Abstract
In iron overload conditions, plasma contains non-transferrin bound iron species, collectively referred to as plasma NTBI. These include iron-citrate species, some of which are protein bound. Because NTBI is taken into tissues susceptible to iron loading, its removal by chelation is desirable but only partial using standard deferoxamine (DFO) therapy. Speciation plots suggest that, at clinically achievable concentrations, deferiprone (DFP) will shuttle iron onto DFO to form feroxamine (FO), but whether NTBI chelation is enhanced to therapeutically relevant rates is unknown. As FO is highly stable, kinetic measurements of FO formation by HPLC or by stopped-flow spectrometry is achievable. In serum from thalassemia major patients, supplemented with 10µM DFO, FO formation paralleled NTBI removal but never exceeded 50% of potentially available NTBI: approximately one third of NTBI was chelated rapidly but only 15% of the remainder at 20h. Addition of DFP increased the magnitude of the slower component, with increments in FO formation equivalent to complete NTBI removal by 8h. This shuttling effect was absent in serum from healthy control subjects, indicating no transferrin iron removal. Studies with iron-citrate solutions also showed biphasic chelation by DFO, the slow component being accelerated by the addition of DFP, with optimal enhancement at 30µM. Physiological concentrations of albumin also enhanced DFO chelation from iron citrate, and co-addition of DFP further accelerated this effect. We conclude that at clinically relevant concentrations, DFP enhances plasma NTBI chelation with DFO by rapidly accessing and shuttling NTBI fractions that are otherwise only slowly available to DFO.
INTRODUCTION
Plasma non-transferrin bound iron (NTBI), is a heterogeneous collection of iron species, typically found in iron overload conditions at 1–10µM when transferrin saturation approaches 100% 1. NTBI is important because it is thought to be the main mechanism by which the myocardium and endocrine tissues become overloaded with iron in conditions associated with excess body iron 2. Conventional chelation treatment with deferoxamine (DFO) infusion achieves steady state DFO concentrations no greater than 10µM, clearing only a fraction of NTBI during the infusion 3, with NTBI rapidly returning to pre-chelation levels within minutes of the infusion ending 3, 4. Incomplete NTBI removal during infusion is not simply related to the plasma concentration of DFO achieved as in vitro studies have shown that only a sub-fraction of plasma NTBI can be ‘directly’ chelated by DFO even at higher DFO concentrations 5. This may reflect the relative unavailability of oligomeric and polymeric species of iron-citrate 6, 7 or albumin bound species 6, 8 to direct chelation by DFO. Incomplete NTBI removal is also seen with other chelation monotherapies. For example, deferiprone (DFP) monotherapy has shown only partial NTBI removal 9, 10 as well as transient and incomplete removal of a redox active subfraction of NTBI termed ‘labile plasma iron’ (LPI) 11, 12. Patients treated with deferasirox monotherapy also show incomplete removal of NTBI 13, even though LPI is progressively removed partly due the long plasma residency of this drug 12.
There is therefore considerable interest in designing chelation regimens that remove NTBI more effectively, so as to minimize uptake into target tissues. In principle, by combining DFO with DFP, improved removal of NTBI could be achieved. While sequential use of DFO and DFP has been shown to decrease the duration of exposure to LPI 11, the shuttling of NTBI onto DFO by DFP has not been directly demonstrated, nor have the conditions under which all NTBI species can be cleared from plasma been elucidated. Mixed ligand therapy (MLT) is an attractive approach however, because a marked synergism of metal chelation can occur when a small kinetically labile ligand, such as DFP, is combined with a larger hexadentate chelator with a greater stability for iron binding, such as DFO. The effective combination of two ligands to enhance chelation rates (MLT) has been demonstrated for a range of metals 14. Typical examples are nitrilotriacetate (NTA) iron shuttling from transferrin to DFO 15, penicillamine/diethylene triamine pentaacetic acid for copper removal 16 and salicylic acid/EDTA for plutonium removal 17.
MLT for iron overload using DFP with DFO, often referred to as ‘combination therapy’ has been used clinically and benefits to iron balance 18 and myocardial iron deposition 19 have been demonstrated. However it is not known whether true ‘shuttling’ of iron occurs between DFP and DFO and how this influences NTBI removal within the plasma compartment. Combinations of these drugs can be applied in two broad ways. Firstly DFP can be administered orally by day with DFO infused subcutaneously over 8–10h at night, thus achieving exposure to chelation for nearly 24 hours each day. However, this is not true MLT, as little or no direct interaction between the two chelators will occur because of their short plasma half-lives. A second approach is to allow the chelators to mix, either in the plasma or in tissues, by administering them simultaneously. Improved chelation with this second approach relies on the principle of the low molecular weight bidentate DFP rapidly accessing chelatable iron pools unavailable to DFO and subsequently ‘shuttling’ the chelated iron onto a DFO ‘sink’ 20, 21. In principle, iron shuttling may occur in the plasma compartment or within cells, where more rapid access to intracellular iron pools by DFP may facilitate this process. In this paper we focus on the potential for shuttling in the plasma compartment as different (cellular and animal) models would be necessary to examine intracellular shuttling mechanisms.
The relative stabilities of DFO and DFP for iron can be represented by the pM values, where the pM of a given chelator for a metal (M), here iron (III), is −log of the uncoordinated metal concentration under defined conditions 22. This is higher for DFO (pM =27.6 23) than for DFP (pM=19.9 24) and is reflected in speciation plots for mixtures of the two chelators, which predict that iron(III) will to bind preferentially to DFO at equilibrium under clinically relevant concentrations of DFO and DFP. However this analysis does not predict the rate at which equilibrium is reached and a rapid rate will be required for clinical impact. Shuttling of iron between DFP and DFO has not been unequivocally demonstrated however. For instance, in animal studies, there is evidence for an additive rather than a synergistic effect on iron excretion 25.
One reason that the kinetics of NTBI removal have not been previously reported with simultaneous use of DFP and DFO is because measurement of total plasma NTBI is technically difficult in the presence of two chelators, where shuttling may continue in vitro after a blood sample has been taken 3, 26. One way around this is to measure ‘labile plasma iron’ using methodology that does not perturb the speciation of NTBI 11, 27. However LPI is only a subfraction of total NTBI and other NTBI species that are not detected in the LPI assay may be critical to tissue iron uptake. It is therefore important to understand how much iron is actually chelated in the plasma compartment with any given regime and whether the iron is derived from NTBI. In this work we have examined the kinetics of total plasma NTBI chelation by DFO, in the presence and absence of DFP, by measuring the rate of formation of the iron complex feroxamine (FO), exploiting the high stability of this complex during assay procedures3. FO formation has been investigated over time periods of hours (by HPLC) in iron overloaded and normal plasma or over seconds (by stopped flow spectrometry) in defined iron solutions, modeled to reflect the heterogeneous nature of NTBI 6. We have also related the total FO formation, with and without addition of DFP, to the total measurable plasma NTBI prior to chelation. The mechanisms and kinetics of the processes have been examined in order to determine whether DFP does indeed act as in intermediary ‘shuttle’ for plasma NTBI onto DFO and whether this occurs at a useful rate. Elucidation of the optimal conditions for iron shuttling in plasma would provide a rationale for optimizing co-administration of these iron chelators clinically.
METHODS
Materials
Deferoxamine (Desferal ® DFO) was purchased from Novartis (Basel, Switzerland). DFP was synthesized as previously described 28. 3-[N-Morpholino]propanesulfonic acid (MOPS), human serum albumin, fraction V and (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS) were purchased from Sigma-Aldrich (Poole, UK). HPLC grade acetonitrile, citric acid and potassium dihydrogen orthophosphate were obtained from VWR International (Lutterworth, UK). Iron atomic absorption standard solution was from Sigma-Aldrich. Chelex® 100 Resin was from Bio-Rad laboratories (CA, USA) and 30 KDa Molecular Weight cut-off Polysulphone Micro Vectaspin filtration devices were obtained from Whatman (Maidstone, UK). Vision spectrophotometric software was from Spectronic Unicam, Cambridge, UK. Deionized water was produced by a Millipore system (Simplicity 185, Millipore, USA) and was used throughout the study.
Speciation Plot
An essential prerequisite for DFP to shuttle iron to DFO is that the molar ratios and iron binding constants favor this process under physiologically relevant conditions. In order to understand the conditions and molar proportions under which iron would be donated from DFP to DFO speciation plots revealing the theoretical proportions of iron complexed to DFP and DFO at steady state under increasing concentrations of DFP were prepared. The speciation plot showing the molar fraction of iron bound to DFO or to DFP at steady state was calculated using the Hyperquad Simulation and Speciation program (HYSS) 29. The affinity constants of DFP, DFO and hydroxide ion for protons and iron(III) used in the speciation plot calculations were from published data 24.
Serum samples from thalassemia and healthy control subjects
Blood samples for in vitro studies were obtained from adult patients (mean age, 33.2 range 31–36y) with thalassemia major (receiving > 8 transfusion episodes/year; 3 male, 3 female) attending the thalassemia clinic at University College Hospital, UK (UCLH). All patients were receiving regular chelation therapy with DFO but samples were only drawn in those who had not received iron chelation for ≥48h. The mean patient serum ferritin value was 1790µg/L, range 550–2934µg/L. 10ml of venous blood was taken into glass tubes, free of anticoagulant, and after clot formation samples were centrifuged at 4°C for 10 min at 1000g and the serum decanted. Serum was then rapidly frozen in aliquots and stored at −80°C until time of analysis. Serum samples were screened for the absence of DFO prior to conducting the experiment. Serum was prepared from healthy controls in the same manner. Informed consent was obtained for collection of samples and this was approved by the institutional review body for University College Hospital, UK. Research was conducted according to the principles of the Declaration of Helsinki.
Preparation of iron complexes
Stock iron citrate (100:1000 µM) was prepared by mixing iron atomic absorption standard with citric acid in water and adjusting the pH to 7.4 with 0.25 M NaOH. When ageing of iron citrate was required, the mixture was left for 24 h either at room temperature (RT) or 37°C. For experiments, the mixture was diluted in 20mM MOPS pH 7.4 to give a final concentration of 10 µM iron:100 µM citrate. Iron-citrate-albumin complex was prepared by the same method except that albumin was carefully mixed with an iron-citric acid mixture to give a solution containing 0.05 mM iron: 0.5 mM citrate: 200 g/L albumin at pH 7. No pH adjustment was necessary here as the high concentration of albumin acted as a buffer. When aged, this mixture was left for 24 h at RT or 37°C. For use in experiments, the mixture was diluted five-fold in 20mM MOPS pH 7.4 to give a final concentration of 10 µM iron: 100 µM citrate: 40 g/L albumin. Where indicated, some complexes were prepared using albumin that had been passed through Chelex®100 anion exchange resin to remove residual contaminating iron.
Time course incubations
In serum from healthy control subjects or from thalassemia major patients, the rate of FO formation from DFO was examined by HPLC (as described below), in the presence and absence of clinically relevant concentrations of DFP. Serum samples were incubated with 10 µM DFO either alone or with DFP (30 µM), and were deproteinized using Whatman Vectaspin ultracentrifugation devices (Molecular Cut-off 30 Kda) at 12320g 4°C for 20 min prior to injection onto the column and with CHAPS (10 mM final concentration) added to each sample prior to filtration in the ultracentrifugation device. NTBI in the sera from thalassemia major patients was also measured, using the method of Singh and co-workers 30 and incorporating previously described minor modifications 4, 31. By comparing these baseline NTBI values with the FO concentration in the same samples at equilibrium, the proportion of NTBI that is chelatable by DFO could be calculated both with and without DFP.
Time course experiments were also undertaken with iron citrate, the fraction of plasma NTBI that is thought to predominate in iron-overloaded patients 6, 32. Physiologically relevant concentrations of iron and citrate were chosen, with relevant ratios of iron to citrate (Fe: 10µM : citrate:100µM), as the behavior of iron citrate complexes is critically dependent on this ratio 6. 10µM iron was chosen, as NTBI is typically found in plasma at concentrations up to 10µM 4. Concentrations of DFO and DFP that were used were also clinically relevant: under clinical conditions of DFO infusion, plasma DFO is typically present at concentrations less than 10µM 3, 33, whereas plasma concentrations of DFP lie between 30 and 300µM 34–36. Albumin was added in selected experiments at physiologically relevant concentrations (40g/L).
Three methods were used to study rates of FO formation (or FP formation) in these iron citrate solutions. For the slower phases of the reaction time course, HPLC and standard spectrophotometry were used, whereas stopped-flow spectrophotometry was used to examine the fastest phases. In time course experiments where FO formation rates were determined by HPLC, DFO (10 µM) was incubated with iron-citrate (10 µM: 100 µM) or iron-citrate-albumin (10 µM: 100 µM: 40 g/L) complexes in 20mM MOPS buffer at pH 7.4, either alone or in the presence of DFP (30 µM) directly in HPLC vials at RT or 37°C. As the sequence of DFP and DFO addition was found not to change the results, DFO was therefore added 5min after DFP in all experiments. Samples of the iron-citrate reaction mixtures were then taken at regular time intervals and injected immediately onto an HPLC column for feroxamine (FO) determination (details follow). Albumin-containing samples were first deproteinized using Whatman Vectaspin ultracentrifugation devices (Molecular Cut-off 30 Kda) at 12320g 4°C for 20 min prior to injection onto the column.
With time course experiments that determined feroxamine (FO) and/or feriprone (FP) formation rates spectrophometrically over periods up to 19.5h, serial spectral scans were run on identical iron citrate reaction mixtures to those used in the HPLC, scanning from 350 to 650 nm every 0.5 h at RT using Vision scanning software and a Unicam UV2 uv/vis spectrophotometer. Absorbances were converted to µM concentrations of chelate complex, using E 1 cm M = 2392 for FO and 4133 for the iron-DFP complex respectively* (P.Evans, unpublished results), after subtraction of the control absorbance of the iron citrate solution monitored over the same time period under identical conditions. In practice, this subtraction had a negligible effect on the rate profiles. With time course experiments that determined the fast phase kinetics, a stopped flow spectrophotometer (SF 51 instrument, Hi-Tech Scientific, Salisbury, UK) was used. Light from a Quartz Halide lamp was passed through the monochromator to give light at 460 nm. The cell path-length was 1 cm. Concentrations quoted are those in the syringes and hence mixing chamber concentrations are half these values.
Feroxamine (FO) determination by HPLC
FO was measured by a simple isocratic HPLC system. A metal-free HPLC system with non-metallic polyether-ethylketone tubing throughout was used (Waters Bio-System 625 LC, non-metallic gradient module with 996 Photodiode Array Detector and W717 autosampler). Samples were injected onto a Chrompak (ChromSpher-ODS, 5 µm, 3 mm × 10cm) glass column fitted with a Chrom Sep guard column (SS, 10 x 2 mm, reversed phase from Varian, Ltd.). Samples (50 µl) were either directly injected onto the HPLC column (for incubations with iron citrate only) or injected after deproteinization (iron citrate albumin and serum samples, method previously described). The isocratic chromatographic conditions were as follows: mobile phase 6% acetonitrile in 20 mM phosphate buffer at pH 7, flow rate 0.8 ml/min, and detection wavelength 430nm. FO levels were determined from a standard curve showing the peak areas corresponding to known serial dilutions of a freshly prepared 200 µM FO solution in 20mM MOPS. The method was validated by co-elution of spiked authentic FO and comparison of peak spectra with the spectrum of FO. There was no chromatographic interference from DFP-iron complexes that were not retained by the column under the conditions used.
Statistical analysis
2- Way Anova using Prism software was used to compare time courses without curve fitting. This was then used to determine whether treatment and time were significant sources of variation (usually at the p<0.0001 level). If this was the case, a Bonferroni post-test was performed to determine whether there were significant differences in iron complex formation between treatments (ie different chelators/chelators in combination) at particular time points. The first order rate constants for kinetic reactions in the stopped-flow were calculated by the Hi-Tech software (KS1, Kinetic Studio Software, UK) using non-linear fit models.
RESULTS
Conditions of iron donation from DFP to DFO
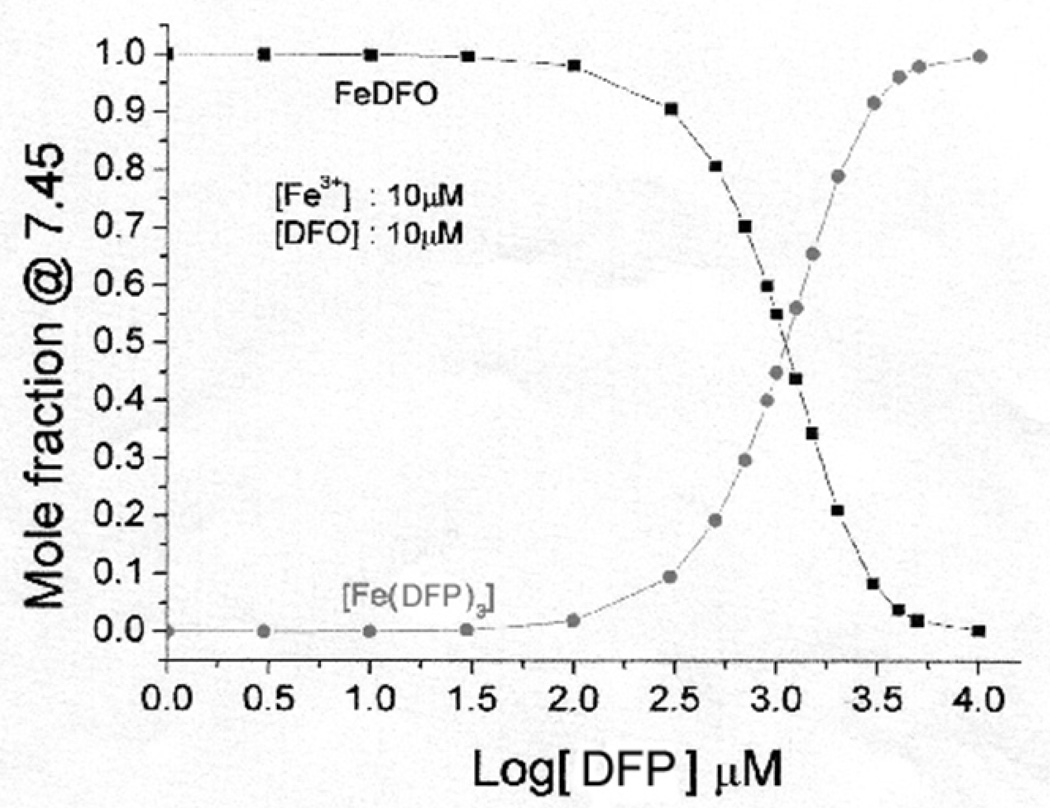
Speciation plot analysis shows that at 10µM iron (III) and 10µM DFO, the proportion of iron present as FO at equilibrium is critically dependent on the concentration of DFP (Figure 1) when these two chelators are present simultaneously. At DFP concentrations between 10µM and 30µM, over 99% of the iron is bound to DFO whereas even at 100µM DFP, this proportion only rises to about 3% of the iron bound to DFP. At 1 mM DFP, about 50% of the iron will be bound to the DFP and 50% to DFO, however this is well above the peak concentration of DFP found in plasma. Thus at clinically relevant concentrations of DFO of approximately 10µM and at clinically relevant concentrations of DFP (up to 100 µM), over 95% of iron will be bound to DFO as FO (Figure 1).
Fig 1.
Speciation plot showing the molar fraction of iron bound to DFO or to DFP at steady state. The speciation plot was calculated using HYSS 29, where the concentrations of iron and DFO are constant at 10µM, and the concentration of DFP was varied. The stability constants used for the calculations were from published data 24.
The distribution of iron in mixtures of DFP and DFO
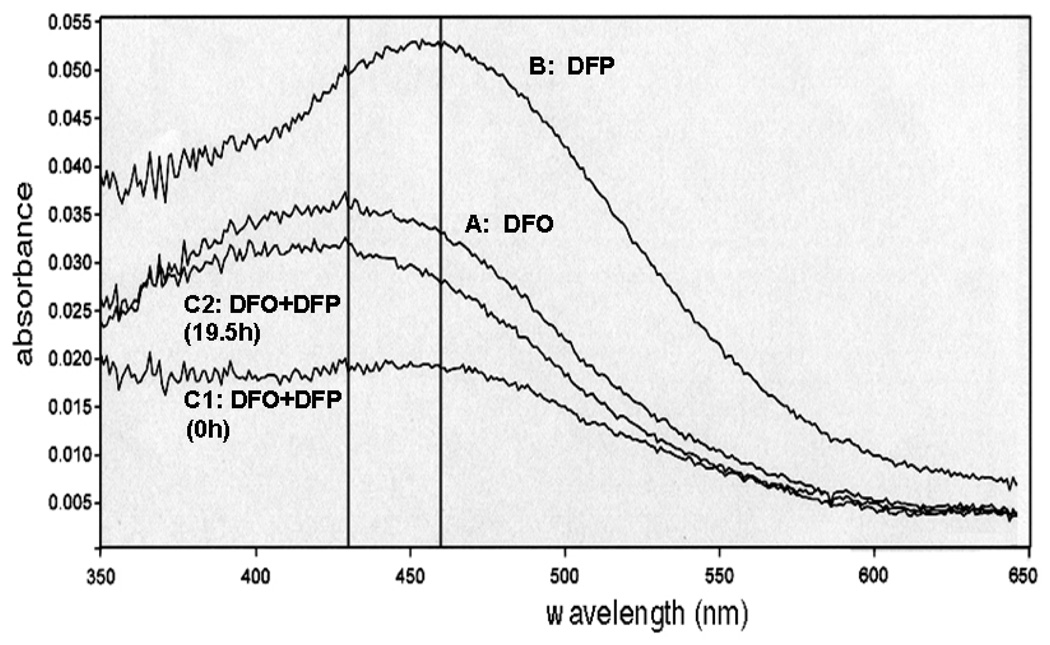
When DFO (10 µM) was incubated alone with iron citrate (10 µM Fe, 100 µM citrate), the spectral plot showed a peak for FO at 430 nm rising to its maximal level of A 430 = 0.035 (12.5 µM FO) over 19.5 hours at RT (Figure 2, trace A), final reaction mixture after 19.5 h incubation. For the same incubation but replacing DFO by an equivalent concentration of DFP (30 µM, ie 10µM iron binding equivalents), the maximum absorption of the DFP-iron complex was red-shifted to 460 nm and the amplitude of reaction appears higher due to the different molar absorption coefficients of the two respective iron complexes (Figure 2, trace B, final reaction mixture scanned after 19.5 h incubation). The reaction was however more rapid, being complete after 10h (A460 = 0.0525, 13.3 µM iron complex). When mixtures of iron citrate with both DFP and DFO were serially scanned between 350 and 650 nm for 19.5 h at RT, the absorption maximum shifted from 460nm immediately after mixing (Figure 2, trace C1) to 430nm being almost identical to the trace obtained with DFO alone at 19.5h (Figure 2, trace C2). During the incubation process, there was thus a sequential change from an absorption maximum at 460 nm to one at 430 nm when both chelators were present simultaneously. Intermediate spectral scans have been omitted for the purposes of clarity. The rate of change in absorbance (both at 430 and 460 nm) for the chelator mixture paralleled that for DFP alone rather than DFO, which was much slower.
Fig 2.
Demonstration of iron shuttling from DFP iron complex to deferoxamine (DFO) when iron citrate is co-incubated with DFO and DFP. The rates of formation of complexes of DFO or DFP from iron-citrate (10:100µM) in 20mM MOPS (pH 7.4) were compared by spectrophotometric scanning of the complexes formed between 350 and 700 nm every 0.5 hour for 19.5 h. For clarity, only selected traces of the final reaction mixtures are shown as follows: A: DFO (10 µM) and iron citrate (10:100 µM), B: DFP (30 µM) and iron citrate (10:100 µM), C1: DFO (10 µM) and DFP (30 µM) with iron citrate (10:100 µM) scanned immediately after mixing, C2: scan of final reaction mixture of C1. The reaction was initiated by addition of iron citrate.
Rates of FO formation from DFO in thalassemic serum containing NTBI, in the presence and absence of DFP
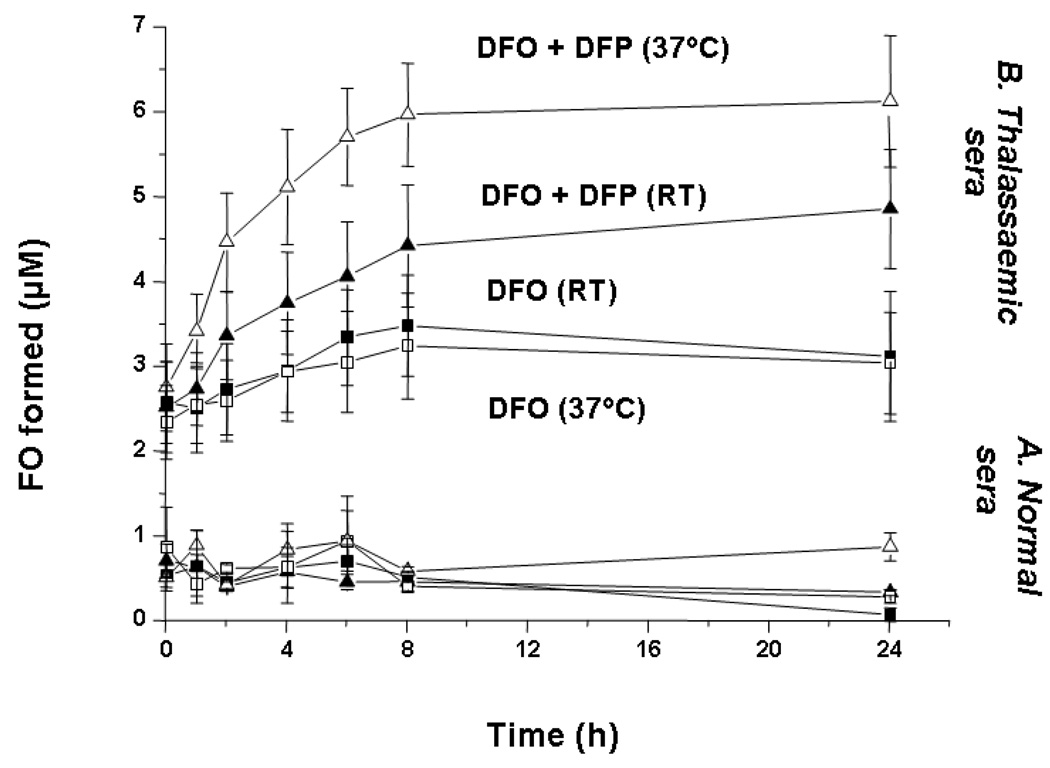
Serum of healthy donors or patients with thalassemia major was incubated with DFO with or without DFP at either room temperature or at 37°C and the rate of FO formation measured by HPLC as described in the methods section. When sera from six thalassemic patients, with a range of NTBI content between 3.5 and 5.4 µM, (mean NTBI 4.6 µM), were individually incubated with DFO (10µM) alone, a proportion of NTBI (34 ± 8%, calculated from the original data) was rapidly chelated, resulting in a mean of ~2.5 µM FO formation at the first time point ascribed as ‘time zero’ (Figure 3), with the temperature having no significant influence on the amount of FO formed. However, the subsequent kinetics of iron removal by DFO were slow, with only 3.2 µM FO formation by 8h (an additional 15%) and no further FO formation up to 24h either at room temperature or at 37°C (Figure 3, lower two traces for thalassemic sera).
Fig 3.
Rate of FO formation from normal and thalassemic plasma incubated with DFO. DFO (10 µM) was incubated with serum obtained from 5 normal subjects and six thalassemic patients whose serum contained 3.5–5.4 µM NTBI either alone (■) or in the presence of DFP (▲, 30 µM) both at RT (closed symbols) and 37°C (open symbols). Samples of the reaction mixtures were taken at regular time intervals, deproteinized through 30 Kda molecular weight cut-off filters and 50 µl of filtrate injected onto the HPLC column for FO determination using isocratic HPLC conditions. FO concentrations were determined using a standard curve showing the peak areas corresponding to known dilutions of a freshly prepared 200 µM FO mixture in 20mM MOPS pH 7.4. The data shown are the mean ± SE for the two study groups.
When DFP was included in the reaction mixture (30µM final concentration, same iron binding equivalents as 10µM DFO), this had no apparent effect on the fast phase of FO formation, with the amplitude of the rapid phase remaining at about 2.5 µM, but the kinetics of the subsequent iron removal were significantly increased (Figure 3, upper two traces for thalassemic sera). This effect was temperature dependent with 5.8 µM FO formation at 37°C and 4.3µM FO at RT after 8h incubation. All values for FO formation at 37°C with combined DFO and DFP were statistically different (p < 0.05) from those with DFO alone with the exception of time points 0 and 1 hour. FO formation was complete by 8h at 37°C. Under these conditions, very little iron was removed from control serum (Figure 3, lower traces, n=5) demonstrating that the increased formation of FO with combined chelators is not achieved by accessing transferrin-bound iron but by binding NTBI species. The initial rise in FO formation at zero time of around 0.75 µM FO in normal sera could be accounted for in terms of iron contamination in reagents: injection of the same reaction mixture but omitting serum also gave immediate FO formation at this same level. Thus DFP increases the availability of the slow phase component of NTBI to chelation by DFO in thalassemia patients. It appears that DFP is allowing the chelation of a fraction of NTBI (41 ± 10% at RT, 61 ± 8 % at 37°C after 24h incubation, calculated from the original data), which would otherwise be unavailable for chelation by DFO. Thus the magnitude of the chelatable NTBI pool available to DFO, in serum of thalassemia major patients, is increased by approximately 50% by addition of clinically relevant concentrations of DFP over a period of 24h, most of this increase occurring within the first 8h of incubation.
Rates of iron complex formation from iron citrate species using DFO and DFP alone or in combination
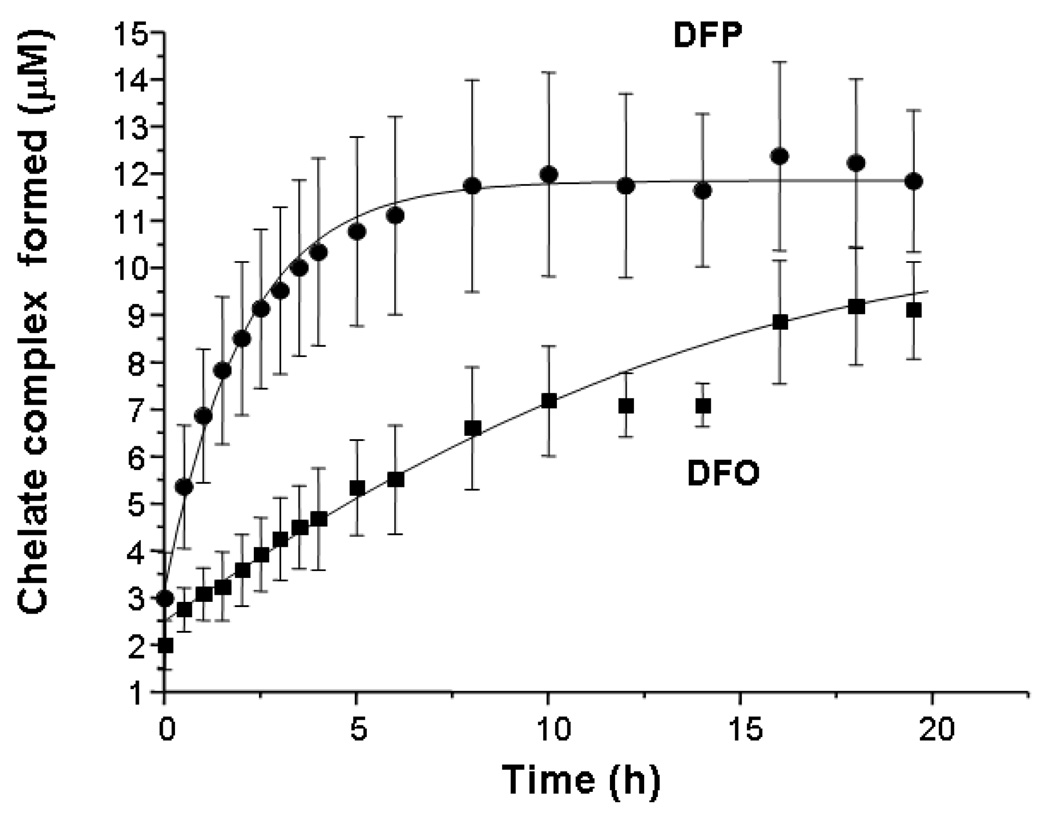
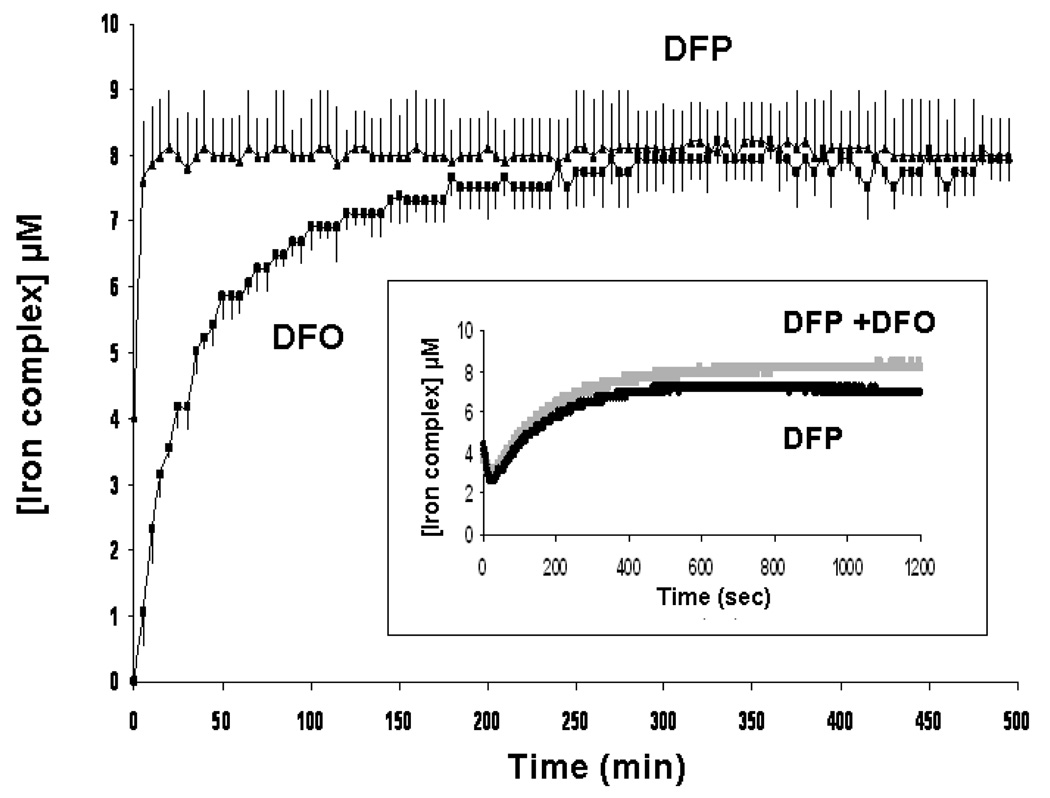
The rates at which DFP and DFO access iron citrate (10:100µM) were initially compared by monitoring formation of iron complexes continuously by spectrophotometry at room temperature (RT, Figure 4) and calculating their concentrations from the molar extinction coefficients (see Materials and Methods). It can be seen that there is a very rapid phase of chelation that has occurred by ‘time zero’ accounting for 2.5µM iron chelated with DFO and 3µM with DFP with no significant difference observed between the two chelators. The overall reaction was complete by 8h with DFP but was still incomplete by 19.5h with DFO at RT (amplitude 9.0µM at 19.5 h). Thus DFP accesses iron citrate species significantly more rapidly than DFO, during the slower second phase of this reaction.
Fig 4.
Rates of iron complex formation from iron citrate incubated with DFO or DFP alone. The rates of formation of iron complexes of DFO or DFP from iron citrate (10:100 µM) in 20mM MOPS (pH 7.4) were compared at equimolar iron binding equivalents of the two chelators, using spectrophotometry. The reaction was monitored continuously for 19.5h at 460nm at RT with DFO (■, 10 µM) or DFP (▲, 30 µM). Iron complex concentrations were calculated using previously determined extinction coefficients for both iron complexes. The data shown are the mean ± SE of 3 independent experiments.
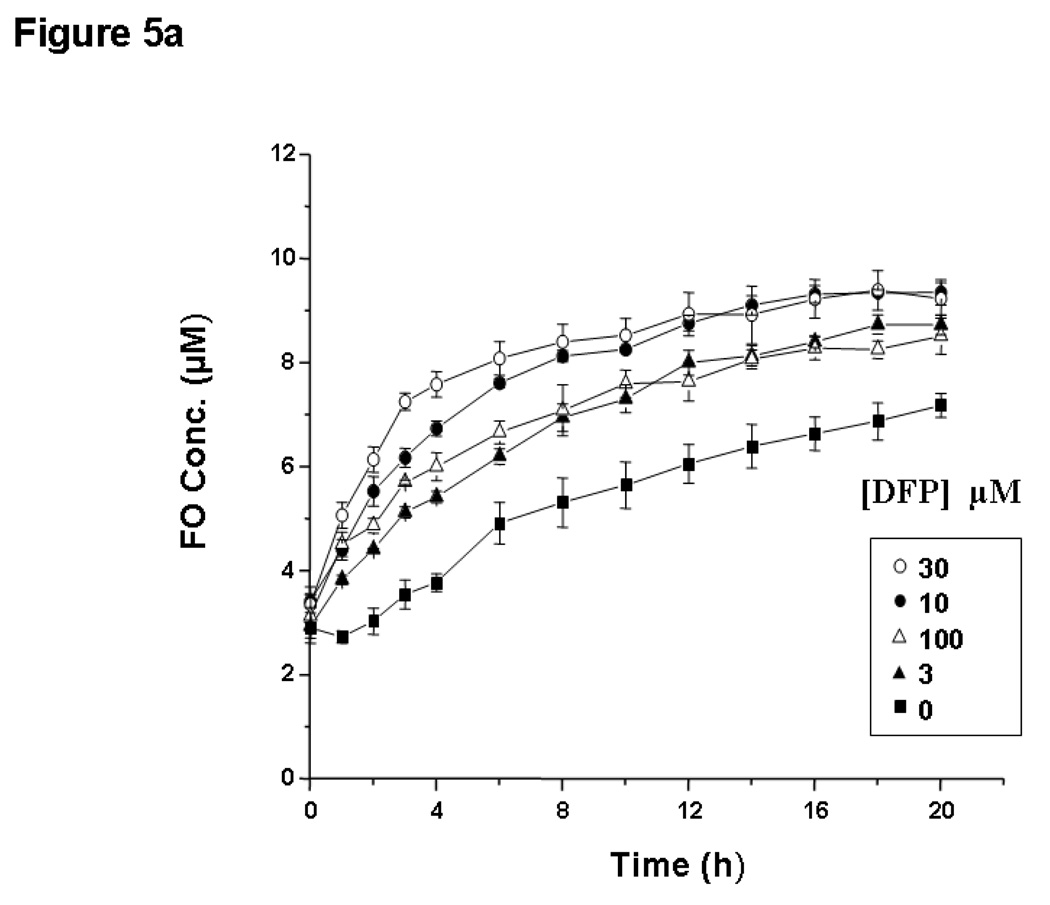
The time, temperature and concentration dependent effects of combining DFP with DFO on FO formation from iron: citrate were next examined using HPLC, which allows specific identification of the FO complex when mixtures of the two chelators are used. When DFO (10 µM) was incubated with iron citrate (10:100 µM) at RT for up to 24h, FO formation was again biphasic, taking over 24h to reach completion (Figure 5a, lowest trace), consistent with the spectrophotometrically determined kinetics of Figure 4. The fast phase had an amplitude of 3 µM FO and was too fast to measure by this method. It can be seen that DFP enhanced the rate of the slower second phase in a concentration-dependent manner, with the maximum effect at 30µM DFP (Figure 5a). However, even low concentrations of DFP (3µM) increase the rate of FO formation, consistent with the concept of DFP acting as a ‘shuttle’ at low concentrations. While the rate of FO formation was maximally increased at 30µM DFP, a further increase in DFP concentration to 100 µM showed a small decrease in the rate of FO formation compared to that observed with 10 or 30 µM DFP (Figure 5a), suggesting that DFP at higher concentrations will retain the chelated iron and subsequently slow its rate of shuttling to DFO. There was no significant difference between any of the FO concentrations measured at zero time for any combination of DFO and DFP when compared to DFO alone. Significant differences (p<0.05) between DFO alone and DFO plus all concentrations of DFP occurred in FO formation at all subsequent time points except where DFP was 3 µM. Here a significant difference was observed after 2 h and at all subsequent time points. It can be seen (Figure 5b) that the rate of the second phase of FO formation is temperature dependent both in the presence and absence of DFP. Thus FO concentrations reach a maximal 9.4 µM after 8h at 37°C, whereas at RT this was 6.4 µM after 8 h and only 9.0 µM after 24h (data for DFO alone). In contrast to the slow phase, the amplitude of FO formation in the fast phase (2–3 µM) was not significantly affected by any of the DFP concentrations tested (Figure 5a, p > 0.05). This phase could not be accounted for by iron contamination in any of the reagents used, which was determined as 0.75 µM by injection of reaction mixtures where iron was omitted.
Fig 5.
Enhancement of FO formation from iron citrate by DFP using HPLC: effects of DFP concentration and temperature. Fig 5a: DFP-concentration dependence of enhancement of FO formation from iron citrate and DFO is shown. DFO (10 µM) was incubated with iron:citrate (10:100 µM) in 20mM MOPS (pH 7.4), either alone or in the presence of various concentrations of DFP (3–100 µM) at RT. Samples of the reaction mixtures were taken at regular time intervals and injected onto the HPLC column for FO determination using isocratic elution. FO concentrations were determined as described for Figure 3. The data shown are the mean ± SE of 3 independent experiments. Fig 5b: Temperature dependence of rate of FO formation from iron citrate and DFO in the presence and absence of DFP. DFO (■, 10 µM) was incubated with iron-citrate (10:100 µM) in 20mM MOPS (pH 7.4), either alone or in the presence of DFP (▲, 30 µM) at RT (closed symbols) and 37°C (open symbols). Samples of the reaction mixtures were then taken at regular time intervals and injected onto the HPLC column for FO determination as described above. The data shown are the mean ± SE of 4 independent experiments.
Fast phase rates of iron complex formation from iron citrate using DFO and DFP alone or in combination
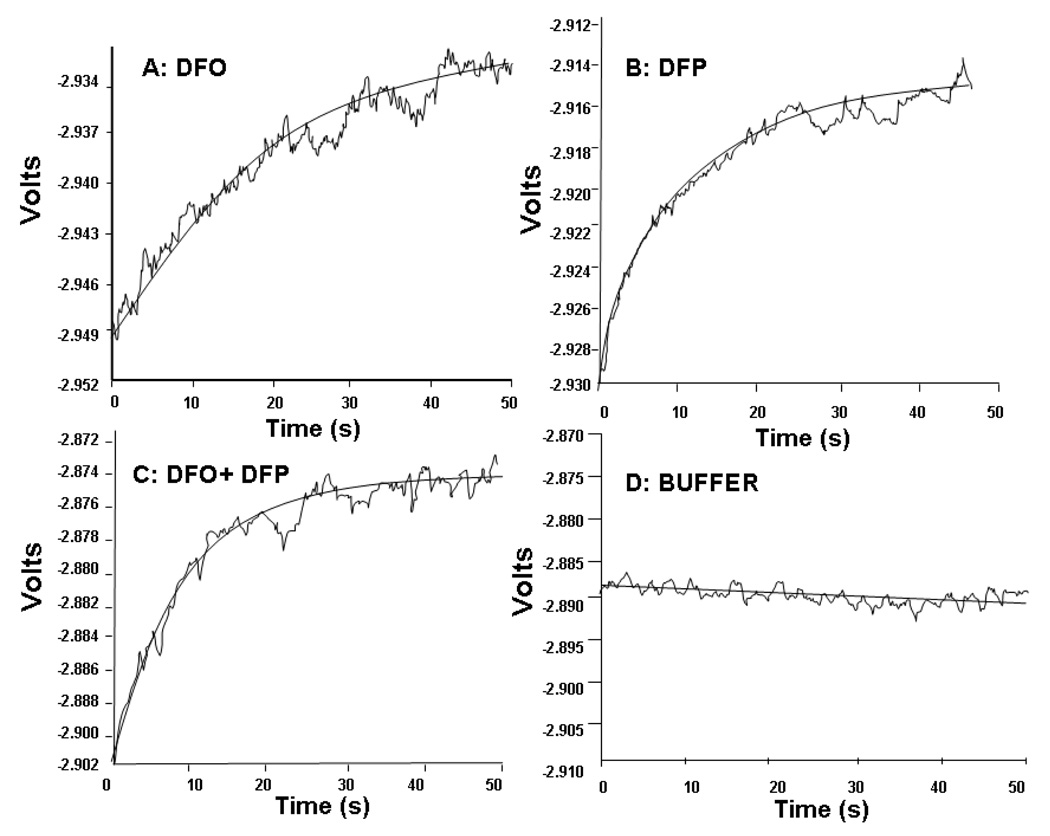
As neither HPLC nor conventional spectrophotometry are suitable to examine the fast phase of FO formation, the rate of this faster process was investigated over the first 50 seconds of reaction using a stopped flow spectrometer (Figure 6). This covers the time range inaccessible in the conventional spectrophotometer and HPLC, representing the mixing and injection time for incubations carried out in these instruments. Reactions of DFP (30µM) or DFO (10µM) with iron citrate (10:100 µM) gave clean exponential absorbance rises equivalent to the fast phase of reaction observed with the HPLC and spectrophotometric methods (Figure 6A and B). The rate of this fast phase was more rapid for DFP iron complex formation (rate constant for process 339.5h−1) than for DFO (160.6 h−1) but the amplitude of iron chelation was similar at 50 seconds showing a similar proportion of total available iron chelated by either chelator (Figure 6A and B). When DFP and DFO were used in combination, the rate of iron complex formation was not significantly faster than with DFP alone (Figure 6C, rate constant 358 h−1). The beneficial effect of DFP on chelation of iron: citrate by DFO is therefore due to a faster chelation in the slow phase of reaction. Confirmation that the fast phase of reaction is a real process and not due to iron contamination in the reagents is shown by the stopped-flow trace in Figure 6D where DFO was mixed with all the reagents excluding the iron.
Fig 6.
Fast phase kinetics of iron chelation from iron citrate by DFP and DFO alone or in combination using stopped flow. Reactions between iron citrate and either DFO (A), DFP (B) or DFO plus DFP (C) are shown. Iron citrate (20:200 µM) in 20 mM MOPS pH 7.4 and DFO (trace A: 20 µM) or DFP (trace B: 60 µM) or DFP plus DFO (trace C: 20 µM DFO, 60 µM DFP) were placed into the two syringes of a stopped flow apparatus. On autotrigger, the syringes ejected an equal volume of both solutions into the reaction chamber where the reaction was monitored by measuring changes in absorbance at 460 nm. Reaction chamber concentrations are half those given for the syringes.
Rates of iron complex formation in the presence of albumin, using DFO and DFP alone or in combination
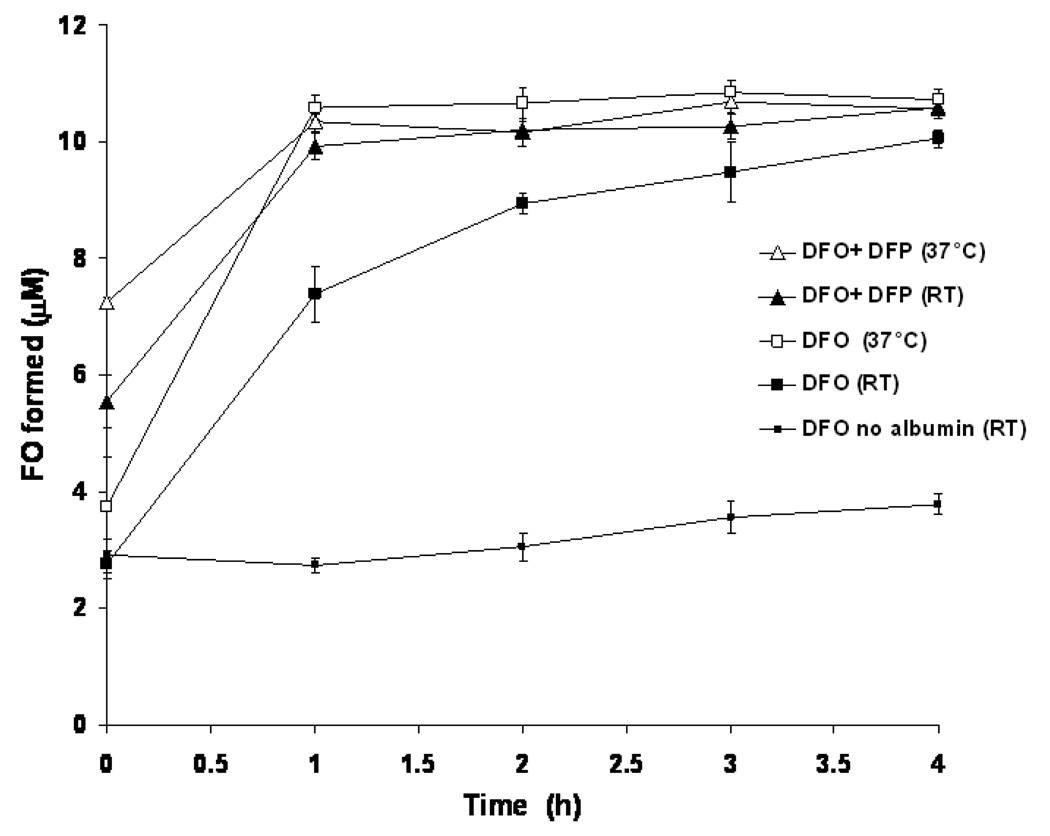
A significant proportion of plasma NTBI may be bound to or loosely associated with albumin, both on account of the high plasma albumin concentration of 40 g/L and also its putative iron binding sites 6. Thus it is important to determine how the presence of this major plasma protein affects chelation of iron citrate species by DFO either alone or in combination with DFP. When iron citrate (10:100µM) was mixed with physiologically relevant concentrations of albumin (40 g/L), the iron was bound to the albumin within the mixing time 6. When the kinetics of iron chelation by DFO in iron citrate albumin mixtures were examined by the HPLC method for detection of FO, it became clear that when iron citrate was mixed with albumin, chelation of iron by DFO was significantly faster than with iron citrate alone (Figure 7, p< 0.05 at all time points except at time zero). Chelation of iron by DFO in the presence of albumin was virtually complete in 4h at RT, in contrast to over 20 h when albumin was absent suggesting a significant interaction of albumin with iron citrate species, thereby increasing the iron pool available for chelation by DFO.
Fig 7.
Rate of FO formation from DFO and iron citrate with albumin at RT and 37°C in the presence and absence of DFP. DFO (10 µM) was incubated with iron citrate albumin (10 µM:100 µM:40 g/l albumin) in 20mM MOPS (pH 7.4), either alone (■) or in the presence of DFP (▲, 30 µM) both at RT (closed symbols) and 37°C (open symbols). Samples of the reaction mixtures were then taken at regular time intervals, deproteinized through 30 Kda molecular weight cut-off filters and 50 µl of filtrate injected onto the HPLC column for FO determination as described for Fig 3. A trace of the reaction between DFO and iron citrate in the absence of albumin at RT (▪, lower trace) is shown for comparison. FO concentrations were determined using a standard curve showing the peak areas corresponding to known dilutions of a freshly prepared 200 µM FO mixture in 20mM MOPS pH 7.4 containing 40g/l albumin. The data shown are the mean ± SE of 4 independent experiments.
Addition of DFP further enhanced the rate of FO formation: 5.5 µM FO was detected at RT immediately after mixing in the presence of 30 µM DFP compared to 2.85 µM FO when DFO was present alone (p< 0.05). FO formation was complete in 1h when DFP was present whereas it was still incomplete with DFO alone after 4h (Figure 7). Chelator iron access is more rapid at 37°C with DFO alone or in combination with DFP. The rate of FO formation was also monitored at RT and at 37°C using chelexed albumin but chelexing the albumin did not show any significant effect on the rate or amplitude of FO formation (data not shown). Although the kinetics in the presence of albumin appear biphasic, the reactions are much more rapid than those without albumin. The initial jump in FO formation may simply be due to loss of a significant proportion of the reaction profile due to the speed of reaction. The initial phases of FO formation were therefore further examined spectrophotometrically and with stopped flow, as the ‘time zero’ using the HPLC technique includes the time for sample deproteinization and loading onto the column (about 30 minutes in total). At ‘time zero’, no immediate formation of FO was seen using the spectrophotometer (Figure 8) in contrast to observations with iron citrate using the same method (Figure 4). Using stopped flow; the reaction kinetics showed that there was in fact no discrete fast phase like that shown in the reaction between the chelators and iron citrate (data not shown). However, even though the very fast kinetics were absent in the presence of albumin, the net rate of iron loading from iron albumin onto either DFO or DFP alone (figure 8) was substantially faster than from iron citrate (1:10 ratio, Figures 4, 5a and 5b). Thus, for example with DFO, FO formation is complete from albumin by 5h (Figure 8) but is still incomplete at 19.5h from iron citrate (Figures 4, 5a and 5b). Likewise iron complexation by DFP from iron albumin is complete within 60 minutes but takes 8h from iron citrate (Figure 4). DFP significantly increases the rate of chelation of iron from iron albumin by DFO (Figures 7, p< 0.05 over the first 90 min of reaction) and 8 inset, DFP + DFO, no significant differences between any paired time points on these two curves, here iron complex concentrations were calculated using the extinction coefficient for FO) to that seen with DFP alone (Figure 8, inset DFP, calculated using the extinction coefficient for the iron complex of DFP).
Fig 8.
Rate of iron complex formation from iron citrate albumin incubated with DFO or DFP measured by spectrophotometry. DFO (■, 10 µM) or DFP (▲, 30 µM) was incubated with iron citrate albumin (10:100 µM:40 g/l) in 20mM MOPS (pH 7.4) at RT and the reaction continuously monitored for 8h at 460 nm by spectrophotometer. Iron complex concentrations were calculated using previously determined extinction coefficients for both iron complexes. The data shown are the mean ± SE of 3 independent experiments. Inset: Black trace: incubation of DFP (30µM) with iron citrate albumin was repeated over a shorter time scale (15 minutes) to determine the reaction profile. Grey trace: DFO (10 µM) and DFP (30 µM) were co-incubated with iron citrate albumin for 20 min and the measured absorbance at 460 nm converted to feroxamine concentration.
DISCUSSION
Although the use of two chelators, or mixed ligand therapy, has long been proposed to increase the efficacy of chelation therapy, this is the first study to demonstrate enhanced chelation of plasma NTBI with DFO by using DFP to ‘shuttle’ NTBI to form feroxamine (FO). In principle, iron shuttling between chelators might also occur within cells, in this study however we have focused only on shuttling within the plasma compartment. The concentrations of chelators at which shuttling has been demonstrated in human plasma are clinically relevant and the shuttling process occurs at a rate that allows complete removal of NTBI by 8h at 37°C, whereas with DFO alone only approximately half of serum NTBI is removed at 24h. The kinetics of FO formation in serum are biphasic, either with DFO alone or in combination with DFP. These biphasic kinetics, demonstrated in our in vitro studies using thalassemic sera, are consistent with previous in vivo DFO infusion studies where reduction in serum NTBI shows distinct fast and slow phases 4. As the increased NTBI removal is accounted for by FO formation rather than iron bound to DFP, the increased NTBI removal is achieved by DFP acting as both a recipient of NTBI and as an iron (III) donor to DFO. This ‘shuttling’ is absent in serum from healthy controls, indicating that increased iron chelation is achieved without removal of iron from transferrin. More direct evidence for DFP acting as a shuttling intermediary is provided by experiments with iron citrate, described below.
As plasma NTBI is known to be heterogeneous, the slow and fast components of chelation suggest the chelation of different iron pools, with different susceptibilities to chelation by DFO. Iron-citrate species have been previously identified in thalassemic sera by NMR 32 and we have recently shown that relatively low molecular mass forms of NTBI (<5000 Kda) can be selectively filtered from thalassemic serum 6. These may equate to the directly chelatable 5 or labile plasma iron found in such sera 27. The slower phase of reaction between NTBI and DFO in thalassemic sera in vitro also accords with the slow rate of DFO access to iron citrate observed by Faller and Nick 37. The maximum plasma concentration of NTBI is generally no more than 10µM 3, 4 and that of citrate approximately 100µM 38. At these molar ratios of 1:10 monomers and dimers of iron citrate predominate with some oligomers also present 6, 7 and we predicted that the fast phase of chelation accessible to DFO was derived from chelation of citrate monomers and dimers, some loosely bound to plasma proteins, and that the slower second phase could result from the slower chelation of oligomeric or polymeric forms of iron citrate, or from as yet unidentified protein-bound species. We therefore also undertook studies of chelation kinetics using defined iron solutions containing citrate with or without physiological concentrations of the predominant plasma protein, albumin. An additional advantage of such an approach was that the fast phase of chelation (which was complete by the time of analysis using HPLC) could be studied using stopped flow, this methodology not being practical in plasma due to high background absorbance and tendency for serum proteins to precipitate.
The studies in iron citrate solutions show similarities to those obtained in serum from iron overloaded thalassemic patients, but also some differences. As with thalassemic sera, chelation by DFO is biphasic and enhanced by the presence of DFP. This enhancement also results in formation of FO as the end product rather than iron bound to DFP, consistent with speciation plot predictions. Stopped flow analysis during the first 50 seconds of reaction shows that the rate but not the magnitude of the initial fast phase is increased in the presence of DFP. With respect to the slow phase in iron citrate solutions, both the rate and magnitude of FO formation is enhanced by the presence of DFP, as with chelation in the thalassemic sera. We interpret the increase in chelation rate of the slower phase to DFP accessing iron species that are relatively inaccessible to DFO and ‘shuttling’ them onto the DFO to form the more thermodynamically stable FO complex. This interpretation is possible since the HPLC system unequivocally detects FO and not other iron complexes such as that of DFP under our experimental conditions. Further evidence for shuttling during the slower phase of the reaction has been provided by serially scanning the reaction mixture over wavelengths from 350 to 650 nm: the initial presence of the DFP-iron complex spectrum is later replaced by the spectrum of FO. This mechanism is also supported by observations on the rate of transfer of iron from pre-formed Fe-DFP complexes to 10 µM DFO which show transfer of Fe to be complete in 1.5 hours (data not shown). The concentration dependence of rate enhancement by DFP also supports this conclusion, since relatively low concentrations of DFP (3 µM) caused considerable rate enhancement, consistent with DFP continually cycling or ‘shuttling’ iron onto a DFO ‘sink’. Unlike thalassemic serum however, the slow phase of chelation by DFO continues beyond 8h. This suggests that, although the iron citrate ratios in this in vitro system are similar to those found in thalassemic serum, additional forms of iron might be present in thalassemic serum as NTBI. This is also indicated by differences in the response of the slow rate to temperature change in DFO access to NTBI in serum and in iron citrate.
Previous work suggests that, under the conditions of these experiments, monomers and dimers of ferric citrate will predominate with some small oligomers also present 6. Recent aqueous speciation of ferric citrate using mass spectrometry and EPR spectroscopy has confirmed that the most relevant species are a monoiron dicitrate species and dinuclear and trinuclear oligomeric complexes, the relative concentration of which is dependent on the iron: citric acid molar ratio 7. In iron-overloaded plasma however, the presence of plasma proteins and oxidants could favor a greater polymerization of iron-citrate species, even at these iron : citrate ratios. We have previously shown that DFO interacts more slowly with iron coordinated to proteins and bio-enzymes than the small neutrally charged DFP, by virtue of the larger size and hexadentate coordination chemistry of DFO 39, and these principles may also explain the slower and incomplete access of DFO to NTBI we observed in serum. Evidence for interaction of NTBI with plasma proteins has been obtained by the decreased filterability of iron citrate through 30 Kda molecular weight cut-off filters in the presence of clinically relevant concentrations of albumin 6, 40. Surprisingly however, the experiments undertaken here with human albumin showed that chelation of iron from citrate solutions is actually enhanced by the presence of albumin, reaching completion in 4h with DFO compared to more than 20 h for the iron citrate without albumin. As with iron citrate solutions, the formation of FO is temperature dependent and enhanced by DFP. Furthermore, as with simple iron citrate solutions, co-incubation of DFP markedly enhanced FO formation at a rate that was practically identical to that measured for DFP alone again consistent with DFP shuttling iron onto FO. The lack of biphasic kinetics and the increased availability of iron bound to albumin relative to iron citrate are consistent with albumin itself having a de-polymerizing effect on iron citrate species, as previously demonstrated 6. This does not explain why NTBI from the serum from thalassemia patients is relatively inaccessible to chelation by DFO. This apparent paradox may be explained by recent work suggesting that in plasma from patients with iron overload or diabetes, non-enzymic modifications to albumin occur, forming glycated adducts that bind iron more tightly than unmodified plasma albumin 8. Irrespective of the nature of such plasma factors retarding the availability of plasma NTBI to chelation by DFO, it is clear that enhanced formation of FO in the presence of DFP is achieved predominantly by increasing the rate and magnitude of the slow kinetic phase of FO formation and that this feature is also shared with FO formation in iron citrate solutions.
In conclusion, this study shows for the first time that the combined presence of DFP with DFO can access NTBI species that are otherwise unavailable to DFO, at clinically achievable concentrations and that this occurs through the shuttling of iron by DFP to form FO. Using DFO alone, comparison of FO formation kinetics in serum, or iron citrate solutions, show biphasic kinetics. Iron that is rapidly available to DFO when used alone is likely to be monomeric or dimeric iron citrate representing no more than about one third of total plasma NTBI. Slowly chelated iron, or that which is unavailable to DFO without the addition of DFP, is likely to be heterogeneous including oligomeric and polymeric iron citrate species and iron bound to modified plasma proteins. Enhanced access of these iron species to DFO can be achieved at low concentrations of DFP (3µM), the maximum effect being seen at 30µM DFP. These studies provide a rationale for simultaneous use of DFO and DFP in the treatment of iron overload conditions by removing plasma NTBI and thus minimizing the predominant mechanism by which iron accumulates in tissues susceptible to iron overload.
ACKNOWLEDGEMENTS
The work was supported by grants from NIH, Bethesda, Maryland USA: DK57645-01 (Hider and Porter): IR01DK55462-02 (Porter). We are grateful to Dr Xiao Kong, Department of Pharmacy, King’s College, London, for developing the speciation plot.
ABBREVIATIONS
- NTBI
non-transferrin bound iron
- DFO
deferoxamine
- DFP
deferiprone
- FO
feroxamine
- LPI
labile plasma iron
- MLT
mixed ligand therapy
- NTA
nitrilotriacetic acid
- HPLC
high performance liquid chromatography
- MOPS
3-[N-Morpholino]propanesulfonic acid, CHAPS, (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate
- HYSS
Hyperquad Simulation and Speciation program
- EDTA
ethylenediaminetetraacetate
- RT
room temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hershko C, Graham G, Bates GW, Rachmilewitz EA. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978;40:255–263. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 2.Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 3.Porter JB, Rafique R, Srichairatanakool S, et al. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: implications for clinical use. Ann N Y Acad Sci. 2005;1054:155–168. doi: 10.1196/annals.1345.018. [DOI] [PubMed] [Google Scholar]
- 4.Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- 5.Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, Cabantchik ZI. Desferrioxaminechelatable iron, a component of serum non-transferrin- bound iron, used for assessing chelation therapy. Blood. 2001;97:792–798. doi: 10.1182/blood.v97.3.792. [DOI] [PubMed] [Google Scholar]
- 6.Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, Porter JB, Hider RC. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem. 2008;13:57–74. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 7.Silva AM, Kong X, Parkin MC, Cammack R, Hider RC. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009:8616–8625. doi: 10.1039/b910970f. [DOI] [PubMed] [Google Scholar]
- 8.Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim Biophys Acta. 2009;1794:1449–1458. doi: 10.1016/j.bbapap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.al-Refaie FN, Wickens DG, Wonke B, Kontoghiorghes GJ, Hoffbrand AV. Serum non-transferrin-bound iron in beta-thalassaemia major patients treated with desferrioxamine and L1. Br J Haematol. 1992;82:431–436. doi: 10.1111/j.1365-2141.1992.tb06441.x. [DOI] [PubMed] [Google Scholar]
- 10.Pootrakul P, Sirankapracha P, Sankote J, et al. Clinical trial of deferiprone iron chelation therapy in beta-thalassaemia/haemoglobin E patients in Thailand. Br J Haematol. 2003;122:305–310. doi: 10.1046/j.1365-2141.2003.04412.x. [DOI] [PubMed] [Google Scholar]
- 11.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Zanninelli G, Breuer W, Cabantchik ZI. Daily labile plasma iron as an indicator of chelator activity in Thalassaemia major patients. Br J Haematol. 2009;147:744–751. doi: 10.1111/j.1365-2141.2009.07907.x. [DOI] [PubMed] [Google Scholar]
- 13.Walter PB, Macklin EA, Porter J, et al. Inflammation and oxidant-stress in {beta}-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–825. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]
- 14.May P, Williams D. Synergistic chelation therapy or mixed ligand complexes for plutonium or cadmium poisoning. Nature. 1979;278:581. doi: 10.1038/278581b0. [DOI] [PubMed] [Google Scholar]
- 15.Pollack S, Aisen P, Lasky FD, Vanderhoff G. Chelate mediated transfer of iron from transferrin to desferrioxamine. Br J Haematol. 1976;34:231–235. doi: 10.1111/j.1365-2141.1976.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson GE, May PM, Williams DR. The action of chelating agents in the removal of copper from ceruloplasmin: an in vitro study. FEBS Lett. 1978;90:173–177. doi: 10.1016/0014-5793(78)80323-8. [DOI] [PubMed] [Google Scholar]
- 17.Schubert J, Derr SK. Mixed ligand chelate therapy for plutonium and cadmium poisoning. Nature. 1978;275:311–313. doi: 10.1038/275311a0. [DOI] [PubMed] [Google Scholar]
- 18.Aydinok Y, Ulger Z, Nart D, et al. A randomized controlled 1-year study of daily deferiprone plus twice weekly desferrioxamine compared with daily deferiprone monotherapy in patients with thalassemia major. Haematologica. 2007;92:1599–1606. doi: 10.3324/haematol.11414. [DOI] [PubMed] [Google Scholar]
- 19.Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 20.Giardina PJ, Grady RW. Chelation therapy in beta-thalassemia: an optimistic update. Semin Hematol. 2001;38:360–366. doi: 10.1016/s0037-1963(01)90030-7. [DOI] [PubMed] [Google Scholar]
- 21.Grady RW, Giardina PJ. Iron chelation with oral deferiprone in patients with thalassemia. N Engl J Med. 1998;339:1712–1713. author reply 3–4. [PubMed] [Google Scholar]
- 22.Martell A. The design and synthesis of chelating agents. Elsevier North Holland Inc; 1981. [Google Scholar]
- 23.Ihnat P, Vennerstrom J, Robinson D. Solution equilibria of deferoxamine amides. J Pharm Sci. 2002;91 doi: 10.1002/jps.10168. [DOI] [PubMed] [Google Scholar]
- 24.Motekaitis R, Martell A. Stabilities of the iron(III) chelates of 1,2-dimethyl-3-hydroxy-4-pyridinone and related ligands. Inorganica Chimica Acta. 1991;183:71–80. [Google Scholar]
- 25.Link G, Konijn AM, Breuer W, Cabantchik ZI, Hershko C. Exploring the "iron shuttle" hypothesis in chelation therapy: effects of combined deferoxamine and deferiprone treatment in hypertransfused rats with labeled iron stores and in iron-loaded rat heart cells in culture. J Lab Clin Med. 2001;138:130–138. doi: 10.1067/mlc.2001.116487. [DOI] [PubMed] [Google Scholar]
- 26.Srichairatanakool S, Kemp P, Porter JB. Evidence for "shuttle" effect of NTBI onto desferrioxamine in thalassaemic plasma in the presence of NTA; International Symposium: Iron in Biology and Medicine, 1997; France: St. Malo; 1997. p. 210. (abstract) [Google Scholar]
- 27.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 28.Dobbin PS, Hider RC, Hall AD, et al. Synthesis, physicochemical properties, and biological evaluation of N- substituted 2-alkyl-3-hydroxy-4(1H)-pyridinones: orally active iron chelators with clinical potential. J Med Chem. 1993;36:2448–2458. doi: 10.1021/jm00069a002. [DOI] [PubMed] [Google Scholar]
- 29.Alderighi L, Gans P, Ienco A, Peters D, Sabatini A, Vacca A. Hyperquad simulation and speciation (hyss): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coordination Chemistry Reviews. 1999;184:311–318. [Google Scholar]
- 30.Singh S, Hider RC, Porter JB. A direct method for quantification of non-transferrin-bound iron. Anal Biochem. 1990;186:320–323. doi: 10.1016/0003-2697(90)90088-q. [DOI] [PubMed] [Google Scholar]
- 31.Gosriwatana I, Loreal O, Lu S, Brissot P, Porter J, Hider RC. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem. 1999;273:212–220. doi: 10.1006/abio.1999.4216. [DOI] [PubMed] [Google Scholar]
- 32.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989;264:4417–4422. [PubMed] [Google Scholar]
- 33.Porter JB. Deferoxamine pharmacokinetics. Semin Hematol. 2001;38:63–68. doi: 10.1016/s0037-1963(01)90061-7. [DOI] [PubMed] [Google Scholar]
- 34.al-Refaie FN, Sheppard LN, Nortey P, Wonke B, Hoffbrand AV. Pharmacokinetics of the oral iron chelator deferiprone (L1) in patients with iron overload. Br J Haematol. 1995;89:403–408. doi: 10.1111/j.1365-2141.1995.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 35.Kontoghiorghes GJ, Goddard JG, Bartlett AN, Sheppard L. Pharmacokinetic studies in humans with the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Clinincal Pharmacology. 1990;48:255–261. doi: 10.1038/clpt.1990.147. [DOI] [PubMed] [Google Scholar]
- 36.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology (Am Soc Hematol Educ Program) 2001:47–61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Faller B, Nick H. Kinetics and mechanism of iron(III) removal from citrate by desferrioxamine B and 3-hydroxy-1,2-dimethyl-4-pyridone. J Am Chem Soc. 1994;116:3860–3865. [Google Scholar]
- 38.Lentner CE. Basle, Switzerland: Ciba-Geigy Limited; 1984. [Google Scholar]
- 39.Cooper CE, Lynagh GR, Hoyes KP, Hider RC, Cammack R, Porter JB. The relationship of intracellular iron chelation to the inhibition and regeneration of human ribonucleotide reductase. J Biol Chem. 1996;271:20291–20299. doi: 10.1074/jbc.271.34.20291. [DOI] [PubMed] [Google Scholar]
- 40.Hider R. Nature of non-transferrin-bound iron. Eur J Clin Invest. 2002;32 Suppl 1:50–54. doi: 10.1046/j.1365-2362.2002.0320s1050.x. [DOI] [PubMed] [Google Scholar]