Abstract
The thymus is a bi-lobed lymphatic organ located in the anterior portion of the ventral thoracic cavity, just behind the sternum. Because the thymus is the site of development of T lymphocytes (T cells), it is frequently targeted in research studies that involve the immune system. Furthermore, the rapid expansion of transgenic and gene-targeted mouse models of immune disorders has enabled a concomitant increase in the number of studies of T-cell development. Such studies may require the administration of intrathymic injections, which have traditionally been done using a surgical approach. Surgical manipulation can result in pain or distress to the animal, which may affect the immune system, potentially confounding experimental results. Here, the authors describe a nonsurgical, ultrasound-guided approach for intrathymic injection in the mouse that results in negligible distress to the animal.
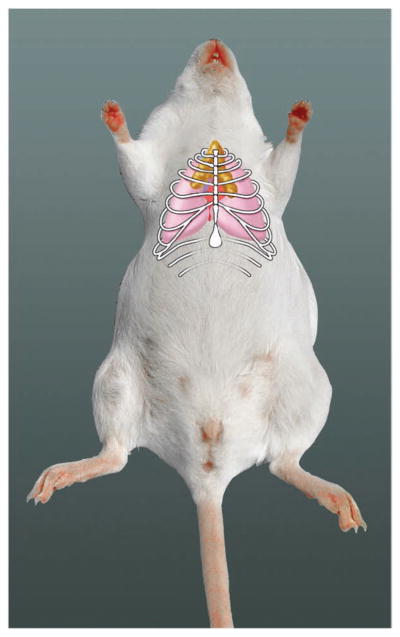
The thymus is a specialized organ of the immune system that is responsible for the production of T cells. The thymus consists of two lobes covered by a thin connective tissue capsule. It is located primarily in the thoracic area (Fig. 1), though a small portion may also be found in the ventral cervical area, extending between the larynx cranially and the heart caudally. The thymus retains its size into early adulthood and regresses thereafter by atrophy. The size of the thymus varies greatly in different mouse strains, but on average, the thymus weighs 42.9 mg in a 10-week-old female mouse (average body weight = 18.9 g) and 40.2 mg in a 10-week-old male mouse (average body weight = 23.7 g)1.
FIGURE 1.
Location of mouse thymus (yellow) in anterior ventral thorax.
T cells, or T lymphocytes, are important in cell-mediated immunity. There are several different types of T cells, including T helper cells, regulatory T cells and natural killer T cells. Many studies have been undertaken to better understand the roles of these subsets of T cells in immunity. One technique that has been used to study T-cell development is intrathymic injection of molecules to perturb T-cell development2 or to label thymocytes and follow their development3. Intrathymic injection has also been used for T cell–specific gene therapy4 and tumor induction studies5.
Intrathymic injection has traditionally been done using surgical procedures to open the thoracic cavity for direct visualization and injection into the thymus2–4. This technique subjects the mouse to substantial pain and distress, which can alter T-cell development and potentially increase mortality. Intrathymic injections can also be done by blind injection into the thoracic cavity6, but this technique requires a skin incision and placement (and later removal) of wound closures. Furthermore, the success rate for this technique can be low, because the thymus is not positively identified before injection.
To avoid using procedures that require opening the thorax and to improve success rates with nonsurgical intrathymic injections, we developed a technique for administering intrathymic injections in anesthetized mice using ultrasound-mediated guidance. Our success rate using this technique has been high.
The procedures described below were approved by the Institutional Animal Care and Use Committee of the National Human Genome Research Institute and were carried out in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the Guide for Care and Use of Laboratory Animals7.
INTRATHYMIC INJECTION
Mice and study design
We carried out intrathymic injections in 85 mice of various strains. Initially, we used 25 mice to verify the accuracy of the technique. We terminally anesthetized these mice, administered intrathymic injections of Trypan blue, euthanized the mice and carried out thoracotomies to verify the accuracy of the injections. Fifty-five mice were given injections of fluorescein isothiocyanate to locally label the thymocytes so that we could follow their fate as they exited the thymus and entered peripheral circulation. The remaining five mice were given sham injections of normal saline and used as controls.
Preparation for injection
We anesthetized each mouse with isoflurane (Abbott Labs., Chicago, IL) and medical air (21% oxygen, 79% nitrogen). We induced anesthesia using an acrylic chamber (VetEquip, Pleasanton, CA) with 4% isoflurane in medical air administered at 300 ml/min and maintained anesthesia using a facemask (fashioned from the finger of a surgical glove) with approximately 1.5–1.75% isoflurane in medical air administered at 300 ml/min.
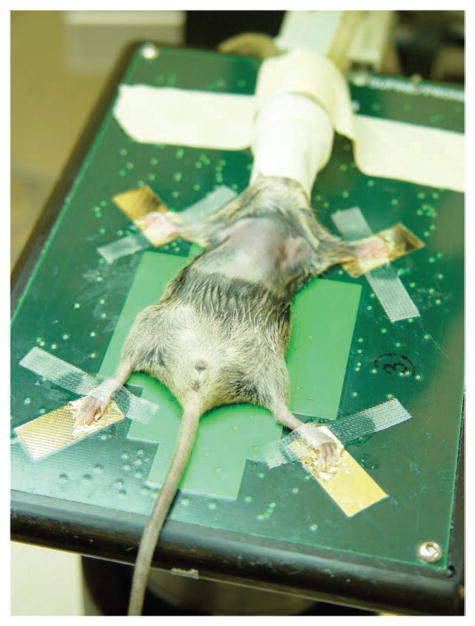
We placed the anesthetized mouse in dorsal recumbency on the ultrasound platform, which is warmed and is capable of detecting both cardiac and respiratory waveforms. For the first few procedures, we also supplied auxiliary heat using an infrared light (carefully placed to avoid overheating the mouse) and used a rectal probe to monitor body temperature. Once we had gained confidence and speed in carrying out the injections, these measures were no longer necessary. To ensure good contact with the detection pads and facilitate detection of cardiac waveforms, we covered the mouse’s paws in ultrasound gel (Aquasonic, Parker, Fairfield, NJ) and taped them to the platform with ‘ouchless’ tape (Transpore, 3M, St. Paul, MN; Fig. 2).
FIGURE 2.
Prepared mouse on ultrasound platform. The mouse’s paws have been covered in ultrasound gel and taped to the platform. The anesthesia mask was fashioned from the finger of a latex glove.
Once the mouse was positioned on the platform, we used a depilatory (Nair, Church & Dwight Co., Princeton, NJ) to remove the hair from the ventral thorax. To ensure good hair removal, we applied a thick layer of the depilatory and allowed at least 4–5 min of contact time before removing the depilatory with a water-moistened gauze pad swept in the direction of hair growth. We removed remaining stray hairs by sweeping a second moistened gauze pad or cotton-tipped applicator against the hair growth. It is important to remove all the hair from the ventral thorax, because hair interferes with the ultrasound waves, decreasing image clarity on the viewing screen and, in turn, making accurate injection more difficult. Hair removal by shaving is not appropriate because this method creates stubble that causes interference.
Injection technique
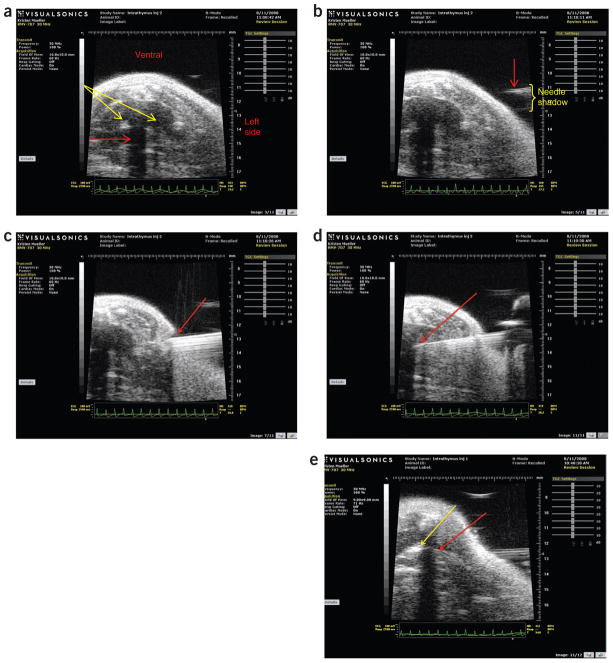
Once the hair was removed, we applied generous amounts of warmed ultrasound gel to the ventral thorax (Fig. 3). Gel should be applied gently and evenly to avoid creating any air bubbles, which also cause interference. We used a 30-mHz ultrasound probe (Visualsonics, Toronto, Canada) to locate the thickest portion of the thymus (Fig. 4a). The probe is attached to a rail system that allowed us to advance the probe to the proper location using the adjustments for the x, y and z axes. Once we located the thymus and fixed the probe and platform in place, we advanced a 30-gauge, 0.3-ml ultra-fine insulin syringe (BD Ultra-Fine II, Becton, Dickenson, Franklin Lakes, NJ) into the thymus and injected approximately 10 μl of material, without aspirating prior to injection (Fig. 4b–e). We then removed the gel with a clean gauze pad, removed the tape restraints from the mouse’s paws, discontinued administration of isoflurane and placed the mouse in a warmed cage for recovery. Mice recovered to sternal recumbency within 30–45 s; within 2–3 min, they were running around in their cages with normal behavior and could not be distinguished from mice that had not been given injections. The 55 mice that were given injections of fluorescein isothiocyanate were euthanized 24 h after injection, the study’s endpoint. These mice were all clinically normal at that time.
FIGURE 3.
The mouse’s ventral thorax has been covered with ultrasound gel in preparation for injection. Bubbles in the ultrasound gel (arrows) should be avoided, as they cause interference.
FIGURE 4.
Ultrasound imaging of thymus and intrathymic injection. (a) The ultrasound probe is positioned over the thickest portion of the thymus (yellow arrows). The sternal shadow is visible (red arrow). (b) The tip of the needle (red arrow) approaching the thorax. The needle shadow is also visible (yellow bracket). (c) The needle (red arrow) penetrating the chest wall. (d) The tip of the needle (red arrow) in the thymus. (e) Successful injection (yellow arrow) of fluid from the tip of the needle (red arrow).
Verification of accuracy
Before using this technique in our study of T-cell development, we verified its accuracy in 25 mice that were not being used in other experiments using the National Human Genome Research Institute’s training protocol under the supervision of the Institute’s veterinarian. We terminally anesthetized these mice, prepared them for injections and administered Trypan blue by intrathymic injection as described above so that we could observe, on post-mortem examination, where the injected material was located. Other dyes could be used instead.
RESULTS
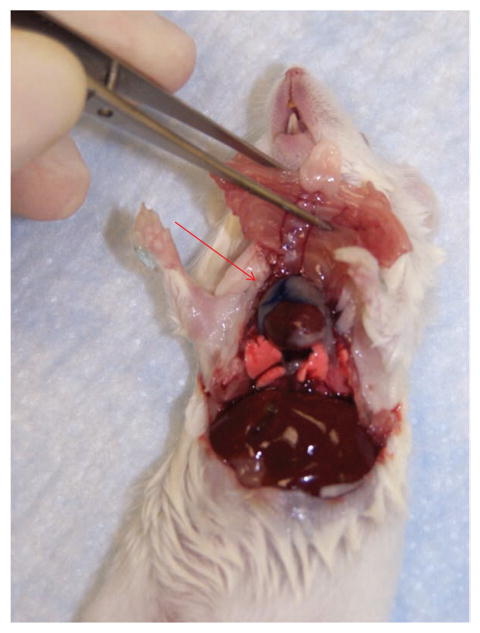
We used this technique to successfully administer intrathymic injections to 85 mice. Complications were very rare; the only one was the accidental laceration of an intrathoracic vessel (presumably the internal mammary artery) that resulted in the euthanasia of one mouse. We observed no signs of distress in any of the mice. Post-mortem examination of the 25 mice that were administered Trypan blue by intrathymic injection allowed us to confirm that the injected material had been successfully delivered to the thymus (Fig. 5). In the 55 mice that were given fluorescein isothiocyanate, we also observed good thymocyte labeling (~85%).
FIGURE 5.
Post-mortem examination confirms that Trypan blue (red arrow) was successfully injected into the thymus.
DISCUSSION
We found ultrasound-guided intrathymic injection to be rapid, accurate and easy to carry out. In our opinion, this technique represents a refinement to previously described procedures. Using ultrasound allows visualization of both the needle and the injected material as they enter the thymus. Using a high-frequency, 30-mHz ultrasound probe allows for increased resolution of the mouse’s thymus. In larger animals and humans, lower-frequency ultrasound probes of 2–10 mHz are typically used to achieve an increased imaging depth. This increased depth comes at the cost of decreased resolution, and in the mouse, the increased depth is not needed.
Once the hair is removed, the injection procedure takes only seconds. The contact time required for the depilatory to take effect cannot be reduced, but the overall procedure time could be expedited by anesthetizing several mice in succession so that they are at different stages of preparation and injection.
Because the injection volume is small and the needle is a very fine gauge, in our experience, post-procedural analgesia was not necessary. The mice rapidly recovered from the anesthesia and seemed clinically normal within minutes. Complications were very rare. Although one mouse had to be euthanized after accidental laceration of an intrathoracic vessel, the ultrasound equipment allowed us to see the immediate hemorrhage and to euthanize the mouse while it was still anesthetized, avoiding any pain or distress.
Ultrasound guidance represents a refinement of the intrathymic injection technique because it causes less distress to the mice. When we used this technique, mice appeared normal within minutes of recovering from anesthesia. Other intrathymic injection techniques require an incision6 or a thoracotomy, which is associated with pain and a high degree of morbidity8. In addition, this morbidity and the resultant release of stress hormones may be a confounding factor in studies of immunity8. In comparison, the use of ultrasound guidance did not cause distress in mice. The lack of distress could mean there are fewer confounding factors in a study and, potentially, fewer animals needed.
Our goal in developing this technique was to provide a useful alternative to injection techniques that require surgical incisions with or without a thoracotomy. We successfully administered materials to 85 mice using this intrathymic injection technique. We verified accurate delivery of materials to the thymus in 25 mice by injection of Trypan blue dye and post-mortem examination. In addition, we observed good thymocyte labeling (~85%) in 55 mice that were given fluorescein isothiocyanate using this injection technique. We believe that greater thymocyte labeling could be achieved with additional experience using the technique and with greater expertise using the ultrasound equipment.
We realize that some investigators may not have access to ultrasound equipment for the implementation of this procedure. When ultrasound equipment is available, however, our results suggest that ultrasound-mediated guidance of injection techniques is a refinement that can help to reduce distress and animal usage in experiments involving intrathymic injection. Although we have only used this procedure in mice, we believe that it would be equally successful in a variety of animal models.
Acknowledgments
We thank Daryl Despres from the National Institutes of Health Mouse Imaging Facility for his assistance with this project. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Deschepper CF, Olson JL, Otis M, Gallo-Payet N. Characterization of blood pressure and morphological traits in cardiovascular-related organs in 13 different inbred mouse strains. J Appl Physiol. 2004;97:369–376. doi: 10.1152/japplphysiol.00073.2004. [DOI] [PubMed] [Google Scholar]
- 2.Guidos CJ, Weissman IL, Adkins B. Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci USA. 1989;86:7542–7546. doi: 10.1073/pnas.86.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scollay R, Butcher E, Weissman I. Thymus cell migration: quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 4.Seggewiss R, Dunbar CE. A new direction for gene therapy: intrathymic T cell-specific lentiviral gene transfer. J Clin Invest. 2005;115:2064–2067. doi: 10.1172/JCI26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto M, Kubo E, Sado T. Development of prelymphoma cells committed to thymic lymphomas during radiation-induced thymic lymphomagenesis in B10 mice. Cancer Res. 1987;47:3469–3472. [PubMed] [Google Scholar]
- 6.de la Cueva T, Naranjo A, de la Cueva E, Rubio D. Refinement of intrathymic injection in mice. Lab Anim (NY) 2007;36:27–32. doi: 10.1038/laban0507-27. [DOI] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- 8.Institute for Laboratory Animal Resources. Recognition and Alleviation of Pain in Laboratory Animals. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]