Abstract
Background
Activation of human basophils results in the release of many different mediators and the expression of new cell surface proteins. The markers CD63 and CD203c have been used in recent years to assess basophil activation but there have been many studies that demonstrate that expression of these markers can be dissociated from histamine release.
Objective
To determine the signal transduction requirements for CD203c and CD63 expression.
Methods
The current study began by exploring the dependency of CD203c and CD63 expression on protein kinase C (PKC) using known selective inhibitors of PKC.
Results
Between 30-300 nM, Ro-31-8220 and Bisindoylmaleimide II had no effect on FMLP- or anti-IgE-induced CD63 or CD203c but enhanced IgE-mediated expression of CD63 by an average of 15 fold at concentrations greater than 1 μM. These results led to the suggestion that these inhibitors altered the normal pathways of degranulation (by a non-PKC dependent mechanism), shifting the normal presence of piecemeal degranulation (PMD) to the process termed anaphylactic degranulation (AND). Morphological studies demonstrated that concentrations of Ro-31-8220 and Bis II >1 μM dramatically increased the presence of degranulation sacs, a morphological feature of AND.
Conclusion
It is proposed that CD63 expression results from only the AND form of histamine release.
Keywords: activation marker, histamine release, calcium, protein kinase C
Introduction
In recent years, new tools have been developed to assess human basophil activation. The basophil is readily available from subjects and provides a sensitive bioassay for the presence of antigen-specific responsiveness in a given subject [1]. Assays which assess the activation of basophils by the changes in cell surface markers are now in routine use [2-12].
The first of these activation markers to be studied in detail was CD63 [13-15]. CD63 has been determined to be a membrane protein of the LAMP family, a family of tetraspanin proteins often found to be involved in vesicle fusion events [16]. Electron micrographs of RBL cells and human basophils suggest that CD63 is associated with histamine containing granules [13-15]. If this association is valid, then the appearance of CD63 would be an indirect measure of histamine granule fusion events and reflect histamine release. However, a variety of studies have dissociated the appearance of CD63 from histamine release [6, 17-19]. This does not invalidate its use as a marker of basophil activation, but it does require that its use as a research tool be given further consideration.
The cell surface marker, CD203c, has been identified as specific for basophils and mast cells [20] among cells of hematopoietic lineage. CD203c is an ecto-nucleotide pyrophosphatase/phosphodiesterase whose purpose is unknown. CD203c is expressed on resting cells at low levels and its expression is rapidly up-regulated following activation. The activation is transient and more rapid than expression of CD63, so assays which use CD203c require careful consideration of the timing for detection, but the selectivity of this marker for basophils (among all circulating leukocytes) make this an attractive marker of activation. With further study, CD203c, like CD63, has been found to not strictly reflect histamine release [5, 8, 21].
A synthesis of the available information on expression of CD63 and CD203c leads to a proposal that as pre-stored markers they are derived from 2 distinct compartments and these compartments are also distinct from histamine containing granules. Table 1 (found in the online repository) summarizes published experience with CD63 and CD203c, as well as histamine release. This table also highlights four distinct patterns of behavior if 3 groups of activation markers are postulated (histamine containing granules, CD63 pre-release storage depots and CD203c pre-release storage depots). This grouping was originally motivated by a recent study by Hennersdorf et al. [5]. These investigators began a classification of basophil activation markers and made the observation that phorbol esters could induce expression of both CD63 and CD203c but with markedly different kinetics. The expression of CD203c was very rapid while expression of CD63 was slow. This result raised the possibility that a mechanistic distinction between expression of these two activation markers may lie in the role of protein kinase C (PKC) during stimulation. Although there is considerable evidence from the study of RBL cells that PKC plays multiple roles, both activating and de-activating during IgE-mediated histamine release [22-24], the supporting evidence for human basophils is weak [25-27]. For some PKC isozymes, translocation to the membrane is a component of the initial steps of their involvement. In human basophils, it was possible to demonstrate translocation of PKC β1, PKC β2 , and PKCδ (human basophils express little or no PKCα) following stimulation with FMLP but not with anti-IgE antibody [27]. More importantly, second and third generation inhibitors of PKC isozymes, did not inhibit IgE-mediated release. A unique characteristic of human basophils, especially in contrast to human mast cells, is their ability to degranulate in response to treatment with phorbol esters alone [28, 29]. However, histamine release is slow to develop, there is no change in cytosolic calcium concentrations following stimulation with PMA (phorbol 12-myristate 13-acetate) [30], and there is no release of LTC4 or IL-4. There is however, very strong translocation of PKC isozymes and inhibition by all classes of PKC inhibitors at appropriate concentrations (IC50 ≈ 100 nM). The observation that expression of CD63 is slow but present [5] puts this activation marker in a class with histamine granules and the rapid expression of CD203c indicates that this process of expression may be sensitive to a different set of PKC isozymes. Since receptor-mediated expression of CD203c requires a calcium response, a synthesis of the observations suggested that expression of CD203c required the activity of a calcium-sensitive PKC isozyme (such as PKCβ1 or β2) but if its activation is strong, such as the strong activation that follows treatment with phorbol esters, then the resting cytosolic calcium levels are sufficient to rapidly drive the activity of the PKC and induce CD203c expression. If expression of CD203c required the activation of an isotype of PKC, then inhibitors of PKC may be found to inhibit CD203c expression while not having an influence on the other two activation-related events. We therefore tested the sensitivity of receptor-mediated CD63 and CD203c expression to selective inhibitors of PKC and made an unexpected observation about the expression of CD63 that may resolve the conflicting observations about its relationship to histamine release.
Materials and Methods
Materials
The following were purchased: PIPES, bovine serum albumin (BSA), EGTA, EDTA, D-glucose, fMLP (formyl-met-leu-phe), phorbol myristic acid (Sigma, St. Louis, MO); crystallized human serum albumin (HSA) (Miles Laboratories, Elkhart, IN); Ro-31-8220 and Bisindolylmaleimide II (Bis II) (Calbiochem, EMD BioSciences, LaJolla, CA); Percoll, (Pharmacia, Piscataway, NJ); anti-CD63 antibody (clone H5C6, BD BioSciences, San Jose, CA) and anti-CD203c (clone NP4D6, BioLegend, San Diego, CA). Goat anti-human IgE Ab was prepared as previously described [31], mouse IgM anti-human IgE, HP6061P (Hybridoma Reagent Laboratory, Baltimore, MD); biotinylated anti-gp120 peptide-specific IgE (a monoclonal antibody that was the gift from Tanox, Inc. (formerly Houston, TX).
Buffers
PIPES-albumin-glucose (PAG) buffer consisted of 25 mM PIPES ((piperazine-N,N-bis-2-ethanesulfonic acid), 110 mM NaCl, 5 mM KCl, 0.1% glucose, and 0.003% HSA. PAGCM was PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2. PAG-EDTA (ethylenediamine N, N, N', N'-tetraacetic acid) consisted of PAG supplemented with 4 mM EDTA, elutration buffer, PAG containing 0.25% BSA. Lactic acid buffer for removing endogenous cell bound IgE; 0.01 M lactic acid, 0.14 M NaCl, 0.005 M KCl, pH 3.9 [31, 32]. PBS was 20 mM sodium phosphate, 140 mM NaCl at pH 7.4.
Histamine Release
Cells (20-30,000 per condition) were challenged in PAGCM buffer for 15-30 minutes at 37°C after which the cells were centrifuged and the supernatant recovered. Histamine was measured in supernatants by automated fluorimetry [33].
Basophil purification
While flow cytometry for CD63 or CD203 could have been performed with whole blood or mixed leukocytes, some of the experiments required purified basophils. Therefore, for consistency between the different types of experiments, all experiments were performed with purified basophils. Residual cells of normal donors undergoing leukapheresis were enriched in basophils using a combination of Percoll density gradients and countercurrent-flow elutriation and negative selection, as previously described [34]. Basophil purities generally exceeded 99% purity. HIPPA regulations do not allow identification or classification of the leukapheresis donors, so information regarding the atopic status of the subjects is not available.
IgE Dissociation and Sensitization
For some of the experiments, basophils were treated with lactic acid buffer (above) for 6 seconds to dissociate endogenous IgE from its receptor. Sensitization was performed at 4°C for 1 hour (5, 2.5, 1.25, 0.625, 0.3125, 0.156, 0.078 μg/ml of biotinylated gp120-specific IgE). Viability after lactic acid-induced dissociation was 92±4%, as determined by erythrocin B exclusion. Spontaneous histamine release in lactic-acid treated cells is not different than non-treated cells, typically less than 5% for non-purified cells and 5-10% for purified cells.
Flow cytometry
Purified basophils were stimulated under various conditions in PAGCM buffer and the reactions stopped by adding an equal volume of 2% paraformaldehyde (in PBS) at 37°C and incubating for 10 minutes at 37°C before quenching with 16 volumes of 4% BSA in PBS. This latter suspension was stored at 4°C until labeling with either CD63 (1/1000 dilution from manufacturer's stock solution) or CD203c (1/500 dilution from manufacturer's stock solution). The primary metric of change in the flow cytometric studies was the difference in the medians of flow cytometric distributions between unstimulated and stimulated cells. For CD203c, this is essentially identical to the net mean metric. But for CD63, the 3 ways of assessing the response, net mean, net median or percent positivity, are not equivalent because of the bimodal nature of the CD63 response. However, to provide some consistency between the CD203c and CD63 measurements, we found the median difference metric useful. In some cases, the data is also discussed in terms of net % positivity with a threshold for positivity set at ca. 5% in the unstimulated cells because this is a metric that is in common use in the CD63 literature.
[Ca2+]i measurements
Basophils were labeled with 4 μM fura-2/AM for 30 min at 37 °C in RPMI 1640 containing 2 % FCS (fetal calf serum)(0.3-0.5×106 cells in 200 μl). Changes in [Ca2+]i (cytosolic free calcium concentration) were determined by digital video microscopy [35, 36]. Data were then obtained for 50 – 150 frames at intervals of 1 to 10 s to determine the [Ca2+]i response. Average of all points was plotted and the variability is shown as a gray region around the mean. For studies of single cells, the time-average of the net change for a 15 minute period was calculated.
Degranulation sac formation
In some cases, the morphological measurements were made on the 350 nm excitation image of fura-2 labeled cells but in the Ro-31-8220 experiments, the cells were labeled for 15 minutes with 4 μM carboxyfluorescein and imaged at 488 nm excitation. The algorithms for quantifying degranulation sac formation have been previously described in detail [17, 37]. Briefly, the images were filtered with a so-called Mexican Hat convolution filter, thresholded to remove non-cell data and contours extracted for both the cell perimeter and internal “holes”. The pixel count of the internal “holes” was stored and for a given cell, the time-average (labeled “cumulative holes”) of the normalized “hole” perimeter (“hole” perimeter/resting cell perimeter) was generated. Each experiment generates a time-average change for 20-40 cells. To average the experiments, the mean change for the 20-40 cells of a particular experiment was calculated and the data re-expressed as a fraction of the mean. In this way, all experiments could be presented in one single cell distribution. For the studies of optimal vs. suboptimal stimulation, the mean for optimal stimulation was used for the calculation of the fractional change. This analytical approach is described in detail in previous publications [17, 37].
Statistics
In general, data is shown as the mean ± standard error of the mean, both in text and figures. In some instances, the figure shows mean±SEM but because the data includes stimulation ± drug, the appropriate statistical analysis uses a paired Student's T-test, i.e., the reported p-values are derived from paired analysis.
Results
Role of PKC: Sensitivity to stimulation and PKC inhibition
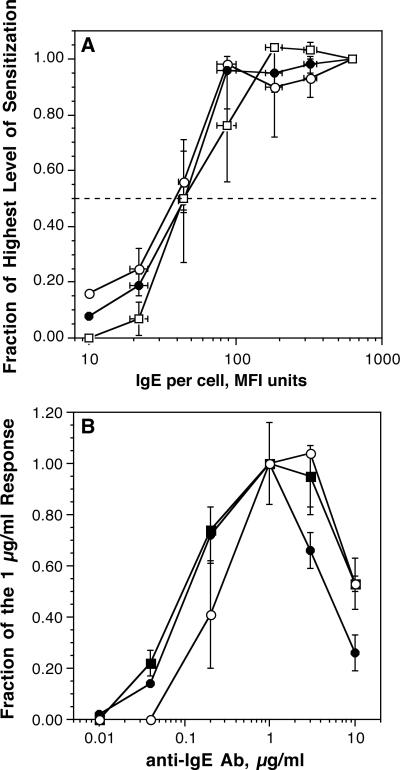
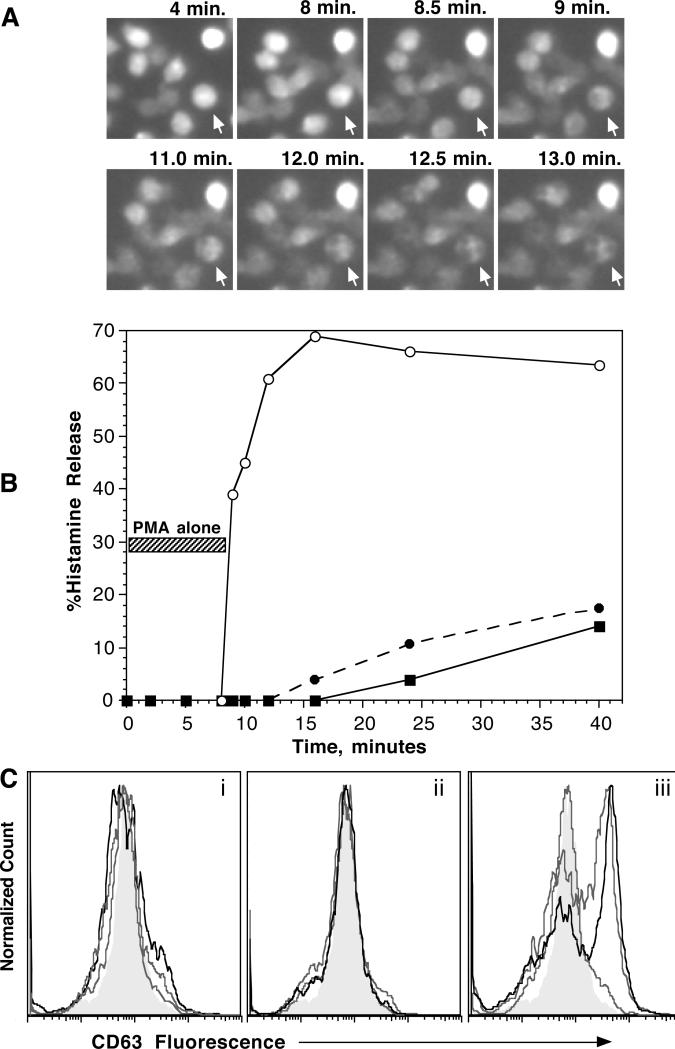
In pilot studies, we verified the results of Hennersdorf et al. [5] and observed that expression of CD203c was rapid relative to the expression of CD63 (see figure 1-OR in the online repository). The distinction between 203c expression kinetics and CD63/histamine release kinetics suggested that the induced expression of CD203c used an activation pathway that was distinct from histamine release and CD63. A difference in activation pathways might be manifested in the relative sensitivity of this activation marker to IgE-mediated activation. One metric to measure sensitivity in basophils is to sensitize basophils with different amounts of antigen-specific IgE and stimulate the cells with a concentration of antigen optimal for histamine release. This metric effectively identifies the number of IgE molecules needed for 50% of the basophils’ maximal response. In the following studies, a biotinylated IgE was used to sensitize basophils and the cells subsequently analyzed by stimulation with streptavidin-alexa647, which is naturally tetrameric. The cells were also analyzed by flow cytometry for the amount of biotnylated IgE bound during sensitization using the same streptavidin-alexa647. Figure 1A shows that there is no difference in the relative sensitivity (EC50) of basophils for all three outcomes, histamine release, CD63 and CD203c expression. However, CD63 expression did drop off more rapidly at the low end of the response.
Figure 1.
Panel A (n=2); Basophils were sensitized with serial concentrations of biotinylated gp-120-specific IgE and challenged with an optimal concentration of streptavidin. Histamine release (○), CD63 (□) and CD203c (•) expression were measured after stimulation for 15 minutes. Panel B (n=4); Basophils were stimulated with the concentrations of polyclonal goat anti-IgE antibody for 15-25 minutes (15 minutes for 3 and 10 μg/ml anti-IgE, 20 minutes for 1 μg/ml anti-IgE antibody and 25 minutes for the remaining concentrations based on kinetic studies of expression rates). For averaging purposes, the data is plotted as a fraction of the response at 1 μg/ml; (•), histamine release, (○), CD63 expression (data analyzed using the ‘net MFI’ metric, see methods), (■), CD203c expression (data analyzed using the ‘net MFI’ metric)
A second way to examine the relative sensitivity of different outcomes is to compare the concentration dependence of the three responses to stimulation with anti-IgE Ab. This has been previously done for CD63 vs. histamine release and some discordance of CD63 and histamine release was observed at supra-optimal concentrations of anti-IgE Ab [17]. This study was repeated using polyclonal anti-IgE Ab and both CD63 and CD203c were measured (figure 1B). As noted previously, at the point where supra-optimal anti-IgE Ab induced a modest decrease in histamine release, CD63 expression was at its optimum. The general effect was to shift the apparent optimum of the concentration-dependence for CD63 expression relative to that for histamine release. Not shown is that CD63 expression at supra-optimal anti-IgE concentrations was faster than at optimal concentrations. Relative to optimal concentrations, supra-optimal concentrations result in a more transient expression of CD63, with the peak time at occurring at 5 minutes (data not shown). The increased rate of expression is a pattern consistent with previous studies of histamine release using supraoptimal concentrations of anti-IgE Ab. The pattern of CD203c expression was intermediate to that of histamine release and CD63 expression.
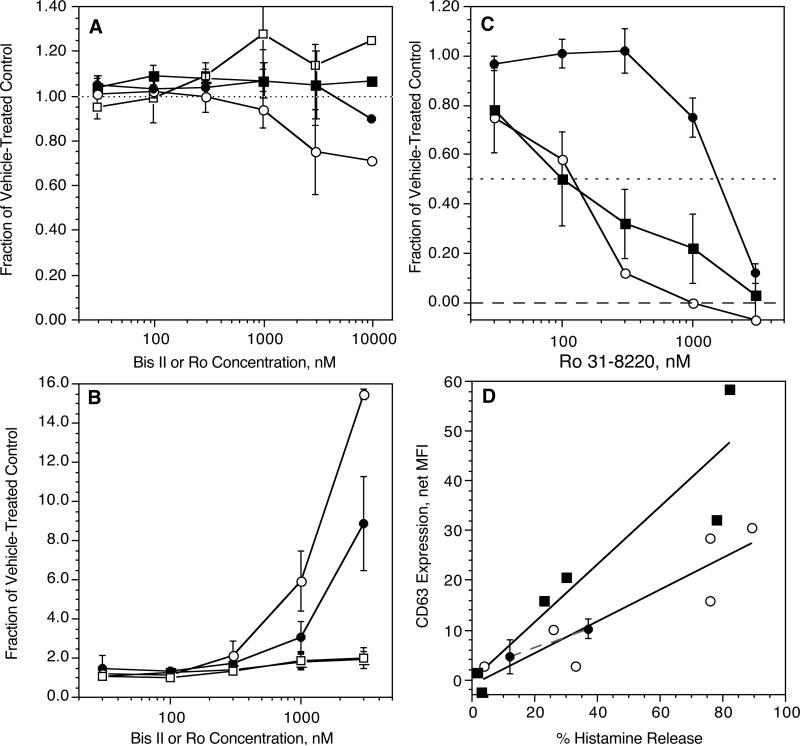
Purified human basophils were then stimulated with an optimal concentration of anti-IgE Ab (0.2 μg/ml) or FMLP (100 nM) in the presence of vehicle control or the PKC inhibitors Bisindoylmaleimide II (Bis II) or Ro 31-8220. Both Ro 31-8220 and Bis II have an IC50of 100 nM for inhibition of 5 different PMA-dependent functions in basophils [27], with maximal inhibition normally apparent at 500 nM. As shown in figure 2A&B, at the expected concentrations, neither inhibitor had significant effects on expression of CD63 or CD203c following stimulation with either FMLP or anti-IgE Ab. There was inhibition of CD63 expression at higher concentrations of Bis II and somewhat less inhibition with Ro-31-8220 with FMLP as the stimulus. A similar pattern was observed for histamine release in previous studies [27]. In contrast, there was enhancement of expression induced by anti-IgE at the higher concentrations of both Bis II and Ro-31-8220. The enhancement of CD203c expression at the highest concentrations was quantitatively similar to those found for histamine release [27] but the levels of enhancement of CD63 expression were marked (12-20 fold). In the region where these inhibitors are considered reasonably selective for PKC isozymes (<500 nM), there was no inhibition of expression of either CD203c or CD63. Figure 2C shows that Ro-31-8220 did not inhibit the PMA- induced expression of CD203c at concentrations below 500 nM while it did inhibit expression of CD63 and histamine release with the expected IC50 of ca. 100 nM. However, it was noted that PMA-induced expression of CD63 seemed quite poor, which made measuring inhibition by Ro-31-8220 difficult. To explore whether the relative expression of CD63 and histamine release using PMA, anti-IgE Ab and FMLP were different, cells were challenged with the three stimuli and both histamine release and CD63 expression determined. Figure 2D shows that qualitatively, the increase in CD63, relative to the histamine release that is induced, was somewhat different for either FMLP or anti-IgE Ab. PMA more closely aligns with the anti-IgE Ab relationship. Despite this rough similarity, PMA induces a qualitatively different flow cytometric profile. The more common pattern for PMA is a general rightward shift of the bulk distribution with only a slight bimodal character. This is in contrast to the mixed bimodal response to either anti-IgE Ab or FMLP (data not shown).
Figure 2.
Inhibition of CD63 and CD203c expression by PKC inhibitors Ro-31-8220 and Bisindolylmalimide II. Panel A (n=3); basophils were incubated with vehicle control (DMSO) or inhibitor for 10 minutes at the concentrations shown, (○) Ro 31-8220 (•) or Bis II and CD63 expression, (□) Ro-31-8220 or (■) Bis II and CD203c expression and then stimulated with 100 nM FMLP for 10 minutes, fixed, and assessed for marker expression. Panel B (n=3); basophils were incubated with vehicle control (DMSO) or inhibitor for 10 minutes at the concentrations shown, (○) Ro 31-8220 (•) or Bis II and CD63 expression, (□) Ro-31-8220 or (■) Bis II and CD203c expression and then stimulated with 0.5 μg/ml anti-IgE Ab for 30 minutes, fixed, and assessed for marker expression. Panel C (n=3); basophils were incubated with vehicle control (DMSO) or Ro-31-8220 for 10 minutes at the concentrations shown, then stimulated with PMA at 50 ng/ml and assessed for (○) CD63 (•) CD203c or histamine release (■). Panel D (n=2); as described in the text, basophils were stimulated with either 3, 10, 100 nM FMLP, 0.01, 0.05 or 0.5 μg.ml anti-IgE Ab or 50 ng/ml PMA in 2 separate experiments and either CD63 or histamine release assessed. The data for each stimulus is plotted with a different symbol and each sample results a separate point on the plot, (■) FMLP, (○) anti-IgE Ab, (•) PMA (with error bars from replication within the experiment). In these figures, the data is based on the net median fluorescence for the marker being examined.
Relationship of CD63 Expression to the Mode of Degranulation
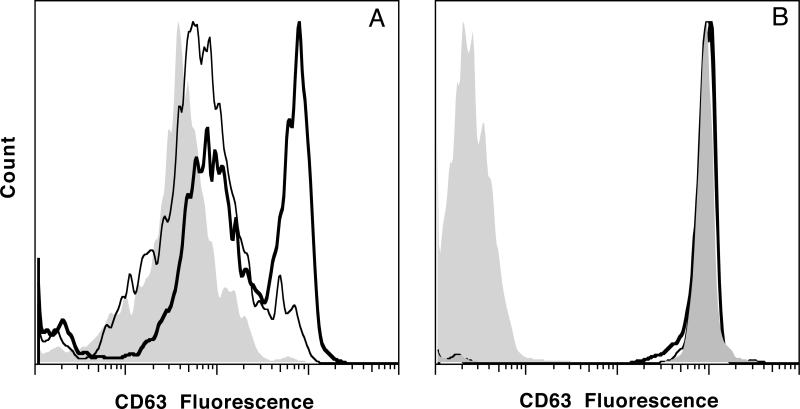
These results suggested that inhibition of PKC, at concentrations considered relatively selective for this family of enzymes (30-300 nM, IC50 ≈ 100 nM), did not distinguish expression of CD203c from either CD63 or histamine release. But at concentrations (>1 μM) where the selectivity of the drugs is in question, the drugs nevertheless revealed a discordance between expression of CD63 and expression of CD203c or histamine release. Focusing on the 3 μM concentration of Ro-81-3220, there is an ca. 2 fold increase in CD203c expression and based on previously published studies of histamine release, an ca. 1.5 fold increase [27]. However, CD63 expression increased ca. 15 fold (when calculated as the net median change, or ca. 5 fold when calculated as the net mean change). Figure 3A shows the flow cytograms for stimulation with anti-IgE Ab with and without 3 μM Ro-31-8220. Figure 3B shows that total CD63, measured in permeabilized cells, does not change with any condition (resting vs stimulated vs. stimulated + Ro-31-8220), i.e., Ro-31-8220 doesn't induce rapid synthesis of CD63. Not shown, but leading to the same conclusion, are similar distributions for all conditions when cells were incubated in the presence of 10 μM cycloheximide to inhibit protein synthesis. The total CD63 exceeds the mean of the right peak observed during stimulation, suggesting that not all internal CD63 is externalized even in these highly expressing cells.
Figure 3.
Example flow cytograms for CD63 expression in basophils stimulated with 0.5 μg/ml 6061P anti-IgE Ab in the presence of vehicle control (DMSO) or Ro-31-8220 at 3 μM (pre-incubation for 10 minutes with drug before stimulation). The samples were fixed after 15 minutes of stimulation. Panel A; the lightest gray distribution is the unstimulated distribution, the thinner black line is the response to anti-IgE Ab with vehicle control, the thicker black-line distribution is the anti-IgE response in the presence of Ro-31-8220. Panel B; using the same conditions of stimulation shown in panel A, the cells were permeabilized during the incubation with anti-CD63 Ab to detect both internal and external CD63 (total). The lightest gray distribution is the isotype control, the darker gray filled-in distribution is the resting cells and overlaid with either thin or thick black lines, but not separable, are the distributions for cells stimulated with anti-IgE Ab ± 3 μM Ro-31-8220.
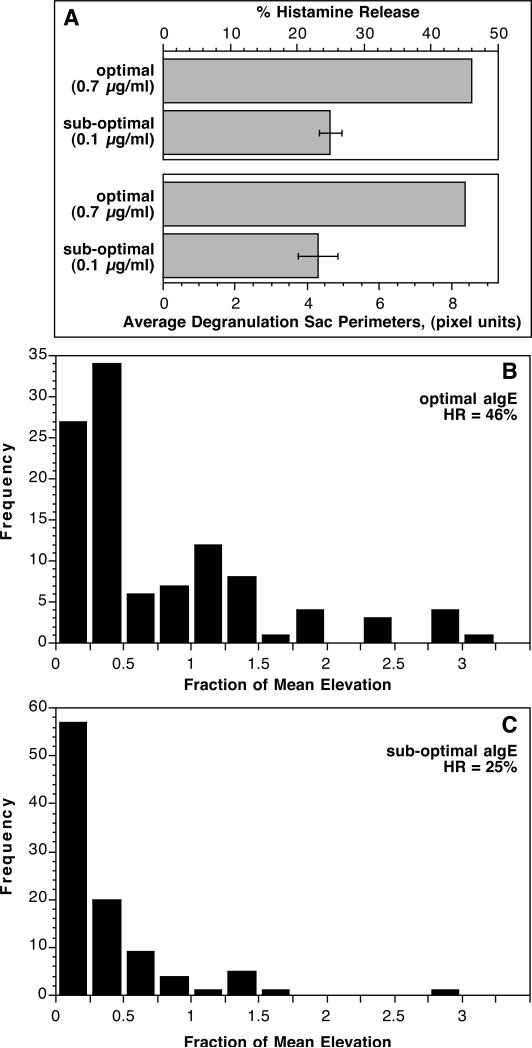
Extensive morphological studies of histamine release from human basophils have identified two pathways for histamine in granules to be transported to the outside of the cell, so-called piecemeal (PMD) and anaphylactic degranulation (AND) [38-44]. In piecemeal degranulation, small vesicles form, or form from the histamine containing granules, and shuttle granule contents to the plasma membrane. In anaphylactic degranulation, the granules fuse with each other and the plasma membrane to expel granule contents. We have previously observed the process of inter-granule fusion and the generation of granule sac formation during measurements of single cell calcium [37]. A metric of degranulation sac formation was developed and described previously [37] but had not been reported with respect to the distributions in populations of single cells. Figure 2-OR in the online repository shows a time lapse example of the formation of these granule sacs during stimulation with FMLP, where they are most apparent. Figure 4A shows that the metric of degranulation sac formation, on average follows histamine release, at least when comparing suboptimal and optimal levels of stimulation. But the single cell behavior is bimodal, with only a fraction of the cells showing significant degranulation sac formation (Figure 4B & C). This skewed distribution had some resemblance to the unique bimodal distribution of CD63 expression and the similarity suggested that there may be a connection between the ability to form degranulation sacs and the ultimate levels of cell surface CD63. The markedly enhanced expression of CD63 in the presence of 3 μM Ro-31-8220 might reflect greater degranulation sac formation. Figures 5A & B show an image of a field of cells stimulated with anti-IgE Ab ± 3 μM Ro 31-8220 and demonstrates a dramatic increase in the degranulation sacs. Not shown in the figure is the observation that these degranulation sacs persist far longer than normally observed for IgE-mediated stimulation. There is great variation in the persistence time but on average an optically identifiable degranulation sac persists for 2-5 minutes when cells are stimulated with anti-IgE Ab without drug. The addition of Ro 31-8220 induces sacs that uniformly persist for >10 minutes. A similar persistence is observed with FMLP-mediated stimulation without drug.
Figure 4.
Panel A; Relationship between histamine release and the average presence of degranulation sacs measured by the algorithm described in the methods (n=3). Panel B; single cell distribution of degranulation sac formation from a basophils stimulated with optimal anti-IgE Ab, histamine release measured from the supernatant of the observation chamber averaged 46±13%. These results were obtained from the same preparations used in panel A. Panel C; single cell distribution of degranulation sac formation from the same preparations of basophils stimulated with sub-optimal anti-IgE Ab, histamine release measured from the supernatant of the observation chamber averaged 25±7%.
Figure 5.
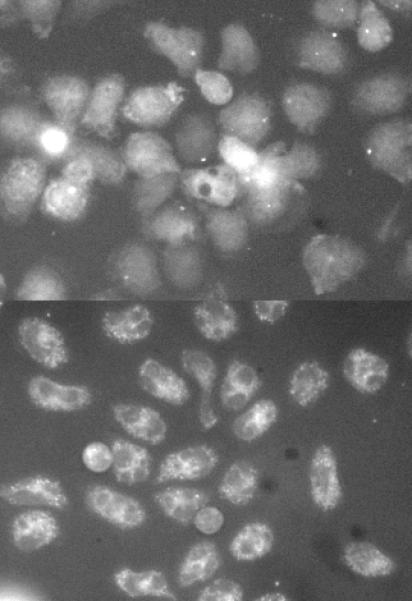
Example images of degranulation sac formation in a field of cells stimulated with anti-IgE Ab (0.5 μg/ml) in the presence of vehicle control (DMSO) or Ro-31-8220 at 3 μM (cells pre-incubated with drug for 10 minutes prior to stimulation. Panel A; anti-IgE Ab with DMSO, image at ca. 10 minutes. Panel B; anti-IgE Ab with Ro-31-8220, image at ca. 10 minutes.
Regulation of the Anaphylactic Degranulation Mechanism
The concentrations of Ro-31-3220 (3 μM) needed to induce this effect suggested that this compound inhibited something besides PKC isozymes. For reasons described in the discussion section, the process of generating optically large degranulation sac is more likely associated with the process of AND rather than PMD. While the specific enzymatic target of the inhibitors is not clear, the effect leads to the suggestion that the drugs alter the nature of histamine release, shifting the response from PMD to AND. A clue to the requirements for inducing AND comes again from studies of PMA-induced histamine release. Studies using electron microscopy suggested that not only does PMA induce small vesicle transport (PMD) but any observed granule fusion appears incomplete [40]. By light microscopy, PMA does not induce significant degranulation sac formation but addition of ionomycin rapidly (within seconds) induces a morphological change that proceeds to degranulation sac formation. Figure 6 demonstrates representative results by three methods. Figure 6A shows the morphological transition, figure 6B shows that rapid acceleration in histamine release and figure 6C shows that change in CD63 expression by flow cytometry. In these experiments, the concentrations of both PMA (10-30 ng/ml) and ionomycin (0.25 μg/ml) were chosen to be suboptimal. In particular, the concentration of ionomycin was chosen to mimic the cytosolic calcium elevations often observed for IgE-mediated secretion. These results suggested that the strength of the cytosolic calcium response may be directing the relative amount of AND vs. PMD. Figure 3-OR in the online repository shows that there is a modest correlation between the magnitude of the anti-IgE-induced cytosolic calcium response and the presence of degranulation sacs in individual cells. The average correlation coefficient for 5 different preparations of basophils was Rs = 0.473 ± 0.112. These experiments led to an experiment to determine if 3 μM Ro 81-3220 enhanced the calcium response to anti-IgE antibody. Lower concentrations of Bis II or Ro-31-8220 have been previously shown to not alter the calcium response [27]. This was true also with the 3 μM concentration where the average calcium elevation was very similar to the vehicle control (with the area under the curve for stimulation in the presence of Ro-31-8220 being 0.81±0.14 of the control (H0 = 1.0; n=3; p=0.14, figure 4-OR in the online repository) (in data not shown, suboptimal stimulation with anti-IgE Ab was also not affected by Ro-31-8220 at 3 μM).
Figure 6.
Ionomycin induces a rapid change in degranulation characterisitics. Panel A; fura-2 labeled basophils were incubated for 8 minutes with 10 ng/ml PMA and after 8 minutes ionomycin at 0.25 μg/ml was added. The images that are selected are at times similar to the those sampled in panels B and C. Panel B: basophils were cultured in PMA at 10 ng/ml (■) or ionomycin at 0.25 μg/ml (•) for various periods of time and the reaction stopped by centrifugation and supernatant recovery. In parallel, a response to PMA at 10 ng/ml was begun and at the 8 minutes time point, ionomycin at 0.25 μg/ml added (○) and samples obtained at various times after the addition of ionomycin. Panel C; a flow cytometric assessment of the rapid change in CD63 expression after the addition of ionomycin to PMA-treated cells. The far left panel (i) shows a progression of times for cells stimulated with PMA at 10 ng/ml; the filled light gray distribution are unstimulated cells at the 15 minute time point, the finest black line distribution is the PMA response at 8 minutes, the next thickest line represents the response after 11 minutes and the thickest black line distribution is the distribution at 15 minutes. A similar time sequence is shown in the middle panel (ii) of Panel C for cells stimulated with 0.25 μg/ml ionomycin and the right panel (iii) shows the distribution for cells stimulated first with PMA for 8 minutes and then ionomycin added at 8 minutes and followed at 11 and 15 minutes.
Discussion
Role of PKC in CD203 and CD63 expression
If PKC isozymes play a role in the activated expression of CD63 or CD203c, the role is modest or balanced by positive and negative effects. Neither PKC inhibitor, at its IC50 for inhibition of PKC (ca. 100 nM), had meaningful effects on expression of either marker. In this respect, the results were similar to those for histamine release at the concentrations where these compounds are considered selective [27]. At higher concentrations, the effects diverge when comparing FMLP vs. anti-IgE Ab, also consistent with histamine release [27]. These effects are probably not related to inhibition of PKC but another serine/threonine kinase or possibly a non-serine/threonine kinase. The absence of an effective inhibition of CD203c expression when stimulating with FMLP or anti-IgE Ab suggests that this activation marker does not normally depend on the activity of a PKC isozyme and that the rapid expression following PMA results from unknown nonspecific effects of this agent. Indeed, the response to PMA doesn't appear to depend on the activation/translocation of PKC since neither PKC inhibitor inhibited this response at concentrations previously shown to inhibit several different endpoints of PMA-induced behavior [27].
Discordance between CD63 Expression and Histamine Release
The marked enhancement of IgE-mediated CD63 expression by relatively high concentrations of Ro-31-8220 was unexpected. On its face, this result provided one more example of discordance between CD63 expression and histamine release. But since CD63 is expressed on granule membranes [13-15], it remains puzzling that CD63 expression and histamine release are not concordant in all situations. However, electron microscopy has revealed the bimodal nature of histamine release [38-44].
Depending on the stimulus, more or less of two forms of granule loss occur in peripheral blood basophils. Anaphylactic degranulation (AND) is characterized by the formation of large degranulation sacs within the cell as granules fuse to each other. It is also characterized by direct granule fusion to the plasma membrane. Piecemeal degranulation (PMD) involves the formation of small vesicles that appear to shuttle granule contents to the plasma membrane. Histamine has been identified in these small granules [44]. The results of this study suggested the hypothesis that anaphylactic degranulation, by allowing direct fusion of granules with the plasma membrane, allows expression of CD63. To complete the proposed hypothesis, the small vesicle transport process characterized as PMD would not also transport the granule membrane proteins like CD63 to the plasma membrane. During IgE-mediated stimulation, electron microscopy has shown that both forms of granule contents loss occur in basophils [45]. In contrast to IgE-mediated and FMLP-mediated degranulation, stimulation with PMA induces predominantly piecemeal degranulation and when granules appear to fuse to the plasma membrane, they produce a so-called forme-fruste fusion, a fusion event that appears incomplete [40].
No previous study has clarified the determinants of the choice between the two forms of granule loss. One of the hallmarks of PMA-induced histamine release is its lack of dependence on an elevation in cytosolic calcium [30]. This result raised the possibility that one of the requirements for effective AND is a cytosolic calcium response. FMLP-induced histamine release appears to start very rapidly (within seconds) and although it appears to begin with PMD, the mode of degranulation rapidly switches to AND [39]. Between the two types of receptor-mediated stimulation, FMLP and anti-IgE, only FMLP induces measurable and rapid translocation of PKC isozymes [27]. However, FMLP also induces a more marked and rapid cytosolic calcium response than IgE-mediated release [30]. Indeed, the peak of the cytosolic calcium response occurs at a time when the initial PMD form of release converts to AND. The peak calcium response is also 2-3 times greater than observed for IgE-mediated release [30]. Taking the various results together, the inability of PKC inhibitors to further enhance FMLP-induced CD63 expression may result from the strong cytosolic calcium response already driving a predominantly AND process of histamine release, but this would need further study.
While the initial peak increase in cytosolic calcium following FMLP stimulation is very strong, IgE-mediated stimulation can also induce strong initial responses. However, these strong responses most readily occur with a polyclonal anti-IgE antibody used at concentrations considered supra-optimal for histamine release [30]. Below optimal concentrations of anti-IgE Ab, the calcium responses are asynchronous between cells and oscillatory in single cells [35] (figures 1A&B show the relatively weak CD63 responses at low levels of stimulation). Therefore, the relatively weaker levels of AND and levels of CD63 expression in basophils stimulated with anti-IgE Ab vs. FMLP (see figure 2D) are consistent with a hypothesis that the strength of cytosolic calcium response regulates the expression of AND and CD63. Figure 3-OR in the online repository shows that for IgE-mediated release, there is a modest correlation between the appearance of degranulation sacs (a hallmark of AND [39, 46]), measured as fluorescence-free regions, (or “holes”) in the basophil and the single cell calcium response. The enhanced rate of both histamine release and CD63 expression, as well as the greater expression of CD63, when using supra-optimal concentrations of polyclonal anti-IgE Ab is also consistent with the enhanced calcium response in this region of the dose-response curve. The high concentration of Ro-31-8220, on average, did not increase the cytosolic calcium response. Therefore, the effect of the PKC inhibitors on the IgE-mediated CD63 response does not appear to result from changes in the levels of cytosolic calcium.
Nevertheless, stimulation in the presence of Ro-31-8220 dramatically enhances the presence of these degranulation sacs. These data suggest that high concentrations of PKC inhibitors, while not necessarily operating on a PKC, inhibit a process that normally limits the cell's ability to engage in the formation of inter-granule fusion and thereby limits the formation of large degranulation sacs. If previous EM studies are a guide and the formation of large degranulation sacs is an indication of the process of AND and the direct granule fusion to the plasma membrane that follows, then the increased expression of granule-associated CD63 on the cell surface might be an expected consequence.
Consistency of the Hypothesis
The results lead to the hypothesis that at any given moment, the total amount of histamine released is the sum of the two processes, AND and PMD and that CD63 expression associates more with the AND form of release. This hypothesis begs a re-analysis of the various published studies of CD63 expression. In table 1 of the online repository, the various studies that help explore mechanisms have been summarized. This table was organized (the table shows four categories of characteristics) with the hypothesis that CD203c, CD63 and histamine reside in different compartments and based on the divisions shown in table 1, there are examples that support this division of compartments. The results of the current study would create a fifth column type in this table, weak to modest enhancement of IgE-mediated histamine release and CD203c expression and marked enhancement of CD63 expression. However, the results of this study's experiments suggest that it may be possible to collapse the number of compartments to two, grouping CD63 and granules into one compartment but with two methods of expressing this combined compartment. This proposed hypothesis still allows histamine release to occur by the PMD mechanism, which would allow little or no CD63 expression.
However, a result that would falsify the hypothesis would be the activated expression of CD63 in the complete absence of histamine release. The only data in the current study that takes a step in this direction is the discordance between CD63 expression and histamine release on the supraoptimal side of the anti-IgE dose response curve, but the difference isn't great enough to draw a strong conclusion. A survey of the literature does raise other possible situations where CD63 expression may occur without evident histamine release. One notable example is the expression of CD63 on apparently resting basophils [47]. If the resting state of expression were the result of some in vivo activation of basophils and not the result of another mechanism of expression this observation might reveal a problem with the hypothesis. However, the problem with this interpretation of the available data is that one cannot know if the circulating basophil, with its history both in the circulation and in the bone marrow during maturation, had not experienced some histamine release (there being no context for evaluating the histamine content of the circulating basophils unless it were extremely reduced). Indeed, recent studies have raised the possibility of chronic aggregation during maturation [48]. There is a study of anti-CD63 antibodies that inhibit IgE-mediated secretion [47]. In this study, pre-treatment of adherent RBL cells with anti-CD63 antibodies inhibited subsequent IgE-mediated release. This may work because there is resting expression of CD63 on the RBL cells [49]. These results have not been replicated on human basophils, so their relevance to the hypothesis being proposed is not clear. A study of honey bee venom PLA2-induced activation of basophils suggested that modest CD63 expression on basophils occurred without histamine release [50]. However, the flow cytometric profile of CD63 expression observed in this study was not bimodal and appeared similar to our experience with PMA. It was also a very modest expression of CD63. It may be that the various roles of CD63 preclude its presence in only a single compartment that is the granule. If there were other compartments for its expression, such a possibility would make it difficult to falsify the hypothesis without a detailed study by electron microscopy. Indeed, validation of the proposed association between AND, but not PMD, with CD63 expression will require a subtle application of electron microscopy, dual labeling of CD63 and histamine, and morphometry.
A synthesis of various observations regarding the nature of histamine release in human basophils led to the proposal that piecemeal and anaphylactic degranulation result in different levels of CD63 expression. The results suggest that two factors may regulate the choice between AND and PMD, the magnitude of the cytosolic calcium response and the activity of an unknown kinase that is sensitive to high concentrations of either Bis II or Ro-31-8220.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health grant AI20253. I would like to thank Valerie Alexander for her technical assistance in these studies.
References
- 1.Lichtenstein LM, Osler AG. Studies on the Mechanisms of Hypersensitivity Phenomena. Ix. Histamine Release from Human Leukocytes by Ragweed Pollen Antigen. J Exp Med. 1964;120:507–30. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, Sainte-Laudy J. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008;146:177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 3.Sudheer PS, Hall JE, Read GF, Rowbottom AW, Williams PE. Flow cytometric investigation of peri-anaesthetic anaphylaxis using CD63 and CD203c. Anaesthesia. 2005;60:251–6. doi: 10.1111/j.1365-2044.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- 4.Sonneck K, Baumgartner C, Rebuzzi L, Marth K, Chen KW, Hauswirth AW, Florian S, Vrtala S, Buhring HJ, Valenta R, Valent P. Recombinant allergens promote expression of aminopeptidase-n (CD13) on basophils in allergic patients. Int J Immunopathol Pharmacol. 2008;21:11–21. doi: 10.1177/039463200802100103. [DOI] [PubMed] [Google Scholar]
- 5.Hennersdorf F, Florian S, Jakob A, Baumgartner K, Sonneck K, Nordheim A, Biedermann T, Valent P, Buhring HJ. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- 6.Aerts NE, Dombrecht EJ, Bridts CH, Hagendorens MM, LS de Clerck, Stevens WJ, Ebo DG. Simultaneous flow cytometric detection of basophil activation marker CD63 and intracellular phosphorylated p38 mitogen-activated protein kinase in birch pollen allergy. Cytometry B Clin Cytom. 2008;76B:8–17. doi: 10.1002/cyto.b.20437. [DOI] [PubMed] [Google Scholar]
- 7.Apostolou E, Deckert K, Puy R, Sandrini A, de Leon MP, Douglass JA, Rolland JM, O'Hehir RE. Anaphylaxis to Gelofusine confirmed by in vitro basophil activation test: a case series. Anaesthesia. 2006;61:264–8. doi: 10.1111/j.1365-2044.2005.04529.x. [DOI] [PubMed] [Google Scholar]
- 8.Lourenco FD, Azor MH, Santos JC, Prearo E, Maruta CW, Rivitti EA, Duarte AJ, Sato MN. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br J Dermatol. 2008;158:979–86. doi: 10.1111/j.1365-2133.2008.08499.x. [DOI] [PubMed] [Google Scholar]
- 9.Chirumbolo S, Vella A, Ortolani R, De Gironcoli M, Solero P, Tridente G, Bellavite P. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy. 2008;6:12. doi: 10.1186/1476-7961-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, Yamaguchi M, Fujisawa T. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146(Suppl 1):47–53. doi: 10.1159/000126061. [DOI] [PubMed] [Google Scholar]
- 11.Buhring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–29. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- 12.Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005;3:9. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knol EF, Mul FPJ, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–338. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 14.Furuno T, Teshima R, Kitani S, Sawada J, Nakanishi M. Surface expression of CD63 antigen (AD1 antigen) in P815 mastocytoma cells by transfected IgE receptors. Biochem Biophys Res Commun. 1996;219:740–4. doi: 10.1006/bbrc.1996.0304. [DOI] [PubMed] [Google Scholar]
- 15.Amano T, Furuno T, Hirashima N, Ohyama N, Nakanishi M. Dynamics of intracellular granules with CD63-GFP in rat basophilic leukemia cells. J Biochem. 2001;129:739–44. doi: 10.1093/oxfordjournals.jbchem.a002914. [DOI] [PubMed] [Google Scholar]
- 16.Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC-peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev. 2002;189:136–51. doi: 10.1034/j.1600-065x.2002.18912.x. [DOI] [PubMed] [Google Scholar]
- 17.MacGlashan DW., Jr. Graded changes in the response of individual human basophils to stimulation: Distributional behavior of events temporally coincident with degranulation. J. Leukocyte Biology. 1995;58:177–188. doi: 10.1002/jlb.58.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, Buhring HJ, Valenta R, Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs BF, Plath KE, Wolff HH, Grabbe J. Regulation of mediator secretion in human basophils by p38 mitogen-activated protein kinase: phosphorylation is sensitive to the effects of phosphatidylinositol 3-kinase inhibitors and calcium mobilization. J Leukoc Biol. 2002;72:391–400. [PubMed] [Google Scholar]
- 20.Buhring HJ, Simmons PJ, Pudney M, Muller R, Jarrossay D, van Agthoven A, Willheim M, Brugger W, Valent P, Kanz L. The monoclonal antibody 97A6 defines a novel surface antigen expressed on human basophils and their multipotent and unipotent progenitors. Blood. 1999;94:2343–56. [PubMed] [Google Scholar]
- 21.Monneret G, Boumiza R, Gravel S, Cossette C, Bienvenu J, Rokach J, Powell WS. Effects of prostaglandin D(2) and 5-lipoxygenase products on the expression of CD203c and CD11b by basophils. J Pharmacol Exp Ther. 2005;312:627–34. doi: 10.1124/jpet.104.074823. [DOI] [PubMed] [Google Scholar]
- 22.Rivera J, Beaven MA. Regulation of secretion from secretory cells by protein kinase C. In: Parker P, Dekker L, editors. Protein Kinase C. Landes Company; Austin. TX: 1997. pp. 131–164. [Google Scholar]
- 23.Chang EY, Szallasi Z, Acs P, Raizada V, Wolfe PC, Fewtrell C, Blumberg PM, Rivera J. Functional effects of overexpression of protein kinase C-α, –β, –δ, –ε, –η in the mast cell line RBL-2H3. J. Immunol. 1997;159:2624–2632. [PubMed] [Google Scholar]
- 24.Song JS, Swann PG, Szallasi Z, Blank U, Blumberg PM, Rivera J. Tyrosine phosphorylation-dependent and -independent associations of protein kinase C-delta with Src family kinases in the RBL-2H3 mast cell line: regulation of Src family kinase activity by protein kinase C-delta. Oncogene. 1998;16:3357–68. doi: 10.1038/sj.onc.1201886. [DOI] [PubMed] [Google Scholar]
- 25.Knol EF, Koenderman L, Mul FPJ, Verhoeven AJ, Roos D. Differential activation of human basophils by anti-IgE and formyl-methionyl-leucyl-phenylalanine. Indications for protein kinase C-dependent and -independent activation pathways. Eur. J. Immunol. 1991;21:881–5. doi: 10.1002/eji.1830210404. [DOI] [PubMed] [Google Scholar]
- 26.Warner JA, MacGlashan DW., Jr. Protein kinase C (PKC) changes in human basophils. IgE-mediated activation is accompanied by an increase in total PKC activity. J Immunol. 1989;142:1669–77. [PubMed] [Google Scholar]
- 27.Miura K, MacGlashan DW., Jr. Expression of protein kinase C isozymes in human basophils: Regulation by physiological and non-physiological stimuli. Blood. 1998;92:1206–1218. [PubMed] [Google Scholar]
- 28.Schleimer RP, Gillespie E, Lichtenstein LM. Release of histamine from human leukocytes stimulated with the tumor promoting phorbol esters. I. Characterization of the response. J. Immunol. 1981;126:570–574. [PubMed] [Google Scholar]
- 29.Massey WA, Cohen VL, MacGlashan DW, Jr., Gittlen SW, Kagey-Sobotka A, Lichtenstein LM, Warner JA. Protein Kinase C modulates IgE-mediated activation of human mast cells from lung and skin. I. Pharmacologic inhibition. J Pharm Exp Ther. 1991;258:824–829. [PubMed] [Google Scholar]
- 30.Warner JA, MacGlashan DW., Jr. Signal transduction events in human basophils - A comparative study of the role of protein kinase-C in basophils activated by anti-IgE antibody and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1990;145:1897–1905. [PubMed] [Google Scholar]
- 31.MacGlashan DW, Jr, Peters SP, Warner J, Lichtenstein LM. Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol. 1986;136:2231–9. [PubMed] [Google Scholar]
- 32.Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983;131:1949. [PubMed] [Google Scholar]
- 33.Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–94. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 34.MacGlashan DW, Jr, White JM, Huang SK, Ono SJ, Schroeder J, Lichtenstein LM. Secretion of interleukin-4 from human basophils: The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- 35.MacGlashan DW, Jr., Guo CB. Oscillations in free cytosolic calcium during IgE-mediated stimulation distinguish human basophils from human mast cells. J Immunol. 1991;147:2259–2269. [PubMed] [Google Scholar]
- 36.MacGlashan DW, Jr, Botana L. Biphasic Ca++ responses in human basophils: Evidence that the initial transient elevation associated with mobilization of intracellular calcium is an insufficient signal for degranulation. J Immunol. 1993;150:980–991. [PubMed] [Google Scholar]
- 37.MacGlashan DW, Jr, Bochner B, Warner JA. Graded changes in the response of individual human basophils to stimulation: Distributional behavior of early activation events. J Leuk Biol. 1994;55:13–23. doi: 10.1002/jlb.55.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Dvorak AM, Galli SJ, Schulman ES, Lichtenstein LM, Dvorak HF. Basophil and mast cell degranulation: ultrastructural analysis of mechanisms of mediator release. Fed Proc. 1983;42:2510–5. [PubMed] [Google Scholar]
- 39.Dvorak AM, Warner JA, Kissell S, Lichtenstein LM, MacGlashan DW., Jr F-met peptide-induced degranulation of human basophils. Lab Invest. 1991;64:234–253. [PubMed] [Google Scholar]
- 40.Dvorak AM, Warner JA, Morgan E, Kissell-Rainville S, Lichtenstein LM, MacGlashan DW., Jr An ultrastructural analysis of tumor-promoting phorbol diester-induced degranulation in human basophils. Am. J. Pathology. 1992;141:1309–1322. [PMC free article] [PubMed] [Google Scholar]
- 41.Dvorak AM. Basophils and mast cells: Piecemeal degranulation in situ and ex vivo: A possible mechanism for cytokine-induced function in disease. In: Coffey RG, editor. Granulocyte Responses to Cytokines. Marcel Dekker; New York: 1992. [PubMed] [Google Scholar]
- 42.Dvorak AM, Morgan ES, Lichtenstein LM, MacGlashan DW., Jr Activated human basophils contain histamine in cytoplasmic vesicles. Int Arch Allergy Immunol. 1994;105:8–11. doi: 10.1159/000236796. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak AM, MacGlashan DW, Jr, Morgan ES. Lichtenstein LM. Histamine distribution in human basophil secretory granules undergoing FMLP-stimulated secretion and recovery. Blood. 1995;86:3560–3566. [PubMed] [Google Scholar]
- 44.Dvorak AM, MacGlashan DW, Jr., Morgan ES, Lichtenstein LM. Vesicular transport of histamine in stimulated human basophils. Blood. 1996;88:4090–4101. [PubMed] [Google Scholar]
- 45.Dvorak AM, Schroeder JT, MacGlashan DW, Jr, Bryan KP, Morgan ES, Lichtenstein LM, MacDonald SM. Comparative ultrastructural morphology of human basophils stimulated to release histamine by anti-IgE, recombinant IgE-dependent histamine-releasing factor, or monocyte chemotactic protein-1. J Allergy Clin Immunol. 1996;98:355–370. doi: 10.1016/s0091-6749(96)70160-4. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak AM. Blood Cell Biochemistry. Vol. 4. Plenum Press; New York: 1991. Basophil and mast cell Degranulation and recovery. [Google Scholar]
- 47.Kraft S, Fleming T, Billingsley JM, Lin SY, Jouvin MH, Storz P, Kinet JP. Anti-CD63 antibodies suppress IgE-dependent allergic reactions in vitro and in vivo. J Exp Med. 2005;201:385–96. doi: 10.1084/jem.20042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishmael SS, MacGlashan DW., Jr Syk expression in peripheral blood leukocytes, CD34+ Progenitors and CD34-derived Basophils. J Leukoc Biol. 2010;87:291–300. doi: 10.1189/jlb.0509336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishikata H, Oliver C, Mergenhagen SE, Siraganian RP. The rat mast cell antigen AD1 (homologue to human CD63 or melanoma antigen ME491) is expressed in other cells in culture. J Immunol. 1992;149:862–70. [PubMed] [Google Scholar]
- 50.Mustafa FB, Ng FS, Nguyen TH, Lim LH. Honeybee venom secretory phospholipase A2 induces leukotriene production but not histamine release from human basophils. Clin Exp Immunol. 2008;151:94–100. doi: 10.1111/j.1365-2249.2007.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.