Abstract
Nine spiral nerve cuff electrodes were implanted in two human subjects for up to three years with no adverse functional effects. The objective of this study was to look at the long term nerve and muscle response to stimulation through nerve cuff electrodes. The nerve conduction velocity remained within the clinically accepted range for the entire testing period. The stimulation thresholds stabilized after approximately 20 weeks. The variability in the activation over time was not different from muscle-based electrodes used in implanted functional electrical stimulation systems. Three electrodes had multiple, independent contacts to evaluate selective recruitment of muscles. A single muscle could be selectively activated from each electrode using single-contact stimulation and the selectivity was increased with the use of field steering techniques. The selectivity after three years was consistent with selectivity measured during the implant surgery. Nerve cuff electrodes are effective for chronic muscle activation and multichannel functional electrical stimulation in humans.
Keywords: Functional electrical stimulation (FES), nerve electrodes, implantable electrodes, peripheral nerve, selective stimulation, upper extremity neuroprostheses
I. Introduction
MOTOR neuroprostheses activate paralyzed muscles and restore function to people with neurological deficits. Most existing neuroprostheses use muscle-based electrodes such as epimysial, intramuscular, and/or surface electrodes [3]-[5]. In these systems, activation of each muscle requires at least one electrode [6], [7]. As the number of muscles to be activated increases, a method of activating more than one muscle from each electrode will be necessary. Nerve cuff electrodes are usually placed on the nerve trunk, proximal to the nerve branches to the individual muscles. Such a single nerve cuff electrode can activate multiple muscles in two ways. First, most axons in the nerve can be stimulated together to activate broad muscles or multiple synergistic muscles that are difficult to stimulate using a single muscle-based electrode. Second, several stimulating contacts within one electrode can activate different portions of the nerve, potentially activating different functions and/or muscles independently [8]-[13]. In a neuroprosthesis application, this would reduce the number of implant sites needed and could decrease the length and complexity of the implant surgery. Also, since nerve electrodes are in direct contact with the nerve, less current is required than when using muscle-based electrodes [14]. Lower stimulating currents will reduce the power required and may extend the life of an implanted battery.
A neuroprosthesis for restoration of shoulder and elbow function was chosen as a suitable initial application for implementation of nerve electrodes to meet the need of activating many muscles and completely activate the large, proximal muscles. The results of this study, however, are broadly applicable to all instances of peripheral motor nerve stimulation.
The Case Western Reserve University (CWRU) spiral nerve cuff electrode [15], which was chosen for the upper extremity neuroprosthesis, contained multiple, potentially selective, contacts around the nerve and had demonstrated stable chronic nerve activation in cats [10], [15]-[18]. Single contact, monopolar stimulation is the most common type of stimulation in implanted devices and has been shown to activate 64% of the fascicles selectively in the cat sciatic nerve [12]. Simultaneous stimulation through multiple contacts, or field steering, has been shown to increase the selective activation capabilities using the spiral nerve cuff electrode [8], [10], [12].
There is also clinical experience with the spiral nerve cuff electrode. It has been implanted on the human optic nerve in one blind volunteer for over six years [19]-[22]. Intraoperative testing in the human upper extremity [14] found recruitment thresholds similar to those reported in cat studies and single contact stimulation was capable of selectively activating at least one muscle independently from each nerve tested [14]. These prior studies support the chronic implant of the spiral nerve cuff electrode in human subjects.
In this paper, we report the results of testing three hypotheses. First, that implanted nerve cuff electrodes do not cause adverse changes in nerve physiology as measured by recruitment thresholds and conduction velocity. Second, selectivity of muscles increases with the use of field steering compared to single contact stimulation. Third, spiral nerve electrodes produce functionally repeatable activation over time.
II. Methods
A. Study Design
The overall project goal was to provide the subjects with a neuroprosthesis to augment or restore shoulder, elbow, and hand function. This study focuses on the use of nerve cuff electrodes in proximal locations. Since this was the first clinical experience with implanted nerve cuff electrodes in the upper extremity, the study was conducted in two phases: a percutaneous phase which utilized an external stimulator with extensive stimulation choices and an implant phase where the implanted stimulator had a finite number of stimulation channels and limited stimulation parameters.
Initially, spiral nerve cuff electrodes were surgically implanted on three to four nerves in each subject (Table I). Selective electrodes, with multiple, individually controlled contacts were placed on nerves innervating nonagonist muscles to evaluate selective recruitment capabilities. Nonselective electrodes with a single contact or with all contacts connected together were used to fully activate all muscles innervated by the nerve and reduce the number of stimulation channels needed in the implanted stimulator. The electrode leads were routed subcutaneously down the lateral chest to just beneath the ribs. Percutaneous leads were attached to the implanted electrode leads and exited the skin near the site where the permanent implanted stimulator would later be implanted in the abdomen. Postsurgery, the subjects’ shoulders were immobilized for three weeks to allow for the electrode encapsulation to stabilize. During the initial stimulation session, stimulation was gradually increased to estimate stimulation threshold and saturation values. These values were used to set up an exercise regime that the subjects performed daily for 2–3 h throughout the entire testing period. The purpose of the exercise was to increase the strength and endurance of muscles that had been paralyzed for over ten years [23], [24]. Data collection using the nerve cuff electrodes began after 2–3 weeks of exercise (5–6 weeks post implant).
TABLE I.
Summary of Implanted Nerves
| Multi-contact Selective |
Multi-contact Non-selective |
Single-contact Non-selective |
||
|---|---|---|---|---|
| Subject 1 | ||||
|
| ||||
| Radial | ✓ | |||
| Musculocutaneous | ✓ | Percutaneous | ||
| Axillary | ✓ | Phase | ||
| Suprascapular | ✓ | |||
| Long Thoracic | ✓ | Implant | ||
| Thoracodorsal | ✓ | Phase | ||
| Subject 2 | ||||
|
| ||||
| Radial | ✓ | |||
| Suprascapular | ✓ | Percutaneous | ||
| Thoracodorsal (R) | ✓ | Phase | ||
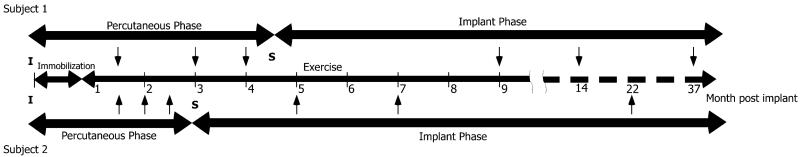
Following three to four months of percutaneous testing, a second surgery was performed. Additional stimulating electrodes, including nonselective nerve cuff electrodes and muscle-based electrodes, were implanted and all electrodes were connected to an implantable stimulator-telemeter (IST) [25]. The IST had limited stimulation channels so only electrode contacts that produced a desired function in the percutaneous phase were selected and used in the implant phase. Long-term testing with the limited stimulation values and stimulation channels continued during the implant phase (Fig. 1). Each subject attended three experimental sessions in both the percutaneous phase and the implant phase. The timing of the sessions varied between subjects due to their personal schedules.
Fig. 1.
Experimental timeline. The initial implant (I) was followed by three weeks of immobilization and then exercise was performed for the duration of the study. During the percutaneous phase, there were three experimental sessions (arrows) for each subject. The second implant surgery (S) marked the beginning of the implant phase which included three more experimental sessions (arrows). The upper portion the timeline is for subject 1 and the lower portion for subject 2.
B. Spiral Nerve Cuff Electrode
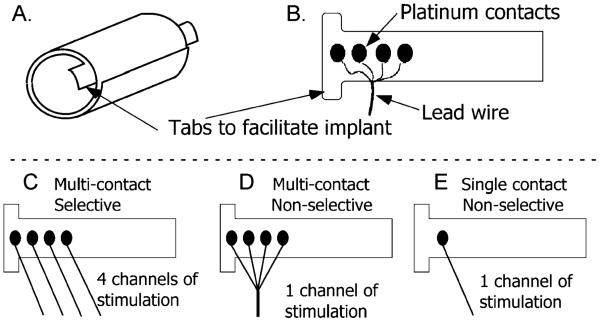
The spiral nerve cuff electrodes for this study [Fig. 2(a)] were fabricated at the Technical Development Laboratory of the Cleveland FES VA Center of Excellence and Case Western Reserve University. Fabrication has been previously described by Naples et al. [15]. Briefly, the cuff electrodes consist of four ovoid pieces of platinum foil connected to seven-strand, 316LVM stainless-steel, perfluoroalkoxy (PFA) coated wire between two layers of silicone sheeting. The first layer of sheeting was stretched and then bonded to an unstretched sheet. Once cured, the unequal tension curled the sheet to produce a spiral. The final diameter was determined by the amount of stretch in the first layer. Up to four stimulating contacts were spaced evenly about the circumference [Fig. 2(b)]. The natural coiling of these electrodes results in a spiral with an intimate fit between the nerve and the contacts, but allows expansion and contraction to reduce constriction of blood flow within the nerve [26].
Fig. 2.
Schematic of spiral nerve cuff electrodes. (a) Spiral electrode coiled, resulting in two full wraps. (b) Electrode unwrapped to show contact layout and lead position. The four independent contacts are located at 90° around the nerve. (c) Multicontact selective electrode with four individually controlled contacts. (d) Multicontact nonselective electrode with a single stimulation channel divided between the four contacts (e) Single contact nonselective electrode for smaller nerves (<2 mm diameter).
Three different electrode configurations were used in this study. Multicontact selective electrodes [Fig. 2(c)] had four independent contacts that were used to selectively activate portions of the nerve. Multicontact nonselective electrodes [Fig. 2(d)] were used for complete activation of larger diameter nerves (4–6 mm). All four contacts were electrically connected and activated simultaneously. Single contact electrodes [Fig. 2(e)] were used for nonselective recruitment of smaller diameter nerves (2–3 mm).
C. Subject Description
Two subjects were selected for participation in this study, subject 1 with high tetraplegia and subject 2 with midlevel tetraplegia. Subject 1 had a Brown–Séquard injury at the C1 level. The subject had motor paralysis below C1 with normal to hypersensitive sensation on the implant side. The only voluntary function on the implant side was shoulder shrug (upper trapezius). Subject 2 had an injury at the C5 level. The subject had some voluntary shoulder movement and elbow flexion (including brachioradialis) with a limited range of motion. The triceps and wrist extensors of subject 2 were partially denervated.
A summary of implanted electrodes is shown in Table I. Multicontact selective electrodes [Fig. 2(c)] were used on the radial nerves of both subjects and the musculocutaneous nerve of subject 1. Multicontact nonselective electrodes [Fig. 2(d)] were used on the suprascapular nerves of both subjects and the axillary nerve of subject 1. Single-contact electrodes [Fig. 2(e)] were used on the thoracodorsal and long thoracic nerves of both subjects. Target muscles and consequent nerve cuff locations and type were chosen based on prior musculoskeletal modeling studies [1], [2]. The MetroHealth Medical Center Institutional Review Board approved the study and subjects gave informed consent prior to participation. An investigational device exemption was obtained from the FDA prior to initiation of the study.
D. EMG Recruitment
Muscle recruitment was quantified by the normalized level of EMG activity at different stimulation values. Twitch recruitment curves were generated by sending single stimulation pulses and recording the muscle electromyograms (EMG). In neuroprostheses, tetanic stimulation is used to produce fused muscle contractions but it has been shown that twitch stimulation provides a similar characterization of muscle recruitment [16], [27] while reducing the muscle fatigue and the duration of testing compared with tetanic stimulation.
An automated data collection algorithm [14] was used to generate pulse width and pulse amplitude modulated recruitment curves. The inputs to the algorithm were subthreshold and supramaximal stimulation values, usually chosen as the minimum and maximum values of the stimulator, while keeping the injected charge density below 1.0 μC/mm2. The twitch response, or activation level, was defined as the area under the rectified EMG response between 5 and 40 ms poststimulus. The limits of 5 ms and 40 ms were chosen to eliminate stimulation artifact and any reflexive contribution to the EMG signal, respectively. Five twitch responses were averaged together for each point on the recruitment curve [14].
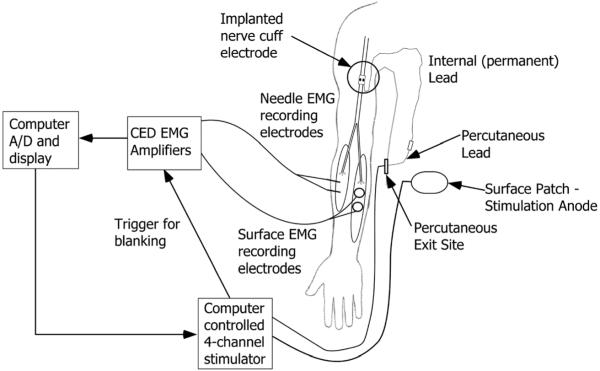
A schematic of the experimental setup during the percutaneous phase is shown in Fig. 3. First, the EMG signal was amplified and filtered (Model 1902, Cambridge Electronic Design, Cambridge, U.K.). The amplifiers were set to clamp the signal during the stimulus pulse to prevent saturation. The signal was ac coupled and low-pass filtered at 1000 Hz. The gain for each channel was set to maximize the size of a supra-maximal twitch response without saturation. Then, the signals were acquired by a PCMCIA A/D card (DAQCard-6036E, National Instruments, Austin, TX) attached to a Dell Latitude laptop PC at a sampling rate of 2.4 kHz per channel. During the percutaneous phase, a custom, computer-controlled stimulator (Crishtronics, Cleveland, OH) delivered charge balanced, biphasic pulses with an amplitude range of 0.1–5 mA (resolution of 0.02 mA), and pulse width range of 10–500 μs (resolution of 2 μs). A 5 cm × 5 cm surface electrode, positioned on the abdomen provided the return current path. The data collection interface software was written in Matlab (Mathworks, Inc., Natick, MA).
Fig. 3.
Schematic of experimental setup during the percutaneous testing showing one of several implanted electrodes. After the percutaneous phase, the percutaneous leads were removed and the permanent leads were attached to an implanted stimulator, located in the abdomen. The experimental setup was the same except for the computer controlled stimulator was replaced by the implantable stimulator telemeter.
During the implant phase, an implanted stimulator telemeter (IST, Technical Development Lab, Cleveland, OH) delivered charge balanced, biphasic pulses at preset amplitude values of 0.1 mA, 0.8 mA, 1.4 mA and 2.1 mA and a pulse width range of 1–200 μs with a 1 μs resolution [25]. For testing during the implant phase, the external Crishtronics stimulator was replaced with the IST, which was controlled using a universal external control unit (UECU-developed at the Technical Development Laboratory, Cleveland, OH) running with the Matlab real-time operating system (XPC Target, Mathworks, Natick, MA).
E. Field-Steering Protocol
In addition to single contact stimulation, field steering was used as a possible method to increase the selective stimulation capabilities of the electrode. During experiments using field steering, simultaneous subthreshold anodic stimulation was used on adjacent contacts. The anodic current was set to be 90% of the anodic stimulation threshold, a level found to be the most effective by previous studies [8]-[10]. One, two and three adjacent anodic contacts were evaluated for each contact. All field steering trials were performed using pulse amplitude modulation with a pulse width of 100 μs for the radial nerves and 50 μs for the musculocutaneous nerve.
Fine-wire, percutaneous EMG recording electrodes (0.5-mm-diameter, bipolar, Viasys Neurocare, Madison, WI) sensed the EMG signals to increase the spatial selectivity of the recordings at the sessions where field steering was evaluated. EMG signals were recorded from the following muscles: triceps, brachioradialis, extensor carpi radialis (ECR), extensor digitorum (ED), supinator, extensor indices (EI), extensor pollicis longus (EPL), biceps, and brachialis. The electrodes were implanted and removed by a physician and the subjects’ blood pressure, pulse and respiration rate were recorded before and after insertion and removal to monitor potential autonomic responses.
F. Nerve Conduction Velocity
The nerve conduction velocity of sensory fibers was measured using the neuromax 1002 (XLTEK, Oakville, ON, Canada), a standard clinical measurement system, once before and after implantation of the nerve cuff electrodes. For the radial nerve, stimulation was performed on the thumb for subject 1 and the index finger for subject 2. The evoked signal was recorded proximal to the wrist. The distance between the stimulating and recording electrodes was 10 cm in each case. For the musculocutaneous nerve, stimulation was performed at the forearm and the signal was recorded from the elbow. The distance between stimulating and recording electrodes was 12 cm. These measures are distal to the implanted electrodes. This tested for Wallerian degeneration of the sensory system, but did not indicate whether or not there were changes in the electrophysiology of the nerve within the cuff.
The motor nerve conduction velocity was calculated from the latency between stimulus and the EMG response at a pulse width of 100 μs and a pulse amplitude of 0.8 mA. Five measurements were obtained at each experimental session and the twitch onset was defined as 10% of the maximum twitch amplitude. The nerve conduction velocity was calculated as follows:
where d is the estimated distance from the nerve cuff electrode to the EMG recording electrodes, tL is the measured latency and tMS is the estimated time for synaptic transmission at the neuromuscular junction, which was assumed to be 1.6 ms for all trials [28].
G. Recruitment Analysis
Recruitment curves were normalized to the maximum EMG value recorded during that experimental session. The recruitment threshold was defined as the stimulus required to evoke 10% of the maximum activation [13]. Selective recruitment was quantified as the percent activation of one muscle before any other muscle reached threshold [14].
Activation Stability
Pulse width modulation recruitment curves at 0.8 mA were generated at four to six different sessions post implant. The recruitment curves were interpolated at a 1 μs resolution and the activation at each pulse width was averaged together over all weeks. The standard deviation was used to quantify the variability over the sessions.
H. Stability of Selective Recruitment
To look at the stability of selective recruitment, the relative activation was defined as the ratio of the activation of one muscle to the sum of the activations of all muscles. This is similar to a selectivity index used previously [10]. This ratio was calculated for each muscle using the most effective stimulation channel. The ratio at 50% activation of the muscle of interest was used to compare across sessions.
III. Results
Testing was performed over 37 months for subject 1 and 22 months for subject 2.
A. Nerve Conduction Velocity
The nerve conduction velocity for both subjects was calculated for both sensory and motor fibers (Table II). The preimplant sensory nerve conduction velocity ranged from 41.1 to 47.6 m/s. Post implant, the nerve conduction ranged from 45.5 to 52.6 m/s. The mean motor nerve conduction velocity post implant ranged from 40.6 to 44.9 m/s.
TABLE II.
Nerve Conduction Velocity
| Sensory Conduction | Motor Conduction | ||
|---|---|---|---|
| Pre-implant NCV (m/s) |
Post-implant NCV (m/s) |
Mean post-implant NCV (m/s) |
|
| Subject 1 | 18 Jul 05 | 2 Nov 05 | |
| Radial | 41.1 | 45.5 | 40.6 ± 5.7 |
| Musculocutaneous | 43.9 | 50.0 | 44.9 ± 7.3 |
|
| |||
| Subject 2 | 1 May 06 | 19 Sept 06 | |
| Radial | 47.6 | 52.6 | 42.6 ± 15.5 |
B. Recruitment Threshold
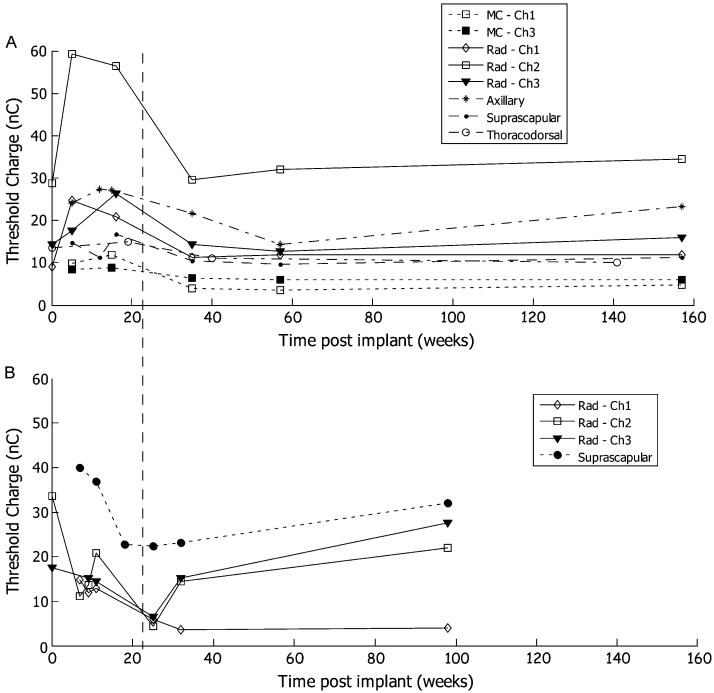
The average threshold over all measurement sessions during both the percutaneous phase and the implant phase was 19 ± 15 nC (Fig. 4). Some contacts exhibited a transient increase in threshold in the first five months post implant. The radial and axillary nerves of subject 1 displayed a prominent increase in recruitment threshold with a subsequent decrease and leveling off by 40 weeks. There was no clear trend in subject 2 but the thresholds remained in the normal range throughout the testing period. For both subjects, there was less variability after 4–5 months of implantation. The average threshold over the final three measurement sessions was 14 ± 9 nC.
Fig. 4.
The threshold charge for all measurement sessions from each contact that was included in the implant phase. (a) Subject 1. (b) Subject 2. The dotted line illustrates the five month point when the thresholds stabilize. MC is musculocutaneous nerve. Rad is radical nerve..
C. Recruitment Stability
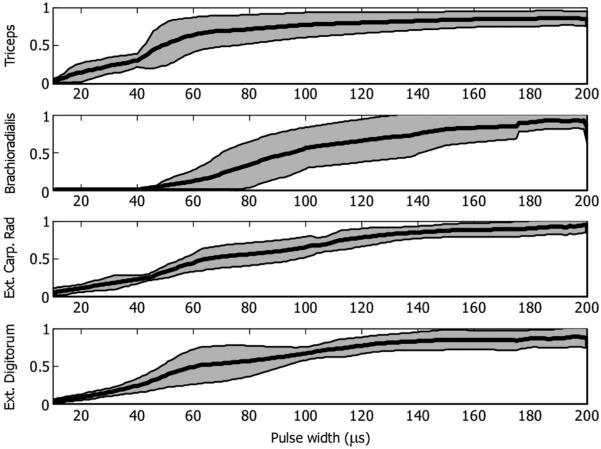
The similarity of muscle activation across sessions is indicated by the mean and standard deviation of the recruitment curves across all sessions (Fig. 5). For subject 1, the radial nerve data was from five sessions and the remaining nerves data was from four sessions. For subject 2, the suprascapular nerve data was from six sessions and the radial nerve data was from five sessions for the extensor digitorum and the extensor carpi radialis and four sessions from the triceps.
Fig. 5.
Mean recruitment curves from all five sessions of radial nerve stimulation in subject 1. The shaded areas depict the standard deviation.
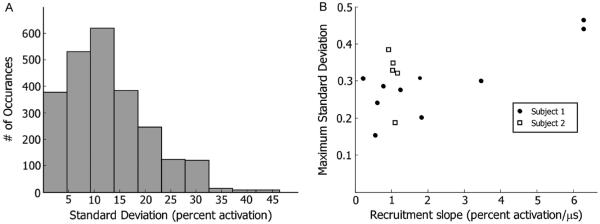
To summarize the data across all six nerves, the standard deviation at each point of the recruitment curves was calculated (Fig. 6). The standard deviation ranged from nearly zero to 0.46. Sixteen percent of the points had a standard deviation of less than 5% activation while 67% of the points had a standard deviation of less than 15% activation. There was a weak correlation (p = 0.09, Spearman’s rank correlation) between the slope of the recruitment curve and the maximum standard deviation for subject 1 and no correlation [p > 0.1, Spearman’s rank correlation] for subject 2 [Fig. 6(b)].
Fig. 6.
Analysis of variability. (a) Histogram of standard deviations of all pulse width from all muscles across all trials. (b) Relationship between maximum standard deviation and slope.
D. Selective Recruitment
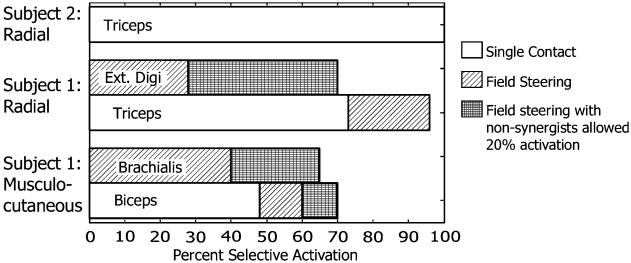
Selective recruitment of one muscle to at least 48% was seen with single contact stimulation in each of the three nerves with multicontact selective nerve cuff electrodes (Fig. 7). Field steering was evaluated during a single experimental session for each subject and produced selective recruitment of two additional muscles: The brachialis and extensor indices were selectively activated to at least 28% when using field steering. If the criterion for selective recruitment was relaxed to allow for 20% activation of another muscle, there was an increase in the amount that the muscles could be selectively activated to at least 65%.
Fig. 7.
Selective recruitment capabilities from each multicontact selective nerve cuff electrode. In each electrode, one muscle could be selectively activated to at least 48% before any other muscle reached threshold using single contact stimulation (white bars). Field steering (diagonal hatched bars) allowed selective recruitment of two additional muscles and increased the selective action of one muscle. When the criterion for selectivity was relaxed to allow for 20% activation of other muscles (cross hatched bars), the selectivity improved again.
E. Stability of Selective Recruitment
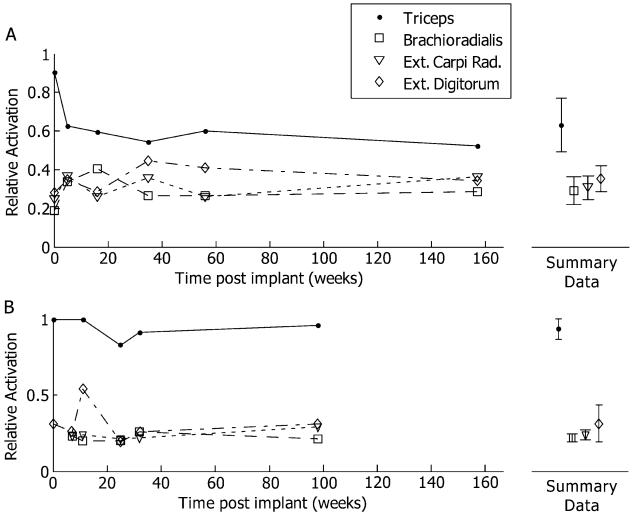
The relative activations of each muscle over time are shown in Fig. 8. The standard deviations between the sessions (displayed on the right of Fig. 8) were less than 10% activation for six of the eight muscles. For S1, the triceps relative activation had a standard deviation of 14%. This large variability was mostly due to a decrease in the relative activation between the implant and the first postoperative session. The standard deviation between the final five sessions was less than 5%. Even with this drop in relative activation, this contact remained the most effective stimulation contact for triceps activation. For S2, the extensor digitorum relative activation had a standard deviation of 12%. Once again, this variability seemed to be due to a single point. In all cases, the most effective contact for each muscle did not change.
Fig. 8.
The relative activation calculated at 50% activation of each muscle (PWM, 0.8 mA) from the radial nerve for each measurement session. (a) Subject 1. (b) Subject 2. The mean and standard deviation across all weeks is presented to the right of each plot. Overall, the best contact for each muscle remained constant, even with some variability in relative activation.
IV. Discussion
Nine spiral nerve cuff electrodes were implanted in two human subjects for up to three years with stable recruitment and no adverse functional effects. The nerve conduction velocity remained within the normal range for the entire testing period. The stimulation thresholds stabilized after approximately 20 weeks. The variability in the activation over time was not different from muscle-based electrodes used in implanted FES systems [29]. Three electrodes had multiple, independent contacts to evaluate selective recruitment of muscles. A single muscle could be selectively activated from each electrode using single-contact stimulation and the selectivity was increased with the use of field steering. The selectivity after three years was consistent with selectivity measured during the implant surgery.
A. Electrophysiology
The recruitment thresholds were stable and in the normal range, and the nerve conduction velocities did not change between preimplant testing and post implant testing. This suggests that the presence of the nerve cuff electrode on the nerve does not adversely affect the nerve function. Both the motor and sensory conduction velocities were slightly below normal. Studies of the radial nerve in healthy young adults found nerve conduction velocity of 62 ± 5.1 m/s in the forearm and slightly faster, 69 ± 5.0 m/s in the upper arm with slightly faster sensory conduction [30]. The motor conduction from the present study ranges from 40–45 m/s. This calculation assumes that the EMG recording electrodes are over the nerve entry point. The latency may include signal conduction along the muscle at 3–5 m/s [31] which could lower the calculated conduction velocities. However, the sensory conduction was uniformly low across both subjects before and after the implant. This suggests that some decrease in conduction velocity may be expected with long-term paralysis. These data do not reflect the nerve conduction through the nerve cuff electrode. But, over the course of the three years of testing, any degeneration would have reached the distal portions of the nerve and significantly affect the conduction and recruitment threshold.
For the radial and axillary nerves of subject 1, there was a transient increase in recruitment threshold over the first five months. Muscle activation was reliably achieved during the period of transient increase and there were no functional abnormalities. This response is similar to the increase seen previously in cats [17]. A transient threshold increase was seen with some of the electrodes but appeared to stabilize after the first eight weeks in cats. The increase in the present human study appears to last approximately 20 weeks, longer than predicted from the animal study. However, threshold data from pacemaker leads implanted in human subjects showed a similar increase in thresholds with a gradual return to baseline after four to seven months [32]. These findings in the human heart agree with our findings from human peripheral nerves.
B. Functional Effects of Variability
There is variability in muscle activation over time using both nerve-based stimulation and muscle-based stimulation. Variability in chronic thresholds using muscle-based stimulating electrodes is similar to the data reported here. The mean thresholds for muscle-based electrodes ranged from 78 to 164 nC with standard deviations ranging from 5 to 166 nC [29]. If these data and the nerve electrode data are both normalized to the maximum threshold mean, the maximum normalized standard deviation is 1.01 using muscle-based electrodes and 0.37 using nerve-based electrodes. Akers also reported that 88% of the muscle electrodes had a standard deviation less than 0.37 [29], which was the maximum standard deviation found using nerve-based electrodes. Therefore, the use of nerve cuff electrodes does not increase variability of the recruitment thresholds.
Presently, neuroprosthesis users can manage this day to day variability since systems have a single degree of freedom. Users are able to adjust their command level to obtain the desired function. Multiple degree of freedom systems are much more challenging to control using an open loop system because the variability discussed above is magnified with each additional degree of freedom. Future neuroprostheses to control the many degrees of freedom at the shoulder, elbow, and hand simultaneously are already being designed with feedback to take this burden of correction off of the user [34].
Finally, since muscles with steep recruitment are difficult to control, stimulus parameters should be selected to minimize the recruitment slope. When using pulse width modulation, decreasing the pulse amplitude has been shown to decrease the recruitment slope [33]. In this study, the lowest pulse amplitude value was 0.1 mA. For most muscles, stimulation at 0.1 mA and 200 μs, the maximum pulse width of the implanted stimulator, resulted in little or no activation. Stimulation at 0.8 mA was reasonable for most muscles but additional amplitudes between 0.1 and 0.8 mA would have been useful for the suprascapular and thoracodorsal nerves.
C. Selective Recruitment
In all contacts of all three selective nerve cuff electrodes, field steering did have an effect on the recruitment of muscles and it was functionally useful on three of the 12 contacts. On these three contacts, an additional muscle could be selectively recruited that was not possible using single contact stimulation or the selective recruitment was increased compared to single contact stimulation. On the other nine contacts, the effect of steering on recruitment was noticeable and did, in some cases, change the order of recruitment, but not sufficiently to affect the selectivity. This study was not an exhaustive search of the parameter space due to limited experimental time. However, the parameters for this study have been used extensively in prior experiments [8]-[10], [12], [16]. It is more probable that the number of stimulating contacts was the limiting factor. The radial nerve innervates 13 muscles and the nerve cuff electrodes had only four stimulation positions. Prior studies employing field steering used electrodes with four stimulation positions on nerves controlling four muscles/functions [10], [12], [16]. Experiments on the cat sciatic nerve using a similar four-contact spiral nerve cuff electrode selectively activated 67% of the fascicles using single-contact stimulation [12], significantly more than in the present study. One muscle could be selectively activated using single-contact stimulation in the present study, which is consistent with results obtained from human upper extremity nerves intraoperatively [14]. Based on these data, nerve electrode designs with a number of contacts equal to the number of function are recommended. A similar study on the cat sciatic nerve using electrodes with up to 12 stimulation positions could selectively activate all four functions without using field steering [13]. This electrode, the flat interface nerve electrode (FINE), also realigns the fascicles within a nerve to a flat cross-section, facilitating access to previously central fascicles. Based on these results, multicontact stimulation is suggested as an augmentation to stimulation with an equal number of contacts and desired functions.
D. Recruitment Stability
The selectivity did not change noticeably from the implant surgery to the chronic experimental sessions. The “best” channel for each muscle remained constant from the implant surgery through the final experimental session. This suggests that the electrode did not move or shift on the nerve during the conclusion of the surgery nor immediately after surgery, prior to encapsulation. One of the reasons for using percutaneous stimulating leads during the initial testing period was to choose which stimulating contacts provided the best responses. Since there were a limited number of stimulation channels available in the implanted stimulator, only these best contacts were used in the implant phase. These results from two subjects indicate that intraoperative testing is a valid predictor of chronic performance, possibly negating the need for a percutaneous phase.
E. Implant Considerations
Surface stimulation was used to test for lower motor neuron injury prior to nerve cuff electrode implantation. Electrode implant locations were chosen for ease of surgical access and leads were routed with slack to allow for joint movements without putting tension on the nerve. Eight of the eleven implanted electrodes were fully functional during the entire testing period. During preimplant testing for subject 2 the long thoracic and left thoracodorsal nerve exhibited signs of lower motor neuron injury. Since these nerves innervated essential musculature for stable arm movements, electrodes were placed on the nerves to determine if viable axons remained and could strengthen remaining motor units. Surface potential recordings verified that the electrodes were delivering current, however, the initial indications were confirmed and no muscle contractions were obtained.
The right thoracodorsal nerve cuff electrode in subject 2 stopped functioning approximately 20 weeks post implant. This electrode was in a position of high mechanical stress behind the shoulder which we hypothesize contributed to the electrode failure. The nerve, however, was still fully functional as tested during the second implant surgery. This highlighted the importance of proper lead routing and electrode placement, especially in consideration of mechanical stress.
V. Conclusion
This study is the first to chronically implant stimulating nerve cuff electrodes on upper extremity human peripheral nerves. Nerve stimulation was able to provide full and graded muscle contraction in all target muscles. One muscle could be selectively activated from each of the three multicontact selective nerve cuff electrodes and the selectivity was increased with the use of field steering techniques. In these two subjects, there are almost 22 accumulated “cuff years.” No adverse changes were observed in recruitment properties nor were any adverse sensory effects noted.
Acknowledgment
The authors would like to thank both subjects who patiently sat through hours of testing, C. Sams for assistance with clinical care of the subjects, Dr. J. Chae for implanting the percutaneous electrodes, and W. Memberg and J. G. Hincapie for support during experiments.
This work was supported in part by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) under Grant N01-NS-1-2333. The work of K. H. Polasek was supported by the U.S. Department of Education under the Graduate Assistances in Areas of National Need (GAANN) Fellowship.
Biography

Katharine H. Polasek received the B.S.E. degree in mechanical engineering from the University of Michigan, Ann Arbor, in 2001 and the M.S. and Ph.D. degrees in biomedical engineering from Case Western Reserve University (CWRU), Cleveland, OH, in 2004 and 2007, respectively. She has been an NIH postdoctoral fellow at CWRU since 2008 and has recently been awarded a Career Development Award through the VA Rehabilitation Research and Development service.
Her research interests include restoration of sensation for individuals with neurological disorders, especially in the upper extremity, and electrical nerve stimulation.

Harry A. Hoyen received the B.S. degree from the University of Wisconsin, Milwaukee and the M.D. degree from the Medical College of Wisconsin, Milwaukee. He completed his internship and residency at Case Western Reserve University, Cleveland, OH.
Since 1997, he has been a hand surgeon in the Department of Orthopaedics, University Hospitals of Cleveland and MetroHealth Medical Center, Cleveland, OH. He is also an Assistant Professor of Orthopaedic Surgery at Case Western Reserve University. His research interests include surgery of the hand and upper extremity, nerve regeneration, and spinal cord injury.

Michael W. Keith received the A.B. degree from Case Western Reserve University, Cleveland, OH, the M.D. degree from Ohio State University, Columbus, with general surgical training from Yale-New Haven Hospital, New Haven, CT, and orthopedic residency at Case Western Reserve University.
Following completion of a hand fellowship at Thomas Jefferson University, Philadelphia, PA, he joined the faculty at Case Western Reserve University. He is currently Professor of Orthopaedics and Biomedical Engineering at Case Western Reserve University. He is a Hand Surgeon at MetroHealth Medical Center, and a consultant in Orthopaedics at the Louis Stokes Cleveland VA Medical Center in Cleveland, OH. His research interests include surgery of the hand and spinal cord injury patients, upper extremity prosthetics, and functional neuromuscular stimulation..

Robert F. Kirsch (M’82) received the B.S. degree in electrical engineering from the University of Cincinnati, Cincinnati, OH, in 1982, and the M.S. and Ph.D. degrees in biomedical engineering from Northwestern University, Evanston, IL, in 1986 and 1990, respectively. He was a postdoctoral fellow in the Department of Biomedical Engineering at McGill University, Montréal, QC, Canada, from 1990 to 1993.
He is currently a Professor of Biomedical Engineering at Case Western Reserve University and Associate Director for Research in the Cleveland VA FES Center. His research focuses on restoring movement to disabled individuals using functional electrical stimulation (FES) and controlling FES actions via natural neural commands. Computer-based models of the human upper extremity are used to develop new FES approaches. FES user interfaces, including ones based on brain recordings, are being developed to provide FES users with the ability to command movements of their own arm.

Dustin J. Tyler (S’92–M’99) received the B.S. degree in electrical engineering from Michigan Technological University in Houghton, in 1992 and Ph.D. degree in biomedical engineering from Case Western Reserve University in Cleveland, OH, in 1999.
He worked from 1998 to 2002 in research and development in the industrial sector designing functional electrical stimulation products for spinal cord injured and stroke patients. In 2002 he returned to research as a Principal Investigator at the Louis-Stokes Cleveland Department of Veterans’ Affairs Medical Center (LSCDVAMC) as part of the Functional Electrical Stimulation Center of Excellence. He received a tenure-track Assistant Professor appointment in the Case Western Reserve University Biomedical Engineering Department in August, 2004 and Associate Professor with tenure July, 2009. In 2005, he was a founding member and currently serves as an Associate Director of the Advanced Platform Technology Center, a Center of Excellence in the VA Rehabilitation Research and Development service. His research interests include clinical implementation of neural interfaces; neuromimetic devices; neural modeling; neuroprostheses for restoration of lost function in neurologically impaired individuals; neuroprostheses for restoration of sensation in amputees; and neuroprostheses for head and neck applications.
Dr. Tyler is a member of MRS, BMES, AAAS, and Tau Beta Pi societies. In 2006, he was awarded the Nord Distinguish Assistant Professor endowed chair in the Case School of Engineering and in 2008 the Case School of Engineering Awards for Excellence in both Teaching and Research. .
Contributor Information
Katharine H. Polasek, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA (katharine.polasek@case.edu)
Harry A. Hoyen, Department of Orthopaedics and Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA; MetroHealth Medical Center, Cleveland, OH 44106 USA
Michael W. Keith, Department of Orthopaedics and Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA; MetroHealth Medical Center, Cleveland, OH 44106 USA
Robert F. Kirsch, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA; Cleveland Louis Stokes Department of Veterans Affairs Medical Center, Cleveland, OH 44106 USA.
Dustin J. Tyler, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH 44106 USA; Cleveland Louis Stokes Department of Veterans Affairs Medical Center, Cleveland, OH 44106 USA (dustin.tyler@case.edu).
References
- [1].Hincapie JG, Blana D, Chadwick EK, Kirsch RF. Musculoskeletal model-guided, customizable selection of shoulder and elbow muscles for a C5 SCI neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2008 Jun.16(3):255–263. doi: 10.1109/TNSRE.2008.922681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blana D, Hincapie JG, Chadwick EK, Kirsch RF. A musculoskeletal model of the upper extremity for use in the development of neuroprosthetic systems. J. Biomech. 2008;41:1714–1721. doi: 10.1016/j.jbiomech.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grandjean PA, Mortimer JT. Recruitment properties of monopolar and bipolar epimysial electrodes. Ann. Biomed. Eng. 1986;14:53–66. doi: 10.1007/BF02364648. [DOI] [PubMed] [Google Scholar]
- [4].Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans. Neural Syst. Rehabil. Eng. 1994 Jun.2(2):80–91. [Google Scholar]
- [5].Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: An electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch. Phys. Med. Rehabil. 1997;78:608–614. doi: 10.1016/s0003-9993(97)90426-3. [DOI] [PubMed] [Google Scholar]
- [6].Kilgore KL, Peckham PH, Keith MW, Thrope GB, Wuolle KS, Bryden AM, Hart RL. An implanted upper-extremity neuroprosthesis. Follow-up of five patients. J. Bone Joint Surg. Am. 1997;79:533–541. doi: 10.2106/00004623-199704000-00008. [DOI] [PubMed] [Google Scholar]
- [7].Davis JA, Jr., Triolo RJ, Uhlir J, Bieri C, Rohde L, Lissy D, Kukke S. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers—Where do we stand? J. Rehabil. Res. Develop. 2001;38:609–617. [PubMed] [Google Scholar]
- [8].Sweeney JD, Crawford NR, Brandon TA. Neuromuscular stimulation selectivity of multiple-contact nerve cuff electrode arrays. Med. Biol. Eng. Comput. 1995;33:418–425. doi: 10.1007/BF02510525. [DOI] [PubMed] [Google Scholar]
- [9].Sweeney JD, Ksienski DA, Mortimer JT. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans. Biomed. Eng. 1990 Jul.37(7):706–715. doi: 10.1109/10.55681. [DOI] [PubMed] [Google Scholar]
- [10].Veraart C, Grill WM, Mortimer JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1993 Jul.40(7):640–653. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- [11].Goodall EV, de Breij JF, Holsheimer J. Position-selective activation of peripheral nerve fibers with a cuff electrode. IEEE Trans. Biomed. Eng. 1996 Aug.43(8):851–856. doi: 10.1109/10.508548. [DOI] [PubMed] [Google Scholar]
- [12].Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2004 Jun.12(2):251–257. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]
- [13].Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2002 Dec.10(4):294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- [14].Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2007 Mar.15(1):76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- [15].Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 1988 Nov.35(11):905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- [16].Grill WM, Jr., Mortimer JT. Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Trans. Rehabil. Eng. 1996 Jun.4(2):49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- [17].Grill WM, Mortimer JT. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans. Rehabil. Eng. 1998 Dec.6(4):364–373. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- [18].Grill WM, Mortimer JT. Neural and connective tissue response to long-term implantation of multiple contact nerve cuff electrodes. J. Biomed. Mater. Res. 2000;50:215–226. doi: 10.1002/(sici)1097-4636(200005)50:2<215::aid-jbm17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [19].Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S, Wanet-Defalque MC. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res. 1998;813:181–186. doi: 10.1016/s0006-8993(98)00977-9. [DOI] [PubMed] [Google Scholar]
- [20].Delbeke J, Oozeer M, Veraart C. Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vis. Res. 2003;43:1091–1102. doi: 10.1016/s0042-6989(03)00013-0. [DOI] [PubMed] [Google Scholar]
- [21].Brelen ME, Duret F, Gerard B, Delbeke J, Veraart C. Creating a meaningful visual perception in blind volunteers by optic nerve stimulation. J. Neural Eng. 2005;2:S22–S28. doi: 10.1088/1741-2560/2/1/004. [DOI] [PubMed] [Google Scholar]
- [22].Veraart C, Wanet-Defalque MC, Gerard B, Vanlierde A, Delbeke J. Pattern recognition with the optic nerve visual prosthesis. Artif. Organs. 2003;27:996–1004. doi: 10.1046/j.1525-1594.2003.07305.x. [DOI] [PubMed] [Google Scholar]
- [23].Peckham PH, Mortimer JT, Marsolais EB. Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin. Orthop. Related Res. 1976:326–333. [PubMed] [Google Scholar]
- [24].Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4:94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- [25].Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, Peckham PH. An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans. Biomed. Eng. 1998;45:463–475. doi: 10.1109/10.664202. [DOI] [PubMed] [Google Scholar]
- [26].Cuoco FA, Jr., Durand DM. Measurement of external pressures generated by nerve cuff electrodes. IEEE Trans. Rehabil. Eng. 2000 Mar.8(1):35–41. doi: 10.1109/86.830947. [DOI] [PubMed] [Google Scholar]
- [27].Durfee WK, MacLean KE. Methods for estimating isometric recruitment curves of electrically stimulated muscle. IEEE Trans. Biomed. Eng. 1989 Jul.36(7):654–667. doi: 10.1109/10.32097. [DOI] [PubMed] [Google Scholar]
- [28].Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc. R. Soc. Lond. B Biol. Sci. 1965;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- [29].Akers JM, Peckham PH, Keith MW, Merritt K. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans. Rehabil. Eng. 1997 Jun.5(2):207–220. doi: 10.1109/86.593301. [DOI] [PubMed] [Google Scholar]
- [30].Trojaborg W, Sindrup EH. Motor and sensory conduction in different segments of the radial nerve in normal subjects. J. Neurol. Neurosurg. Psychiatry. 1969;32:354–359. doi: 10.1136/jnnp.32.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. 4th ed. McGraw-Hill; New York: 2000. The motor unit and muscle action; pp. 674–694. [Google Scholar]
- [32].Platia EV, Brinker JA. Time course of transvenous pacemaker stimulation impedance, capture threshold, and electrogram amplitude. Pacing Clin. Electrophysiol. 1986;9:620–625. doi: 10.1111/j.1540-8159.1986.tb05408.x. [DOI] [PubMed] [Google Scholar]
- [33].Gorman PH, Mortimer JT. The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans. Biomed. Eng. 1983 Jul.30(7):407–414. doi: 10.1109/tbme.1983.325041. [DOI] [PubMed] [Google Scholar]
- [34].Blana D, Kirsch RF, Chadwick EK. Combined feedforward and feedback control of a redundant, nonlinear, dynamic musculoskeletal system. Med. Biol. Eng. Comput. 2009;47:533–542. doi: 10.1007/s11517-009-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]