Abstract
Controlled rainfall experiments utilizing drop forming rainfall simulators were conducted to study various factors contributing to off-target transport of off-the-shelf formulated pyrethroid insecticides from concrete surfaces. Factors evaluated included active ingredient, product formulation, time between application and rainfall (set time), and rainfall intensity. As much as 60% and as little as 0.8% of pyrethroid applied could be recovered in surface runoff depending primarily on product formulation, and to a lesser extent on product set time. Resulting wash-off profiles during one-hour storm simulations could be categorized based on formulation, with formulations utilizing emulsifying surfactants rather than organic solvents resulting in unique wash-off profiles with overall higher wash-off efficiency. These higher wash-off efficiency profiles were qualitatively replicated by applying formulation-free neat pyrethroid in the presence of independently applied linear alkyl benzene sulfonate (LAS) surfactant, suggesting that the surfactant component of some formulated products may be influential in pyrethroid wash-off from urban hard surfaces.
INTRODUCTION
Studies conducted throughout the nation have documented pyrethroid insecticide concentrations in urban waterways that exceed known toxicity thresholds for the protection of aquatic life (1-3). In a specific urban watershed study conducted in the Central Valley of California, pyrethroid insecticides detected in urban creek sediments were found to significantly correlate with acute mortality in amphipod-based toxicity bioassays, with a strong proximal relationship to residential development and associated stormwater outfalls (1). Pyrethroid-related sediment toxicity has similarly been observed from single-family home dominated urban watersheds in central Texas (3). In a targeted urban monitoring study, chemical analysis of both dry- and wet-weather runoff from single-family homes in California’s central valley consistently detected pyrethroid insecticides, with frequent detections of bifenthrin, permethrin, and cyfluthrin (4). These studies point to applications on residential landscapes and structures as likely sources of pyrethroid insecticides in urban streams.
An improved understanding of the particular sources and mechanisms by which pyrethroid insecticides are transported away from their point of application is necessary to more effectively consider strategies to reduce or eliminate their impact on the aquatic environment. When considering the fate and transport of a pesticide in the environment, engineers, scientists and resource managers first consider the physical and chemical properties of the active ingredient. It is often the practice, however, to neglect the possible effects of pesticide product formulation and the components that make up that mixture. Formulated pesticide products are complex mixtures comprising numerous substances that may improve active ingredient efficacy, product delivery and product application.
Numerous small-scale pesticide wash-off studies have been conducted on turf and bare soils and have identified factors of active ingredient chemical/physical property, product formulation, rainfall/runoff intensity, and product set time as important variables controlling pesticide wash-off (5-9). Chief among these controlling variables are the physical and chemical properties of the active ingredients themselves. Intuitively, those active ingredients with lower aqueous solubility and greater organic matter normalized partition coefficients (Koc) routinely result in lower wash-off fractions and lower total mass wash-off in comparison to those active ingredients with higher aqueous solubility and lower organic matter partitioning.
Comparatively few studies have looked at pesticide wash-off from mineral-based and essentially impervious hard surfaces such as concrete. In Ramwell (10), runoff of herbicide from concrete and asphalt was examined. In that study, Koc was a poor predictor of actual sorption of herbicide to concrete, with actual sorption being fairly limited. Many differences exist between pyrethroid insecticides and the relatively water soluble herbicides tested by Ramwell, but a model environmental system where pesticide wash-off is maximized can be imagined as one where the application surface is virtually devoid of organic matter and virtually impervious to infiltrating water, such as concrete sprayed in the course of post-construction residential pest control.
In this study our principal objectives were to quantify the transport of pyrethroid insecticides from concrete treated with a variety of commercially formulated liquid products available off-the-shelf for residential structural pest control and to identify the key factors controlling the extent of wash-off. At the outset of this study we anticipated that pyrethroid wash-off from concrete would principally correlate with the chemical properties of the specific active ingredients such that individual product wash-off fraction would be correlated with the octanol-water partition coefficient (Kow), with some additional variability attributable to product specific formulation and precipitation event characteristics. Our observation of distinctly different wash-off profiles and wash-off masses among the formulated products led to our interest in formulation components commonly referred to as ‘inert’ or ‘other’ ingredients, and to the specific possibility that surfactants could enhance or facilitate transport of pyrethroid insecticide from concrete surfaces. The four commercial products tested in this study include different active ingredients, different formulation bases, and comparatively different weight percentages and classes of surfactant (e.g., anionic and/or nonionic surfactants). These differences, combined with associated studies with model systems containing selected active ingredients and formulation components, were used to draw conclusions about the factors controlling the extent of insecticide wash-off.
EXPERIMENTAL SECTION
Rainfall Simulations
Drop forming rainfall simulators were designed and calibrated to specifications outlined by Battany and Grismer (11). Surface runoff from simulations was collected as volume intervals and time recorded for calculation of runoff rate (see the supporting information section for more detail on this and other aspects of the experimental procedures).
Simulations were designed to control for five variables, including rainfall intensity, slope, product set time, pyrethroid active ingredient, and formulation type. Slope was held constant at 4 degrees from the horizontal. Rainfall intensity was controlled at either 25 mm/hr or 50 mm/hr, and simulations were performed for 60 minutes. Product set times investigated were 1.5 hours, 24 hours, and seven days from the time of product application. On some surfaces, successive simulations were conducted without intervening product application, starting at 1.5 h, 7 d, 21 d and 49 d after the initial application (1.5h, 7d 2nd, 21d 3rd, 49d 4th). Concrete surfaces were washed prior to product application and stored outdoors.
Determination of Pyrethroid and Surfactants
Neat standards of pyrethroids and C10-C16 LAS were obtained from ChemService, Inc. (West Chester, PA). It was determined by fast atom bombardment mass spectroscopy (FAB-MS) that three of the four products tested in this study contained LAS at varying concentrations, therefore LAS was selected as a model surfactant to investigate the role of particular formulation components on pyrethroid wash off. The CMC of the LAS was determined to be 250 mg/L using a SensaDyne QC6000 surface tensiometer. Quantitative determination of pyrethroids in simulated stormwater runoff samples was accomplished using an Agilent 6890 gas chromatograph with electron capture detection. Quantitative determination of LAS surfactant and qualitative investigation of formulated pyrethroid pesticide product composition were accomplished using an Agilent 1200 liquid chromatograph with diode array detection and inline with an Agilent 1200 evaporative light scattering detector (ELSD). Separate extraction of both pyrethroid and LAS surfactant was accomplished using an octodecyl (C-18) solid phase extraction cartridge (Supelco ENVI-C18) with a 500 mg sorbent bed. Surfactant class was confirmed by obtaining negative and positive ion fast atom bombardment (FAB) mass spectra for all formulated products utilizing a JEOL MSroute JMS-600h double focusing mass spectrometer and surfactants identified by comparison to mass fragment tables presented in Ventura et. al. (12).
Experiments with Formulated Products
Four off-the-shelf general-use formulated products with labels permitting application to foundations and patios were tested (Table 1). Products were prepared and applied to concrete surfaces (80×80×5 cm; Quickrete 5000) per label specification and at label rates (labels did not specify a measured rate per se, but rather generally specified that the surface be thoroughly “wetted with a coarse spray but without soaking”). In order to normalize surface treatment, application was conducted so that the entire concrete surface was wetted (approximately 55-65 grams of formulated product gravimetrically), with actual application rate recorded. Although application of formulated products were conducted in a similar manner and a similar volume, differences in active ingredient weight percentages resulted in different application masses across products. In those cases where the product was sold ready-to-use, the supplied pump action hand sprayer was utilized. In those cases where the product was sold as a dilutable liquid concentrate, a separately purchased home-and-garden pump action hand sprayer was used (Delta Industries, PA).
TABLE 1.
Formulated residential-use products tested
| Active Ingredient |
Product Type |
Product % a.i. (w/w) |
a.i. log Kow |
a.i. Solubility (μg/L) |
|---|---|---|---|---|
| β cyfluthrin | SC / Dilutable | 2.5 | 5.97 | 2.3 |
| λ cyhalothrin | EC / Dilutable | 0.50 | 7.00 | 5.0 |
| esfenvalerate | EC / RTU | 0.0033 | 5.62 | 6.0 |
| bifenthrin | CE / RTU | 0.050 | 6.40 | 0.014 |
a.i.: active ingredient; SC: suspension concentrate; EC: emulsifiable concentrate; CE: microemulsion; RTU: ready-to-use; Kow and solubility from Laskowski, 2002 (20)
Experiments with Neat Pyrethroids
To investigate wash-off of pyrethroids free of any influence from formulation inerts, neat pyrethroid wash-off experiments were conducted utilizing esfenvalerate and bifenthrin. Neat esfenvalerate and bifenthrin were dissolved in hexane and applied to clean concrete surfaces at similar mass rates of application to their corresponding esfenvalerate EC and bifenthrin CE products. The hexane carrier was allowed to evaporate followed by a 25 mm/hr simulation 1.5 hours after application.
Experiments with Neat Bifenthrin and LAS
To investigate the role of surfactants in the wash-off of pyrethroids, an experiment with neat bifenthrin and LAS was conducted. Bifenthrin was first applied to the concrete surface in hexane at a rate equivalent to the corresponding bifenthrin CE product. The hexane was allowed to evaporate upon which LAS dissolved in deionized water was then applied to the same surface. LAS was applied at a rate of 906 mg/m2. The deionized water carrier was allowed to evaporate followed by a 25 mm/hr simulation 1.5 hours after application.
RESULTS AND DISCUSSION
Formulated Product Rainfall Simulations
Rainfall simulations with formulated pesticide products resulted in distinctive wash-off profiles (Figure 1). As a percentage of the total mass removed from the concrete surface over the course of an experiment, the profiles either took a form characterized by a steep initial dissipation rate followed by a more steady rate, which we refer to throughout this paper as a Type A profile, or the form of a relatively steady dissipation rate over the duration of the experiment, with more constant concentration of pyrethroid in runoff, which we refer to as a Type B profile. In the Type A cases, approximately 70 to 90 percent of the total mass removed over the simulation was contained within the first 2 liters of runoff, whereas the corresponding volume for the Type B cases contained only 30 to 45 percent of the total mass that was ultimately removed. This trend held across experiments, despite factors of rainfall intensity, initial product set time, or application rate. However, upon successive wash-off events of a single initial application, Type A profiles gradually took the form of Type B profiles (e.g., Fig. 1, 25/49d 4th).
FIGURE 1.
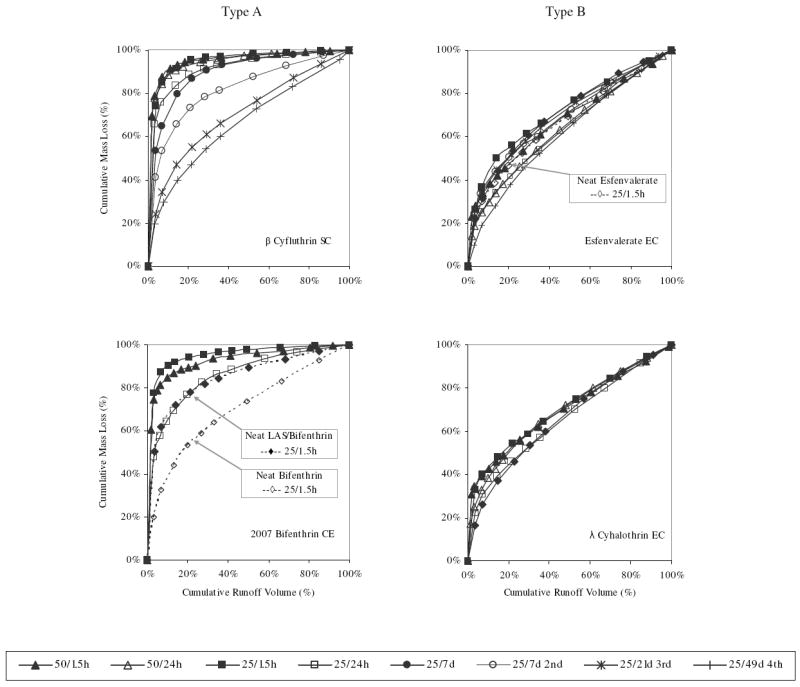
Type A and Type B dissipation profiles of formulated pyrethroid pesticide products as percent of cumulative runoff volume and percent of cumulative mass wash-off divided into those with rapid dissipation (Type A) and those with steady dissipation (Type B). Legend reports intensity (mm/hr)/product set-time (hours or days).
Products applied at label rates to concrete resulted in orders-of-magnitude differences in total mass wash-off and event mean concentration (EMC) among the tested products (Table 2). Event mean concentration nearly reached or far exceeded aqueous solubility thresholds for the pyrethroids in most simulations (18). For the β cyfluthrin suspension concentrate (SC) product at an initial set-time of 1.5h and a 25 mm/hr simulation, the EMC exceeded aqueous solubility by some 280 times. For λ cyhalothrin and esfenvalerate emulsifiable concentrate (EC) products at an initial set-time of 1.5h and a 25 mm/hr simulation, EMC values were approximately one-half and one-third aqueous solubility, respectively. As a percentage of the total mass applied, the β cylfuthrin SC product had the highest wash-off factor, followed by 2007 bifenthrin micro-emulsion (CE), λ cyhalothrin EC, and esfenvalerate EC products.
TABLE 2.
Applied mass (μg), total mass wash-off (μg), 10 liter mass wash-off (μg), and EMC (μg/L) for rainfall simulations utilizing formulated product sand neat-grade pyrethroids and surfactant. Mean result of two simultaneous replicates and (standard deviation)
| Formulated Product Experiment (Intensity/Set Time) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 25/1.5h |
25/24h |
25/7d |
25/7d 2nd |
25/21d 3rd |
25/49d 4th |
50/1.5h |
50/24h |
|
| β Cyfluthrin SC | ||||||||
| Applied Mass | 15,300 (247) | 14,600 (233) | 12,200 (27.2) | a | a | a | 13,900 (166) | 15,300 (552) |
| Total Mass Washoff | 8,290 (5,340) | 2,650 (776) | 998 (8.64) | 151 (66.3) | 41.6 (11.3) | 15.1 (0.106) | 4,830 (139) | 4,150 (134) |
| Mass Washoff at 10 L | 8,200 (5,340) | 2,590 (758) | 977 (8.77) | 140 (64.9) | 35.9 (10.8) | 12.5 (0.144) | 4,660 (149) | 3,970 (137) |
| EMC | 587 (387) | 180 (51.6) | 71.3 (1.1) | 10.4 (4.71) | 2.95 (0.811) | 1.07 (0.00210) | 174 (5.45) | 143 (1.56) |
| Esfenvalerate EC | ||||||||
| Applied Mass | 1,750 (60.7) | 1,860 (88.7) | 2,010 (51.3) | a | a | a | 1,790 (84.0) | 1,920 (4.67) |
| Total Mass Washoff | 25.5 (9.95) | 22.6 (0.109) | 15.1 (1.84) | 17.9 (8.2) | 8.04 (2.79) | 1.44 (0.0677) | 26.4 (0.483) | 41.4 (1.61) |
| Mass Washoff at 10 L | 22.3 (8.83) | 18.0 (0.359) | 13.4 (1.33) | 14.8 (7.11) | 6.89 (2.59) | 1.16 (0.111) | 16.2 (1.22) | 22.6 (1.46) |
| EMC | 1.87 (0.741) | 1.57 (0.0321) | 1.10 (0.0695) | 1.22 (0.561) | 0.578 (0.202) | 0.101 (0.00435) | 0.955 (0.0121) | 1.44 (0.0632) |
| λ Cyhalothrin EC | ||||||||
| Applied Mass | 1,090 (6.67) | 1,470 (1.63) | 1,170 (18.7) | b | b | b | 1,060 (26.4) | 1,410 (13.3) |
| Total Mass Washoff | 46.1 (4.13) | 35.7 (3.12) | 2.83 (0.0908) | 50.7 (7.56) | 26.2 (3.23) | |||
| Mass Washoff at 10 L | 40.5 (4.42) | 28.5 (2.37) | 2.47 (0.119) | 31.4 (5.36) | 16.6 (1.99) | |||
| EMC | 3.45 (0.388) | 2.47 (0.218) | 0.214 (0.0130) | 1.75 (0.243) | 0.897 (0.104) | |||
| 2007 Bifenthrin CE | ||||||||
| Applied Mass | 30,000 (247) | 31,300 (742) | b | b | b | b | 31,200 (1,630) c | b |
| Total Mass Washoff | 1,510 (484) | 456 (147) | 646 (531) c | |||||
| Mass Washoff at 10 L | 1,500 (484) | 438 (151) | 607 (530) c | |||||
| EMC | 111 (41.9) | 33.1 (11.6) | 22.7 (29.3) c | |||||
| 2009 Bifenthrin CE | ||||||||
| Applied Mass | 32,600 (813) | b | b | b | b | b | 32,000 (389) | b |
| Total Mass Washoff | 490 (353) | 702 (139) | ||||||
| Mass Washoff at 10 L | 465 (347) | 642 (131) | ||||||
| EMC | 33.4 (24.3) | 24.0 (4.93) | ||||||
| Neat-Grade Experiments (Intensity/Set Time) | ||||||||
| 25/1.5h | 25/1.5h | 25/1.5h | ||||||
| Bifenthrin & LAS Neat Grade | Bifenthrin Neat Grade | Esfenvalerate Neat Grade | ||||||
| Applied Mass | 30,000 (0.0) | 29,200 (0.0) | 1,890 (0.0) | |||||
| Total Mass Washoff | 257 (15.1) | 73.1 (19.2) | 65.5 (1.28) | |||||
| Mass Washoff at 10 L | 243 (10.1) | 61.4 (18.1) | 53.5 (0.0784) | |||||
| EMC | 19.2 (0.598) | 4.98 (1.22) | 4.46 (0.00320) | |||||
initial application was that of corresponding 25/1.5h experiment
experiment not conducted
result of 4 replicates
Prior to product application, concrete surfaces were washed to remove settled material that had accumulated on the surface. As an uncontrolled experimental covariate total suspended solids (TSS) was measured for all volume intervals. No positive correlation was observed between TSS and total pyrethroid wash-off.
For all products tested, increased product set-time resulted in decreased wash-off. For the β cyfluthrin SC product an approximate 9-fold decrease in total mass wash-off was observed for comparable 25 mm/hr simulations between the 1.5h and 7d set-time intervals. Decrease in wash-off was further amplified with the 7d 2nd, 21d 3rd, and 49d 4th repeated simulations, despite the fact that 40 percent of the applied β cyfluthrin mass was not washed off during the initial 1.5h set-time simulation, much of which presumably remained on the surface. Attempts to recover the residual product using solvent washes failed to close the mass balance, presumably because the product had infiltrated the concrete surface. At least some active ingredient persisted on the concrete surfaces for long periods, with measurable masses washed off even after set times of 238 days (Fig. S2 in supporting information).
It is interesting to note that doubling rainfall intensity did not necessarily lead to greater mass wash-off for all products tested. For the β cyfluthrin SC and 2007 bifenthrin CE 1.5h set-time experiments, greater mass wash-off was observed for the lower intensity 25 mm/hr simulations. Inherent replicate variability in early set-time experiments precludes making determinations of statistical significance, yet mean point estimates follow other expected trends lending validity to the unintuitive observation.
Variability between replicates, as measured by standard deviation in Table 2, generally decreased with increasing set-time and successive simulation. This is not unexpected given the limited replication coupled with amplifying physical factors such as variable surface microtopology, crudeness of application technique, and variable product specific drying rates. Although 1.5 hours was sufficient for the surfaces to appear visually dry after an application, the spreading and final disposition of spray droplets likely remained dynamic. Due to the use of simple pump-action bottle sprayers, the spray droplets and spray pattern were not homogeneous; a condition expected in the field because numerous residential pesticide products are sold in pump-action spray containers. These factors taken together contributed to measured variability between replicates. It is worth noting, however, that regardless of product, application rate, rainfall intensity, and initial set time, runoff concentration in the static latter half of the dissipation profile were generally within a factor of ten from each other, ranging from 0.11 to 6.8 μg/L. Only the β cyfluthrin SC product deviated from this trend, and only for the 25 mm/hr, 1.5 and 24 hour set-time simulations. In these two simulations, final concentrations were 19 and 11 μg/L, respectively.
Pearson’s correlation coefficients indicated weak relationship between total mass wash-off and application rate (r=0.186) and total mass wash-off and active ingredient KOW (r=-0.313). To further test the contributing factors of application rate and product formulation, simulations were conducted utilizing bifenthrin and esfenvalerate applied to concrete surfaces free of any chemical formulation. Pyrethroids were applied at mass loadings equivalent to those produced by their respective formulated products, but were applied to the surface in hexane. As shown in Fig. 1, dissipation assumed the Type B profile. Differences between the total mass wash-off for neat bifenthrin and neat esfenvalerate were statistically insignificant (two-tailed t-test: p = 0.50; p = 0.24), despite the 15-fold difference in their application rates (Table 2). Contrary to what we originally hypothesized, application rate and physical/chemical property differences between specific pyrethroid active ingredients could not sufficiently explain the differences in dissipation profile or total mass wash-off between the formulated products.
Investigation into Formulation Differences
Commercially formulated liquid products are often complex mixtures of numerous inactive ingredients, including emulsifying agents, solvents, adjuvants, defoaming agents, antimicrobial agents, pH stabilizers, and water. Surfactants and solvents are known to be useful cosolvents of hydrophobic organic compounds and rise to immediate interest when we consider possible formulation effects on pyrethroid wash-off.
In this particular study, we used commercially formulated pyrethroid products that could be classified as a suspension concentrate, a micro-emulsion, or an emulsifiable concentrate. As depicted in Fig 1, suspension concentrates and micro-emulsions yield Type A dissipation profiles and comparatively high fractional wash-off, whereas emulsifiable concentrates yielded Type B dissipation profiles and comparatively low fractional wash-off. Furthermore, products formulated as suspension concentrates and micro-emulsions contain high mass percentages of surfactant and little to no organic solvent whereas emulsifiable concentrates utilize high mass percentages of organic solvent and comparatively lower mass percentages of surfactant. When a hypothetical spray droplet intercepts a surface, the volatile components such as water and solvent will evaporate leaving a residue of active ingredient, surfactant, and other low volatility components. With this picture in mind, we hypothesized that the residual surfactant co-applied with the pyrethroid active ingredient may have contributed to the enhanced wash-off we had observed.
Through their amphiphilic nature, surfactants find their utility in many commercial products by reducing interfacial tension. Below the critical micelle concentration (CMC), surfactant molecules in an aqueous solution are found in freely dissolved monomeric form (13), while above the CMC, the total amount of surfactant can be described as divided between monomeric and micellar forms. While surfactant in non-aggregated monomeric form can increase the aqueous solubility of sparingly soluble hydrophobic compounds (14), much the same way as is postulated for aqueous systems containing colloidal organic macromolecules (15), it is the presence of micelles that largely endows a surfactant with the characteristic of a unique, and at times powerful, cosolvent (13-14, 16).
The usage of surfactants as emulsifiers in liquid pesticide formulations can be rather complex. In the particular case of suspension concentrates and micro-emulsions, these formulations often involve mixtures of different surfactants and/or surfactant classes. We wished to better understand the surfactant composition of our products, so we subjected them to analysis by HPLC-ELSD and FAB-MS. Through the assistance of these analytical tools, we were able to elucidate surfactant classes, and in some cases unequivocally identify the surfactant present (Table 3).
TABLE 3.
Commercial product surfactant composition
| Commercial Product |
Surfactant Class |
Surfactant Type |
|---|---|---|
| β cyfluthrin SC | nonionic | unknown polyethoxylate |
| λ cyhalothrin EC | anionic | C8-C16 LAS |
| esfenvalerate EC | anionic | C10-C15 LAS |
| 2007 bifenthrin CE | nonionic/anionic | C10-C14 LAS; PEO-PPO block copolymer |
| 2009 bifenthrin CE | nonionic/anionic | C10-C14 LAS; PEO-PPO block copolymer |
SC: suspension concentrate; EC: emulsifiable concentrate; CE: microemulsion; LAS: linear alkylbenzene sulfonate; PEO-PPO: polyethylene oxide-polypropylene oxide
To illustrate the complexity of these formulations, Figure 3 presents the ELSD chromatogram for our bifenthrin CE product. Over the course of this study the original bifenthrin CE product purchased late in 2007 was completely consumed. A second identically labeled product was purchased in early 2009. By ELSD, the 2007 bifenthrin CE product was shown to contain LAS at an equivalent concentration of 1.59 g/L (identified through comparison to our LAS standard) and some unknown multi-homologue nonionic surfactant (identified by relative retention time), whereas LAS was not detected in the 2009 bifenthrin CE product, but the peak area in the nonionic surfactant retention time window nearly doubled. Analysis of product samples by FAB was unable to ascertain relative concentration of identified components but did confirm the presence of LAS in both product samples, despite its apparent absence based on the ELSD analysis of the 2009 bifenthrin CE product (the effective instrument quantification limit of our ELSD for LAS is approximately 50 mg/L, and there was little available product sample for concentration purposes). Both products also contained a complex mass signature similar to that of some polyethylene oxide (PEO) and polypropylene oxide (PPO) block co-polymers. While the specific nature of the hydrophobic polymeric unit could not be identified, FAB analysis established that the nature of this hydrophobe changed between products, as evidenced by a consistent base-shift in the mass spectrum between products.
FIGURE 3.
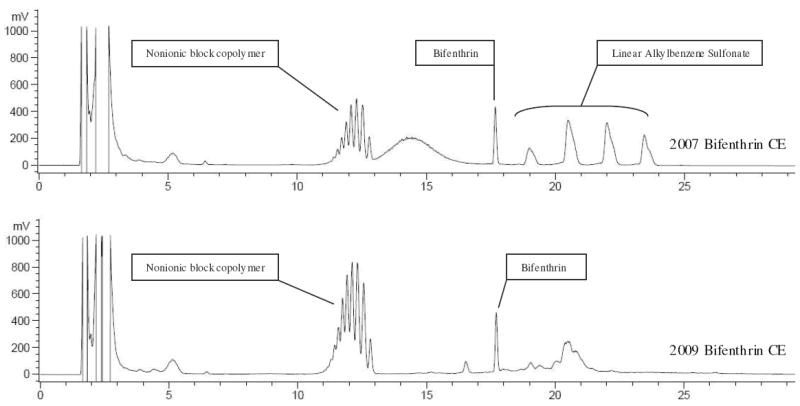
LC-ELSD chromatograms of 2007 and 2009 bifenthrin CE products at equivalent product concentration, clearly indicating change in formulation composition.
As illustrated by our experience with the bifenthrin CE product, formulation of commercial end-use products can be a moving target. What is interesting about this apparent reformulation, however, is our observation regarding corresponding total mass wash-off between 2007 and 2009 products. As shown in Table 2, at the lower rainfall intensity of 25 mm/hr and a set time of 1.5 hours, the 2009 product yielded 3-fold less total wash-off and EMC than its 2007 counterpart (two-tailed t-test; p=0.138). Although replicate variability precludes findings of statistical significance, it does bring us to question the role of product formulation - specifically the type and concentration of surfactant blend – on the corresponding nature of how and how much pyrethroid mass is removed from a concrete surface by rainfall.
While it is evident in the results of Table 2 that formulation is a contributing factor to wash-off, formulation is not a truly decomposed and singly isolatable contributing factor. As previously noted, working with commercially formulated products presents a number of challenges and our desire was to test a model system that would include only active ingredient and surfactant, free of any other convoluting formulation components. To test the role of surfactant in determining the observed dissipation profile and total mass wash-off, the simulation utilizing neat bifenthrin on concrete was repeated with the addition of LAS (Table 3). In the presence of LAS, bifenthrin dissipation assumed a Type A profile (Figure 1) with a nearly seven-fold increase in total mass wash-off and EMC (Table 2). In this LAS co-solvated bifenthrin simulation, LAS was applied at a rate of 906 mg/m2. As shown in Figure 2, average initial LAS concentration in runoff was 57.6 mg/L, or approximately one-fifth the measured aqueous CMC. At this concentration LAS would entirely be represented in monomeric form, yet evidently still sufficient to enhance the dissolution and solubilization of bifenthrin. This period of enhancement is rather short-lived, however, with concentration of LAS co-solvated bifenthrin reaching that of neat bifenthrin after approximately the first 5 liters of runoff. At this point, 19.1 mg/L LAS appears a relatively ineffective cosolvent of bifenthrin.
FIGURE 2.
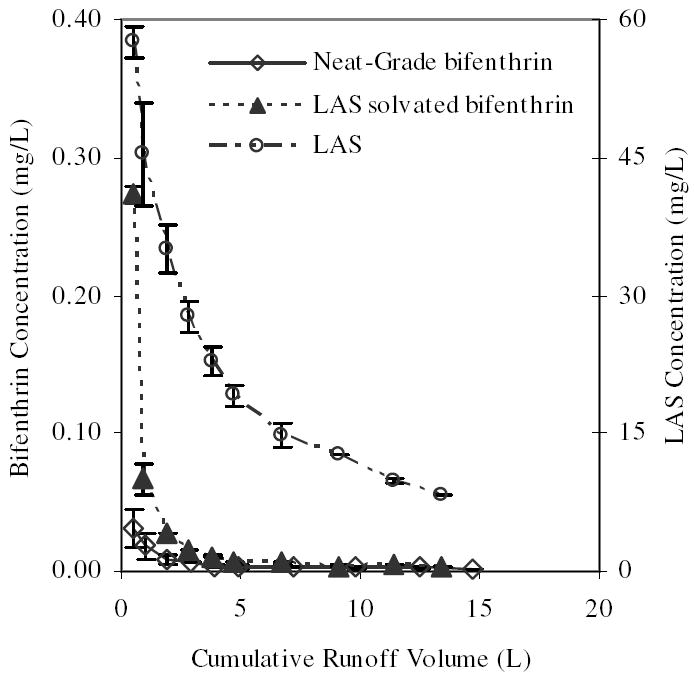
Average concentration of bifenthrin and LAS in simulated runoff from LAS/bifenthrin treated concrete surfaces at 25 mm/hr rainfall intensity and 1.5h set-time. Error bars represent a single standard deviation.
Although use of LAS resulted in a Type A dissipation profile and enhanced mass removal, the total mass removed did not approach the magnitude of that removed for the β cyfluthrin SC product or the 2007 bifenthrin CE product. Although no experiment was conducted to specifically examine this phenomenon, such an outcome is likely due to the complex nature of the SC and CE formulations relative to the simple two component system of bifenthrin and LAS. While formulated active ingredients dry to the concrete surfaces as a homogeneous mixed residue of pyrethroid and surfactant, in our model system bifenthrin and LAS, which were applied independently, would have dried as heterogeneous isolated residues. Furthermore, as is the case with SC formulations where the active ingredient is a milled solid typically in the 2 to 8 μm range, the active ingredients may be in different physical forms (17). Surfactants are adsorbed to the surface of these particles, allowing for particle dispersion in water. After application and evaporation of the aqueous medium, surfactants may remain adsorbed to the particle surface allowing the particle to be more readily rewetted and dispersed in overlying runoff during a precipitation event. Such a case could plausibly lead to large mass transport due not only to the dynamics of surfactant movement, but also the physics of particle movement. In contrast, formulated micro-emulsions are thermodynamically stable three-component systems comprised of water, oil and surfactant. The oil represents a liquid technical grade active ingredient, or a solid technical grade active ingredient dissolved in a small amount of solvent, whereas the emulsifying surfactants are typically a blend of nonionic and anionic surfactants (18).
To fully understand the role of product formulation, one must consider the specific components comprising a particular formulation. Although we have specifically focused on the contribution of surfactants as emulsifying agents, surfactants and oils, if present, can additionally serve the role as adjuvants. Spray drop retention, spreading, and rainfastness are often important properties of formulated pesticide products, particularly in application to leaf surfaces (19). In agricultural applications, the role of formulation adjuvants in a purposefully mixed package is well understood, but there is scant information in the literature as to effect of adjuvants on the environmental fate of active ingredients. As we have demonstrated in this study with LAS, it is possible for surfactant, if applied in sufficient quantity, to facilitate enhanced wash-off, but it is also a possibility that other components in the formulation package may contribute in some antagonistic way as well.
An investigation into such a possibility would require a greater understanding and disclosure of the various ingredients of a formulation than we have enjoyed in this study given our use of trade-secret protected commercial products. However, operation of such a component may possibly have been observed in the esfenvalerate EC experiments. In comparable 25 mm/hr and 1.5h set-time experiments, the difference in formulated esfenvalerate wash-off is significantly less in comparison to neat-grade esfenvalerate applied in hexane (two-tailed t-test; p = 0.030), despite the similarity in applied masses and experimental conditions. As the product is principally marketed for application to garden and landscape, it is possible some operative adjuvant is present, affording a level of “rainfastness”.
It can be concluded that differences in product formulation can lead to order-of-magnitude differences in pyrethroid mass wash-off from impervious concrete surfaces. The effect of formulation, however, cannot simply be described in general aggregate terms, such as suspension concentrate, micro-emulsion, or emuslifiable concentrate. As was demonstrated through controlled rainfall simulations, although residues of surfactant can act as co-solvents and facilitators of increased solubilization and wash-off, the physical form of the active ingredient and other product adjuvants can possibly lead to enhanced or retarded wash-off as well. In the particular case of LAS co-solvated bifenthrin, enhanced bifenthrin desorption was possible at aqueous LAS concentrations below the CMC. These controlled experimental observations have a bearing on the permitted uses of specific pyrethroid formulation types, and provides needed initial information for the development of wash-off coefficients from pyrethroid treated concrete surfaces.
Supplementary Material
Acknowledgments
The research described in this paper was supported by funding from the California Department of Pesticide Regulation under contract number 06-0086C and by the National Institute of Environmental Health Sciences under award number P42E5004699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the California Department of Pesticide Regulation, the National Institute of Environmental Health Sciences, or the National Institutes of Health. The authors thank Drs. Jennifer Field and Jeff Morre of Oregon State University for help with the FAB-MS analysis and Russell Jones, Bayer Crop Sciences; Drs. Frank Spurlock and Kean Goh, California Department of Pesticide Regulation; Dr. Herbert Scher, UC Davis Biological and Agricultural Engineering; and Chris Wissel-Tyson, UC Davis Civil and Environmental Engineering for their assistance in executing this study.
Footnotes
SUPPORTING INFORMATION AVAILABLE Expanded discussion of experimental procedures and pyrethroid and surfactant analysis are provided in supporting information. Additionally, TSS results and long-term measurements of pyrethroid leaching from concrete are provided. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Weston DP, Holmes RW, You J, Lydy JM. Aquatic toxicity due to residential use of pyrethroid insecticides. Environ Sci Technol. 2005;39:9778–9784. doi: 10.1021/es0506354. [DOI] [PubMed] [Google Scholar]
- 2.Holmes RW, Anderson BS, Phillips BM, Hunt JW, Crane DB, Mekebri A, Connor V. Statewide investigation of the role of pyrethroid pesticides in sediment toxicity in California’s urban waterways. Environ Sci Technol. 2008;42:7003–7009. doi: 10.1021/es801346g. [DOI] [PubMed] [Google Scholar]
- 3.Hintzen EP, Lydy MJ, Belden JB. Occurrence of potential toxicity of pyrethroids and other insecticides in bed sediments of urban streams in central Texas. Environ Pollut. 2009;157:110–116. doi: 10.1016/j.envpol.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Weston DP, Holmes RW, Lydy MJ. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut. 2009;157:287–294. doi: 10.1016/j.envpol.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Armbrust K, Peeler H. Effects of formulation on the run-off of imidacloprid from turf. Pest Manag Sci. 2002;58:702–706. doi: 10.1002/ps.518. [DOI] [PubMed] [Google Scholar]
- 6.Cole JT, Baird JH, Basta NT, Huhnke RL, Storm DE, Johnson GV, Payton ME, Smolen MD, Martin DL, Cole JC. Influence of buffers on pesticide and nutrient runoff from bermudagrass turf. J Environ Qual. 1997;26:1589–1598. [Google Scholar]
- 7.Evans JR, Edwards DR, Workman SR, Williams RM. Response of runoff diazinon concentration to formulation and post-application irrigation. Trans ASAE. 1998;41:1323–1329. [Google Scholar]
- 8.Wauchope RD, Williams RG, Marti LR. Runoff of sulfometuron-methyl and cyanazine from small plots: Effects of formulation and grass cover. J Environ Qual. 1990;19:119–125. [Google Scholar]
- 9.Gouy V, Dur J, Calvet R, Belamie R, Chaplain V. Influence of adsorption-desorption phenomena on pesticide run-off from soil using simulated rainfall. Pestic Sci. 1999;55:175–182. [Google Scholar]
- 10.Ramwell CT. Herbicide sorption to concrete and asphalt. Pest Manag Sci. 2005;61:144–150. doi: 10.1002/ps.959. [DOI] [PubMed] [Google Scholar]
- 11.Battany MC, Grismer ME. Development of a portable field rainfall simulator for use in hillside vineyard runoff and erosion studies. Hydrol Process. 2000;14:1119–1129. [Google Scholar]
- 12.Ventura F, Caixach J, Figueras A, Espalder I, Fraisse D, Rivera J. Identification of surfactants in water by FAB mass spectrometry. Water Res. 1989;23:1191–1203. [Google Scholar]
- 13.Edwards DA, Luthy RG, Zhongbao L. Solublization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ Sci Technol. 1991;25:127–133. [Google Scholar]
- 14.Kile DE, Chiou CT. Water solubility enhancements of DDT and trichlobenzene by some surfactants below and above the critical micelle concentration. Environ Sci Technol. 1989;23:832–838. [Google Scholar]
- 15.Chiou CT, Malcolm RL, Brinton TI, Kile DE. Water solubility enhancement of some organic pollutants and pesticides by dissolved humic and fulvic acids. Environ Sci Technol. 1986;20:502–508. doi: 10.1021/es00147a010. [DOI] [PubMed] [Google Scholar]
- 16.Rosen MJ. In: Surfactant and Interfacial Phenomena. Rosen MJ, editor. John Wiley & Sons; New York: 1989. pp. 170–205. [Google Scholar]
- 17.Halliday CG. Flowable pesticide formulations: development, process, and the need for standard testing procedures. In: Seymour KG, editor. Pesticide Formulations and Application Systems: Second Conference, ASTM STP 795. American Society for Testing and Materials; Philadelphia: 1983. pp. 45–52. [Google Scholar]
- 18.Skelton PR, Munk BH, Collins HM. Formulation of pesticide microemulsions. In: Hovde DA, Beestman GB, editors. Pesticide Formulations and Application Systems: 8th Volume, ASTM STP 980. American Society for Testing and Materials; Philadelphia: 1988. pp. 36–45. [Google Scholar]
- 19.Bukovac MJ, Cooper JA, Whitmoyer RE, Brazee RD. Pesticide delivery: multiple role of adjuvants in foliar application of systemic compounds. In: Downer RA, Mueninghoff JC, Volgas GC, editors. Pesticide Formulations and Delivery Systems, ASTM STP 1430. American Society for Testing and Materials International; West Conshohocken: 2003. pp. 91–107. [Google Scholar]
- 20.Laskowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol. 2002;174:49–170. doi: 10.1007/978-1-4757-4260-2_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


