Abstract
Previously, pretreatment with estradiol benzoate (EB) was found to modulate response of HPA axis and gene expression in several catecholaminergic neuronal locations in ovariectomized (OVX) rats exposed to single immobilization stress (IMO). Here, we investigated the role of estrogen receptor (ER) subtypes, using selective agonists for ERα (PPT) or ERβ (WAY-200070) in two major central noradrenergic systems and the HPA axis after exposure to single and repeated IMO. OVX female rats received 21 daily injections of either EB (25 μg/kg), PPT (10 mg/kg), WAY-200070 (10 mg/kg) or vehicle. Injections of EB and PPT, but not WAY-200070, elicited reduced body weight and increased uterine weight, showing their selectivity. Both EB and PPT increased corticosterone levels about 2-3 fold, but prevented any further rise with either single or repeated IMO, indicating an ERα, but not ERβ, mediated mechanism. In the locus coeruleus (LC), the rise in dopamine-β-hydroxylase (DBH) mRNA with both stress paradigms was abrogated in EB or PPT injected animals. However, WAY-200070 blocked response of DBH mRNA to single but not repeated IMO. In the nucleus of the solitary tract (NTS), the rise in tyrosine hydroxylase (TH) and DBH mRNAs with both IMOs was absent, or greatly attenuated, in EB or PPT treated rats. In most cases, WAY-200070 inhibited the response to single but not repeated IMO. The results demonstrate that pretreatment with estradiol, or ER selective agonists, modulate the stress-triggered induction of gene expression of norepinephrine biosynthetic enzymes in LC and NTS, with ER selectivity depending on duration of the stress.
Keywords: estradiol, estrogen receptor, tyrosine hydroxylase, dopamine β-hydroxylase, stress
Introduction
It is now widely appreciated that there are distinct gender related differences in stress responses and in susceptibility to stress associated disorders (Baum and Grunberg 1991; Figueiredo et al. 2002; Kajantie and Phillips 2006). Women are more susceptible than men to stress related neuropsychiatric diseases. It is crucially important to determine the cause for this differential susceptibility. Gonadal steroids, such as estradiol, are proposed to play an important role in influencing these gender related differences (Walf and Frye 2005; Scharfman and MacLusky 2008; Stovall and Pinkentorn 2008). Estrogens have been found to modulate functions of several physiological systems and their responses to stress in humans and animals. The stress-induced elevation of hormones of hypothalamic-pituitary-adrenal (HPA) system, ACTH and corticosterone, is higher in females than males (Critchlow et al. 1963; Burgess and Handa 1992; Patchev et al. 1995; Dayas et al. 2000; Young et al. 2001; Serova et al. 2005). Ovariectomy (OVX) of adult rats reduces plasma ACTH and corticosterone levels, an effect reversed by estradiol replacement (Burgess and Handa 1992). Treatment of OVX rats with estrogen also reduces the induction of c-Fos in the paraventricular nucleus (PVN) of the hypothalamus in response to footshock (Ter Horst et al. 2009). In women, cortisol levels are significantly decreased after menopause and can be restored with estrogen replacement therapy (Helgason et al. 1981). Long-term estradiol supplementations lead to faster restoration of increased blood pressure during stress and tend to reduce the immobilization-induced cardiac dysfunction in OVX rats (Serova et al. 2005; Ueyama et al. 2007). Estrogens also modulate functions of catecholaminergic (CA) systems under basal conditions and in response to stress, affecting gene expression for catecholamine biosynthetic enzymes in central and peripheral noradrenergic systems (Liaw et al. 1992; Arbogast and Hyde 2000; Pau et al. 2000; Serova et al. 2005). We previously have shown that in OVX female rats, estradiol benzoate (EB) can differentially modulate response of HPA axis and catecholamine biosynthetic enzyme genes to single immobilization (IMO) stress in several CA regions, including the brain noradrenergic nuclei of locus coeruleus (LC) and the nucleus of solitary tract (NTS) as well as in adrenal medulla (Serova et al. 2005). Several of the responses were attenuated, while some were actually opposite those of control animals. The IMO stress triggered elevation in plasma ACTH levels was lessened in EB pretreated animals. Similarly, with stress in EB treated animals there was no further change or even decline for mRNA levels of tyrosine hydroxylase (TH), the initial and generally rate limiting enzyme of CA biosynthesis, in adrenal medulla and the NTS, and dopamine β-hydroxylase (DBH) mRNA levels in the LC.
However, the responses to estrogens are tissue specific and may depend on the specific estrogen receptor (ER) subtype expressed. The biological actions of estrogens are mediated by two different, but related, estrogen receptor (ER) subtypes, ERα and ERβ, belonging to the nuclear hormone receptor superfamily [rev. in (McKenna et al. 1999a; Klinge 2001)]. Estrogens activate multiple intracellular signaling pathways depending on the receptor subtype and on its subcellular localization, which can be in the membrane or nucleus (Enmark and Gustafsson 1999; McKenna et al. 1999b; Patrone et al. 2000). The characterization of mice lacking ERα, ERβ or both ERs, revealed that they have overlapping, and also unique roles, in estrogen dependent functions in vivo [rev in (Pfaff et al. 2002; Matthews and Gustafsson 2003; Harris 2007)]. ERβ is suggested to be crucially involved in regulating non-reproductive behaviors and brain development. Female βERKO mice display increased anxiety and reduced cognitive function (Krezel et al. 2001). The distribution of the ER subtypes is such that many of the CA specific regions express both ERα and ERβ although the ratio varies with location and gender (Laflamme et al. 1998; Shughrue and Merchenthaler 2001).
Studies in cell culture revealed that transcriptional regulation of the TH gene, by estrogens differed depending on ER subtype. In PC12 cells, 17β-estradiol triggered an elevation of TH transcription with ERα and a reduction with ERβ (Maharjan et al. 2005). In contrast to the TH gene, estradiol up-regulates DBH gene expression and promoter activity with both ER subtypes (Serova et al. 2002; Sabban et al. 2009).
It remains to be determined in vivo, if selective activation of ER specific subtypes also have differential effects on CA biosynthetic enzyme gene expression. Therefore, ER selective agonists were administered in this study. We determined both basal effects and response to stress. The studies so far were restricted to examining effects of single exposure to IMO, while many of the harmful effects of stress are associated with prolonged or repeated exposure [reviewed in (Sabban and Kvetnansky 2001; Sabban 2007; Kvetnansky et al. 2009)]. Here we examine whether some of the variation in the ability of EB to modulate the response to stress depends on the selective ER subtypes, which are activated in the specific CA location. To address this issue, we have administered EB or ER subtype selective agonists and determined their effect on the response to single as well as repeated stress.
Materials and Methods
Animals
All experiments described were performed in accordance with the National Institute of Health Guide and Use of Laboratory Animals (NIH Publications No. 80-23) and were approved by the New York Medical College Animal Care and Use Committee.
Adult ovariectomized (OVX) Sprague-Dawley female rats (230-250g) were obtained from Taconic Farms (Germantown, NY) eight days after the surgery. Rat chow and water were available ad libitum and the rats were maintained four per cage under controlled conditions on a 12-h light/night cycle at 23 ± 2°C. Six days later they were randomly divided into four subgroups (24 rats each) and treated with either 25 μg/kg of estradiol benzoate (EB, Sigma, St. Louis, USA), 10 mg/kg of ERα agonist propyl pyrazole triol (PPT, Wyeth Research, Collegevill, USA), 10 mg/kg of ERβ agonist WAY-200070 (Wyeth Research, Collegevill, USA) or vehicle (10%DMSO/90% sesame oil, Sigma, St. Louis, USA) once daily for 21 days by 50 μl subcutaneous injections into the nape of the neck.
The dose of EB has been shown in our previous studies to effectively modulate response of noradrenergic neurons to single IMO stress (Serova et al. 2004; Serova et al. 2005) and increased plasma estradiol levels to 800 ± 61 pg/ml three hours after the injection (Serova et al. 2004). The doses of PPT and WAY 200070 are based on previously published studies with mature rats (Harris et al. 2002; Miller et al. 2005). For example, a dose of 10 mg/kg PPT daily injected to rats for 21 days elicited lordosis induced negative feedback inhibition of LH release and prevented weight gain by OVX. It also provided protection against ischemia induced cell death in hippocampal neurons (Miller et al. 2005). The dose of 15 mg/kg significantly reduced hot flushes in OVX females (Harris et al. 2002). A similar dose of WAY 200070 or DPN was found to significantly decrease anxiety related behavior (Lund et al. 2005; Weiser et al. 2009).
All animals were weighed on the 15th day. On the 16th day, all treatment groups were subdivided into three subgroups of 8 animals each and they continued to receive the daily drug injections as before. One subgroup was subjected to repeated daily immobilization stress (IMO) for 2 hours each for six consecutive days (days 16-21). A second subgroup received a single 2 hr IMO, one hour after the injection on day 21. These animals were euthanized by decapitation on the 21st day immediately after the IMO. The third subgroup was not immobilized and was euthanized on the same day as the other animals from the same treatment and served as a control for the stress.
After euthanasia, blood was collected into EDTA containing prechilled tubes. Uteri were isolated and weighted. The brain was removed and the LC, as well as the rostral-medial and caudal parts of the NTS were immediately dissected using a tissue slicer with digital micrometer (Stoelting Co, Wood Dale, IL). Frontal sections 13.2 -14.2 mm and 14.2 – 15 mm from bregma were taken for rostral-medial and caudal portions of the NTS, respectively; and 9.2 - 10.4 mm for the LC. The slices were placed in ice-cold saline, and the bilateral regions of the LC and NTS were punched out and immediately frozen in liquid nitrogen.
Isolation of RNA and Quantitative RT-PCR
Total RNA was isolated and analyzed as previously described (Serova et al. 1999; Serova et al. 2005). Briefly, the tissue from each animal was homogenized in RNA-Stat-60 (Tel-Test, Inc., Friendswood, TX) and purified with RNAqueous-Micro RNA Isolation Kit (Ambion, Austin, TX) (Serova et al. 2004; Serova et al. 2005). The amount of total RNA from each sample was quantified using Ribo-Green fluorescent dye (Molecular Probes, Eugene, OR).
Quantitative analysis of TH and DBH mRNA levels was performed by Real-time RT-PCR with SYBR Green I with LightCycler (Roche Molecular Biochemicals, Indianapolis, USA) as previously described (Serova et al. 2005). The RT reactions were performed separately with TH or DBH specific primers 5′-TCAGGCTCCTCTGACAG-3′ and 5′-AGGCTGCAAGGCTTCTGTGATGGC-3′ respectively, in 5μl PCR mixture (1×AMV buffer, 10mM dNTP, 8 units RNAse inhibitor, 1.25 units AMV, 10 μM reverse primer, and 1μg of template RNA). 20μl PCR reactions were set up with a final concentration of 1× LightCycler DNA Master SYBR Green I, 0.5 μM of each of the forward and reverse primers, 5 mM MgCl2 and 2 μl of the standard cDNA or cDNA with unknown concentration. Standard curves plotted using serial dilutions from 2 ng to 0.2 pg of a TH or DBH cDNA was used for the quantification by Fit Points Method. The following primers were used: for TH, 5′-TGAACCAATTCCCCATGTGG-3′, 5′-TGAACCAGTACACCGTGGAGAG-3′; for DBH, 5′-CCACGCCATGCAGTTCTTCACCA-3′, 5′-AGGCTGCAAGGCTTCT-GTGATGGC-3′. The expected sizes of target sequences were confirmed with melting curve analysis by comparing its melting temperature with the melting temperatures of the standards as a positive control. The values for TH and DBH mRNA were normalized to levels of total RNA.
Determination of plasma corticosterone levels
Corticosterone levels were determined by using the 125I corticosterone RIA Kit (ICN, Costa Mesa, USA) according to the manufacture's protocol. Plasma was diluted 1:200 and incubated with 125I labeled corticosterone and antiserum to corticosterone at room temperature for 2 hours. After centrifugation, the 125I in precipitates were measured in gamma counter and compared with the standard curve for quantification. The intra- and inter-assay coefficients of variation for corticosterone assays were 17.2 and 11.2%, respectively.
Statistical Analysis
The results were evaluated by one and two way ANOVA. For two way ANOVA, we determined the significance of the main factors, treatment and stress, and their interaction, followed by Bonferroni's Multiple Comparison Test Using GraphPad and InStat programs. A value of p ≤ 0.05 was considered significant.
Results
Body and uterine weight
The body and uterine weight in the different treatment groups are shown in Table 1. As expected long-term treatment of the OVX females with EB led to significantly reduced body weight compared to the vehicle treated group of rats. The effects of selective agonists for ERα (PPT) and ERβ (WAY-200070) on body weight were different. Injections of PPT, similar to EB, reduced body weight. In contrast, there was no change in body weight with WAY-200070. The mean weight of the uterus was about ten fold greater in EB treated, than in the control vehicle treated animals. PPT, treatment also led to elevation of uterine weight, but to a lesser extent than with EB. The uterine weights of rats treated with WAY-200070 did not differ from those of the vehicle treated group.
Table 1.
Body and uterine weight in OVX female rats with different treatments
| Treatment | Uterine weight (g) | Body weight (g) |
|---|---|---|
| Vehicle | 0.08 ± 0.004 | 309.5 ± 3.7 |
| EB | 0.85 ± 0.191* | 238.3 ± 2.4* |
| PPT | 0.51 ± 0.054*++ | 248.1 ± 3.6* |
| WAY 200070 | 0.097 ± 0.009 | 295 ± 5.0 |
p < 0.001 versus Vehicle,
p < 0.01 versus EB
Changes in corticosterone levels
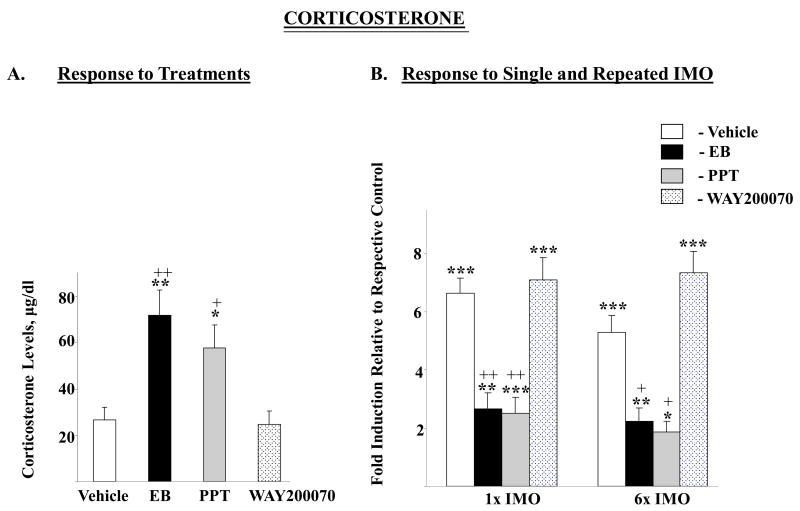
Plasma levels of corticosterone were significantly changed by the treatments (F=9.14, p<0.0002). Administration of EB or PPT led to about 2.5 fold higher corticosterone levels compared to treatment with vehicle (p< 0.01, p<0.05 respectively) or WAY-200070 (p< 0.01, p<0.05 respectively) (Fig. 1A). The changes in response to stress for the different groups were analyzed by two way ANOVA. There were significant effects of stress (F84,2=19.6, p<0.0001), and treatment (F84,3=10.2 p<0.0001), as well as interaction (F84,6=2.7, p<0.02). Although the concentrations of plasma corticosterone in stressed rats in the various groups were similar, the magnitude of rise above levels of their respective unstressed controls varied. The pattern of the changes with single and repeated IMO was similar (Fig. 1B). With vehicle or WAY-200070, there was a significant, about seven-fold, elevation of plasma corticosterone levels after single (1×) IMO. Similar results were obtained with repeated (6×) IMO. Rats receiving injections of EB or PPT had much smaller increase, of about 2 fold above levels in unstressed animals with the same treatments. The responses of EB and PPT treated rats to single stress were significantly (p≤0.01) different from the response of the vehicle treated animals. Similar results were obtained with exposure to 6× IMO (p≤0.05).
Figure 1.
Effect of long-term administration of EB or ER subtype selective agonists to OVX female rats on plasma corticosterone levels following single or repeated immobilization stress. A. Plasma corticosterone levels after long-term injections of vehicle, EB, PPT or WAY 200070. Data are expressed as mean ± S.E.M with 6-8 samples per group. *P < 0.05, ** p< 0.01 vs Vehicle, +p < 0.05, ++p < 0.01 vs WAY 200070 by Bonferroni's multiple comparison test. B. Response to single (1×) or repeated (6×) IMO stress. Data are expressed as mean ± S.E.M. with the levels of corticosterone in the respective treatment taken as 1. * p < 0.05, ** p < 0.01, *** p < 0.001 vs respective unstressed controls; +p < 0.05, ++p < 0.01 vs 1× IMO/Vehicle or 6× IMO/Vehicle by Bonferroni's multiple comparison test.
Gene expression of catecholamine biosynthetic enzymes
Locus Coeruleus (LC)
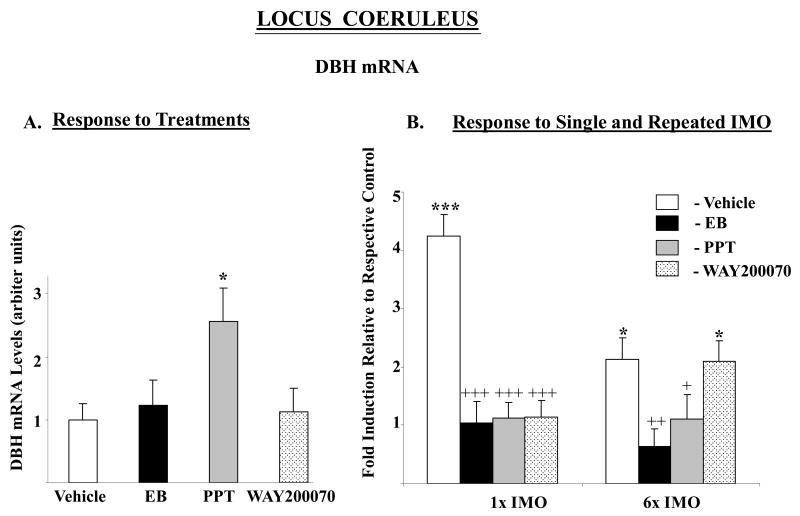
There was a significant effect of stress (F84,2=11.1, p<0.0001) and treatment (F84,3=15.3 p<0.0001), as well as interaction (F84,6=9.9, p<0.0001) on DBH mRNA levels. DBH mRNA levels in control animals which received the vehicle were elevated about 4-fold with single IMO, and about 2-fold above levels in unstressed rats with repeated IMO (Fig. 2). Injections with EB or either of the ER agonists effectively prevented the response of DBH mRNA to single IMO. Moreover, treatment with EB and PPT abolished the response to repeated IMO as well. In rats treated with WAY-200070, repeated IMO still elicited a significant rise in DBH mRNA and did not differ significantly from the response of the vehicle treated rats. As previously shown (Serova et al. 2005), we did not find changes in TH mRNA levels in the LC of OVX rats in response to stress (data not shown).
Figure 2.
Effect of long-term administration of EB or ER subtype selective agonists to OVX female rats on DBH mRNA levels in the LC following single or repeated immobilization stress. A. Relative DBH mRNA levels expressed as mean ± S.E.M. (6-8 samples per group) with levels in Vehicle treated rats taken as 1. *p < 0.05 vs Vehicle. B. Response to single (1×) or repeated (6×) IMO stress. Data are expressed as mean ± S.E.M. with levels of DBH mRNA with respective treatment in unstressed animals taken as 1. *p < 0.05, ***p < 0.001 vs respective unstressed controls; +p < 0.05, ++p < 0.01, +++p < 0.001 vs 1× IMO/Vehicle or 6× IMO/Vehicle by Bonferroni's multiple comparison test.
Nucleus of the Solitary Tract (NTS)
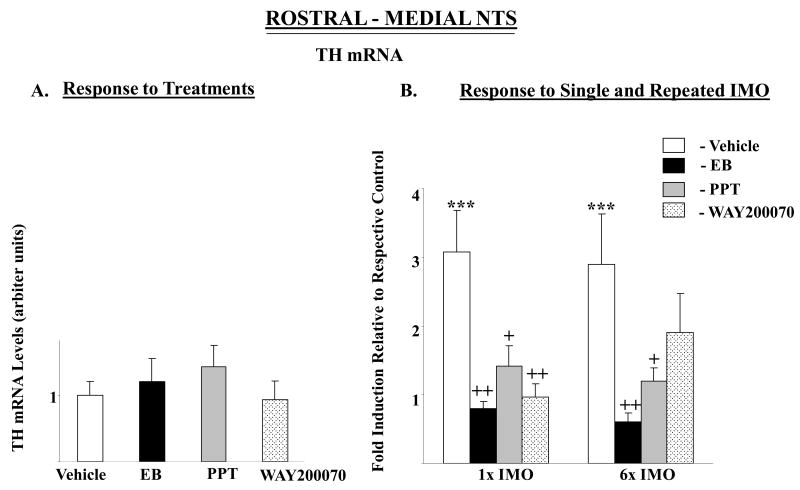
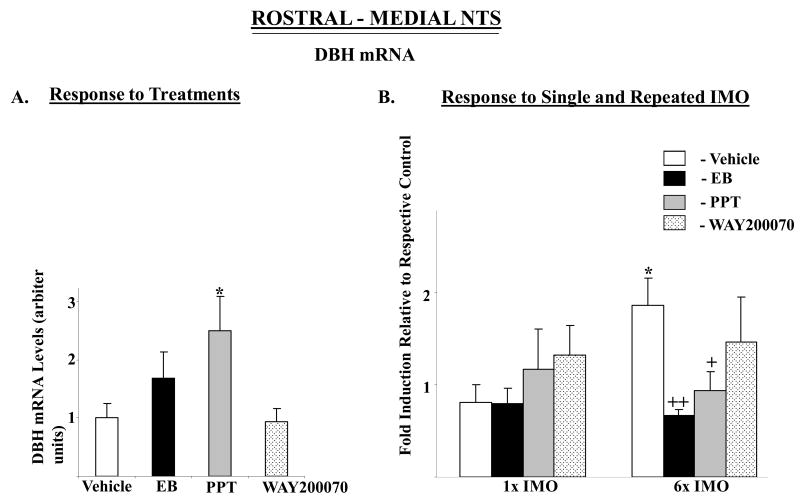
None of the drugs administrated significantly changed TH mRNA levels in the rostral-medial nor caudal NTS, except for a small, but significant, rise in TH mRNA in the caudal portion of the NTS in the PPT treated group (Fig. 3A, C). In both rostral-medial and caudal regions, there were significant main effects of stress (F84,2=3.9, p<0.02 and 14.8, p≤0.0001) and treatment (F84,3=10.6 p<0.0001, 17.6 p<0.0001),_respectively. The interaction was also significant (F84,6=3.2, p<0.01, and 6.3, p<0.0001). The pattern of changes with single IMO in the different groups was similar in both parts of the NTS (Fig. 3B, D). Single IMO triggered about three-fold elevation in TH mRNA levels in rostral-medial and 2.5–fold rise in the caudal NTS of the vehicle treated rats. In rats treated with PPT, WAY-200070, or EB, exposure to single IMO stress did not elevate TH mRNA. With repeated stress only the EB and PPT treated groups differed from the vehicle treated controls.
Figure 3.
Effect of long-term administration of EB or ER subtype selective agonists on TH mRNA levels in rostral-medial and caudal parts of the NTS of OVX female rats following single or repeated immobilization stress. Changes in TH mRNA in rostral-medial (A, B) and caudal (C, D) parts of the NTS are shown. (A, C): TH mRNA levels expressed as mean ± S.E.M. (6-8 samples per group) with levels of TH in Vehicle treated rats taken as 1. *p < 0.05 vs Vehicle. (B, D): Response to single (1×) or repeated (6×) IMO stress. Data are expressed as mean ± S.E.M. with levels of TH mRNA with respective treatment in unstressed rats taken as 1. * p < 0.05, *** p < 0.001 vs respective unstressed controls; +p < 0.05, ++p < 0.01 vs 1× IMO/Vehicle or 6× IMO/Vehicle.
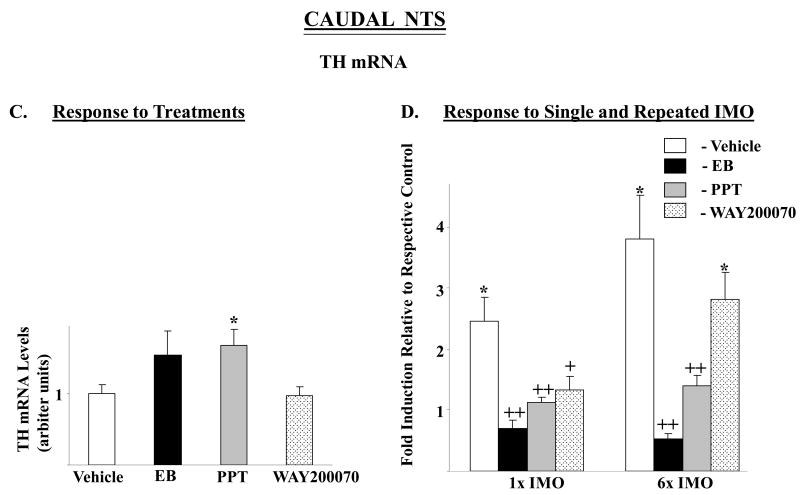
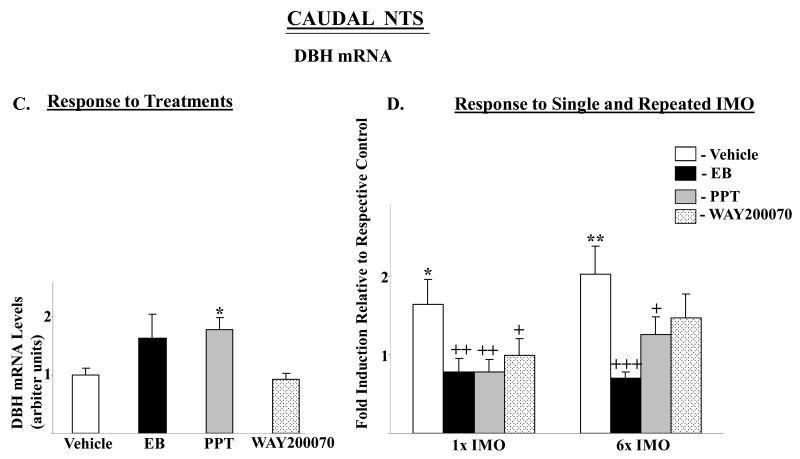
DBH mRNA in the rostral-medial and caudal NTS were affected by the treatments (F= 3.9, p<0.02, and 3.4, p<0.03, respectively) with levels significantly elevated above vehicle only in rats treated with PPT (Fig. 4A, C). In the rostral medial NTS, no main effects or interaction were found by 2-way ANOVA (Fig. 4B). In the caudal NTS, there was significant main effects of stress (F84,2=4.2, p<0.02) and treatment (F84,3=7.6 p<0.0002), with significant interaction (F84,6=2.4, p<0.03). In the caudal NTS, both single and repeated IMO stress elicited about 2-fold induction of DBH mRNA levels in vehicle treated rats, but not in any of the other groups (Fig. 4D).
Figure 4.
Effect of long-term administration of EB or ER subtype selective agonists to OVX female rats on DBH mRNA levels in rostral-medial and caudal parts of the NTS following single or repeated immobilization stress. Changes in DBH mRNA in rostral-medial (A, B) and caudal parts (C, D) of the NTS are shown. (A, C): DBH mRNA levels expressed as mean ± S.E.M. (6-8 samples per group) with levels of DBH in Vehicle treated rats taken as 1. *p < 0.05 vs Vehicle. (B, D): Response to single (1×) or repeated (6×) IMO stress. Data are expressed as mean ± S.E.M. with levels of DBH mRNA with respective treatment in unstressed rats taken as 1. * p < 0.05, **P < 0.01 vs respective unstressed controls; +p < 0.05, ++p < 0.01, +++p < 0.001 vs 1× IMO/Vehicle or 6× IMO/Vehicle.
Discussion
This is the first study to our knowledge to investigate the estrogen receptor selectivity on the regulation of gene expression of catecholamine biosynthetic enzymes in the brain noradrenergic neurons by estrogens and their role in modulation of the response to acute and chronic stress. Overall, the results indicate that the ERα is especially important in mediating the effects of estradiol on basal expression of TH and DBH and on modulation of the response to both single and repeated stress in OVX female rats. However, ERβ is involved mostly in modulation of acute stress reaction. The findings indicate that these treatments can prevent excessive response of TH and DBH genes to stress.
The results of this study revealed, for the first time, that long-term activation of eitherERα or ERβ changed the response of DBH mRNA levels in the LC to acute stress. In rats injected with the ERα agonist PPT, the response to both durations of IMO stress was absent, and DBH mRNA was unchanged beyond the higher basal level. Pretreatment with the ERβ agonist, WAY20070, which in contrast to PPT, did not change basal unstressed levels, also abolished the response of DBH mRNA to a single IMO. However, long-term stimulation of ERβ failed to reduce elevation of DBH mRNA in the LC with repeated stress. This has important implications for stress mediated behavior, and can influence vigilance and attention in potentially threatening environments. In male animals, various types of stress including IMO, were shown to trigger marked elevations in TH and DBH gene expression in the LC [(Smith et al. 1991; Melia et al. 1992; Rusnak et al. 1998; Wang et al. 1998; Serova et al. 1999; Osterhout et al. 2005) reviewed in (Sabban and Kvetnansky 2001; Kvetnansky et al. 2009)]. In contrast, in the OVX female rats with low estradiol levels, IMO stress is not effective to trigger an elevation in the LC of TH mRNA and only DBH mRNA levels are increased.
The LC is sexually dimorphic with more TH and DBH positive cells observed in female mice and rats (Luque et al. 1992; Pendergast et al. 2008). In female rats, both ER subtypes are found within the LC, with predominate expression of ERβ (Shughrue et al. 1997; Mitra et al. 2003). However, the findings reveal that ERβ is more involved with single, rather than repeated IMO. The effect of an ERβ agonist on the response of the LC to stress is especially important since functions of these receptors are linked to affective disorders and decreased levels of ERβ were found in persons committing suicide (Ostlund et al. 2003). The anti-depressant role of ERβ has been demonstrated in ERβ knockout mice and with specific pharmacological manipulations (Walf and Frye 2008; Walf et al. 2008). Significantly decreased anxiety-like and depressive-like behavior were found in OVX females injected with WAY-200070 (Weiser et al. 2008). Potential changes in ER subtypes expressed in the LC induced by selective agonists and stress or in combination remain to be determined. We can speculate that a chronic strong stress, such as IMO, may decrease expression of ERβ and raise incidence of stress related depression.
This study is also the first showing that in noradrenergic neurons of the NTS, gene expression of TH significantly increases in response to repeated stress, as previously seen for single IMO (Serova et al. 2005). DBH gene expression was elevated by IMO stress with both durations in caudal NTS but in the rostral-medial NTS only with repeated IMO. The NTS is involved in the assimilation and integration of multiple viscerosensory processes, including cardiovascular control mechanisms, and also in osmoregulatory functions that control body fluid homeostasis (Hochstenbach and Ciriello 1995; Lawrence and Jarrott 1996). Noradrenergic neurons located throughout the rostral-medial and most of the caudal (commissuralis) subnuclei of the NTS are barosensitive, and respond dynamically to alterations in blood pressure as part of the homeostatic cardiovascular control process. However their functional role is not identical. The rostral-medial portion of the NTS exerts more control on the arterial system while the caudal NTS is more selective for the cardiac system. Thus, it will be important to perform a more detailed response of subpopulations of catecholaminergic neurons of the NTS to IMO stress.
Estradiol benzoate, as the findings indicate, can modulate functional activity of noradrenergic neurons of the NTS during single IMO stress through ERα as well as ERβ, since administration of EB and both selective agonists have similar ameliorative effects on TH in rostral-medial and caudal, and DBH in caudal part of the NTS. A high density of ERα and ERβ has been demonstrated from caudal to medial regions of the NTS (Shughrue et al. 1997; Laflamme et al. 1998; Shughrue and Merchenthaler 2001; Schlenker and Hansen 2006). However, caudal NTS expression of ERα is greater than that for ERβ (Schlenker and Hansen 2006). This might be a reason why pretreatment with EB as well as PPT were especially effective in preventing or attenuating changes in TH and DBH gene expression elicited by repeated IMO. In addition, the expression of ER subtypes might be selectively regulated by stress. Earlier studies revealed significant elevation ER-immunoreactivity in the NTS of intact female rats after one hour of IMO stress (Estacio et al. 1996).
The selectivity the ER compounds were confirmed by changes in body and uterine weight. EB and PPT, but not WAY-200070, led to about 20% reduction in body weight and increased the uterus weight, showing their association with activation of ERα. These data are in agreement with other studies concluding that prevention of body weight gain is associated with activation of predominantly ERα in adipose tissue (Heine et al. 2000; Wegorzewska et al. 2008). The effect of EB and the ERα agonist on body weight may be at least partially regulated by suppression of food intake in the NTS, since gastrointestinal signals generated by ingested food are first processed within the NTS (Sutton et al. 2004; Travagli et al. 2006; Valassi et al. 2008). Tracing studies revealed substantial axonal projections from the caudal NTS neurons to the immediately subjacent dorsal motor nucleus of the vagus and that they are part of a gastro-gastric vago-vagal reflex (Rogers and McCann 1993). Thus, estrogens via ERα may modulate brainstem circuits regulating gastric function.
It is interesting to note that in several areas basal levels of TH and DBH were significantly elevated with PPT but not with EB treatment. It might be explained by the ying-yang relationship between the ERα and ERβ. Both ERs possess fairly similar binding affinities for 17β-estradiol, 0.2 nM for ERα and 0.6 nM for ERβ (Gustafsson and Warner 2000). Compared to ERα, generally ERβ has a weaker transcriptional activity and it often functions oppositely or acts as a dominant negative regulator of estrogen signaling (Paech et al. 1997; Cowley and Parker 1999). When coexpressed with ERα, it causes a concentration dependent reduction of ERα mediated transcriptional activation and the repression of ERα mediated effects (Hall and McDonnell 1999).
Our results also clearly show that ERα, but not ERβ, is involved in estradiol mediated elevation in corticosterone and attenuation of corticosterone response to IMO stress. Similar to our earlier studies, we demonstrated that EB could attenuate elevation of corticosterone levels in response to single IMO (Serova et al. 2005). Here we show for the first time that it can also modulate response to repeated IMO stress. In this regard, women receiving estradiol through extradermal patch (0.1 mg) for one month had an attenuated elevation of cortisol to single endotoxin injection (Puder et al. 2001). The effects of EB on corticosterone levels with and without stress were similar in rats treated with PPT, indicating the involvement of ERα. Recently ERα was also found to mediate estrogen mediated impairment of glucocorticoid negative feedback on the HPA axis (Weiser and Handa 2009).
ERβ does not appear to mediate the corticosterone response shown in the present study as pretreatment with WAY-200070 for 21 days did not alter the rise in corticosterone with single 2 hr IMO, or repeated 2 hr IMO for 6 consecutive days. It also did not alter corticosterone levels in the absence of stress. In contrast, WAY-200070 given during seven days is reported to reduce corticosterone levels 20 min after single forced swim test, indicating the importance of ERβ (Weiser et al. 2009). These differences may be due to different duration and type of stress, as the neuronal pathways mediating the activation of the HPA axis depend on stress paradigms, stressor modality and intensity [reviewed in (Kvetnansky et al. 2009; Ulrich-Lai and Herman 2009)].
The changes in plasma corticosterone with EB and PPT could be due to a direct effect or results from modulation of the HPA axis at the levels of ACTH or corticotrophin releasing hormone (CRH). Transcriptional activity of CRH promoter was found to be stimulated more strongly with ERβ than with ERα and ERβ is co-localized with CRH in rat PVN (Miller et al. 2004). However, recent data suggest that estrogens can also regulate CRH promoter activity through ERα (Lalmansingh and Uht 2008). In addition, ERα and ERβ differentially regulate transcription of the human CRH-binding protein gene leading to down regulation of the HPA axis and reduction of corticosterone levels in stress conditions.
Are the estradiol or ER agonist mediated changes observed in the noradrenergic neurons in the response to stress mediated by effects on the HPA axis? Since we used subcutaneous but not local administration we cannot rule out the possibility that their effects on gene expression in the LC and NTS might be indirect. CRH neurons innervate the LC and can increase electrophysiological activity of noradrenergic neurons, noradrenaline synthesis and release in prefrontal cortex and hypothalamus (Valentino and Van Bockstaele 2001). Moreover, the LC and NTS express glucocorticoid receptors (Fuxe et al. 1987). Glucocorticoids can influence expression of norepinephrine biosynthetic enzymes, at least in cell culture (Lewis et al. 1987; McMahon and Sabban 1992; Hwang and Joh 1993; Kim et al. 1993). It is possible that the attenuation of the induction of IMO triggered elevations in the LC or NTS in EB and PPT treated rats are partially due to the inhibition of the corticosterone response to stress. This could also be due to alterations in activation of NTS neurons in response to stress by input from PVN since its lesions significantly suppressed the stress-related induction of c-Fos in NTS catecholaminergic cells. Furthermore, tracer deposits in the NTS retrogradely labeled substantial numbers of PVN cells that were also Fos-positive after stress (Dayas et al. 2004). However, the effectiveness of WAY-200070 in blocking the IMO elicited rise in TH and DBH in the noradrenergic neurons, but not plasma corticosterone, argue against solely a glucocorticoid mediated mechanism.
Conversely, the catecholaminergic neurons from the LC as well as the NTS may contribute to the changes in the response of the HPA axis with stress observed in estradiol or PPT treated animals, since the hypothalamic PVN receives a major input from noradrenergic neurons in the NTS and innervation of lesser magnitude from the LC. Thus, noradrenaline can excite CRH-containing cells in the hypothalamic PVN to regulate the HPA axis [reviewed in (Dunn and Swiergiel 2008; Ulrich-Lai and Herman 2009)].
A number of important questions remain such as whether a higher concentration of WAY20070 than used in this study might have a more potent effect on repeated IMO. Future dose response curves would be informative. It also remains to be determined if the effects of estradiol and the ER agonists are mediated by membrane or nuclear ERs; whether they are direct or indirect; and whether the expression of ER subtypes are changed by the stress. It is still unclear whether chronic pretreatments are necessary to elicit the changes observed.
In sum, the findings of this study demonstrate that estradiol and ER selective agonists can markedly attenuate the response of norepinephrine biosynthetic enzymes in the LC and NTS and activation of the HPA axis, with ER selectivity depending on the duration of the stress. These findings demonstrate mechanisms that likely contribute to differences in susceptibility to stress related disorders depending on hormonal status.
Acknowledgments
This work was partially supported by grant NS 28869 from the National Institutes of Health (ES) and by Wyeth Research.
References
- Arbogast LA, Hyde JF. Estradiol attenuates the forskolin-induced increase in hypothalamic tyrosine hydroxylase activity. Neuroendocrinology. 2000;71:219–227. doi: 10.1159/000054539. [DOI] [PubMed] [Google Scholar]
- Baum A, Grunberg NE. Gender, stress, and health. Health Psychol. 1991;10:80–85. doi: 10.1037//0278-6133.10.2.80. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. J Comp Neurol. 2004;478:22–34. doi: 10.1002/cne.20259. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Gustafsson JA. Oestrogen receptors - an overview. J Intern Med. 1999;246:133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- Estacio MA, Yamada S, Tsukamura H, Hirunagi K, Maeda K. Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 1996;717:55–61. doi: 10.1016/0006-8993(96)00022-4. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Cintra A, Agnati LF, Harfstrand A, Wikstrom AC, Okret S, Zoli M, Miller LS, Greene JL, Gustafsson JA. Studies on the cellular localization and distribution of glucocorticoid receptor and estrogen receptor immunoreactivity in the central nervous system of the rat and their relationship to the monoaminergic and peptidergic neurons of the brain. J Steroid Biochem. 1987;27:159–170. doi: 10.1016/0022-4731(87)90306-2. [DOI] [PubMed] [Google Scholar]
- Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason S, Carlstrom K, Damber MG, Damber JE, Selstam G, von Schoultz B. Effects of various oestrogens on circulating androgens and cortisol during replacement therapy in post-menopausal women. Maturitas. 1981;3:301–308. doi: 10.1016/0378-5122(81)90038-4. [DOI] [PubMed] [Google Scholar]
- Hochstenbach SL, Ciriello J. Plasma hypernatremia induces c-fos activity in medullary catecholaminergic neurons. Brain Res. 1995;674:46–54. doi: 10.1016/0006-8993(94)01434-j. [DOI] [PubMed] [Google Scholar]
- Hwang O, Joh TH. Effects of cAMP, glucocorticoids, and calcium on dopamine beta-hydroxylase gene expression in bovine chromaffin cells. J Mol Neurosci. 1993;4:173–183. doi: 10.1007/BF02782500. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kim KT, Park DH, Joh TH. Parallel up-regulation of catecholamine biosynthetic enzymes by dexamethasone in PC12 cells. J Neurochem. 1993;60:946–951. doi: 10.1111/j.1471-4159.1993.tb03241.x. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3′,5′-cyclic adenosine 5′-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Harrington CA, Chikaraishi DM. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987;84:3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw JJ, He JR, Hartman RD, Barraclough CA. Changes in tyrosine hydroxylase mRNA levels in medullary A1 and A2 neurons and locus coeruleus following castration and estrogen replacement in rats. Brain Res Mol Brain Res. 1992;13:231–238. doi: 10.1016/0169-328x(92)90031-6. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Luque JM, de Blas MR, Segovia S, Guillamon A. Sexual dimorphism of the dopamine-beta-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res. 1992;67:211–215. doi: 10.1016/0165-3806(92)90221-h. [DOI] [PubMed] [Google Scholar]
- Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors alpha and beta and interactions with cyclic AMP. J Neurochem. 2005;93:1502–1514. doi: 10.1111/j.1471-4159.2005.03142.x. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999a;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999b;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- McMahon A, Sabban EL. Regulation of expression of dopamine beta-hydroxylase in PC12 cells by glucocorticoids and cyclic AMP analogues. J Neurochem. 1992;59:2040–2047. doi: 10.1111/j.1471-4159.1992.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Osterhout CA, Sterling CR, Chikaraishi DM, Tank AW. Induction of tyrosine hydroxylase in the locus coeruleus of transgenic mice in response to stress or nicotine treatment: lack of activation of tyrosine hydroxylase promoter activity. J Neurochem. 2005;94:731–741. doi: 10.1111/j.1471-4159.2005.03222.x. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. Faseb J. 1995;9:419–423. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- Patrone C, Pollio G, Vegeto E, Enmark E, de Curtis I, Gustafsson JA, Maggi A. Estradiol induces differential neuronal phenotypes by activating estrogen receptor alpha or beta. Endocrinology. 2000;141:1839–1845. doi: 10.1210/endo.141.5.7443. [DOI] [PubMed] [Google Scholar]
- Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol. 2000;12:899–909. doi: 10.1046/j.1365-2826.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Tuesta LM, Bethea JR. Oestrogen receptor beta contributes to the transient sex difference in tyrosine hydroxylase expression in the mouse locus coeruleus. J Neuroendocrinol. 2008;20:1155–1164. doi: 10.1111/j.1365-2826.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. Trends Neurosci. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- Puder JJ, Freda PU, Goland RS, Wardlaw SL. Estrogen modulates the hypothalamic-pituitary-adrenal and inflammatory cytokine responses to endotoxin in women. J Clin Endocrinol Metab. 2001;86:2403–2408. doi: 10.1210/jcem.86.6.7528. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Zorad S, Buckendahl P, Sabban EL, Kvetnansky R. Tyrosine hydroxylase mRNA levels in locus ceruleus of rats during adaptation to long-term immobilization stress exposure. Mol Chem Neuropathol. 1998;33:249–258. doi: 10.1007/BF02815186. [DOI] [PubMed] [Google Scholar]
- Sabban E, Maharjan S, Nastromo G, Serova L. Divergent Effects of Estradiol on Gene Expression of Catecholamine Biosynthetic Enzymes. Phisiology and Behavior. 2009 doi: 10.1016/j.physbeh.2009.07.011. in press. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–98. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache. 2008;48 Suppl 2:S77–89. doi: 10.1111/j.1526-4610.2008.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006;1123:89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience. 2005;132:249–259. doi: 10.1016/j.neuroscience.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry. 1999;45:853–862. doi: 10.1016/s0006-3223(98)90360-2. [DOI] [PubMed] [Google Scholar]
- Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus ceruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- Stovall DW, Pinkentorn JAV. Estrogen agonist/antagonists in combination with estrogen for prevention and treatment of menopause-associated signs and symptoms. Women's Health. 2008;4(3):257–268. doi: 10.2217/17455057.4.3.257. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci. 2004;24:10240–10247. doi: 10.1523/JNEUROSCI.2764-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Ishikura F, Matsuda A, Asanuma T, Ueda K, Ichinose M, Kasamatsu K, Hano T, Akasaka T, Tsuruo Y, Morimoto K, Beppu S. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ J. 2007;71:565–573. doi: 10.1253/circj.71.565. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158:331–342. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Adult female wildtype, but not oestrogen receptor {beta} knockout, mice have decreased depression-like behaviour during prooestrus and following administration of oestradiol or diarylpropionitrile. J Psychopharmacol. 2008 doi: 10.1177/0269881108089598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Kitayama I, Nomura J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998;62:2083–2092. doi: 10.1016/s0024-3205(98)00183-0. [DOI] [PubMed] [Google Scholar]
- Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, Handa RJ, Wu TJ. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am J Obstet Gynecol. 2008;199:67 e61–65. doi: 10.1016/j.ajog.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor beta (ER{beta}) agonist diarylpropionitrile (DPN): biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2008 doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]