Abstract
Evaluation of the Case Western Reserve University spiral nerve cuff electrode on the femoral nerve trunk was performed intraoperatively in four subjects undergoing femoral-popliteal bypass surgery. The threshold, nerve size and selective activation capabilities of the electrode were examined. The activation thresholds for the first muscle to be recruited were 6.3, 9, 10.6, and 37.4 nC with pulse amplitudes ranging from 0.3 to 1 mA. The femoral nerve was found to have an elliptical cross-section with a major axis average length of 9 mm (8–12 mm) and a minor axis length of 1.5 mm. In all four subjects selective activation of the sartorius was obtained. In two subjects, the rectus femoris could also be selectively activated and in one subject the vastus medialis was selectively activated. Each electrode had four independent contacts that were evaluated separately. Small air bubbles were formed in the space over some contacts, preventing stimulation. This occurred in one contact in each electrode, leaving three effective stimulation channels. This issue has been corrected for future studies.
1. Introduction
Functional electrical stimulation (FES) is used to elicit contractions in paralyzed muscles and increase the independence of people with impaired neurological function. In most existing systems, intramuscular and epimysial electrodes [1–3] are used to activate the paralyzed muscles. Nerve cuff electrodes are an alternative method of muscle activation.
The femoral nerve innervates knee extensors (vastus lateralis, medialis and intermedius) as well as hip flexors (sartorius and rectus femoris). Fisher et al [4] placed a spiral nerve cuff electrode distal to the branching of the femoral nerve, excluding most branches to the hip flexors, to activate the uni-articulate heads of the quadriceps and elicit standing. To simplify the surgical implant and facilitate walking, electrodes could be placed prior to the branching and the desired functions, hip flexion or knee extension depending on the phase of gait, could be obtained using selective stimulation.
The ability of the Case Western Reserve University (CWRU) self-sizing spiral nerve cuff electrode [5] to selectively activate knee extensors and hip flexors from a site on the femoral nerve trunk was evaluated in this study. In animal studies [6, 7] it was possible to control multiple muscles or actions with a single multi-contact electrode placed on a compound nerve trunk. Studies in the human upper extremity using the spiral nerve cuff electrode have shown selective stimulation of a single muscle from a proximal location [8]. The purpose of this study was to intraoperatively evaluate the function of the spiral nerve cuff electrode on the femoral nerve trunk and determine nerve size and stimulation thresholds in preparation for chronic implant.
2. Methods
2.1. Subject recruitment
Subjects were recruited from non-paralyzed individuals scheduled for femoral-popliteal bypass surgery. Since the femoral nerve and artery are in close proximity and typically exposed together, this was a suitable population for femoral nerve stimulation. The nerve stimulation did not contribute to the outcome of the surgery. The study was approved by the institutional review board of the Louis Stokes Cleveland Department of Veteran Affairs Medical Center and all subjects gave informed consent prior to participation.
2.2. Spiral nerve cuff electrode
The CWRU spiral nerve cuff electrodes for this study (figure 1) were fabricated at the Technical Development Laboratory, part of the Cleveland FES VA Center of Excellence and Case Western Reserve University. The cuff electrodes consist of four ovoid pieces of platinum foil connected to seven-strand, stainless-steel, perfluoroalkoxy-coated wires between two layers of silicone sheeting. One layer of sheeting is stretched before being bonded to the unstretched sheeting. This unequal tension produces a spiral of a predetermined diameter with the four stimulation contacts spaced evenly about the circumference of the nerve [5].
Figure 1.
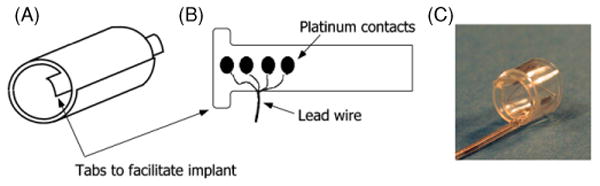
Schematic of spiral cuff electrode. (A) Spiral electrode coiled, resulting in two full wraps. (B) Electrode unwrapped to show contact layout. The four independent contacts are located at 90° around the nerve. (C) Photo of the electrode. Notice tabs added to the leading edge of the cuff to facilitate implantation.
Electrodes sized with an inner diameter from 6–10 mm were available for each surgery. After exposure of the femoral artery, the surgeon exposed 2 cm of the femoral nerve and measured the nerve using a surgical ruler. Since the femoral nerve in this location has an elliptical cross-section, measurements of the major and minor axes were taken. An electrode of the appropriate cross-sectional area was installed on the nerve trunk, distal to the inguinal ligament but prior to nerve branching.
2.3. Data collection
A schematic of the experimental setup is shown in figure 2. A battery powered, computer-controlled stimulator (Crishtronics, Cleveland, OH) delivered charge balanced, biphasic, rectangular pulses with an amplitude range of 0.02–5 mA (resolution 0.02 mA), and pulse width range of 10–500 μs (resolution of 2 μs). A 13 mm, 27 gauge sub-dermal needle (Axon Systems, Inc.) was used as the stimulation anode and inserted in the tissue near the cuff electrode. Bipolar needle EMG recording electrodes (Nicolet-VIASYS, Madison, WI) were placed within the vastus medialis, vastus lateralis (knee extensors), rectus femoris (knee extensor and hip flexor) and sartorius (hip flexor) [9, 10]. The muscle responses were preamplified near the implant site (B&L Engineering, Tustin, CA) and additional amplification, AC coupling and low-pass filtering at 1 kHz were performed by CED amplifiers (Model 1902, Cambridge Electronic Design, Cambridge, UK). The amplifiers were set to clamp the input signal during the stimulus pulse to prevent saturation of the amplifiers and remove stimulus artifact. The gain for each muscle was adjusted to maximize the size of a supra-maximal twitch response without saturation of the amplifiers (±8 V). The signals were acquired by a 16 bit, PCMCIA A/D card (DAQCard-6036E, National Instruments, Austin, TX) in a Dell Latitude laptop PC at 2.4 kHz.
Figure 2.
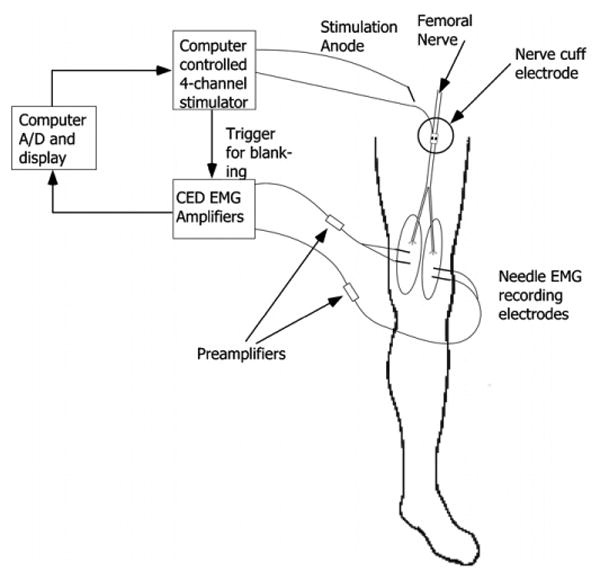
Schematic of the experimental setup. An EMG reference patch electrode was placed on the opposite hip.
The data collection algorithm (written in Matlab-Mathworks, Inc., Natick, MA) used a binary search routine to generate pulse width and pulse amplitude modulated recruitment curves. For each recruitment curve, one parameter (pulse amplitude or pulse width) was held constant while the other was varied by the algorithm, within the limits of the stimulator, and keeping the charge density below 0.5 μC mm−2. Twitch recruitment was used to minimize muscle fatigue and the duration of testing. The stimulation frequency was 4 Hz to minimize the experiment time without fusing of muscle twitches. To quantify each twitch, the area under the rectified M-wave was calculated between 5 and 40 ms post-stimulus [8]. The limits of 5 ms and 40 ms were chosen to eliminate stimulation artifact and any reflexes, respectively. Three twitch responses were averaged together for each point on the recruitment curve.
For each subject, an initial recruitment curve was generated using pulse amplitude modulation at 100 μs to compare across all subjects. Subsequent curves were generated using pulse width modulation, while lowering the pulse amplitude to reduce the recruitment slope and potentially increase the selective activation. Due to limited experimental time, two to four recruitment curves were collected from each subject on each stimulation channel. The experimental procedure added approximately 30 min to the 4 h surgery.
In addition to the surgical testing, a therapist evaluated the motor and sensory status of the subjects prior to surgery and 2 weeks after the procedure. Muscle strength of the quadriceps was assessed using the manual muscle test and one and two point discrimination and sensitivity to touch on the upper thigh of the limb to be tested were evaluated. This was to confirm that the experimental application and removal of the spiral nerve cuff electrode and stimulation of the nerve did not result in changes to the underlying neurophysiology.
2.4. Data analysis
The recruitment curves were processed and analyzed to determine the stimulation thresholds and selectivity of each electrode. The percent activation was calculated by normalizing each data point by the maximum quantified M-wave recorded for that subject. The muscle threshold was defined as 10% of this maximum value. The nerve threshold for each subject was reported as the lowest threshold charge across all contacts and muscles. Selective activation was quantified as the percent activation of one muscle before any other muscle reached threshold. Preferential activation was defined as the percent activation of one muscle before any other muscle reached 20% activation.
3. Results
Five male subjects undergoing a femoral bypass surgery participated in this study. The manual muscle tests, light touch and pin prick sensory exams from all subjects showed no significant post-operative changes, indicating no change in femoral nerve physiology.
3.1. Nerve dimension
The size of the nerve was measured in each subject (table 1). The length of the major axis ranged from 8 to 12 mm with a mean width of 9.2 ± 1.8 mm while the length of the minor axis was 1.5 mm for all nerves.
Table 1.
Nerve size and stimulation thresholds across subjects.
| Nerve size | Thresholda (nC (mA)) | ||||
|---|---|---|---|---|---|
| Subject no | (major × minor) (mm) | Sartorius | Rectus femoris | Vastus lateralis | Vastus medialis |
| 1 | 8 × 1.5 | No data recorded | |||
| 2 | 8 × 1.5 | 9(0.9) | 51 (1) | 63.5 (1) | 46.5 (1) |
| 3 | 12 × 1.5 | 6.3(0.3) | 34 (0.5) | 40.5 (0.5) | 30 (0.3) |
| 4 | 10 × 1.5 | 40.4 (0.4) | 37.4(0.4) | 61 (0.6) | 72.6 (0.7) |
| 5 | 8 × 1.5 | 17.4 (0.3) | 11.2 (0.4) | 12 (0.3) | 10.6(0.4) |
Thresholds are reported by the charge in nC with the pulse amplitude in mA in parentheses.
3.2. Activation thresholds
The lowest activation threshold from each nerve ranged from 6.3 nC to 37.4 nC (table 1—boxed values). This is similar to results from the human upper extremity where thresholds ranged from 3 nC to 62.5 nC [8]. In all cases, the muscle with the lowest threshold could be selectively activated (figure 4). Subject 1 had very high activation thresholds and no usable data were recorded.
Figure 4.
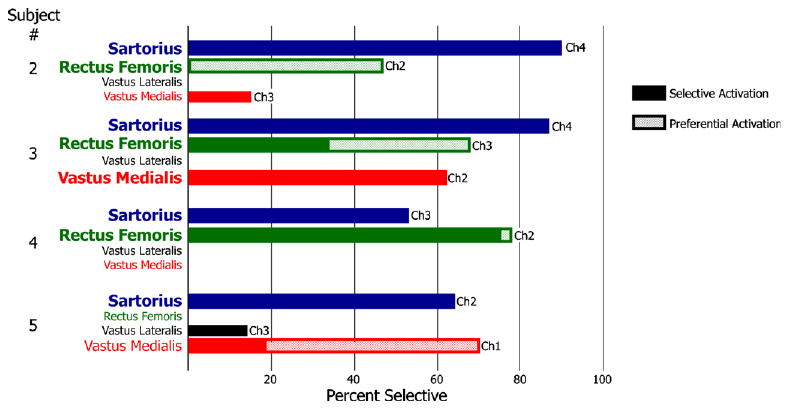
Selective activation from each subject (solid bars). A single muscle could be selectively activated to at least 50% in all subjects. The hatched bars represent preferential activation of muscles.
3.3. Selective activation
Full recruitment curves of the four muscles were generated to determine selective and preferential activation (figure 3). On channel 4 of subject 3, the sartorius was selectively activated to 65% before any other muscle reached threshold (figure 3(A)). On channel 3 of the same subject, the rectus femoris could be selectively activated to 34% and preferentially activated to 68% (figure 3(B)).
Figure 3.
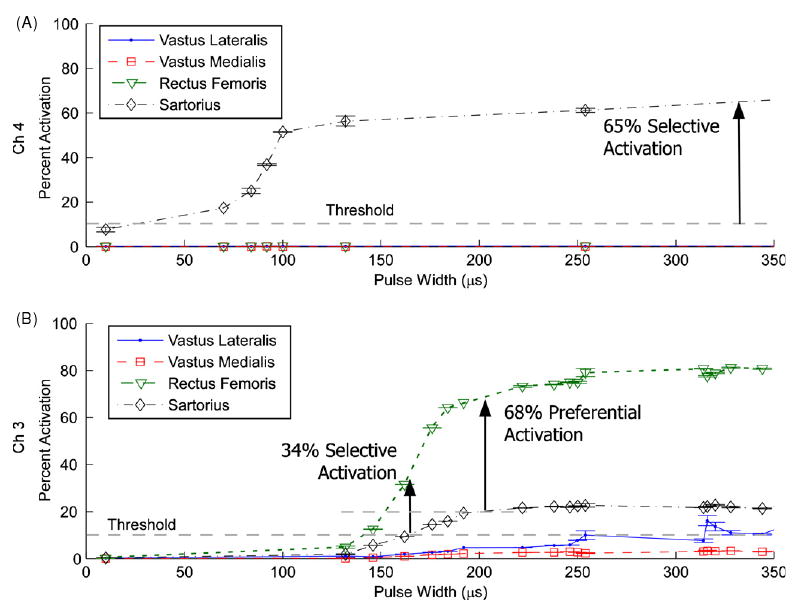
Pulse width modulation recruitment with a pulse amplitude of 0.3 mA from subject 3. (A) Stimulation on channel 4. Sartorius was recruited to 65% activation before any other muscle reached threshold. (B) Stimulation on channel 3. Rectus femoris was recruited to 34% activation before any other muscle reached threshold but to 68% if 20% activation of other muscle was allowed. This was defined as preferential activation. Error bars show the standard deviation of each set of three twitches.
At least one muscle could be selectively activated over 50% in each subject (figure 4) and two muscles could be selectively activated over 50% in subjects 3 and 4. In two subjects, muscles that could not be selectively activated could be preferentially activated: the rectus femoris to 47% in subject 2 and the vastus medialis to 70% in subject 5. In subject 3, the preferential activation of the rectus femoris was 60% compared to a selective activation of 28% (figure 4-hatched bars). Hip flexors could be selectively activated in every subject while knee extensors were selectively activated in subject 3 and preferentially activated in subject 5.
3.4. Functionally selective knee extension
Two of the four recorded muscles, the vastus medialis and vastus lateralis, were functionally synergistic and only extended the knee. In subject 3, these two muscles were activated before either hip flexor using pulse width modulation at 0.5 mA (figure 5). The vastus medialis reached 37% and the vastus lateralis reached 42% before the sartorius reached threshold. Even though there was no selective muscle activation, greater activation of the knee extensors was obtained compared to the vastus lateralis alone. In the other subjects, both knee extensors were not recruited before the hip flexors.
Figure 5.
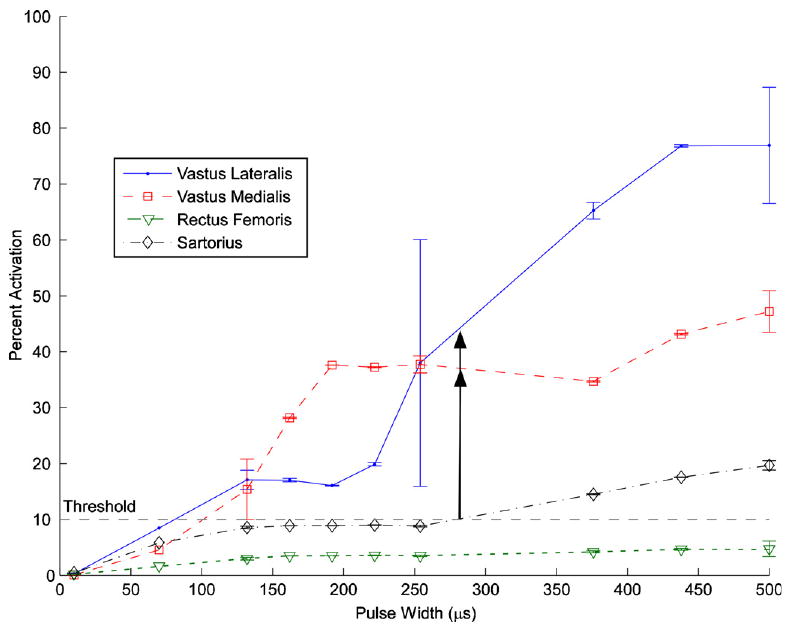
Example of functional selectivity: selective knee extension from activation of both the vastus lateralis and the vastus medialis in subject 3 using a pulse width modulation of 0.5 mA on channel 2. The vastus medialis reached 37% and the vastus lateralis reached 42% before either hip flexor reached threshold. Error bars show the standard deviation of each set of twitches.
4. Discussion
The CWRU spiral nerve cuff electrode was tested intraoperatively on the human femoral nerve trunk. Stimulation thresholds were similar to values found during upper extremity nerve stimulation. Selective activation of at least one muscle was found in each subject. Hip flexors could be selectively activated in every subject. In one subject, selective activation of the knee extensors was obtained.
The dimensions of the femoral nerve measured intraoperatively can be compared with similar measurements from eight femoral nerve cross-sections, taken from four human cadavers fixed in formalin [11, 12]. In these fixed cross-sections, the mean major axis length was 10.5 ± 1.52 mm and the mean minor axis length was 2.3 ± 0.63 mm. The major axis was not significantly different than the present study (p = 0.74, two sample t-test) but the minor axis was significantly different (p = 0.03, two sample t-test). This agrees with the previous experience where cadaver measurements have over-estimated nerve cuff electrode sizes (unpublished observation).
One muscle could be selectively activated in every subject. This was consistent with results from intraoperative testing of nerves in the upper extremity where the muscle that was selectively activated was the first to branch distal to the nerve cuff electrode [8]. It is reported [13] that branching axons remain closely grouped together proximal from the branch, sometimes for a considerable distance. This proximity of axons innervating the same muscle may explain why the first muscle to branch is always selectively activated. In the femoral nerve, the first muscle to branch is the sartorius [12], which could be selectively activated in all subjects to at least 53%.
This was an acute, intraoperative study, but the long-term stability of spiral nerve cuff electrode stimulation has been reported elsewhere. Prior studies in the upper extremity looked at the stability of the selective activation and found that the selectivity after 3 years was consistent with the selectivity measured during the implant surgery [14]. The chronic activation thresholds of spiral nerve cuffs have also been found to be stable for electrodes implanted on individual branches of the femoral nerve [4].
In this study, all the electrodes had four independent stimulating contacts but in each case one contact did not produce muscle contraction, leaving only three available contacts. All contacts in the electrode had intact electrical continuity, tested before and after the surgery. The contacts are inset by 0.1 mm, the thickness of the sheeting, with a round opening centered over the contact. An air bubble in this ‘well’ will occlude the contact, creating a path of high impedance that prevents delivery of stimulation. Following this study, a testing procedure was instituted and performed prior to electrode use. First, air bubbles are removed by injecting saline over the surface of the electrode, using a needle-less syringe. Then, a test current is passed through each electrode contact in a saline bath to insure that all contacts are free of air bubbles and saline is injected again as needed.
The femoral nerve has an oblong cross-section [11]. When the spiral nerve cuff electrode was placed on the femoral nerve, the nerve was reshaped to have a circular cross-section. The details of the resultant fascicle movement are unknown but it is possible that the fascicles that had previously been grouped by muscle became mixed. Others have shown that an electrode with a more oblong cross-section, similar to the natural shape of the nerve, could be beneficial to selectivity [12, 15, 16].
5. Conclusion
Stimulation on the femoral nerve trunk using the spiral nerve cuff electrode resulted in selective activation of one to three muscles from each nerve, consistent with previous results using the spiral nerve cuff electrode in the upper extremity. Selective activation of muscles that flex the hip followed by knee extensors could be obtained in all subjects. This would be beneficial for a walking neuroprosthesis.
Acknowledgments
This work was supported by grant EB001899 from the National Institutes of Health, and with resources and the use of facilities at the Louis Stokes Cleveland Department of Veterans' Affairs Medical Center.
References
- 1.Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Rehabil Eng. 1994;2:80–91. [Google Scholar]
- 2.Akers JM, Peckham PH, Keith MW, Merritt K. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1997;5:207–20. doi: 10.1109/86.593301. [DOI] [PubMed] [Google Scholar]
- 3.Uhlir JP, Triolo RJ, Davis JA, Jr, Bieri C. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:279–87. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- 4.Fisher LE, Tyler DJ, Anderson JS, Triolo RJ. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. J Neural Eng. 2009;6:046010. doi: 10.1088/1741-2560/6/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng. 1988;35:905–16. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 6.Grill WM, Jr, Mortimer JT. Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Trans Rehabil Eng. 1996;4:49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- 7.Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2004;12:251–7. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]
- 8.Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans Neural Syst Rehabil Eng. 2007;15:76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 9.Delagi EF, Perot A, Thomas CC. Anatomic Guide for the Electromyographer: The Limbs. 2nd. Springfield, IL: Thomas; 1981. [Google Scholar]
- 10.Lee HJ, DeLisa JA. Surface Anatomy for Clinical Needle Electromyography. New York: Demos; 2000. [Google Scholar]
- 11.Gustafson KJ, Neville JJ, Syed I, Davis JA, Jr, Triolo RJ. Fascicular anatomy of the human femoral nerve: implications for neural prostheses utilizing nerve cuff electrodes. J Rehabil Res Dev. 2009;46(7) doi: 10.1682/jrrd.2008.08.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart JD. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve. 2003;28:525–41. doi: 10.1002/mus.10454. [DOI] [PubMed] [Google Scholar]
- 14.Polasek KH, Hoyen HA, Keith MW, Kirsch RF, Tyler DJ. Stimulation stability and selectivity of chronically implanted multi-contact nerve cuff electrodes in the human upper extremity. IEEE Trans Neural Syst Rehabil Eng. 2009;17(5) doi: 10.1109/TNSRE.2009.2032603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 16.Leventhal DK, Durand DM. Subfascicle stimulation selectivity with the flat interface nerve electrode. Ann Biomed Eng. 2003;31:643–52. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]


