA defined using the CSQ (RR = 1.01 (0.75–1.36) or for RA defined by unrefuted self-report (RR = 0.96 (0.83–1.12).
Discussion
In this large randomized, double-blind, placebo-controlled study of 600 IU of natural source vitamin E on alternate days among 39,876 female health professionals age 45 years and older, we found no significant associations with the primary endpoint, definite RA, or most secondary endpoints, defined using several different case definitions. There was a suggestion of inverse association between vitamin E and seropositive RA that did not reach statistical significance. This finding is of interest since seropositive RA is a more severe phenotype, and epidemiologic risk factors for RA such as cigarette smoking are more strongly associated with seropositive RA than with seronegative RA.13
Previous observational studies have suggested that antioxidants may protect against the development of RA, but the results for individual antioxidants are conflicting. In the Iowa Women’s Health Study, a prospective cohort study, antioxidant intake and risk of elderly onset RA was examined. There was a strong inverse association for β-cryptoxanthin and supplemental zinc, but only a weak inverse association for vitamin C, and no association for vitamin E and risk of RA.35 The risk of inflammatory polyarthritis, a precursor of RA, was studied in another population-based prospective study. Vitamin C demonstrated a strong inverse association with inflammatory polyarthritis, but β-cryptoxanthin and zeaxanthin antioxidants showed only weak inverse associations after adjustment for Vitamin C.36, 37 In a case-control study in which subjects were asked to recall diet from five years prior to the diagnosis of RA, or the reference date for controls, no difference was found in vitamin E intake between 324 incident cases with rheumatoid arthritis and 1,245 population controls.32 However, the reliability and validity of assessing vitamin E intake using a 5-year recall is uncertain.
Biomarkers of antioxidant nutrient intake also have previously been investigated in relation to risk of RA. In a nested case-control study within a Finnish cohort of 18,709 adult men and women who had no history of arthritis at baseline, 122 incident RA cases occurred over seventeen years of follow-up.33 Elevated risk of RA was seen among adults with low serum levels of selenium and α-tocopherol at baseline. In another nested case-control study, serum levels of α-tocopherol, β carotene, and retinol were determined from stored samples from a serum bank of blood donated 2–15 years prior to the onset of RA. Twenty-one incident cases of RA were diagnosed during follow-up, and cases had lower serum concentrations of αtocopherol, β-carotene and retinol at baseline than their matched controls, but the only statistically significant difference was for β-carotene.34 The Women’s Health Study thus adds further information from a randomized, controlled trial demonstrating no significant association between vitamin E supplementation and the primary endpoint, risk of RA.
One limitation of the present study is the relatively small number of incident RA cases, which would limit the power to detect modest reductions in risk, particularly of definite RA (whether total, seropositive, or seronegative) and inflammatory polyarthritis. Previous observational studies have reported a 28–56% reduction in risk of RA with vitamin E supplement use35, or among those with high serum levels.33 Post-hoc power calculations showed that in the present study, with 106 cases of definite RA, we would have 40% power to detect a 30% reduction in risk, and 86% power to detect a 50% reduction in risk. For the other RA endpoints, with larger numbers, power would be accordingly higher. In addition, we ascertained RA cases based on self-reports, a validated screening questionnaire (CSQ), and medical record review, instead of direct history and physical examination. Our use of ACR criteria when reviewing medical records may have resulted in misclassification of some RA cases as non-cases, however, analysis of less specific categories of diagnosis demonstrated consistent null associations. The annual RA incidence rate in this study, 27.1 per 100,000 person-years was substantially lower than rates reported by other studies.1, 2, 33 Incidence rates in the present study may be lower because of the “healthy participant” effect – individuals who choose to participate in such studies tend to be healthier than the general population. In addition, 28% of women who self-reported RA were non-responders after 3 mailings to our request for completing a CSQ. The numbers of definite RA among the non-responders is unknown. However, we did include all of the non-responders in our analysis of self-reported RA where we demonstrated no association with vitamin E. There were a large number of women who initially reported a diagnosis of RA, then refuted this diagnosis upon second contact, which is comparable to a similar study, the Iowa Women’s Health Cohort Study.47 This may be due to diagnoses of possible RA by primary care physicians with subsequent determination by rheumatologists that the symptoms were not due to RA. In the Nurses’ Health Study, only 7% of original self-reports are confirmed as definite RA using similar validation methods, with the great majority of original reports found to be osteoarthritis, not RA upon medical record review.48 We also did not take into account dietary intake of vitamin E or other dietary anti-oxidants. However, because the Women’s Health Study is a randomized clinical trial, we would not expect vitamin E dietary intake to differ between the vitamin E and placebo groups, as supported by the similarity of other potential confounders between these two groups in Table 1. Moreover, the typical dietary intake of vitamin E among women who do not take supplements is 11.4 IU/day, well below the dose given in the trial of 600 IU every other day.49 Finally, the Women’s Health Study comprises female health professionals who are better educated and have higher socioeconomic status compared with the general population. Nevertheless, we would not expect the biological effects of vitamin E to differ among women in different educational and socioeconomic strata.
In conclusion, nutritional factors of interest in the etiology of RA have included fatty acids from oils50, 51, alcohol 52–54, coffee 47, 48, 55, red meat56, 57, and Vitamin D.58 Results have been inconsistent across studies, and it is not clear that diet strongly influences RA risk.59 Despite plausible biological mechanisms, as well as data from some previous observational studies showing that diets high in antioxidants are associated with lower RA risk, the present randomized controlled trial does not show that long-term use of vitamin E supplements significantly decreases the risk of developing the primary endpoint definite RA.
Acknowledgments
Supported by NIH grants HL43851, CA47988, AR47782, AR49880, AR0524
We are indebted to the 39,876 participants in the Women’s Health Study for their dedicated and conscientious collaboration; to the entire staff of the Women’s Health Study, under the leadership of David Gordon, Maria Andrade, Susan Burt, Mary Breen, Marilyn Chown, Lisa Fields-Johnson, Georgina Friedenberg, Inge Judge, Jean MacFadyen, Geneva McNair, Laura Pestana, Philomena Quinn, Claire Ridge, Harriet Samuelson, and Fred Schwerin. We also would like to thank Lynda Rose and Lori Chibnik for programming and statistical support.
References
- 1.Linos A, Worthington JW, O’Fallon WM, Kurland LT. The epidemiology of rheumatoid arthritis in Rochester, Minnesota: a study of incidence, prevalence, and mortality. Am J Epidemiol. 1980;111(1):87–98. doi: 10.1093/oxfordjournals.aje.a112878. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42(3):415–20. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis & Rheumatism. 1987;30(11):1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 4.Begovich AB, Carlton VE, Honigberg LA, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–7. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a Risk Locus for Rheumatoid Arthritis -- A Genomewide Study. N Engl J Med. 2007 doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmers EF, Plenge RM, Lee AT, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357(10):977–86. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plenge RM, Cotsapas C, Davies L, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39(12):1477–82. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlino LA, Cerhan JR, Criswell LA, Mikuls TR, Saag KG. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin Arthritis Rheum. 2003;33(2):72–82. doi: 10.1016/s0049-0172(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 9.Doran MF, Crowson CS, O’Fallon WM, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol. 2004;31(2):207–13. [PubMed] [Google Scholar]
- 10.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50(11):3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 11.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis & Rheumatism. 1999;42(5):910–7. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465–71. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 13.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503, e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Merry P, Winyard PG, Morris CJ, Grootveld M, Blake DR. Oxygen free radicals, inflammation, and synovitis: and synovitis: the current status. Ann Rheum Dis. 1989;48(10):864–70. doi: 10.1136/ard.48.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantapaa-Dahlqvist S, Wallberg-Jonsson S, Dahlen G. Lipoprotein (a), lipids, and lipoproteins in patients with rheumatoid arthritis. AnnRheumDis. 1991;50(6):366–8. doi: 10.1136/ard.50.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winyard PG, Tatzber F, Esterbauer H, Kus ML, Blake DR, Morris CJ. Presence of foam cells containing oxidised low density lipoprotein in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52(9):677–80. doi: 10.1136/ard.52.9.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunec J, Halloran SP, White AG, Dormandy TL. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981;8(2):233–45. [PubMed] [Google Scholar]
- 18.Selley ML, Bourne DJ, Bartlett MR, et al. Occurrence of (E)-4-hydroxy-2-nonenal in plasma and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1992;51(4):481–4. doi: 10.1136/ard.51.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato M, Miyazaki T, Nagaya T, et al. Antioxidants inhibit tumor necrosis factor-alpha mediated stimulation of interleukin-8, monocyte chemoattractant protein-1, and collagenase expression in cultured human synovial cells. J Rheumatol. 1996;23(3):432–8. [PubMed] [Google Scholar]
- 20.Aaseth J, Haugen M, Forre O. Rheumatoid arthritis and metal compounds--perspectives on the role of oxygen radical detoxification. Analyst. 1998;123(1):3–6. doi: 10.1039/a704840h. [DOI] [PubMed] [Google Scholar]
- 21.Edmonds SE, Winyard PG, Guo R, et al. Putative analgesic activity of repeated oral doses of vitamin E in the treatment of rheumatoid arthritis. Results of a prospective placebo controlled double blind trial. Ann Rheum Dis. 1997;56(11):649–55. doi: 10.1136/ard.56.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangge H, Hermann J, Schauenstein K. Diet and rheumatoid arthritis--a review. Scand J Rheumatol. 1999;28(4):201–9. doi: 10.1080/03009749950155553. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto A, Hayashi I, Murakami Y, et al. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J Rheumatol. 2007;34(11):2144–53. [PubMed] [Google Scholar]
- 24.Fairburn K, Grootveld M, Ward RJ, et al. Alpha-tocopherol, lipids and lipoproteins in knee-joint synovial fluid and serum from patients with inflammatory joint disease. Clin Sci (Lond) 1992;83(6):657–64. doi: 10.1042/cs0830657. [DOI] [PubMed] [Google Scholar]
- 25.Wasil M, Hutchison DC, Cheeseman P, Baum H. Alpha-tocopherol status in patients with rheumatoid arthritis: relationship to antioxidant activity. Biochem SocTrans. 1992;20(3):277S. doi: 10.1042/bst020277s. [DOI] [PubMed] [Google Scholar]
- 26.Kiziltunc A, Cogalgil S, Cerrahoglu L. Carnitine and antioxidants levels in patients with rheumatoid arthritis. Scand J Rheumatol. 1998;27(6):441–5. doi: 10.1080/030097498442271. [DOI] [PubMed] [Google Scholar]
- 27.Paredes S, Girona J, Hurt-Camejo E, et al. Antioxidant vitamins and lipid peroxidation in patients with rheumatoid arthritis: association with inflammatory markers. J Rheumatol. 2002;29(11):2271–7. [PubMed] [Google Scholar]
- 28.Honkanen V, Konttinen YT, Mussalo-Rauhamaa H. Vitamins A and E, retinol binding protein and zinc in rheumatoid arthritis [see comments] Clin Exp Rheumatol. 1989;7(5):465–9. [PubMed] [Google Scholar]
- 29.Honkanen VE, Pelkonen P, Konttinen YT, Mussalo-Rauhamaa H, Lehto J, Westermarck T. Serum cholesterol and vitamins A and E in juvenile chronic arthritis. Clin Exp Rheumatol. 1990;8(2):187–91. [PubMed] [Google Scholar]
- 30.Sklodowska M, Gromadzinska J, Biernacka M, et al. Vitamin E, thiobarbituric acid reactive substance concentrations and superoxide dismutase activity in the blood of children with juvenile rheumatoid arthritis. Clin Exp Rheumatol. 1996;14(4):433–9. [PubMed] [Google Scholar]
- 31.Heliovaara M, Knekt P, Aho K, Aaran RK, Alfthan G, Aromaa A. Serum antioxidants and risk of rheumatoid arthritis. Ann Rheum Dis. 1994;53(1):51–3. doi: 10.1136/ard.53.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro JA, Koepsell TD, Voigt LF, Dugowson CE, Kestin M, Nelson JL. Diet and rheumatoid arthritis in women: a possible protective effect of fish consumption. Epidemiology. 1996;7(3):256–63. doi: 10.1097/00001648-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Knekt P, Heliovaara M, Aho K, Alfthan G, Marniemi J, Aromaa A. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology. 2000;11(4):402–5. doi: 10.1097/00001648-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Comstock GW, Burke AE, Hoffman SC, et al. Serum concentrations of alpha tocopherol, beta carotene, and retinol preceding the diagnosis of rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1997;56(5):323–5. doi: 10.1136/ard.56.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157(4):345–54. doi: 10.1093/aje/kwf205. [DOI] [PubMed] [Google Scholar]
- 36.Pattison DJ, Silman AJ, Goodson NJ, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis. 2004;63(7):843–7. doi: 10.1136/ard.2003.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattison DJ, Symmons DP, Lunt M, et al. Dietary beta-cryptoxanthin and inflammatory polyarthritis: results from a population-based prospective study. Am J Clin Nutr. 2005;82(2):451–5. doi: 10.1093/ajcn.82.2.451. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 39.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 40.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 41.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. 1999;91(24):2102–6. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 42.Buring JE, Hennekens CH Group ftWsHSR. The Women’s Health Study: Summary of the study design. J Myocardial Ischemia. 1992;4:27–9. [Google Scholar]
- 43.Buring JE, Hennekens CH Group ftWsHSR. The Women’s Health Study: Rationale and background. J Myocardial Ischemia. 1992;4:30–40. [Google Scholar]
- 44.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 45.Karlson EW, Costenbader KH, McAlindon TE, et al. High sensitivity, specificity and predictive value of the Connective Tissue Disease Screening Questionnaire among urban African-American women. Lupus. 2005;14(10):832–6. doi: 10.1191/0961203305lu2227oa. [DOI] [PubMed] [Google Scholar]
- 46.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 47.Mikuls TR, Cerhan JR, Criswell LA, et al. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum JID -0370605. 2002;46(1):83–91. doi: 10.1002/1529-0131(200201)46:1<83::AID-ART10042>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Karlson EW, Mandl LA, Aweh GN, Grodstein F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. 2003;48(11):3055–60. doi: 10.1002/art.11306. [DOI] [PubMed] [Google Scholar]
- 49.Wu K, Willett WC, Chan JM, et al. A prospective study on supplemental vitamin e intake and risk of colon cancer in women and men. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1298–304. [PubMed] [Google Scholar]
- 50.Linos A, Kaklamanis E, Kontomerkos A, et al. The effect of olive oil and fish consumption on rheumatoid arthritis--a case control study. Scand J Rheumatol. 1991;20(6):419–26. doi: 10.3109/03009749109096821. [DOI] [PubMed] [Google Scholar]
- 51.Linos A, Kaklamani VG, Kaklamani E, et al. Dietary factors in relation to rheumatoid arthritis: a role for olive oil and cooked vegetables? Am J Clin Nutr. 1999;70(6):1077–82. doi: 10.1093/ajcn/70.6.1077. [DOI] [PubMed] [Google Scholar]
- 52.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5(5):525–32. [PubMed] [Google Scholar]
- 53.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. AnnRheumDis. 1990;49(12):980–2. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerhan JR, Saag KG, Criswell LA, Merlino LA, Mikuls TR. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J Rheumatol. 2002;29(2):246–54. [PubMed] [Google Scholar]
- 55.Heliovaara M, Aho K, Knekt P, Impivaara O, Reunanen A, Aromaa A. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann Rheum Dis. 2000;59(8):631–5. doi: 10.1136/ard.59.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattison DJ, Symmons DP, Lunt M, et al. Dietary risk factors for the development of inflammatory polyarthritis: evidence for a role of high level of red meat consumption. Arthritis Rheum. 2004;50(12):3804–12. doi: 10.1002/art.20731. [DOI] [PubMed] [Google Scholar]
- 57.Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2007;9(1):R16. doi: 10.1186/ar2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 59.Pattison DJ, Harrison RA, Symmons DP. The role of diet in susceptibility to rheumatoid arthritis: a systematic review. J Rheumatol. 2004;31(7):1310–9. [PubMed] [Google Scholar]