Abstract
Blood vessels have long been known to respond to hemodynamic force, and several mechanotransduction pathways have been identified. However, only recently have we begun to understand the effects of hemodynamic force on embryonic development. In this review, we will discuss specific examples illustrating the role of hemodynamic force during the development of the embryo, with particular focus on the development of the vascular system and the morphogenesis of the heart. We will also discuss the important functions served by mechanotransduction and hemodynamic force during placentation, as well as in regulating the maintenance and division of embryonic, hematopoietic, neural, and mesenchymal stem cells. Pathological misregulation of mechanosensitive pathways during pregnancy and embryonic development may contribute to the occurrence of cardiovascular birth defects, as well as to a variety of other diseases, including preeclampsia. Thus, there is a need for future studies focusing on better understanding the physiological effects of hemodynamic force during embryonic development and their role in the pathogenesis of disease.
Keywords: Hemodynamic force, vascular development, cardiac morphogenesis, hematopoiesis, stem cells
Mechanisms of mechanotransduction
The idea that the physical forces imparted by flowing blood play important physiological roles was first postulated over a century ago by Thoma [136]. His experiments demonstrated that blood vessels morphologically remodel over time and either widen or regress in order to adapt to the amount of flow that they carry [59, 122, 136]. Early explanations of this phenomenon by Murray and others held that vessels do this by sensing shear stress in order to remodel and change size as heart rates and blood volumes change [85, 105]. The basic premise of Murray’s law is that the cost of moving blood can be minimized by expanding the diameter of the vessels and is balanced by the metabolic cost of enlarging the vessels and of blood production itself [85, 105, 116]. Murray’s law has now been validated in many systems, from mammals to sponges, but how these principles influence morphogenesis in developing vascular systems is just beginning to be understood [57, 58, 105, 116].
The molecular basis of mechanosensitivity
Flowing blood exerts several forces on vessels (Figure 1A). These include: the normal force exerted on the vessel wall by blood pressure; the associated circumferential stress that occurs as the vessel stretches in response to that pressure; and finally, the frictional force exerted by flowing blood as it drags along the vessel wall, also known as shear stress [15]. By virtue of their direct contact with flowing blood, vascular endothelial cells, which comprise the inner layer of blood vessels, are able to sense these forces, and are thought to do so by making use of a variety of membrane-localized molecules [15, 61, 96]. These endothelial cells then respond to such force by initiating cytoskeletal rearrangements that help them align in a direction parallel with flow [15]. When exposed to laminar flow, in which fluid flows in parallel layers and in a steady and orderly fashion, endothelial cells also downregulate genes that promote proliferation and the inflammatory response [15]; however, disturbed flow, in which fluid flows in a disorderly fashion, is known to have the opposite effect and induces increased proliferation [15, 19]. Particular levels of cyclic pressure have also been shown to upregulate endothelial cell proliferation in a VEGF-C (vascular endothelial growth factor-C) dependent manner [118, 119].
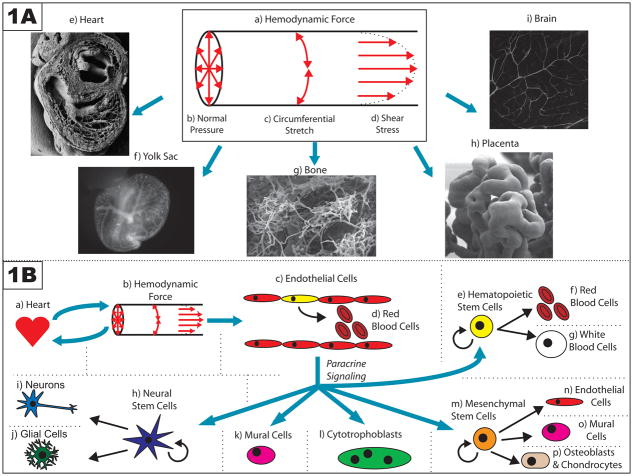
Figure 1. Summary of the effects of hemodynamic force on embryonic development.
(A) Three mutually perpendicular hemodynamic forces are exerted onto the vessel wall by flowing blood (a). First, blood pressure exerts a normal force against the vessel wall (b). Second, as a result of this normal force, the cells comprising the wall also experience circumferential stretch (c). Finally, because flowing blood exerts drag on the vessel wall, the endothelial cells that line the vessel experience a frictional force known as shear stress (d). These hemodynamic forces are important at a variety of developmental time points and in a diverse range of vessel architectures, including those seen in: the heart (e – adapted with permission from Oxford University Press: <Hogers B, et al. (1999). Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res 41:94. Figure 6a.>) [45]; the extraembryonic yolk sac (f); the bone (g – adapted with kind permission from Springer Science+Business Media: <Morini S, et al. (2006). Microvascular adaptation to growth in rat humeral head. Anat Embryol (Berl) 211:407. Figure 8.>) [82]; the placenta (h – © Society for Reproduction and Fertility (2009). Reproduced by permission. Adapted from: <Burton GJ, et al. (2009). Regulation of vascular growth and function in the human placenta. Reproduction 138:897. Figure 3b.>) [11]; and the brain (i).
(B) Hemodynamic force can have effects on a variety of cell types. The heart (a) creates hemodynamic force (b) by pumping blood; reciprocally, this force affects the morphogenesis of the heart as it develops. This hemodynamic force is also sensed by endothelial cells that line the walls of vessels (c), and thereby directs a variety of intrinsic cellular responses, including those that are important for vascular remodeling and arterial/venous specification. Some of these endothelial cells, particularly in the aorta-gonads-mesonephros, may be hemogenic endothelial cells (depicted in yellow) that divide to produce blood cells in a flow-dependent manner (d). Hemodynamic force may also further regulate the self-renewal and differentiation of hematopoietic stem cells that are not part of the vessel wall (e) by using paracrine signals released by the endothelium. Other paracrine signals released by the endothelium in response to hemodynamic force may also influence the self-renewal and differentiation of progenitor cells in other stem cell niches, including neural stem cells (h) and mesenchymal stem cells (m). Finally, flow-dependent release of paracrine signals by endothelial cells have also been implicated in the recruitment of mural cells (k) to the walls of developing vessels, and in the invasion of maternal vessels in the endometrium by cytotrophoblasts (l) during placentation.
In some cases, in vitro experiments have yielded evidence suggesting that some molecules are preferentially activated by one particular type of force over another. For example, it has recently been shown that a molecular complex of PECAM-1 (platelet endothelial cell adhesion molecule-1), VEGFR2 (vascular endothelial growth factor receptor 2), and VE-cadherin (vascular endothelial cell cadherin) is necessary for cultured endothelial cells to respond specifically to shear stress [139]. Normally, endothelial cells initiate a cascade of intracellular signaling events in response to laminar shear through VEGFR2-dependent activation of PI3K (phosphatidylinositol-3-OH kinase) [139]. However, endothelial cell lines deficient in either Pecam-1 or Ve-cadherin expression are unable to do so; furthermore, ectopic expression of these two genes along with Vegfr2 is sufficient to confer this mechanosensitivity to cell lines that are otherwise unresponsive to shear stress [139]. The ability of endothelial cells to respond specifically to circumferential stretch, on the other hand, is thought to preferentially involve integrin-mediated interactions with the extracellular matrix [3, 13]. In particular, it has been shown that integrins can interact with FAK (focal adhesion kinase) to activate MAPK (mitogen-activated protein kinase) in response to stretch [3].
However, in practice, it is difficult to distinguish between these distinct forces in vivo, and so they are usually not considered separately; instead, they are often referred to collectively as hemodynamic force. The subtle roles played by each distinct force in the live animal have also been further obscured by the difficulty of extrapolating in vitro data to predict physiological circumstances in vivo. For instance, cell culture work suggests PECAM-1 may be necessary for the response of the endothelium to shear stress [139]; however, Pecam-1 knockout mice are able to survive through adulthood with only minor cardiovascular defects [14, 22, 112]. This may be explained by genetic compensation, or by the existence of overlapping and potentially redundant mechanosensory mechanisms. Another possibility is that survival of these knockout mice during embryonic development might be explained by a difference in the magnitude of hemodynamic force that is experienced by adult vessels as compared to embryonic vessels. Our relative lack of understanding of these differences is reflected in the fact that, despite notable successes in measuring hemodynamic forces during development [52], a detailed understanding of how these forces change over time remains elusive. Finally, although it is clear that endothelial cells respond to hemodynamic force during development [53, 59, 68], it may also be true that different mechanisms are used in developing cells than are used in adult cells. The unanswered questions related to this one example therefore illustrate the challenges inherent to the study of these complex molecular pathways in vivo.
The large number of molecular mechanisms that have been implicated in conferring mechanosensitivity to endothelial cells serves as another indication of the complexity of the cellular response to hemodynamic force [61]. For example, the non-motile (9+0) microtubular primary cilia of endothelial cells have been shown in some cases to protrude into the lumen of the vessel and to mediate the opening of calcium channels in response to shear stress [20, 88, 140]; interestingly, mice lacking essential components of this mechanosensitive primary cilium are dysfunctional in cardiovascular development and suffer from focal hemorrhaging and defects in cardiac morphogenesis [9, 121]. Other lines of evidence have shown that cell surface components in particular, including several G-protein coupled receptors [12, 61, 73], TRP (transient receptor potential) ion channels [10, 97], and the endothelial glycocalyx [151] are also important constituents of mechanosensory pathways. However, at present, the importance of each of these components during development is unclear. The mechanisms by which endothelial cells respond to hemodynamic force may in fact be dependent on a combinatorial activation of any number of these molecules, and may change during development depending on context or on the availability of different intracellular signaling pathways. Future efforts in the field may therefore focus on elucidating the complex interactions between these different pathways. Exactly how some of these molecules are biomechanically activated also remains largely unknown.
In contrast, the downstream effects of hemodynamic force on intracellular pathways are better understood. A number of major signaling pathways are quickly activated after the onset of flow, including notably the PI3K/AKT pathway and the MAPK pathway [15, 35, 139]. A variety of transcription factors also show responsiveness to hemodynamic force, including Klf2 (Krüppel-like factor 2), which is necessary for heart valve formation [36, 37, 60, 142], and SP1, which is important for arterial specification [93]. Furthermore, histone methylation and acetylation, which can directly affect transcription, have been shown to be induced by shear stress [50, 51].
In addition to regulating these cell-intrinsic signaling events, hemodynamic force also regulates endothelial cell paracrine signaling to other cell types. For example, the secretion of a variety of growth factors, including VEGF, PDGF (platelet-derived growth factor), and TGFβ (transforming growth factor beta), is tightly regulated in endothelial cells by hemodynamic force [17, 47, 77, 94]. Nitric oxide release, an important mediator of vascular tone that is regulated by eNOS (endothelial nitric oxide synthase), is also sensitively controlled by shear stress [31, 36, 37, 68, 135]. Finally, hemodynamic force has been observed to regulate the expression of cell adhesion molecules on endothelial cells [16]. In these ways, endothelial cells are able to respond to blood flow by transducing downstream signaling events in a paracrine manner. The exposure of endothelial cells to shear stress therefore initiates a series of cell-cell signaling events that can influence the development of adjacent tissues. This broad effect is reflected in the wide-range of roles played by mechanical force, not only in the cardiovascular system, but in other systems as well.
The physiological effects of mechanical force
What we have learned about the effects of hemodynamic force has been surprisingly applicable to the study of the mechanotransduction of other forces as well. The nervous system, for example, relies on mechanosensitive pathways very similar to those seen in endothelial cells [40, 92, 96]. Such mechanisms serve as the molecular basis of hearing and touch [25, 92, 96]. Surprisingly, kidney development is also dependent on mechanotransduction pathways [20, 87]. The same primary cilia that have recently been implicated in the cardiovascular response to shear stress were originally shown to sense similar forces exerted by fluid flow in the kidney tubule and to thereby regulate the cell cycle; pathological misregulation of this mechanism often leads to cyst formation [9, 87, 88, 121].
In the context of both the embryonic and adult cardiovascular systems, however, hemodynamic force is the predominating force. The large body of work that has focused on elucidating the physiological effects of this force has increased our understanding of a variety of pathologies in the adult vascular system [56, 79, 125, 134, 155]. However, some of the newest breakthroughs in our understanding of the physiological roles of hemodynamic force have been in the context of embryogenesis [1, 68, 91]. This review will focus on the current state of what we know about the effects of hemodynamic force during pregnancy and embryonic development (summarized in Figure 1), their roles in regulating the maintenance and division of stem cells, and their implications in a variety of diseases.
The effects of hemodynamic force on cardiovascular development
Vascular remodeling during early development
Shortly after gastrulation, extraembryonic mesodermal cells in the yolk sac are specified to form the blood islands [108]. These blood islands are the first sites of hematopoiesis during development, and also contain angioblasts, which coalesce to form the primitive capillary plexus by E8.5 (embryonic day 8.5) in a process termed vasculogenesis [108, 109]. However, the polygonal arrangement of this initial plexus quickly begins remodeling shortly after the start of the heartbeat (~3 somite stage) and the onset of flow (~6 somite stage) [68, 107, 109]. Within a day, this plexus remodels into a hierarchical network of large vessels carrying high volumetric flow and small vessels carrying low flow (Figure 2) [68, 107]. This finalized, low-resistance vascular network is highly efficient at perfusing the yolk sac and delivering blood to the embryo proper, and aberrant formation of this vessel network results in growth retardation, cardiac failure, and eventual embryonic death [35, 68].
Figure 2. Defective yolk sac vascular remodeling in embryos with deficient heart function.
Shown are littermate mouse embryos at the 7 somite (A, B), 10 somite (C, D), and 23 somite (E, F) stages, which are either heterozygous (A, C, E) or homozygous (B, D, F) for the recessive null allele of Mlc2a. Blood vessels are visualized through use of the fluorescent reporter Tg(ε-globin-KGFP), which is transgenically expressed in primitive erythroblasts. Shortly after gastrulation, extraembryonic mesodermal cells in the yolk sac coalesce to form the blood islands (A). Shortly thereafter, angioblasts in these blood islands form the primitive capillary plexus of the yolk sac (C), and by the 23 somite stage, this early capillary plexus has remodeled into a hierarchical structure of large and small vessels (E). Mice which are homozygous for the Mlc2a null mutation, and therefore have deficient heart function, are still able to form the blood islands (B) and the primitive capillary plexus in the yolk sac (D). However, they are not able to remodel their yolk sac vasculature as the embryo grows (F), and therefore die in mid-gestation. This process of vascular remodeling is thought to depend on the hemodynamic force created by the robust flow of blood through the capillary plexus in wildtype mice (adapted with permission from the Company of Biologists: <Lucitti JL, et al. (2007). Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134:3321. DOI:10.1242/dev.02883. Figure 4.>) [68].
It is now clear that hemodynamic force is an important factor regulating this process of yolk sac vascular remodeling. Dramatic reductions in hemodynamic force during development, through the disruption of heart function for example, are able to prevent proper remodeling. Knocking out either Mlc2a (atrial myosin light chain 2) or Ncx1 (sodium calcium exchanger 1), two molecules that are required for proper heart function and that are expressed only in the heart, abolishes vascular remodeling (Figure 2) and causes mid-gestation embryonic death [48, 145]. The cardiac-specific manner in which these genes are expressed supports a model by which altered heart function can cause secondary effects on the remodeling of the yolk sac vasculature. Genetic rescue experiments have also been reported that further bolster this argument. Vascular remodeling defects in N-cadherin (neuronal cadherin) knockout mice, for instance, can be rescued by cardiac-specific transgenic expression of N-cadherin or E-cadherin (epithelial cadherin) [71]. Similarly, vascular remodeling defects seen in mice lacking the cardiac homeobox transcription factor Nkx2.5 can be rescued in mouse chimeras in which wildtype cells contribute to the heart at rates of 85% or higher [132]. Cardiac specific cellular defects are therefore capable of causing non-cell autonomous failures in vascular remodeling in the yolk sac.
However, only recently has evidence been reported that attributes these secondary effects to the hemodynamic consequences of heart defects, as opposed to the changes in the circulation of oxygen, nutrients, and signaling molecules that also accompany heart failure [68]. By sequestering primitive erythroblasts in the blood islands using polymerized acrylamide, hemodynamic force in a developing embryo can be dramatically reduced by decreasing blood hematocrit, and therefore blood viscosity. When this is done, yolk sac vascular remodeling is abolished, and the embryo no longer undergoes proper turning; however, restoration of blood viscosity by injection of hetastarch, a viscous plant starch, is capable of rescuing both normal embryonic turning and normal vascular remodeling [68]. Together, these data make a strong case for the fact that hemodynamic force is both necessary and sufficient for yolk sac vascular remodeling. Similar conclusions have been made from observing the failure of vascular remodeling in mice which are deficient in primitive and definitive hematopoiesis, and therefore have low blood hematocrit and viscosity [42]. Hemodynamic force may also prove to be important during vascular remodeling in the embryo proper [42]. Endothelial cell migration, which must be highly regulated for proper vascular remodeling to occur [8, 35], is one cellular process that might be regulated by hemodynamic force; however, future studies will be needed to address this possibility.
Vessel maturation and arterial/venous specification
After the initial phase of vascular remodeling in the yolk sac and embryo nears completion, the vessels undergo a process of stabilization that is largely mediated by interactions between endothelial cells and mural cells [4, 30, 109]. During this stage of development, endothelial cells secrete PDGFB (platelet derived growth factor B). PDGF binds to PDGF receptors on pericytes and smooth muscle cells to recruit them to participate in the formation of the vessel wall [4, 30, 109]. At the same time, these recruited mural cells secrete Ang-1 (angiopoitin-1) [30, 109, 127, 146]. Upon binding to Tie2 receptors on endothelial cells, this glycoprotein transduces a number of downstream signaling events that lead to vessel stabilization, a necessary step in the maturation of the vessels which brings them to homeostasis [109, 127, 128]. Without proper function of this paracrine signaling loop, cardiovascular development cannot proceed; this is underscored by the finding that the knockout of either Pdgfb or Ang-1 in mice results in embryonic lethality due to hemorrhaging and defective vascular integrity [64, 128].
In vitro experiments that more closely examine this paracrine loop suggest that it too may be regulated to some extent by hemodynamic force. Shear stress, for example, can stimulate the expression and secretion of PDGFB by endothelial cells [47, 77]. Hemodynamic force may thus strengthen high-flow vessels by leading to mural cell recruitment, thereby reinforcing vessel maturation in an ANG-1 dependent manner. Furthermore, once smooth muscle cells and pericytes are recruited to the vessel wall, hemodynamic force may continue to play a role in regulating the interactions of mural cells with neighboring endothelial cells. Using customized flow chambers, smooth muscle cells have been cocultured with endothelial cells on opposite sides of a porous membrane in a manner such that the endothelial cells can be subjected to flow while the smooth muscle cells are maintained in an environment without shear stress [16, 28, 86]. Interestingly, at different levels of flow, the endothelial cells are able to stimulate significantly different levels of smooth muscle cell proliferation by communicating through the porous membrane [86]. It also seems that endothelial cells are able to direct the cytoskeletal reorganization of cocultured smooth muscle cells so that they align in a direction perpendicular to that of flow; this is the configuration which smooth muscle cells are known to adopt in vivo [16]. Signals are propagated in the opposite direction as well, and smooth muscle cells are able to induce the expression of various genes in endothelial cells; this effect too is modulated by the exertion of shear stress on the endothelium [16]. In these ways, hemodynamic force continues to play a role in the later stages of vascular development, even after vessels have reached homeostasis.
As vessels form, they also become specified to differentiate into either arteries or veins. Predetermined genetic mechanisms largely control this choice between arterial or venous fate through a variety of pathways, including the Notch and ephrin signaling pathways [109, 149, 156]. However, experimental evidence indicates that hemodynamic force is also involved in this differentiation process. In fact, shear stress has been shown to drive endothelial progenitor cells toward an arterial fate [93]. Prolonged exposure to shear stress both in vitro and in vivo causes endothelial cells to upregulate classical arterial markers, such as ephrin-B2, and to downregulate classical venous markers, such as Eph-B4 (the conjugate receptor of ephrin-B2) [59, 93, 149]. These downstream effects of flow are even powerful enough to respecify arteries and veins. For example, the recruitment of perivascular cells to the vessel wall in response to hemodynamic force, as discussed above, appears to play an important part in the process of arterialization [84, 100, 141], a fact which has important implications for the use of vein grafts to replace arteries [33, 65, 66]. Transplantation studies first demonstrated the plasticity of endothelial cells by showing that endothelial cells from the dorsal aorta of the quail are able to colonize both arteries and veins in the developing chick up through late development [83]. Subsequent studies in chick have also shown that changes in shear stress and pressure, brought on either by venous ligation or vascular incision, are capable of inducing changes in arterial/venous fate [36, 37, 45, 59]. These data further emphasize the importance of hemodynamic force in the process of vessel maturation.
Cardiac development and morphogenesis of the aortic arch
Given the extensive role of hemodynamic force in directing vessel morphogenesis, it is not surprising that mechanical forces appear to exert a similar influence over cardiac development [43, 46, 102, 129, 130]. Proper heart morphogenesis is an intricate program by which the early embryonic heart tube remodels into a complex, multi-chambered pump [41]. Disruption of normal flow through the heart, however, disrupts this finely tuned process [36, 43, 45, 48]. As mentioned above, genetic manipulation of heart function via ablation of various cardiac-specific genes can cause failures in vascular remodeling; in a similar fashion, those same manipulations cause defects in cardiac septation and valve formation [48, 132]. Defects in cardiogenesis can also be caused by knocking out Pkd1 (polycystic kidney disease 1) and Pkd2, genes that are involved in ciliary mechanotransduction in the mouse [9, 121]. Together, these data suggest that sufficient blood flow and the ability to sense it are crucial for the heart to form properly.
However, results gleaned from genetic loss-of-function experiments in the heart are hard to interpret. For example, it is difficult to rule out the possibility that cardiac-specific mutations disrupting heart function do not also disrupt intrinsic morphogenic programs. To partially address these problems, alternative methods have been used to examine the role that hemodynamics play in this system. For instance, both pharmacological and surgical techniques, such as the ligation of the atria or the vitelline arteries of the chick, have confirmed that changes in blood flow patterns can result in defects in heart septation and valve formation [36, 37, 45, 69, 113, 142]. The use of these techniques, in combination with similar investigations which instead use aortic banding, suggest that pressure in particular may be important for regulating cardiac morphogenesis [53, 130, 131, 138]. Occluding blood flow through the zebrafish heart by cardiac injection of glass beads has also demonstrated that altering hemodynamic force in the heart can dramatically impair cardiac morphogenesis [46]. Because hemodynamic force and heart function are tightly coupled, however, further investigation will be needed to confirm these results and ensure that shear stress and pressure do indeed directly affect cardiogenesis.
A closely related and likely more tractable question is whether or not hemodynamic forces play a role in the morphogenesis of the aortic arch. Due perhaps to the close proximity of the developing aortic arch to the heart, the asymmetric formation of the great arteries has proven to be particularly sensitive to shear stress [2, 55, 122, 150, 154]. Recent studies have shown that the asymmetric formation of the adult aortic arch is a direct consequence of shear stress-induced remodeling of the embryonic BAA (branchial arch artery) system [122, 154]. Normal development of the heart includes a spiraling of the outflow tract, which then sets up an asymmetric distribution of blood flow through the right and left BAAs; however, deletion of the gene for the paired-like homeodomain transcription factor Pitx2, or deletion of the gene’s asymmetric enhancer, results in abolished spiraling of the outflow tract, and subsequently, symmetric blood flow and randomization of the laterality of the aortic arch in mice [2, 122, 154]. Similar pathologies can be achieved by microsurgical disruption of embryonic blood flow, or by manipulation of the levels of VEGF or PDGFA in the BAA system [154]. The morphogenesis of the aortic arch, therefore, is a process which is dependent on a complex interplay between a multitude of genetic and epigenetic factors, including hemodynamic force. Similarly, future advances in our understanding of cardiac development will need to take into account all of these complex interactions between intrinsic and extrinsic factors during embryogenesis.
Implantation and placental development
Evidence also exists to suggest that placental development is dependent on mechanosensitivity and hemodynamic force. Soon after implantation of the developing embryo into the uterine wall, the maternally-derived, largely avascular deciduum surrounds the embryo [49]. The oxygen gradient created by this hypoxic environment then induces cytotrophoblasts to extend from the embryo toward the more oxygen-rich tissues in the uterus [49, 103]. After spanning this gap, these cytotrophoblasts will invade the uterine wall, and finally reach the spiral arteries of the endometrium; there, they undergo an epithelial to endothelial transition, and change their expression of a wide array of integrins so that they may intercalate into the walls of the maternal vessels [7, 26, 29, 49, 67, 103]. In doing so, these syncytial trophoblast cells thereby invade the maternal vasculature and remodel it so that maternal blood flow is rerouted into the intervillous space of the developing placenta [26, 67, 103].
Several studies suggest that hemodynamic force regulates this process by which the cytotrophoblasts remodel the spiral arteries. First, cytotrophoblasts have been shown to be mechanosensitive in vitro [67, 101, 123]. Intriguingly, shear stress upregulates the expression of β1 integrin in cytotrophoblasts, a molecule which is also known to be upregulated as the cells invade maternal vessels [123]. Shear stress can also induce the motility of cytotrophoblasts in culture [67, 123, 124]. Furthermore, endothelial cells that are cocultured with cytotrophoblasts are able to further enhance the degree of cytotrophoblast migration, particularly in the direction opposite to flow [124]. These data therefore raise the possibility that the hemodynamic force exerted by maternal blood flow plays a role in directing cytotrophoblasts to invade and remodel the maternal arteries in the endometrium. This hypothesis is supported by the in vivo observation that over time, cytotrophoblasts migrate progressively further upstream within the maternal arterioles that they invade; in contrast, after cytotrophoblasts invade venules, they do not migrate any further [7, 67].
Poor cytotrophoblast invasion of the spiral arteries is linked with a high incidence to preeclampsia [29, 49, 103, 147]. Failure of the developing placenta to obtain an adequate blood supply after insufficient vessel invasion leads to the release of vasoactive substances into the maternal bloodstream that are thought to underlie the sudden appearance of hypertension, proteinuria, and edema that is characteristic of preeclampsia [29, 137, 147]. If hemodynamic force is important in regulating cytotrophoblast invasion of the spiral arteries, then defects in cytotrophoblast mechanotransduction may very well play a central role in the pathogenesis of this serious condition [81, 101], highlighting the clinical importance of more fully understanding the mechanisms of mechanotransduction in a variety of systems. It has been suggested [104] that in the future, tetraploid complementation techniques may help us to investigate this question by allowing genetic manipulation of the placenta without affecting the embryo proper.
Hemodynamic force as a regulator of stem cell proliferation and differentiation
One other field in which mechanotransduction is emerging as an important factor is stem cell biology. Various in vitro studies have shown, for instance, that mechanical forces regulate certain aspects of ES cell (embryonic stem cell) differentiation [110, 111, 117], and that laminar shear stress in particular can be used to drive embryonic stem cells toward an endothelial cell lineage [51, 153]. These data, together with evidence showing that blood vessels play important roles in a variety of stem cell niches, suggest that hemodynamic force may play a role in the regulation of stem cell maintenance.
Hematopoiesis
Hematopoietic stem cells comprise one population of cells that are newly recognized as being sensitive to the effects of hemodynamic force. During early development, primitive hematopoiesis begins in the extraembryonic yolk sac, where HSCs (hematopoietic stem cells) arise in the blood islands [24, 72, 108]. These cells produce the primitive erythroblasts that are crucial for early embryonic development; however, their importance is quickly supplanted by the emergence of new hematopoietic sites within the embryo proper [23, 24, 72]. This second wave of what is known as definitive hematopoiesis occurs in a variety of places at different times during development, including the PSp/AGM (para-aortic splanchnopleura/aorta-gonads-mesonephros), fetal liver, spleen, and eventually the adult bone marrow [1, 23, 24]. Though it was originally thought that the HSCs in the yolk sac colonized all of these sites in order to initiate definitive hematopoiesis, quail-chick transplantation studies eventually disproved that idea, demonstrating instead that definitive HSCs in the adult arise from a lineage that is completely independent of the cells that first appear in the blood islands [21, 23]. These sites of definitive hematopoiesis have been the focus of several recent studies [1, 32, 91, 99], and the AGM region in particular has been of considerable interest, due largely to the fact that the AGM is capable of initiating autonomous hematopoiesis even in explant cultures [23, 75, 90, 91, 99].
Recent investigations have suggested that hemodynamic force controls hematopoiesis in the AGM [1, 91], possibly by acting on a specialized population of endothelial cells called the hemogenic endothelium, which is capable of producing blood cells [32, 91]. Changing the in vivo rates of blood flow in zebrafish, either genetically or pharmacologically, can cause changes in the levels of definitive hematopoiesis in the AGM [91]. Experiments performed on mouse AGM explant cultures have also yielded similar results; exposing such cultures to shear stress is sufficient to increase the hematopoietic colony forming potential of the tissue, and to induce its expression of hematopoietic markers [1]. Induction of the runt-related transcription factor Runx1 in these cultures may be of particular importance, due to the fact that Runx1 is required for the emergence and function of HSCs throughout development [1, 89, 90, 95]. NO (nitric oxide) signaling is another important component to AGM hematopoiesis; in fact, overproduction of NO can rescue the defects in hematopoiesis that are observed in flow-compromised sih (silent heart) zebrafish, while reduction in blood flow-dependent NO signaling causes significant reductions in blood production [1, 91].
These observations, in combination with in vitro data, and the fact that mutant mice with cardiac defects often have defects in hematopoiesis as well, have begun to reshape our understanding of both hematopoiesis and mechanotransduction [1, 72, 99, 132]. It appears that hemodynamic force may have even farther reaching effects than previously realized.
Hemodynamic force and the stem cell niche
Each distinct stem cell population is often observed to exist in its own specialized microenvironment, also known as a stem cell niche. NSCs (neural stem cells), for example, which divide to populate the developing brain, reside during embryonic development in specialized microenvironments known as the VZ (ventricular zone) and the SVZ (subventricular zone) [38]. They also continue to function throughout adulthood in specialized environments in the SEZ (subependymal zone), which lines the lateral walls of the lateral ventricles (Figure 3), and in the SGZ (subgranular zone) of the hippocampus [114, 115, 133]. Collectively, these environments comprise what we know as the neural stem cell niche.
Figure 3. The adult neural stem cell niche.

This figure shows a flatmount preparation of the highly vascular subependymal zone of the adult mouse brain. Functional vessels are visualized with a fluorescently labeled dextran that fills the vessel lumen. Neural stem cells persist in this region of the adult brain, which is directly adjacent to the lateral ventricle.
Interestingly, blood vessels seem to play an important role in this stem cell niche. In fact, there is both in vitro and in vivo evidence to suggest that these blood vessels exert some control over the differentiation and expansion of the neural progenitors in the niche [98, 114, 115, 133]. It has been shown in coculture experiments, for example, that endothelial cells secrete trophic factors that induce both symmetric and asymmetric divisions of neural stem cells in vitro [114]. Observations in vivo have also demonstrated a striking physical association between blood vessels and proliferating NSCs in the SEZ and the SGZ [98, 115, 133].
Based on this evidence, some have speculated that NSC maintenance and division in the neurovascular niche may be another process which is regulated by hemodynamic force. Such a mechanism would be consistent with the fact that shear stress regulates the secretion of a number of growth factors by the endothelium, many of which are known to influence neurogenesis [80, 126, 144]. It would also fit with data showing that the changes in cerebral blood flow that are induced in mouse models of stroke result in a stimulation of neural proliferation and neuroblast migration [34, 62, 157]. Actually, another step toward verifying this hypothesis has recently been made with the demonstration that surgical induction of chronic cerebral hypoperfusion increases levels of neurogenesis in the SEZ [78]. If the changes in blood flow and shear stress that are so often experienced by cerebral vessels after brain trauma are indeed a trigger for neurogenesis, then it would also explain how neural stem cells in the SEZ are able to divide in response to non-local trauma [34]. However, future studies will need to carefully separate the effects of hemodynamic force from the effects of hypoxia and cell damage that also occur in these situations.
These promising observations therefore merit further investigation into the possible role of hemodynamic force in the regulation of neurogenesis. Confirmation of such a mechanism could potentially lead to therapies for the stimulation of endogenous brain repair after stroke [5], or for the correction of the histopathological effects of reduced cerebral blood flow during embryonic development [76]. A better understanding of this process could also lead to new treatments for the changes in neurogenesis and cerebral blood flow that are observed in those diagnosed with some mental illnesses such as schizophrenia and depression [63, 70, 74].
Hemodynamic force may also prove to be important in other stem cell niches as well. MSCs (mesenchymal stem cells), for example, reside within the bone marrow in a vascularized niche of their own [18]. These MSCs are known to mediate bone deposition and cartilage formation through a regulated production of osteoblasts and chondrocytes [120, 152] and are also thought to regulate mechanosensitive bone remodeling, possibly through sensitivity to interstitial fluid flows created in response to bone compression [6, 39, 54, 143, 152]. MSCs may be sensitive to hemodynamic forces within the bone as well; in fact, accumulating evidence suggests that MSCs can differentiate into vascular cell types [6, 44, 106, 148], and that shear stress and cyclic strain may regulate this process [6, 44, 106, 148]. The role of matrix stiffness in determining MSC fate further supports the idea that the mechanical environment of the bone marrow niche may be important [27]. Due to their proposed role in producing perivascular cells that contribute to vessel function [6], as well as their close association with HSCs, it would therefore not be surprising if MSCs are also eventually shown to be sensitive to hemodynamic force.
Conclusions
Hemodynamic force was first observed to play a role in flow-induced vascular remodeling over a century ago. Today, those classical observations have come to serve as the foundation of an entire field focused on the roles of hemodynamic force in the body. Even now, we are still continuing to uncover new ways in which hemodynamic force exerts important effects on various aspects of embryonic development. Considering the important roles played by mechanotransduction in the pathogenesis of such a wide variety of diseases, further elucidation of the effects of hemodynamic force on embryonic development should remain a research priority, now and in the future.
Acknowledgments
The authors would like to thank the members of the Dickinson lab for critical reading of the manuscript. The authors also acknowledge support of this work from NIH HL077187, EB005173, and EB007076.
References
- 1.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, García-Cardeña G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai D, Liu W, Ma L, Dong F, Lu MF, Wang D, Verzi MP, Cai C, Gage PJ, Evans S, Black BL, Brown NA, Martin JF. Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol. 2006;296:437–449. doi: 10.1016/j.ydbio.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, Akazawa H, Takano H, Nagai R, Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233–238. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- 4.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blankenship TN, Enders AC. Modification of uterine vasculature during pregnancy in macaques. Microsc Res Tech. 2003;60:390–401. doi: 10.1002/jemt.10277. [DOI] [PubMed] [Google Scholar]
- 8.Blatnik JS, Schmid-Schönbein GW, Sung LA. The influence of fluid shear stress on the remodeling of the embryonic primary capillary plexus. Biomech Model Mechanobiol. 2005;4:211–220. doi: 10.1007/s10237-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 9.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138:895–902. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- 12.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Huang XN, Yan W, Chen K, Guo L, Tummalapali L, Dedhar S, St-Arnaud R, Wu C, Sepulveda JL. Role of the integrin-linked kinase/PINCH1/alpha-parvin complex in cardiac myocyte hypertrophy. Lab Invest. 2005;85:1342–1356. doi: 10.1038/labinvest.3700345. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 16.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667–2674. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 17.Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002;102:13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- 18.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 19.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 22.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 23.Dzierzak E, Medvinsky A. The discovery of a source of adult hematopoietic cells in the embryo. Development. 2008;135:2343–2346. doi: 10.1242/dev.021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enders AC, King BF. Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat. 1991;192:329–346. doi: 10.1002/aja.1001920403. [DOI] [PubMed] [Google Scholar]
- 27.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res. 1997;67:169–178. doi: 10.1006/jsre.1996.4978. [DOI] [PubMed] [Google Scholar]
- 29.Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/vhrm.s4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 31.García-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 32.Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood. 2008;112:3194–3204. doi: 10.1182/blood-2008-02-139055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman J, Zhong L, Liu SQ. Negative regulation of vascular smooth muscle cell migration by blood shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H928–938. doi: 10.1152/ajpheart.00821.2006. [DOI] [PubMed] [Google Scholar]
- 34.Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 36.Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res. 2005;96:1291–1298. doi: 10.1161/01.RES.0000171901.40952.0d. [DOI] [PubMed] [Google Scholar]
- 37.Groenendijk BC, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda) 2007;22:380–389. doi: 10.1152/physiol.00023.2007. [DOI] [PubMed] [Google Scholar]
- 38.Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- 39.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng. 2008;36:1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 40.Haeberle H, Bryan LA, Vadakkan TJ, Dickinson ME, Lumpkin EA. Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PLoS One. 2008;3:e1750. doi: 10.1371/journal.pone.0001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey RP, Rosenthal N. Heart development. Academic Press; San Diego: 1999. [Google Scholar]
- 42.He C, Hu H, Braren R, Fong SY, Trumpp A, Carlson TR, Wang RA. c-myc in the hematopoietic lineage is crucial for its angiogenic function in the mouse embryo. Development. 2008;135:2467–2477. doi: 10.1242/dev.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hierck BP, Van der Heiden K, Poelma C, Westerweel J, Poelmann RE. Fluid shear stress and inner curvature remodeling of the embryonic heart. Choosing the right lane! Scientific World Journal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res. 1999;41:87–99. doi: 10.1016/s0008-6363(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 46.Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh HJ, Li NQ, Frangos JA. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991;260:H642–646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- 48.Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S, Chen J. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development. 2003;130:6111–6119. doi: 10.1242/dev.00831. [DOI] [PubMed] [Google Scholar]
- 49.Huppertz B, Peeters LL. Vascular biology in implantation and placentation. Angiogenesis. 2005;8:157–167. doi: 10.1007/s10456-005-9007-8. [DOI] [PubMed] [Google Scholar]
- 50.Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res. 2003;93:155–161. doi: 10.1161/01.RES.0000080933.82105.29. [DOI] [PubMed] [Google Scholar]
- 51.Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 52.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 53.Keller BB, Liu LJ, Tinney JP, Tobita K. Cardiovascular developmental insights from embryos. Ann N Y Acad Sci. 2007;1101:377–388. doi: 10.1196/annals.1389.012. [DOI] [PubMed] [Google Scholar]
- 54.Kim YJ, Bonassar LJ, Grodzinsky AJ. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech. 1995;28:1055–1066. doi: 10.1016/0021-9290(94)00159-2. [DOI] [PubMed] [Google Scholar]
- 55.Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- 56.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 57.LaBarbera M. Principles of design of fluid transport systems in zoology. Science. 1990;249:992–1000. doi: 10.1126/science.2396104. [DOI] [PubMed] [Google Scholar]
- 58.LaBarbera M, Boyajian GE. The Function of Astrorhizae in Stromatoporoids: Quantitative Tests. Paleobiology. 1991;17:121–132. [Google Scholar]
- 59.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Bréant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 60.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 62.Li WL, Yu SP, Ogle ME, Ding XS, Wei L. Enhanced neurogenesis and cell migration following focal ischemia and peripheral stimulation in mice. Dev Neurobiol. 2008;68:1474–1486. doi: 10.1002/dneu.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 65.Liu SQ, Fung YC. Changes in the organization of the smooth muscle cells in rat vein grafts. Ann Biomed Eng. 1998;26:86–95. doi: 10.1114/1.52. [DOI] [PubMed] [Google Scholar]
- 66.Liu SQ, Ruan YY, Tang D, Li YC, Goldman J, Zhong L. A possible role of initial cell death due to mechanical stretch in the regulation of subsequent cell proliferation in experimental vein grafts. Biomech Model Mechanobiol. 2002;1:17–27. doi: 10.1007/s10237-002-0003-2. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Fan Y, Deng X, Li N, Guan Z. Effect of flow-induced shear stress on migration of human trophoblast cells. Clin Biomech (Bristol, Avon) 2008;23(Suppl 1):S112–117. doi: 10.1016/j.clinbiomech.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lucitti JL, Visconti R, Novak J, Keller BB. Increased arterial load alters aortic structural and functional properties during embryogenesis. Am J Physiol Heart Circ Physiol. 2006;291:H1919–1926. doi: 10.1152/ajpheart.01061.2005. [DOI] [PubMed] [Google Scholar]
- 70.Lui S, Parkes LM, Huang X, Zou K, Chan RC, Yang H, Zou L, Li D, Tang H, Zhang T, Li X, Wei Y, Chen L, Sun X, Kemp GJ, Gong QY. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–484. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- 71.Luo Y, Ferreira-Cornwell M, Baldwin H, Kostetskii I, Lenox J, Lieberman M, Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 2001;128:459–469. doi: 10.1242/dev.128.4.459. [DOI] [PubMed] [Google Scholar]
- 72.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schönbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633–1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 74.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 76.Miller B, Nagy D, Finlay BL, Chance B, Kobayashi A, Nioka S. Consequences of reduced cerebral blood flow in brain development. I. Gross morphology, histology, and callosal connectivity. Exp Neurol. 1993;124:326–342. doi: 10.1006/exnr.1993.1203. [DOI] [PubMed] [Google Scholar]
- 77.Mitsumata M, Fishel RS, Nerem RM, Alexander RW, Berk BC. Fluid shear stress stimulates platelet-derived growth factor expression in endothelial cells. Am J Physiol. 1993;265:H3–8. doi: 10.1152/ajpheart.1993.265.1.H3. [DOI] [PubMed] [Google Scholar]
- 78.Miyamoto N, Tanaka R, Zhang N, Shimura H, Onodera M, Mochizuki H, Hattori N, Urabe T. Crucial role for Ser133-phosphorylated form of cyclic AMP-responsive element binding protein signaling in the differentiation and survival of neural progenitors under chronic cerebral hypoperfusion. Neuroscience. 2009;162:525–536. doi: 10.1016/j.neuroscience.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–319. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 80.Mohapel P, Frielingsdorf H, Häggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 81.Moll W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S19–27. doi: 10.1016/s0301-2115(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 82.Morini S, Pannarale L, Conti D, Gaudio E. Microvascular adaptation to growth in rat humeral head. Anat Embryol (Berl) 2006;211:403–411. doi: 10.1007/s00429-006-0092-2. [DOI] [PubMed] [Google Scholar]
- 83.Moyon D, Pardanaud L, Yuan L, Bréant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- 84.Murfee WL, Van Gieson EJ, Price RJ, Skalak TC. Cell proliferation in mesenteric microvascular network remodeling in response to elevated hemodynamic stress. Ann Biomed Eng. 2004;32:1662–1666. doi: 10.1007/s10439-004-7819-0. [DOI] [PubMed] [Google Scholar]
- 85.Murray CD. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc Natl Acad Sci U S A. 1926;12:207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nackman GB, Fillinger MF, Shafritz R, Wei T, Graham AM. Flow modulates endothelial regulation of smooth muscle cell proliferation: a new model. Surgery. 1998;124:353–360. discussion 360–351. [PubMed] [Google Scholar]
- 87.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 88.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marín-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 90.North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 91.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, Dzierzak E, Zon LI. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 93.Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, Asahara T, Ando J. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106:203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 94.Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 96.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 97.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 98.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 99.Pardanaud L, Eichmann A. Stem cells: The stress of forming blood cells. Nature. 2009;459:1068–1069. doi: 10.1038/4591068a. [DOI] [PubMed] [Google Scholar]
- 100.Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 101.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Poelmann RE, Gittenberger-de Groot AC, Hierck BP. The development of the heart and microcirculation: role of shear stress. Med Biol Eng Comput. 2008;46:479–484. doi: 10.1007/s11517-008-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 104.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Revellin R, Rousset F, Baud D, Bonjour J. Extension of Murray’s law using a non-Newtonian model of blood flow. Theor Biol Med Model. 2009;6:7. doi: 10.1186/1742-4682-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riha GM, Wang X, Wang H, Chai H, Mu H, Lin PH, Lumsden AB, Yao Q, Chen C. Cyclic strain induces vascular smooth muscle cell differentiation from murine embryonic mesenchymal progenitor cells. Surgery. 2007;141:394–402. doi: 10.1016/j.surg.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 107.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 108.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 109.Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 110.Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 111.Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schenkel AR, Chew TW, Chlipala E, Harbord MW, Muller WA. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol. 2006;81:23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schroder EA, Tobita K, Tinney JP, Foldes JK, Keller BB. Microtubule involvement in the adaptation to altered mechanical load in developing chick myocardium. Circ Res. 2002;91:353–359. doi: 10.1161/01.res.0000030179.78135.fa. [DOI] [PubMed] [Google Scholar]
- 114.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 115.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sherman TF. On connecting large vessels to small. The meaning of Murray’s law. J Gen Physiol. 1981;78:431–453. doi: 10.1085/jgp.78.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol. 2008;104:766–772. doi: 10.1152/japplphysiol.00870.2007. [DOI] [PubMed] [Google Scholar]
- 118.Shin HY, Gerritsen ME, Bizios R. Regulation of endothelial cell proliferation and apoptosis by cyclic pressure. Ann Biomed Eng. 2002;30:297–304. doi: 10.1114/1.1458595. [DOI] [PubMed] [Google Scholar]
- 119.Shin HY, Smith ML, Toy KJ, Williams PM, Bizios R, Gerritsen ME. VEGF-C mediates cyclic pressure-induced endothelial cell proliferation. Physiol Genomics. 2002;11:245–251. doi: 10.1152/physiolgenomics.00068.2002. [DOI] [PubMed] [Google Scholar]
- 120.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn. 2008;237:2304–2314. doi: 10.1002/dvdy.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Snider P, Conway SJ. Developmental biology: the power of blood. Nature. 2007;450:180–181. doi: 10.1038/450180a. [DOI] [PubMed] [Google Scholar]
- 123.Soghomonians A, Barakat AI, Thirkill TL, Blankenship TN, Douglas GC. Effect of shear stress on migration and integrin expression in macaque trophoblast cells. Biochim Biophys Acta. 2002;1589:233–246. doi: 10.1016/s0167-4889(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 124.Soghomonians A, Barakat AI, Thirkill TL, Douglas GC. Trophoblast migration under flow is regulated by endothelial cells. Biol Reprod. 2005;73:14–19. doi: 10.1095/biolreprod.104.036509. [DOI] [PubMed] [Google Scholar]
- 125.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 127.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 128.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 129.Taber LA. Mechanical aspects of cardiac development. Prog Biophys Mol Biol. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 130.Taber LA. Biophysical mechanisms of cardiac looping. Int J Dev Biol. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- 131.Taber LA, Chabert S. Theoretical and experimental study of growth and remodeling in the developing heart. Biomech Model Mechanobiol. 2002;1:29–43. doi: 10.1007/s10237-002-0002-3. [DOI] [PubMed] [Google Scholar]
- 132.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 133.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taylor CA, Hughes TJ, Zarins CK. Effect of exercise on hemodynamic conditions in the abdominal aorta. J Vasc Surg. 1999;29:1077–1089. doi: 10.1016/s0741-5214(99)70249-1. [DOI] [PubMed] [Google Scholar]
- 135.Teichert AM, Scott JA, Robb GB, Zhou YQ, Zhu SN, Lem M, Keightley A, Steer BM, Schuh AC, Adamson SL, Cybulsky MI, Marsden PA. Endothelial nitric oxide synthase gene expression during murine embryogenesis: commencement of expression in the embryo occurs with the establishment of a unidirectional circulatory system. Circ Res. 2008;103:24–33. doi: 10.1161/CIRCRESAHA.107.168567. [DOI] [PubMed] [Google Scholar]
- 136.Thoma R. Untersuchungen über die Histogenese und Histomechanik des Gefässsystems. Ferdinand Enke; Stuttgart: 1893. [Google Scholar]
- 137.Thomas CP, Andrews JI, Raikwar NS, Kelley EA, Herse F, Dechend R, Golos TG, Liu KZ. A recently evolved novel trophoblast-enriched secreted form of fms-like tyrosine kinase-1 variant is up-regulated in hypoxia and preeclampsia. J Clin Endocrinol Metab. 2009;94:2524–2530. doi: 10.1210/jc.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tobita K, Garrison JB, Liu LJ, Tinney JP, Keller BB. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol. 2005;283:193–201. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- 139.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 140.Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 141.Van Gieson EJ, Murfee WL, Skalak TC, Price RJ. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ Res. 2003;92:929–936. doi: 10.1161/01.RES.0000068377.01063.79. [DOI] [PubMed] [Google Scholar]
- 142.Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, Fraser SE. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 2009;7:e1000246. doi: 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 144.Wachs FP, Winner B, Couillard-Despres S, Schiller T, Aigner R, Winkler J, Bogdahn U, Aigner L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol. 2006;65:358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- 145.Wakimoto K, Kobayashi K, Kuro OM, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;275:36991–36998. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- 146.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–1184. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- 147.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Li M, Lin PH, Yao Q, Chen C. Fluid shear stress regulates the expression of TGF-beta1 and its signaling molecules in mouse embryo mesenchymal progenitor cells. J Surg Res. 2008;150:266–270. doi: 10.1016/j.jss.2007.12.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, Keller BB, Pekkan K. Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng. 2009;37:1069–1081. doi: 10.1007/s10439-009-9682-5. [DOI] [PubMed] [Google Scholar]
- 151.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wescoe KE, Schugar RC, Chu CR, Deasy BM. The role of the biochemical and biophysical environment in chondrogenic stem cell differentiation assays and cartilage tissue engineering. Cell Biochem Biophys. 2008;52:85–102. doi: 10.1007/s12013-008-9029-0. [DOI] [PubMed] [Google Scholar]
- 153.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 154.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 155.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 156.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 157.Zhu C, Qiu L, Wang X, Xu F, Nilsson M, Cooper-Kuhn C, Kuhn HG, Blomgren K. Age-dependent regenerative responses in the striatum and cortex after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:342–354. doi: 10.1038/jcbfm.2008.124. [DOI] [PubMed] [Google Scholar]