Abstract
Ligand-gated ion channels (ionotropic receptors) link to the cortical cytoskeleton via specialized scaffold proteins and thereby to appropriate signal transduction pathways in the cell. We studied the role of filamentous actin in the regulation of Ca influx through glutamate receptor-activated channels in third-order neurons of salamander retina. Staining by Alexa-Fluor 488-phalloidin, to visualize polymerized actin, we show localization of filamentous actin in neurites, and the membrane surrounding the cell soma. With Ca2+ imaging we found that in dissociated neurons, depolymerization of filamentous actin by latrunculin A, or cytochalasin D significantly reduced glutamate-induced intracellular Ca2+ accumulation to 53±7% of control value. Jasplakinolide, a stabilizer of filamentous actin, by itself slightly increased the glutamate-induced Ca2+ signal and completely attenuated the inhibitory effect when applied in combination with actin depolymerizing agents. These results indicate that in salamander retinal neurons the actin cytoskeleton regulates Ca2+ influx through ionotropic glutamate receptor-activated channels, suggesting regulatory roles for filamentous actin in a number of Ca2+-dependent physiological and pathological processes.
Keywords: actin filament, AMPA, NMDA, latrunculin, channel, receptor
The neuronal cytoskeleton consists of microtubules and microfilaments that can interact with neurotransmitter receptors and ion channels. Studies suggest that postsynaptic glutamate-activated channels form clusters that are anchored in the plasma membrane through interactions with the actin cytoskeleton that play a role in channel function (reviewed in Sheng and Pak, 2000). The dynamic assembly of filamentous actin (F-actin) is essential to a variety of regulatory processes, including filopodial growth, spine motility, modulation of ion channels, and synaptic transmission (reviewed in Oertner and Matus, 2005).
Increasing lines of evidence indicate that the reorganization of the actin cytoskeleton may modulate Ca2+ influx through various sources. Ca2+ entry through voltage-gated, and N-methyl-Daspartic acid (NMDA)-receptor activated channels, as well as endoplasmic Ca2+ release in neurons can all be affected by depolymerization of F-actin (Johnson and Byerly, 1994; Furukawa et al., 1997; Wang et al., 2002; Schubert and Akopian, 2004). In turn, Ca2+ flux from different sources can induce changes in F-actin organization that in some cases appear contradictory. For instance, in neurons of the CNS strong activation of NMDA receptors permits a sufficient influx of Ca2+ to depolymerize F-actin (Rosenmund and Westbrook, 1993; Furuyashiki et al., 2002). In contrast, in bipolar cell terminals of the goldfish retina, Job and Lagnado (1998) showed that Ca2+ influx during light or K+-induced depolarization stimulates growth of an actin network. The interrelation between Ca2+ influx and F-actin organization thus contributes to the mechanisms of certain Ca-dependent regulatory processes in neurons.
Intracellular Ca2+ plays an important role in the regulation of retinal function, including modulation of ion channels and synaptic transmission (reviewed in Akopian and Witkovsky, 2002). In the developing chick retina, neuro-transmitter-evoked Ca2+ release from intracellular stores stabilizes dendrites during the period of synapse formation, a process presumably involving reorganization of the cytoskeletal network (Wong et al., 2000; Lohmann et al., 2002). Both Ca2+ and cytoskeleton play an important role in retinal plasticity (Weiler and Janssen-Bienhold, 1993; Holt et al., 2003). Furthermore, changes in [Ca2+]i are likely to regulate a number of crucial cellular processes, including gene expression, metabolism, spontaneous and evoked release, and development (reviewed in Berridge et al., 2003).
Very little is known about the localization and functional roles of F-actin in retinal ganglion cells. There are at least three different sources of [Ca2+]i in salamander retinal ganglion cells; (i) voltage-gated channels, (ii) glutamate receptor-activated channels (Zhang et al., 1995), and (iii) IP3-, and ryanodine-sensitive internal Ca2+ stores (Shen and Slaughter, 1998) that could be affected by F-actin reorganization. We reported earlier that the disruption of F-actin resulted in an inhibition of high voltage-activated L-type Ca current in ganglion cells of salamander retina (Schubert and Akopian, 2004). Whether the actin cytoskeleton regulates other Ca2+ sources in the retina is unknown. In the present study we first determined the distribution of actin filaments in ganglion cells of salamander retina by confocal laser-scanning microscopy. Next, we ascertained the functional role of the actin network, providing evidence for a prominent coupling between the actin cytoskeleton and glutamate receptor-activated Ca2+-permeable channels in third order (amacrine/ganglion) cells. Our results show that in salamander retinal neurons the disruption of the actin cytoskeleton reduces glutamate-induced Ca2+ influx.
EXPERIMENTAL PROCEDURES
Retinal preparation
The handling and the maintenance of animals met the National Institutes of Health guidelines and were approved by Institutional Care and Use Committee at NYU School of Medicine. The numbers of animals used and their suffering were minimized. Salamanders (Ambystoma tigrinum) were anesthetized using tricaine methanosulfonate (100 mg/ml) until the animal no longer reacted to tactile stimulation, then were decapitated and double pithed. The eyes were cut in half, and the retina was dissected out either for preparation of dissociated cells, or treated as a flat-mount. Eyecups were used for preparation of cryostat sections.
F-actin staining on whole mounts and dissociated cells
Specimens (retinal flat-mounts, or eyecups) were fixed for 30 min at 4 °C, 4% paraformaldehyde+0.1 M PBS (pH 7.4). After fixation, retinal whole mounts were washed and processed free floating in a small vial at room temperature. To visualize actin filaments, specimens were first washed three times with PBS, incubated for 15 min (or 5 min for cells) with 0.5% (0.3% for cells) Triton X in PBS and then preincubated for 30 min with 3% BSA 0.3% Triton X in PBS, and finally incubated for 2 h with Alexa Fluor 488-phalloidin (Molecular Probes, Eugene, OR, USA) diluted in 3% BSA 0.3 Triton-X solution to a final phalloidin concentration of 0.2–0.3 μM. After extensive rinsing, the tissues were mounted with Vectashield (Vector, Burlingame, CA, USA) and observed with a confocal laser-scanning microscope Nikon Eclipse C-1 (Nikon, Japan). Images were acquired using 60× or 100× oil-immersion objective lens and EZ-C1 (Nikon) software. Images for control and latrunculin-treated cells were obtained with the same scanning parameters and were processed using deconvolution software AutoDeblur (AutoQuant Imaging, Inc., Watervliet, NY, USA), and Adobe Photoshop (version 7.0; Adobe Systems Inc., San Jose, CA, USA). To control for variations in staining intensity, latrunculin-, or cytochalasin-treated cells were always compared with control cells prepared the same day under identical fixation, permeabilization, staining, and microscopy conditions.
Labeling of retinal ganglion cells
Tetramethylrhodamine-dextran (Mw 3000, Molecular Probes) was used to label axonal fibers and ganglion cell somas through the cut optic nerve. A few crystals of tracer were placed on a freshly cut optic nerve and the eyecup with attached tracer was stored at 4 °C overnight. The retina with labeled ganglion cells then was stained with Alexa Fluor 488-phalloidin, mounted with Vectashield and observed by confocal microscopy.
Acute retinal cell culture
Isolated retinas were incubated with Papain solution (10 U/ml, Worthington Biochemicals, Lakewood, NJ, USA) in 5 ml Ca-free/4 Mg Ringer solution containing 1 mg cysteine and 10 mM Na-Pyruvate, for 30 min at room temperature with gentle agitation. After rinses in PBS, each retina was cut in small pieces and the cells were gently dissociated in L 15 medium (Gibco, Carlsbad, CA, USA) using a small tip pipette. In some experiments dextran-labeled retina was isolated from the eyecup, treated with papain, and dissociated ganglion cells were stained with phalloidin. After dissociation, the retinal cell suspension was plated on poly-L-lysine-coated coverslips in a 12 well plate and stored overnight at 4 °C. The following day the cells were treated with either 5 μM latrunculin A, or 10 μM cytochalasin D for 30–40 min. Cells were then fixed for 30 min in ice-cold solution of PBS-buffered 4% paraformaldehyde containing 4% sucrose. To stain-actin filaments with Alexa Fluor-phalloidin we used the procedure described above. Cells were then rinsed, coverslips were mounted with Prolong Antifade Mounting Medium (Molecular Probes) and the specimens examined with a confocal laser-scanning microscope. Four independent experiments were carried out with 12 coverslips per experiment, and at least 30 cells were monitored for phalloidin staining each in control, cytochalasin D, and latrunculin A. To quantify the changes in the actin network, Metavue software (Universal Imaging Co., Downington, PA, USA) was used to obtain a profile of intensity along a line drawn through the center of the neurons. The average intensity values were calculated from lines with a scan width of 20 pixels, and data plotted with SigmaPlot software version 7.0 (SPSS Inc., Chicago, IL, USA).
Free [Ca2+]i measurement and data acquisition
The detailed procedures are fully described elsewhere (Krizaj and Copenhagen, 1998). Briefly, cells were loaded with 2–5 μM fura-2 AM (fura 2- acetoxymethylester; Molecular Probes) for 10 min and subsequently washed for 20 min. The fluorescence signals were acquired on an inverted microscope (Nikon Eclipse 200) using a dry 40× objective (N.A.=0.8) or an oil 100× objective (N.A.=1.2). Image acquisition was generally binned at 3×3 by a cooled 12 bit digital CCD camera (Cascade, Photometrics, Tucson, AZ, USA). The acquisition rate was 0.3–1 Hz. The camera and the shutter (Lambda DG-4, Sutter Instruments, Novato, CA, USA) were controlled by commercial software (Metafluor 6.1; Universal Imaging, West Chester, PA, USA). Ratios between the 340 nm and 380 nm excitation wavelengths were calculated after subtraction of the background fluorescence. Free Ca2+ levels were calibrated in vivo with 10 mM ionomycin using the standard relationship; the Kd for Ca2+ binding to fura-2 was taken to be 224 nM. All pooled data are presented as ±S.E.M. with n indicating the number of neurons tested. Levels of significance were assessed using Student's paired t-test. Differences in means were considered significant when the P-value was less than 0.05. For confocal analysis, cells were loaded with 3–5 μM fluo-4 AM (Molecular Probes), incubated for 10 min and washed for another 10 min. Confocal images were collected in the line scan mode using a LSM 5 Pa confocal microscope (Zeiss, Jena, Germany) and a 63× water immersion objective (N.A.=0.8). Fluo-4 fluorescence was excited with the 488 nm band of the Ar laser with transmission set at 1%. The scan interval was 20–50 ms and the pinhole size was set at 1–2 A. The data are presented on an intensity scale in arbitrary units.
RESULTS
Distribution of F-actin in retinal ganglion cells
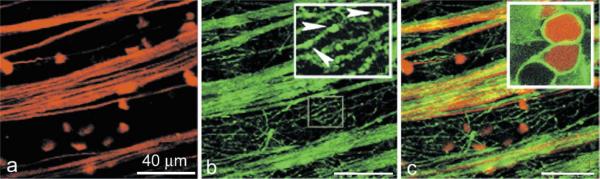
We studied the distribution of F-actin in labeled ganglion cells in retinal flat mounts using Alexa Fluor 488-phalloidin, a fluorescent actin-stabilizing compound used to stain, visualize, and quantify F-actin (Cooper, 1987). Confocal images of Alexa Fluor 488-phalloidin-stained retinal flat mount preparation are illustrated in Fig. 1. Rhodamine-conjugated dextran labeled cell bodies, and axons (Fig. 1a), as well as some unidentified thick processes (not shown). Fig. 1b shows the same preparation double stained with Alexa Fluor 488-phalloidin. In the nerve fiber layer (NFL) prominent phalloidin staining was observed in axon fibers, and numerous processes with bright spots along these processes (Fig. 1b, inset). Superposition of images in a, and b is illustrated in Fig. 1c. High power view of confocal images obtained by focusing on dextran-labeled ganglion cell soma revealed F-actin concentration in a submembranous area at the cell's perimeter (Fig. 1c, inset).
Fig. 1.
Distribution of F-actin in retinal ganglion cells. Ganglion cells and axon fibers were retrogradely labeled with rhodamine-dextran (A), and then flat-mounts stained with Alexa Fluor 488-phalloidin (B). F-actin was present in axonal fibers and numerous processes of ganglion cells; often appearing as bright spots (inset, arrowheads). Merger of two confocal images is illustrated in C. The high-power view of ganglion cells (inset) was obtained by focusing on cell soma labeled with dextran (red), and phalloidin (green).
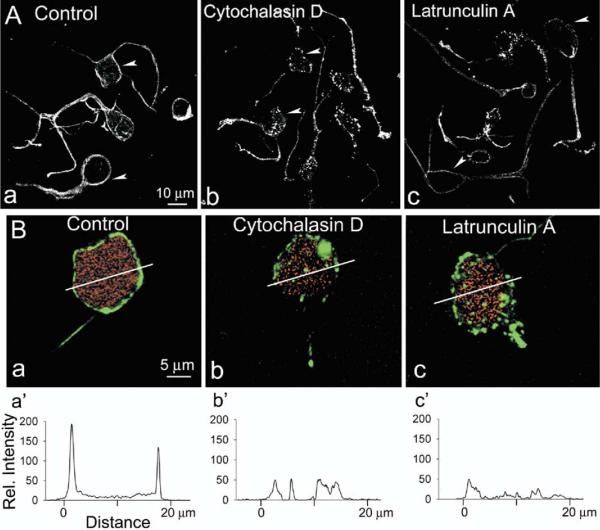
We next studied the distribution of F-actin in acutely dissociated third-order neurons of salamander retina. These neurons, presumably amacrine or ganglion cells, usually retain some thick processes after the dissociation, and could be easily distinguished from photoreceptors, horizontal cells and Muller cells due to their characteristic morphology. Dissociated cells in control solution, and after exposure with cytochalasin D or latrunculin A (Spector et al., 1983), were stained with Alexa Fluor 488-phalloidin and observed with a confocal laser scanning microscope. Representative images of cells from four independent experiments are illustrated in Fig. 2A. In untreated (control) cells intense phalloidin fluorescence was concentrated in a submembranous area at the cell's perimeter, and in neurites. F-actin disruption caused a change in a pattern and the intensity of Alexa Fluor 488-phalloidin fluorescence in soma and neuritis. We used two different F-actin depolymerizing agents: cytochalasin D, which binds to the barbed (plus) ends of actin filaments, preventing further polymerization at that end, and latrunculin A that binds to and sequesters actin monomers, preventing their association into actin filaments (Spector et al., 1983). Cytochalasin typically caused discontinuities of F-actin staining at the surrounding plasma membrane, and the appearance of fluorescence puncta in cell interior (Fig. 2A, b). Treatment with latrunculin A, on the other hand, resulted in an overall loss of fluorescence intensity (Fig. 2A, c).
Fig. 2.
Disruption of the actin cytoskeleton in retinal neurons by latrunculin A. (A) Confocal images of dissociated third-order retinal neurons exposed for 30 min to either vehicle (Control), or 10 μM cytochalasin D, or 5 μM latrunculin A before fixation and staining with Alexa-Fluor488-phalloidin. Note intense fluorescence ring surrounding the cell perimeter (arrowheads) in control cells, and predominantly punctate character of staining, or loss of fluorescence in cytochalasin-, and latrunculin-treated cells, respectively. (B) F-actin staining in dissociated rhodamine-dextran-labeled ganglion cells in control saline (a), and after incubation with cytochalasin D (b), or latrunculin A (c). (a′–c′) Shows the phalloidin fluorescence intensity profile across the white line marked in a–c.
Next, we attempted to visualize F-actin distribution in ganglion cells. Earlier reports indicate that, in rat retina, the microtubule-associated protein MAP1 is localized exclusively to ganglion cells and their processes (Tucker and Matus, 1988). We also found strong MAP1 immunoreactivity in the cells in the ganglion cell layer of the vertical section of salamander retina (not illustrated). However, many cells in the inner nuclear layer also showed immunoreactivity to MAP1; consequently we were unable to use MAP1 as an appropriate ganglion cell marker in our preparation. Instead, we retrogradely labeled ganglion cells in eyecup preparation with rhodamine-dextran, isolated and treated the retina with papain, as described in Experimental Procedures, and then stained the dissociated cells with Alexa-Fluor 488-phalloidin (Fig. 2B). Confocal images of representative ganglion cells double labeled with rhodamine-dextran, and Alexa Fluor 488-phalloidin in control condition, and after F-actin disruption are illustrated in Fig. 2B, a–c. Fig. 2B, d–f shows the intensity profile across the white line marked in Fig. 2B, a–c. F-Actin was concentrated in a submembranous region surrounding cell soma. Two peaks in Fig. 2B, d represent F-actin concentration at either side of the ganglion cell in control saline. Disruption of F-actin led to either a reduction, or a complete elimination of fluorescence intensity peaks.
Depolymerization of F-actin reduces glutamate-induced Ca2+ influx
In the vertebrate retina two types of ionotropic glutamate receptors, α-amino-3-hydroxy-5-methyl-4-isoxasole proprionic acid receptor (AMPAR) and N-methyl-D-aspartic acid receptor (NMDAR), were shown to be Ca2+-permeable (Leinders-Zufall et al.1994; Okada et al., 1999; Thoreson and Witkovsky, 1999). Glutamate induces an elevation of intracellular Ca2+ in salamander retinal third-order neurons by activating AMPA and NMDA ionotropic receptors, as well as metabotropic receptors (Shen and Slaughter, 1998). We examined whether glutamate-induced Ca2+ influx may be affected by the actin cytoskeleton reorganization.
Imaging of the calcium indicator dye fura-2 was used to compare [Ca2+]i responses to glutamate in freshly dissociated neurons before and after the disruption of F-actin. In these series of experiments, we monitored third-order neurons that retained processes after dissociation (morphologically similar to those illustrated in Fig. 2). Glutamate was applied at concentrations of 100–300 μM onto isolated cells. Because actin filaments strongly modulate voltage-activated Ca2+ entry in salamander retinal ganglion cells (Schubert and Akopian, 2004), 100 μM Cd2+ was added to the bath solution to exclude a contribution of Ca2+ influx through voltage-gated channels. We also included thapsigargin (1 μM), a selective inhibitor of the endoplasmic reticulum Ca2+-ATPase (Thastrup et al., 1990), to prevent any contribution of Ca2+ release from internal stores (Leinders-Zufall et al., 1994). Baseline [Ca2+]i in the putative retinal ganglion cells was 46±17 nM (n=20). Glutamate (300 μM) induced a large elevation in intracellular Ca2+ to 277±60 nM (n=20). After removal of glutamate from the bath solution the [Ca2+]i signal recovered almost completely to the baseline level. These experiments suggest that the dynamic range of glutamatergic modulation in retinal ganglion cells encompasses about a six-fold change of [Ca2+]i under our experimental conditions.
Next we examined the type of glutamate receptor involved in Ca2+ signaling. We used AP-5 to block NMDA receptors and measured glutamate-induced intracellular Ca2+ accumulation. The mean glutamate-induced intracellular Ca2+ in the absence and the presence of AP-5 (100 μM) was 210±12 nM, and 165±37 nM, respectively (not illustrated). The small reduction in Ca2+ signal in the presence of AP-5 however, was statistically not significant (n=6, P>0.5). On the other hand, in the presence of 20 μM CNQX, glutamate induced Ca2+ accumulation was significantly reduced from 277±38 nM to 60±5 nM (n=4, P<0.005), indicating that in our experimental condition (1 mM Mg2+ in Ringer solution) the glutamate-induced intracellular Ca2+ accumulation was mediated primarily by AMPA receptor activation.
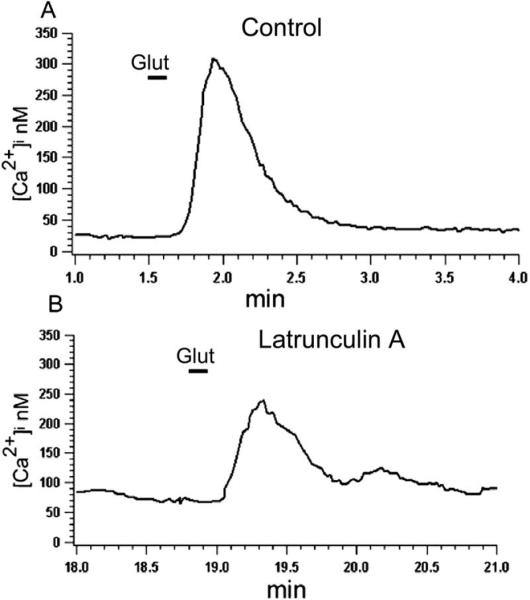
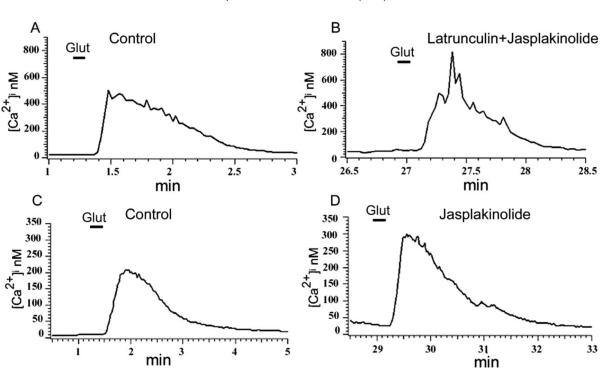
To study the effect of F-actin organization on glutamate-induced Ca2+ influx, neurons were pretreated with the actin-depolymerizing agent latrunculin A. Incubation of neurons with latrunculin A (5 μM) for 20–30 min resulted in a reduction of the glutamate-induced Ca2+ signal to 53±7% (n=11, P<0.005) of the value measured in control, untreated cells (Fig. 3B). Jasplakinolide, a membrane-permeable stabilizer of the actin cytoskeleton that binds to F-actin competitively with phalloidin (Bubb et al., 1994), prevented the inhibitory effect of latrunculin A on the glutamate-induced intracellular Ca2+ accumulation. The mean glutamate-induced [Ca2+]i accumulation in the combined presence of latrunculin A and jasplakinolide (5 μM) was 105±17% (n=5, P>0.3) of the value in control (Fig. 4A and B). By itself jasplakinolide slightly increased the glutamate-induced Ca2+ signal in some cells to 115±3% of control value (Fig. 4C and D); this change, however, was statistically not significant (n=7, P>0.3). In addition, jasplakinolide applied alone had no effect on baseline Ca2+ levels, which were 40±8 nM and 44±10 nM, respectively in the control solution or in the presence of 5 μM jasplakinolide (n=4). Finally, in accordance with our conclusion that AMPA receptors mediated the Ca2+ signal, we found no significant effect for NMDA receptor antagonist AP-5 on the latrunculin inhibition of glutamate-induced intracellular Ca2+ accumulation (n=6, not illustrated).
Fig. 3.
Effect of latrunculin A on the glutamate-induced Ca2+ influx. In control solution glutamate (100 μM) induced Ca2+ influx (A), which was significantly (P<0.05) reduced following 30 min incubation of cells with 5 μM latrunculin A (B).
Fig. 4.
Jasplakinolide attenuates the inhibitory effect of latrunculin A on glutamate-induced Ca2+ influx. Cells were incubated for 30 min in Ringer solution containing 5 μM latrunculin A+5 μM jasplakinolide (B), or jasplakinolide alone (D), and glutamate-induced Ca2+ signals were compared with those measured in control solution (A and C). Neither jasplakinolide alone nor in combination with latrunculin A had statistically significant effect on the glutamate-induced Ca2+ signal (P>0.3 and P>0.1 respectively).
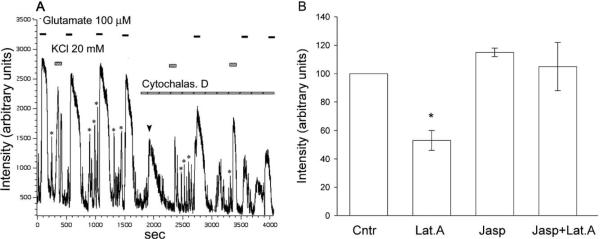
Qualitatively similar results were observed when cells treated with another depolymerizing agent, cytochalasin D. In these experiments we used high-temporal resolution confocal imaging to record Ca2+ signals induced by glutamate, and high potassium. A representative experiment (Fig. 5A), shows that the disruption of F-actin with cytochalasin D (10 μM) resulted in a reduction of glutamate-(100 μM), as well as high-potassium- (20 mM) induced intracellular Ca2+ accumulation. On a slower time base we observed a sustained Ca2+ signal in response to 0.5–1 min applications of glutamate (not shown). In addition, this method allowed us to monitor [Ca2+]i transients as the cell was spontaneously firing, and the amplitude of transients was also reduced by cytochalasin D. These data are consistent with our previous study (Schubert and Akopian, 2004), in which we demonstrated a reduction of voltage-gated L-type Ca current in salamander retinal ganglion cells. The bar graph in Fig. 5B summarizes the effect of F-actin depolymerization on the glutamate-induced Ca2+ influx. Our data strongly support the hypothesis that a reduction in glutamate-induced Ca2+ influx by latrunculin A, and cytochalasin D is associated with the disruption of F-actin.
Fig. 5.
Effect of cytochalasin D on Ca2+ influx via glutamate receptor-activated channels, and voltage-activated Ca2+ channels. (A) High-temporal resolution Ca2+ signals induced by glutamate (300 μM), and KCl (20 mM) were recorded with a confocal microscope before and after incubation of cells with 10 μM cytochalasin D. Cytochalasin D itself caused a transient increase in [Ca2+]i (arrowhead), and reduced both glutamate-, and KCl-induced Ca2+ signals. The amplitude of spontaneous Ca2+ transients (asterisks) was also reduced following exposure to cytochalasin D. (B) Bar graph summarizing the effect of F-actin depolymerization on glutamate-induced Ca2+ accumulation.
DISCUSSION
The major finding of this study is that disruption of the actin cytoskeleton reduces glutamate receptor-activated intracellular Ca2+ accumulation in third-order neurons of salamander retina. Although we did not characterize these neurons by axonal labeling (which would interfere with optical imaging), they presumably were amacrine and/or ganglion cells as they retained some processes after dissociation, and could be easily distinguished from photoreceptors, horizontal, bipolar and Muller cells on morphological grounds.
Using Alexa Fluor 488-phalloidin we, identified an intense band of F-actin in a submembrane region around the cell bodies and in neurites of dissociated retinal third-order neurons. Treatment with F-actin depolymerizing agents resulted in a change of staining pattern (with cytochalasin D), or a substantial loss of phalloidin fluorescence (with latrunculin A) indicative of a disruption of F-actin. F-actin was localized to rhodamine-dextran-labeled ganglion cell axons, and the submembranous region around the cell soma in retinal flat-mounts, and dissociated cells. These results suggest that at least some cells studied in Ca2+ imaging were ganglion cells, although a contribution from amacrine cells cannot be completely excluded.
In neurons of salamander retina, a glutamate-induced [Ca2+]i elevation potentially could arise from multiple Ca2+ sources. Thus, Ca2+ might enter the cell through voltage-gated Ca channels activated by glutamate-induced depolarization, or through channels activated by both AMPA, and NMDA ionotropic glutamate receptors (reviewed in Thoreson and Witkovsky, 1999). In addition, metabotropic glutamate receptor activation might stimulate Ca2+ release from IP3-sensitive internal stores (Shen and Slaughter, 1998). In our study the glutamate-induced Ca2+ signal was measured in the presence of Cd2+, and thapsigargin to exclude contribution of voltage-gated Ca channels, and Ca2+ release from internal stores, respectively. In these circumstances the Ca2+ signal is mediated primarily through ionotropic glutamate receptor-activated channels. Furthermore, we conducted our experiments in the presence of 1mMMg2+, which blocks NMDA receptors in a voltage-dependent manner (Nowak et al., 1984). We could not, however, completely exclude a contribution of the NMDA receptors, as some studies on salamander retina suggested that NMDA receptors are active at resting potentials (−70 mM), even in the presence of 1 mM Mg2+ (Gottesman and Miller, 2003). Therefore we conducted separate experiments to measure glutamate-induced Ca2+ signal in the presence of NMDA, and AMPA receptor antagonists. The data indicate that under our experimental conditions, AMPA receptors were the primary source for Ca2+ entry induced by glutamate. In accord with these data, AP-5 had no significant effect on latrunculin inhibition of the glutamate-induced Ca2+ signal. Finally, the attenuation of the inhibitory effect of latrunculin A in the presence of jasplakinolide supports the idea that depolymerization of F-actin underlies the mechanism of reduction in glutamate-induced Ca2+ influx. These data are in accord with earlier observations in hippocampal neurons (Furukawa et al., 1997; Sattler et al., 2000; Lei et al., 2001).
Although increasing data indicate that actin filaments can influence voltage-, and ligand-gated ion channels in neurons (Johnson and Byerly, 1994; Furukawa et al., 1997; Maguire et al., 1998; Schubert and Akopian, 2004), the molecular interactions involved remain to be established. Some data suggest that actin may interact directly with ion channels (Berdiev et al., 1996). On the other hand, a number of actin-binding proteins that link actin with membranes have been implicated in modulating calcium influx through both voltage- and receptor-activated channels (reviewed in Hartwig, 1994). Moreover, since the actin cytoskeleton is known to anchor postsynaptic receptors, they are implicated in removal of glutamate receptors from the plasma membrane by endocytosis (Carroll et al., 1999). It will be of considerable interest to determine whether the changes in Ca2+ influx observed here are associated with a change in the membrane-associated glutamate receptor number, and/or an alteration in Ca-permeable channel properties.
Functional significance
In the vertebrate retina, as in other parts of the CNS, glutamate receptors and voltage-gated Ca channels play fundamental roles in neurotransmission and in developmental and synaptic plasticity. Activation of both AMPA and NMDA subtypes of glutamate receptor has been implicated in structural plasticity in the retina (Yen et al., 1995; Okada et al., 1999; Wong et al., 2000; Lohmann et al., 2002), thereby contributing to the light-evoked postsynaptic responses of retinal ganglion cells (Diamond and Copenhagen, 1993; Gao and Wu, 1999; Velte et al., 2003). Glutamate also has been shown to act as an endogenous neurotoxin, which exerts its toxic effect on ganglion cells through Ca2+ permeable channels (reviewed in Sucher et al., 1997). Furthermore, elevated intracellular Ca2+ modulates ion channels and light-evoked postsynaptic responses in retinal ganglion cells (reviewed in Akopian and Witkovsky, 2002). Our data suggest that remodeling of the actin network could further regulate Ca2+ entry via glutamate receptors (present report), or L-type Ca channels (Schubert and Akopian, 2004). In this way, F-actin could play a significant role in excitatory signaling of retinal ganglion cells and synaptic transmission (Kim et al., 1999). Given that Ca2+ itself acts as a potent modulator of the balance between G-, and F-actin (Job and Lagnado, 1998; Oertner and Matus, 2005), it is possible that the feedback loop between Ca2+ entry and actin depolymerization regulates the localization and stabilization of glutamate receptors in retinal ganglion cells. Our preliminary results indicate that Ca2+ influx through voltage-gated channels, which can be activated by glutamate-induced depolarization, is sufficient to disrupt F-actin in isolated neurons of salamander retina. Finally, our data suggest that the F-actin network could play a key role in maintaining a balance between Ca2+ entry through voltage-activated and receptor-activated channels, thereby exerting control over a number of Ca2+-dependent physiological and pathological processes.
Acknowledgments
We thank Dr. P. Witkovsky for critically reading the manuscript. This research was supported by the NIH grants EY 12497 to A.A., EY 13870 to D.K., That Man May See, and an unrestricted grant from RPB to the Department of Ophthalmology UCSF, and a Hungarian grant and a fellowship from the Knights Templar Foundation to T.S.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxasole proprionic acid
- F-actin
filamentous actin
- F-actin
filamentous actin
- fura-2
fura 2-acetoxymethylester
- MAP
microtubule-associated protein
- NMDA
N-methyl-D-aspartic acid
REFERENCES
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;2:113–132. doi: 10.1385/MN:25:2:113. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Prat AG, Centiello HF, Ausiello DA, Fuller CM, Jovoy B, Benos DJ, Ismailov II. Regulation of epithelial sodium channels by short actin filaments. J Biol Chem. 1996;271:17704–17710. doi: 10.1074/jbc.271.30.17704. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JA, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron. 1993;4:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17:8178–8186. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyashiki T, Arakawa Y, Takemoto-Kimura S, Bito H, Narumiya S. Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2002;99:14458–14463. doi: 10.1073/pnas.212148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wu SM. Multiple types of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. Brain Res. 1999;821:487–502. doi: 10.1016/s0006-8993(99)01067-7. [DOI] [PubMed] [Google Scholar]
- Gottesman J, Miller RF. N-methyl-D-aspartate receptors contribute to the baseline noise of retinal ganglion cells. Vis Neurosci. 2003;20:329–233. doi: 10.1017/s0952523803203114. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Actin-binding proteins 1: spectrin superfamily. Protein Profile. 1994;1:706–778. [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. Ca2+ channel Ca2+-dependent inactivation in a mammalian central neuron involves the cytoskeleton. Pflugers Arch. 1994;429:14–21. doi: 10.1007/BF02584025. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photo-receptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Czerwinska E, Czerwinski W, Walsh MP, MacDonald JF. Regulation of NMDA receptor activity by F-actin and myosin light chain kinase. J Neurosci. 2001;21:8464–8472. doi: 10.1523/JNEUROSCI.21-21-08464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Waxman SG, Kocsis JD. Differential role of two Ca2+-permeable non-NMDA glutamate channels in rat retinal ganglion cells: kainate-induced cytoplasmic and nuclear Ca2+ signals. J Neurophysiol. 1994;72:2503–2516. doi: 10.1152/jn.1994.72.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Maguire G, Connaughton V, Prat AG, Jackson GR, Jr, Cantiello HF. Actin cytoskeleton regulates ion channel activity in retinal neurons. Neuroreport. 1998;9:665–670. doi: 10.1097/00001756-199803090-00019. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Matus A. Calcium regulation of actin dynamics in dendritic spines. Cell Calcium. 2005;37:477–482. doi: 10.1016/j.ceca.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Okada T, Schultz K, Geurtz W, Hatt H, Weiler R. AMPA-preferring receptors with high Ca2+ permeability mediate dendritic plasticity of retinal horizontal cells. Eur J Neurosci. 1999;3:1085–1095. doi: 10.1046/j.1460-9568.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000;20:22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert T, Akopian A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience. 2004;125:583–590. doi: 10.1016/j.neuroscience.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic and ionotropic glutamate receptors regulate calcium channel currents in salamander retinal ganglion cells. J Physiol. 1998;510:815–828. doi: 10.1111/j.1469-7793.1998.815bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Matus AI. Microtubule-associated proteins characteristic of embryonic brain are found in the adult mammalian retina. Dev Biol. 1988;130:423–434. doi: 10.1016/0012-1606(88)90338-7. [DOI] [PubMed] [Google Scholar]
- Velte TJ, Yu W, Miller RF. Estimating the contributions of NMDA and non-NMDA currents to EPSPs in retinal ganglion cells. Vis Neurosci. 2003;6:999–1014. doi: 10.1017/s0952523800011731. [DOI] [PubMed] [Google Scholar]
- Weiler R, Janssen-Bienhold U. Spinule-type neurite outgrowth from horizontal cells during light adaptation in the carp retina: an actin-dependent process. J Neurocytol. 1993;22:129–139. doi: 10.1007/BF01181576. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mattson MP, Furukawa K. Endoplasmic reticulum calcium release is modulated by actin polymerization. J Neurochem. 2003;82:945–952. doi: 10.1046/j.1471-4159.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen L, Sibley JT, Constantine-Paton M. Analysis of synaptic distribution within single retinal axonal arbors after chronic NMDA treatment. J Neurosci. 1995;6:4712–4725. doi: 10.1523/JNEUROSCI.15-06-04712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Sucher NJ, Lipton SA. Co-expression of AMPA/kainate receptor-operated channels with high and low Ca2+ permeability in single rat retinal ganglion cells. Neuroscience. 1995;67:177–188. doi: 10.1016/0306-4522(94)00627-h. [DOI] [PubMed] [Google Scholar]