Abstract
Multiple organ systems are adversely affected by diabetes, including the brain, which undergoes changes that may increase the risk of cognitive decline. Although diabetes influences the hypothalamic–pituitary–adrenal axis, the role of this neuroendocrine system in diabetes–induced cognitive dysfunction remains unexplored. Here we demonstrate that, in both insulin–deficient rats and insulin–resistant mice, diabetes impairs hippocampus–dependent memory, perforant path synaptic plasticity and adult neurogenesis, and the adrenal steroid corticosterone contributes to these adverse effects. Rats treated with streptozocin have reduced levels of insulin, and exhibit hyperglycemia, increased levels of corticosterone, and impairments in hippocampal neurogenesis, synaptic plasticity and learning. Similar deficits are observed in db/db mice, which are characterized by insulin resistance, elevated corticosterone levels and obesity. Changes in hippocampal plasticity and function in both models are reversed when normal physiological levels of corticosterone are maintained, suggesting that cognitive impairment in diabetes may result from glucocorticoid–mediated deficits in neurogenesis and synaptic plasticity.
Keywords: glucocorticoid, dentate gyrus, streptozotocin, water maze, object recognition
As a result of high calorie diets and sedentary lifestyles, diabetes is rapidly becoming more prevalent in Western societies1. In addition to its well–known adverse effects on the cardiovascular and peripheral nervous systems, diabetes also appears to negatively impact the brain, increasing the risk of depression and dementia2, 3. Human subjects with either type 1 (caused by insulin deficiency) or type 2 (mediated by insulin resistance) diabetes typically exhibit impaired cognitive function compared to age–matched non–diabetic subjects3, 4. Cognitive deficits have also been documented in studies of rodent models of diabetes. For example, rats rendered diabetic by treatment with the pancreatic β–cell toxin streptozocin (STZ; a model of type 1 diabetes) exhibit impaired performance in tests of spatial learning ability5, 6. Similar deficits have been reported in the db/db mouse7, a model of Type 2 diabetes in which obesity, hyperglycemia, and elevations in circulating corticosterone levels arise from a mutation that inactivates the leptin receptor8. However, the mechanism(s) responsible for cognitive dysfunction in diabetes has not been established.
Within the hippocampus, changes in the strength of synapses between groups of neurons play a critical role in certain types of learning and memory. At the level of the dentate gyrus, regulation of synaptic connectivity extends beyond changes in the number and strength of synapses, to the de novo addition of new neurons in adulthood9. Data from studies of animal models suggest impairment of both synaptic plasticity and adult neurogenesis in diabetes. Long–term potentiation (LTP) of synaptic transmission, believed to be a cellular mechanism of learning and memory, is impaired in the dentate gyrus of rats with streptozocin–induced diabetes10. Diabetic animals also exhibit lower rates of adult neurogenesis11, while exercise and dietary energy restriction, which have anti–diabetic effects, can enhance synaptic plasticity12, 13 and neurogenesis12, 14, 15. Because cognitive ability is impaired in subjects with either type 1 or type 2 diabetes, and in animal models of both types of diabetes, it is unlikely that global changes in insulin levels are directly responsible for impaired neuronal plasticity.
Humans with poorly controlled diabetes exhibit hyperactivation of the hypothalamic – pituitary – adrenal (HPA) axis, resulting in elevated circulating levels of cortisol2, 4. Similarly, levels of adrenal glucocorticoids are elevated in rodents with experimental diabetes16-20. The specific mechanism by which diabetes results in hyperactivation of the HPA axis is unknown, but is apparently not the result of the hyperglycemia per se17. Although it is not known whether glucocorticoids are involved in cognitive dysfunction in diabetes, elevated cortisol levels have been associated with poor cognitive ability in humans subjected to psychosocial stress21, during normal aging22 and in Alzheimer's disease23. Studies of rodents have provided evidence that elevated adrenal glucocorticoids mediate deficits in cognitive function caused by chronic stress24, 25. In addition, chronic stress and high levels of corticosterone can impair synaptic plasticity26–29. Moreover, stress levels of corticosterone inhibit neurogenesis in the hippocampus of adult rats30 and lifelong levels of corticosterone are correlated with age–related declines in neurogenesis and memory31. It is therefore possible that elevated corticosterone levels in diabetes may mediate central impairment of neuronal structure and function.
Here we provide direct evidence that elevated glucocorticoid levels contribute to the impairment of synaptic plasticity and neurogenesis, and associated learning and memory deficits, in animal models of both insulin resistant and insulin deficient diabetes. These findings support a role for HPA axis hyperactivity in diabetes–induced cognitive impairment, and suggest novel approaches for improving cognitive function in subjects with diabetes.
Lowering corticosterone levels reverses learning deficits
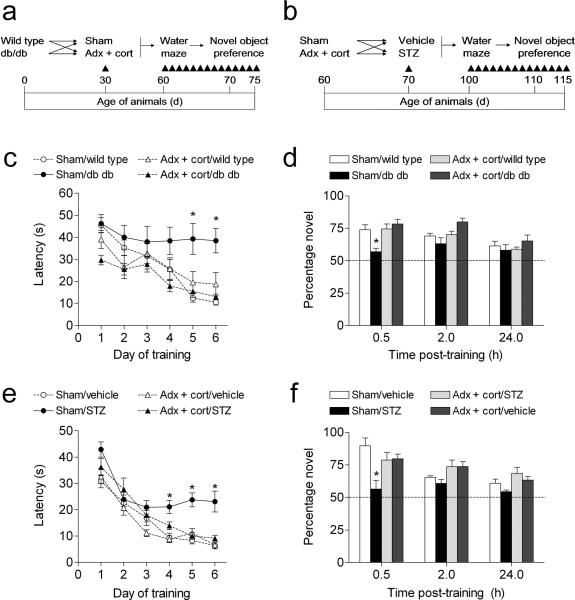
To evaluate whether elevated corticosterone levels are accompanied by alterations in hippocampus–dependent learning in diabetic animals, we tested cognitive function in diabetic and non–diabetic animals that had been adrenalectomized and administered low-dose corticosterone replacement30, or sham–operated (Fig. 1a–b). This intervention has previously been used to both lower and normalize corticosterone levels following stress32. First we evaluated performance in the hippocampus–dependent version of the water maze. In both db/db mice and STZ–treated rats, sham–operated diabetic animals had longer escape latencies, and took a less direct route to the hidden platform (Fig. 1c,e; Supplementary Fig. 1a–b). These findings concur with previous reports5-7. Both of these deficits were reversed in diabetic animals with normal physiological levels of corticosterone (escape latency, db/db mice, F1,32 = 4.91, p = 0.03, STZ–treated rats, F1,46 = 7.19, p = 0.01; path length, db/db mice, F1,33 = 4.52, p = 0.04; path length, STZ–treated rats, F1,31 = 13.62, p = 0.001). There was no effect of adrenalectomy and corticosterone replacement in non–diabetic mice and rats. Additionally, there were no significant differences in swim speed across diabetic and non–diabetic animals with different levels of corticosterone (db/db mice, F1,33 = 1.42, p = 0.24; STZ–treated rats, F1,43 = 0.14, p = 0.71). Although db/db mice that had received adrenalectomy and corticosterone replacement had shorter escape latencies and path lengths on the first day of training (Fig. 1c, Supplementary Fig. 1a), this was primarily due to improvements during the successive trials, as latency and path length during trial 1 were not different from other groups (data not shown).
Figure 1. Maintaining normal physiological levels of corticosterone prevents learning deficits in rodent models of diabetes.
(a), Experimental design for studies in Type 2 diabetic mice. (b), Experimental design for studies in Type 1 diabetic rats. Adrenalectomized animals received corticosterone replacement via the drinking water (25μg ml–1 in 0.9% saline). (c), Sham–operated db/db mice were unable to learn the location of the hidden platform in the Morris water maze. In contrast, db/db mice that received adrenalectomy and corticosterone replacement learned the location of the platform as effectively as non–diabetic mice. Shorter escape latencies on the first day of training in adrenalectomized db/db mice were attributable to performance on successive trials, as escape latencies were similar during the first trial (see Results). (d), db/db mice with intact adrenal glands exhibit impaired object recognition, while db/db mice with levels of corticosterone ‘clamped’ through adrenalectomy and corticosterone replacement show preference for the novel object that is similar to wildtype controls. (e), Learning is impaired in insulin–deficient diabetic rats experiencing elevated corticosterone levels, but not in diabetic rats that received adrenalectomy and corticosterone replacement. (f), Novel object preference is reduced in sham–operated diabetic rats, but preserved in diabetic rats with normal levels of corticosterone. Abbreviations: Adx + cort = adrenalectomized with 25μg ml–1 corticosterone replacement; STZ = streptozocin–treated (70mg kg–1). Error bars = s.e.m.
In a probe trial conducted 24 hours after the last session of acquisition training, sham–operated STZ–diabetic rats spent less time searching in the target quadrant, relative to non–diabetic, sham–operated rats (F1,27 = 5.07, p = 0.03; Supplementary Fig. 1d). This contrasts with the results of the probe trial in the db/db mice, where we observed no significant effect of diabetes or adrenalectomy on the percentage of time spent searching in the target quadrant (F1,33 = 0.85, p = 0.36; Supplementary Fig. 1c). Performance in the visible platform version of the Morris water maze, which is not hippocampus–dependent, was similar across conditions in both models (db/db mice, F1,33 = 0.001, p = 0.96; STZ–treated rats, F1,28 = 0.57, p = 0.45; data not shown).
Next we tested recognition memory in the novel object preference test. This task takes advantage of the natural bias for novelty in rodents, and unlike the water maze, does not depend on aversive motivation. In both models, non–diabetic animals showed robust preference for the novel object, particularly at the shortest post–training interval. However, sham–operated diabetic animals showed less of a preference for the novel object (db/db mice, F1,26 = 10.52, p = 0.003; STZ–treated rats, F1,11 = 11.68, p = 0.006, Fig. 1d,f). In contrast, diabetic animals that had received adrenalectomy and corticosterone replacement preferred to explore the novel object, at levels that were similar to sham–operated and adrenalectomized control animals. We also recorded the latency to begin exploring, and the total time spent exploring both objects during each trial (novel + familiar/duration of behavioral observation; see Supplementary Methods). In db/db mice, diabetic animals spent more time exploring the objects (F1,28 = 22.78, p = 0.001; Supplementary Fig. 2a), and latency to approach either object was not different across groups (Supplementary Fig. 2b). In STZ–treated rats, there were no differences in the amount of time spent exploring the objects (Supplementary Fig. 2c), but sham–operated diabetic animals waited longer before approaching the objects, and adrenalectomized diabetic animals waited less (F1,12 = 6.14, p = 0.001; Supplementary Fig. 2d). The parameters surrounding object exploration are difficult to interpret, because neither total time exploring or the latency to explore was significantly correlated with preference for the novel object (data not shown). However, together with the water maze results, these data suggest that untreated diabetes exerts pervasive negative effects on hippocampus–dependent memory, and that these effects can be reversed by lowering corticosterone levels.
Normalizing corticosterone levels restores LTP
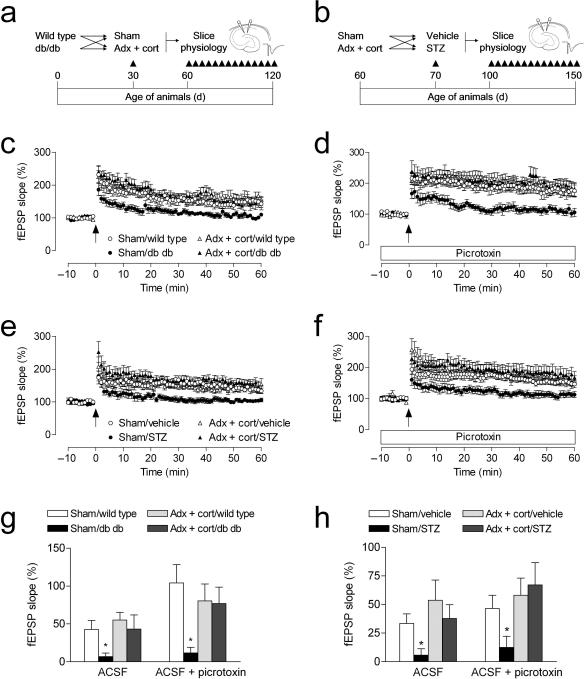
We further examined the role of corticosterone in the diabetes–induced impairment of hippocampal learning by measuring synaptic plasticity at perforant path – dentate gyrus synapses in acute slices from an additional group of adrenalectomized or sham–operated diabetic and non–diabetic rodents (Fig. 2a–b). In agreement with previous studies6, 7, both db/db mice and STZ–diabetic rats show reduced LTP at medial perforant path synapses in the dentate gyrus when recordings are made in the presence of the GABAA receptor antagonist picrotoxin (100μM; Fig. 2d,f). Adrenalectomy and corticosterone replacement prevented LTP impairment in both models (db/db mice, F1,36 = 5.15, p = 0.03; STZ–treated rats, F1,35 = 5.90, p = 0.02). Baseline synaptic transmission was not different in db/db mice and controls, irrespective of corticosterone manipulation (Supplementary Fig. 3a). However, in rats, adrenalectomy and corticosterone replacement reduced baseline synaptic transmission, in both diabetic and non–diabetic animals (F1,39 = 3.65, p = 0.03; Supplementary Fig. 3c). No alterations in the paired–pulse depression that is characteristic of this pathway were observed in either model (Supplementary Fig. 3b,d). Taken together, these findings suggest that diabetes causes a primarily postsynaptic deficit in dentate gyrus plasticity, which is reversible by lowering levels of corticosterone.
Figure 2. Lowering corticosterone levels regulates synaptic plasticity in diabetic rodents.
(a), Design for studies in Type 2 diabetic mice. (b), Design for studies in Type 1 diabetic rats. Adrenalectomized animals received corticosterone replacement via the drinking water (25μg ml–1 in 0.9% saline). (c), Sham–operated db/db mice exhibit reduced dentate gyrus LTP, but db/db mice with normal physiological levels of corticosterone are not impaired. (d), Insulin–resistant mice that had been sham–operated also exhibit impaired LTP in the presence of picrotoxin, which decreases local inhibition and also blocks GABAergic excitation on new neurons34, 35. In contrast, insulin–resistant mice with normal physiological levels of corticosterone show control levels of LTP under these conditions. (e), Sham–operated insulin–deficient rats exhibit reduced LTP; preventing elevation of corticosterone levels prior to induction of experimental diabetes restores LTP. (f), STZ–diabetic rats with intact adrenal glands demonstrate reduced LTP in the presence of picrotoxin. Lowering corticosterone levels also reverses the effect of diabetes on LTP under these conditions. (g–h), Comparison of the amount of potentiation in slices from diabetic and non–diabetic mice (g) and rats (h) with different levels of corticosterone, recorded in ACSF and in ACSF with picrotoxin. Error bars = s.e.m.
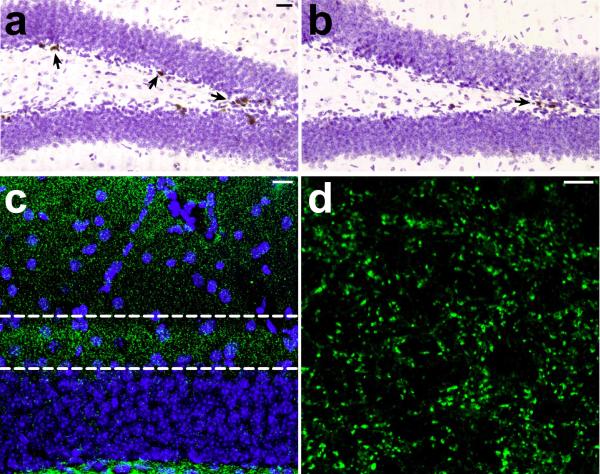
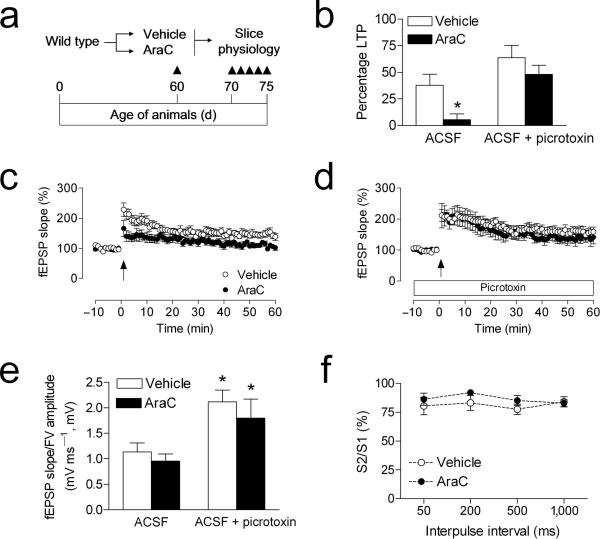
Adult–generated neurons exhibit a number of distinct electrophysiological properties. Among these is the transient capacity for GABAergic excitation33, 34. It was recently demonstrated that changes in adult neurogenesis correlate with changes in medial perforant path LTP in the absence, but not in the presence of picrotoxin35, 36. We confirmed this in slices from wildtype animals that had been infused with the antimitotic drug cytosine arabinoside (AraC), which effectively reduces progenitor cell proliferation (BrdU–labeled cells, vehicle = 4440 ± 822.6, AraC = 696.0 ± 230.9; t10 = 5.08, p = 0.0005; Fig. 3a–b). We also counted pyknotic cell profiles to determine whether antimitotic treatment might influence cell death; there was no significant difference between vehicle– and AraC–treated animals in the number of pyknotic cells in the dentate gyrus (t7 = 1.58, p = 0.16; mean ± s.e.m., vehicle = 216 ± 54; AraC = 450 ± 160).
Figure 3. AraC treatment reduces cell proliferation, without altering synaptic marker immunoreactivity.
(a–b), Following a single injection of 300mg kg–1 BrdU with a 24 hour survival period, we observed a significant reduction in the number of labeled cells in wildtype mice that had been infused with the antimitotic drug AraC for ten to fourteen days. The micrograph in (a) shows the dentate gyrus of a mouse infused with vehicle, and the micrograph in (b) shows the dentate gyrus of a mouse infused with AraC. Arrows indicate labeled cells. (c), We also quantified synaptic marker expression in the inner third of the dentate molecular layer, where the medial perforant path synapses are located. This confocal micrograph shows the anatomical region (outlined) where scans were taken for analysis of synaptophysin labeling, and where electrodes were positioned for electrophysiological recordings in slices. (d), Micrograph taken at the resolution and scale used for analysis of synaptophysin labeling (see Supplementary Methods). We observed no differences in the area or intensity of staining for the synaptic marker synaptophysin, suggesting that AraC treatment does not result in loss of synapses among the larger population of dentate granule neurons.
To evaluate whether AraC treatment might alter synaptic marker expression in the anatomical region where medial perforant path synapses are located, we used immunofluorescence labeling for synaptophysin. There were no differences in the area or intensity of synaptophysin immunofluorescence in AraC– and vehicle–treated animals, suggesting no loss of synapses among the larger population of mature granule neurons (optical intensity index, vehicle = 1.1 × 106 ± 3.1 × 105, AraC =1.2 × 106 ± 2.1 × 105; t10 = 0.21, p = 0.84; Fig. 3c–d). In slices from AraC–treated animals, we observed selective deficits in medial perforant path LTP recorded in plain ACSF (Fig. 4b–c). These deficits were not detected when recordings were made in the presence of picrotoxin (100μM; Fig. 4b,d). Although there was a significant effect of picrotoxin on the input–output curve, there was no effect of AraC treatment (Fig. 4e). There was also no effect of AraC infusion on paired–pulse depression, recorded in plain ACSF (Fig. 4f).
Figure 4. Antimitotic treatment selectively impairs dentate gyrus LTP recorded in the absence of picrotoxin.
(a), Experimental design for studies using minipump delivery of antimitotic drugs. (b), Comparison of the amount of LTP in vehicle– and AraC–infused mice, when recordings were made in the presence or absence of the GABAA antagonist picrotoxin (100μM). Asterisk (*) indicates significance at p < 0.05 following 2 × 2 ANOVA. (c), LTP at medial perforant path synapses in the dentate gyrus is impaired in AraC–infused mice. (d), LTP recordings in the presence of picrotoxin were not influenced by AraC infusion. (e), The relationship between the slope of the dendritic field potential and the amplitude of the axonal fiber volley is influenced by picrotoxin, but not by AraC treatment. (f), Presynaptic paired–pulse depression, measured in plain ACSF, was not altered by treatment with AraC. Error bars = s.e.m.
To measure LTP in diabetic animals under conditions that would permit the activation of newly generated neurons, we induced LTP in the absence of picrotoxin. We found that diabetic animals show impaired LTP, and maintaining low levels of corticosterone through adrenalectomy restores LTP to a level similar to controls (db/db mice, F1,33 = 3.10, p = 0.04; STZ–treated rats, F1,39 = 5.24, p = 0.03; Fig. 2c,e). These results suggest that diabetes alters synaptic plasticity via multiple mechanisms involving both changes in new neurons, and changes in the mature neuronal population.
Corticosterone–mediated impairment of cell proliferation
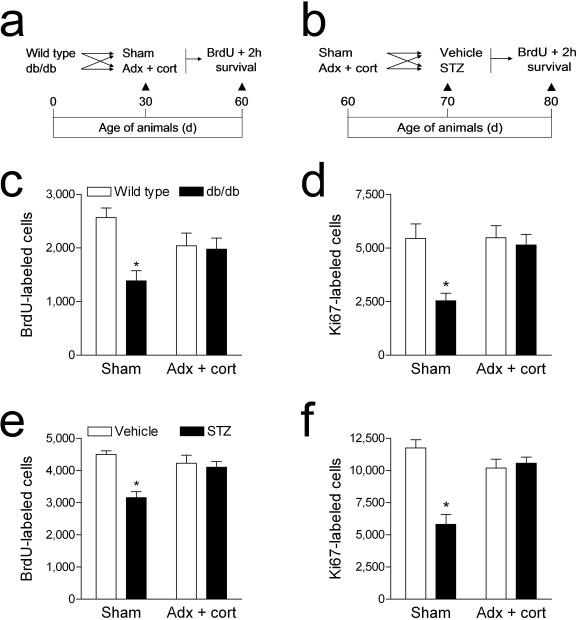
To assess what role elevated corticosterone levels might play in the diabetes–induced suppression of hippocampal cell proliferation, we administered a single injection of the DNA synthesis marker bromodeoxyuridine (BrdU; 300mg kg–1 IP) to adrenalectomized and sham–operated animals, with and without diabetes (Fig. 5a–b). In db/db mice, adrenalectomy and corticosterone replacement prevented the reduction in BrdU labeling in the dentate gyrus that we observed in sham–operated db/db mice at two hours post–injection (F1,31 = 5.82, p = 0.02; Fig. 5c, 6a–b). Similarly, in STZ–diabetic rats, adrenalectomy and corticosterone replacement prior to the induction of experimental diabetes prevented the decrease in BrdU–labeled cell number observed in sham–operated diabetic animals (F1,41 = 10.76, p = 0.002; Fig. 5e, Supplementary Fig. 4a–b). There was no effect of adrenalectomy and corticosterone replacement in vehicle–treated rats, or in wildtype mice.
Figure 5. Elevated corticosterone levels contribute to the suppression of dentate gyrus cell proliferation in diabetic rodents.
(a), Design for studies in Type 2 diabetic mice. (b), Design for studies in Type 1 diabetic rats. Adrenalectomized animals received corticosterone replacement via the drinking water (25μg ml–1 in 0.9% saline). (c), Sham–operated db/db mice exhibit reduced BrdU labeling in the dentate gyrus, while db/db mice that received adrenalectomy and corticosterone replacement were not different from non–diabetic mice. (d), Labeling for the endogenous proliferation marker Ki67 is reduced in sham–operated Type 2 diabetic mice, while Type 2 diabetic mice that had been adrenalectomized and given corticosterone replacement were not different from non–diabetic animals. Legend in (c) applies to (d). (e), Type 1 diabetic rats with intact adrenal glands have fewer BrdU–labeled cells in the dentate gyrus – this reduction is not observed in Type 1 diabetic rats with normal levels of corticosterone. (f), Labeling for the endogenous proliferation marker, Ki67, follows a similar pattern – diabetic rats with intact adrenal glands have significantly fewer Ki67–labeled cells than controls, while diabetic animals that received adrenalectomy and low–dose corticosterone replacement were not significantly different from non–diabetic rats. Legend in (e) applies to (f). Error bars = s.e.m.
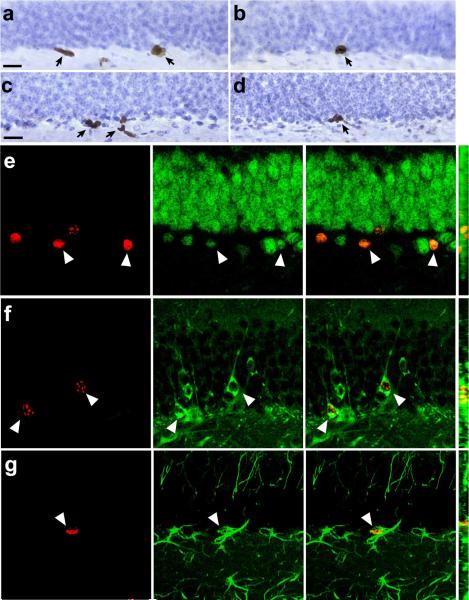
Figure 6. Hippocampal cell proliferation and neurogenesis is reduced in a mouse model of Type 2 diabetes.
(a), BrdU labeled cells in the proliferative dentate subgranular zone of a wildtype mouse at 2 hours post–injection. Arrows indicate labeled cells. (b), BrdU labeled cells in the subgranular zone of a db/db homozygous mouse. (c), Progenitor cells expressing the endogenous proliferation marker Ki67 in the dentate gyrus of a wildtype mouse. (d), Cells labeled with antibodies to Ki67 in the dentate gyrus of a db/db mouse. Scalebar = 20 μm. (e), Cells co–expressing the proliferative marker BrdU (red, left) and the mature neuronal marker NeuN (green, middle) at 3 weeks post–injection (merged image shown at right). (f), Double labeling with BrdU (red) and Tuj1 (green), also at 3 weeks post–injection (merged image shown at right). (g), Cells double–labeled with antibodies to BrdU (red) and the astroglial marker GFAP (green) at 3 weeks post– injection (merged image shown at right). For (e–g), the far right panel shows the merged image on the Z-axis.
Diabetes has been associated with neurovascular pathologies, which could alter the availability of the exogenous marker BrdU. To measure an index of hippocampal cell proliferation that would not be influenced by availability, we used the endogenous proliferative marker, Ki67. Labeling for Ki67 followed the same pattern as labeling for BrdU; sham–operated diabetic animals had fewer Ki67–labeled cells, while diabetic animals that had been adrenalectomized and given corticosterone replacement were not significantly different from non–diabetic animals (db/db mice, F1,20=5.24, p=0.03; STZ–treated rats, F1,35 = 5.89, p = 0.02; Fig. 5d, 5f, Fig. 6c–d, Supplementary Fig. 4c–d). There were no effects of adrenalectomy and corticosterone replacement on Ki67 labeling in non–diabetic wildtype mice or vehicle–treated rats. These results indicate that lowering corticosterone levels prevents the decrease in hippocampal progenitor cell proliferation in insulin resistant and insulin deficient diabetes.
Lasting suppression of adult neurogenesis with diabetes
To evaluate whether suppression of hippocampal cell proliferation in diabetic animals translates into a reduction in adult neurogenesis, we administered a single injection of BrdU (300mg kg–1 IP) to diabetic and non–diabetic rodents and sacrificed them three weeks later. In db/db mice, we observed a reduction in the number of BrdU–labeled cells in the dentate gyrus (mean ± s.e.m., wildtype = 860 ± 69, db/db = 416 ± 85; t8 = 4.05, p = 0.004). There was no difference in the proportion of cells expressing the mature neuronal marker NeuN (wildtype = 98 ± 1.22, db/db = 96 ± 1.87; t8 = 0.89, p = 0.39; Fig. 6e) or the immature neuronal marker Tuj1 (wildtype = 93 ± 0.96, db/db = 93 ± 1.26; t8 = 0.03, p = 0.97; Fig. 6f). There was also no change in the percentage of BrdU–labeled cells expressing the astroglial marker GFAP (wildtype = 7.35 ± 1.01, db/db = 8.92 ± 2.51; t8 = 0.58, p = 0.57; Fig. 6g). Because the analyses were made separately, in adjacent series of stereological sections, values reflect relative expression of each marker among the BrdU–labeled cell population. However, the absence of any proportionate difference in the expression of neuronal and glial markers suggests that differentiation of newly generated cells was not affected.
Similarly, in STZ–diabetic rats, there were fewer cells labeled with BrdU relative to vehicle–treated controls (mean ± s.e.m., vehicle = 3728 ± 412, STZ = 2070 ± 466.6; t9 = 2.66, p = 0.02). There was no change in the proportion of cells co–expressing BrdU and the neuronal markers NeuN (vehicle = 91.33 ± 2.81, STZ = 84 ± 5.21; t9 = 1.30, p = 0.23; Supplementary Fig. 4e) or Tuj1 (vehicle = 87 ± 2.40, STZ = 86.67 ± 1.33; t10 = 0.24, p = 0.81; Supplementary Fig. 4f). The percentage of BrdU–labeled cells co–expressing GFAP, a marker of astrocytes, was not altered in Type 1 diabetic rats (vehicle = 7.33 ± 1.91, STZ = 9.33 ± 1.33; t10 = 0.86, p = 0.41; Supplementary Fig. 4g). However, coincident with the reduction in BrdU–labeled cell number, these data indicate a net reduction in the number of new neurons and astrocytes for both insulin resistant mice and insulin deficient rats.
Effect of corticosterone manipulation on glucose and insulin levels in diabetic animals
To determine whether lowering corticosterone levels might prevent or alter the impact of experimental diabetes, we measured insulin and glucose levels in serum from diabetic and non–diabetic rodents that had been adrenalectomized or sham–operated. In both diabetes models, sham–operated diabetic animals exhibit corticosterone concentrations that are comparable to those reported in non–diabetic animals following an acute stressor (Table 1). In the Type 1 diabetes model, preventing the elevation of corticosterone did not alter STZ–induced hyperglycemia (Table 1). Effects were similar in serum samples from fed and fasted animals (fed glucose levels, F1,41 = 10.19, p = 0.002; fasting glucose levels, F1,13 = 90.36, p < 0.001; Table 1). Likewise, adrenalectomy and corticosterone replacement had no impact on the ability of STZ to reduce levels of insulin (F1,33 = 25.57, p < 0.001; Table 1). There was no long–term effect of STZ diabetes on feeding or body weight (Supplementary Table 1). Because we administered corticosterone replacement through the drinking water, it is important to note that despite the higher volume of solution consumed by adrenalectomized rats in both the STZ–treated and vehicle–treated conditions (F1,15 = 23.78, p < 0.001; Supplementary Table 1), these animals maintained serum corticosterone concentrations that were similar to sham–operated, non–diabetic animals (Table 1).
Table 1. Endocrine characteristics of Type 1 and Type 2 diabetes in rodents with different levels of corticosterone.
Data were analyzed using 2 × 2 ANOVA and asterisk (*) indicates significance at p < 0.05 relative to sham–operated non–diabetic controls. Values shown are means and s.e.m. Abbreviations: STZ = streptozocin; SHAM = sham–operated, ADX + CORT = adrenalectomized with 25 μg ml–1 corticosterone replacement.
| FASTING GLUCOSE (mg dL–1) | FED GLUCOSE (mg dL–1) | INSULIN (ng ml–1) | CORTICOSTERONE (ng ml–1) | ||
|---|---|---|---|---|---|
| wildtype | SHAM | 71.77 (8.63) | 140.65 (15.67) | 1.41 (0.15) | 46.16 (15.99) |
| ADX+CORT | 64.80 (1.87) | 95.95 (20.65) | 1.41 (0.14) | 18.85 (3.95) | |
| db/db | SHAM | 330.27 (21.01)* | 334.12 (41.09)* | 3.16 (0.68)* | 258.55 (43.11)* |
| ADX+CORT | 98.40 (17.51) | 328.88 (19.83)* | 1.03 (0.17) | 11.24 (2.21) | |
| Vehicle | SHAM | 30.19 (5.77) | 129.86 (21.72) | 2.07 (0.27) | 53.08 (17.72) |
| ADX+CORT | 44.74 (2.23) | 170.87 (11.74) | 1.73 (0.27) | 19.87 (4.56) | |
| STZ | SHAM | 290.96 (35.94)* | 318.46 (16.76)* | 0.79 (.09)* | 418.24 (18.26)* |
| ADX+CORT | 221.08 (14.80)* | 263.06 (11.29)* | 0.91 (0.16)* | 30.14 (5.78) |
db/db mice respond differently than STZ–treated rats to adrenalectomy and corticosterone replacement. In this model, adrenalectomy and corticosterone replacement reversed the increase in fasting glucose levels in db/db mice (F1,39 = 21.38, p < 0.001; Table 1). However, postprandial glucose levels in adrenalectomized db/db mice remained higher than those of non–diabetic controls (F1,33 = 47.46, p < 0.001; Table 1). Lowering corticosterone levels also attenuated hyperinsulinemia (F1,23 = 5.69, p = 0.03; Table 1). Both sham–operated and adrenalectomized db/db mice weighed more than wildtype mice, and consumed more food (animal weights, F1,31 = 32.43, p < 0.001; food intake, F1,48 = 51.90, p < 0.001, Supplementary Table 1). db/db mice characteristically exhibit polydipsia, and this was observed in sham–operated db/db mice, but not in db/db mice that had received adrenalectomy and corticosterone replacement (F1,42 = 14.77, p = 0.004; Supplementary Table 1).
To evaluate whether levels of insulin and glucose in the hippocampus were altered in diabetic animals, we measured their levels in whole–hippocampal homogenates from STZ–diabetic rats and db/db mice. We observed no effect of STZ diabetes on hippocampal glucose or insulin concentrations (glucose, mmol mg–1; vehicle = 23.34 ± 4.08, STZ = 24.24 ± 5.96, t10 = 0.12, p = 0.90; insulin, μmol mg–1; vehicle = 1.55 ± 0.15, STZ = 1.81 ± 0.25, t10 = 0.86, p = 0.42). Similarly, levels of glucose and insulin in the hippocampus of db/db mice were not different from wildtype mice (glucose, mmol mg–1; wildtype = 10.73 ± 1.85, db/db = 13.48 ± 1.16, t6 = 1.26, p = 0.25; insulin, μmol mg–1; wildtype = 1.26 ± 0.15, db/db = 1.60 ± 0.21, t6 = 1.32, p = 0.25). While these results do not preclude a change in the availability or sensitivity to glucose and/or insulin at the level of individual cells, they do provide indirect support for the idea that another factor, namely corticosterone, contributes to the impairment of hippocampal plasticity in diabetic animals.
Corticosterone mediates learning impairments in db/db mice
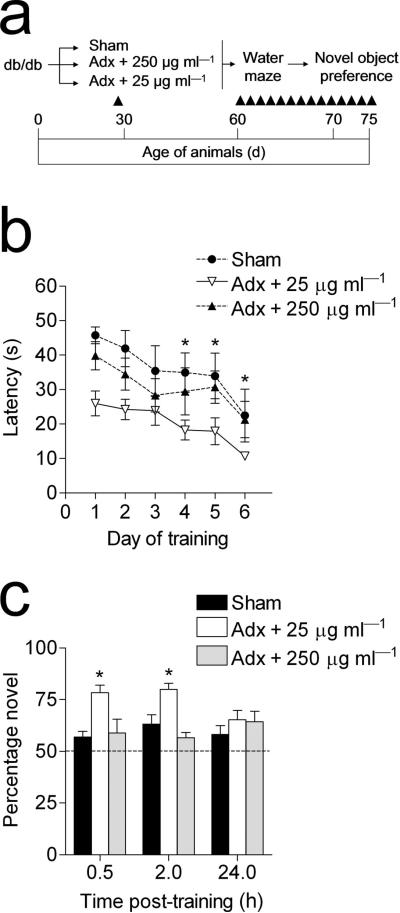
Because adrenalectomy removes not only endogenous corticosterone, but also the primary source of peripheral epinephrine, we performed an internal replication of our previous experimental design with an additional group of db/db mice that were adrenalectomized and given a high replacement dose of corticosterone via the drinking water (250 μg ml–1 in 0.9% saline; Fig. 7a). This regimen resulted in circulating corticosterone levels similar to those of sham–operated db/db mice (ng ml–1; mean ± s.e.m., 333.28 ± 47.38). These animals were tested in the Morris water maze and object recognition tasks. In support of our earlier result, db/db mice that had received adrenalectomy and 25 μg ml–1 corticosterone replacement learned the location of the hidden platform more rapidly than sham–operated db/db mice and adrenalectomized db/db mice receiving 250 μg ml–1 corticosterone replacement (F2,10 = 8.35, p < 0.001; Fig. 7b, Supplementary Fig. 5a). db/db mice that had been adrenalectomized and given low–dose corticosterone replacement also showed greater improvement over successive trials on day 1, but performance on the first trial was not different from sham–operated db/db mice, or db/db mice that had been adrenalectomized and given a higher dose of corticosterone (data not shown). There were no effects of any of the treatments on swim speed (Supplementary Fig. 5b).
Figure 7. A high replacement dose of corticosterone reinstates learning deficits in adrenalectomized db/db mice.
(a), Experimental design – db/db mice were sham–operated or adrenalectomized; adrenalectomized mice received corticosterone replacement at a dose of 25 or 250μg ml–1 in 0.9% saline. One month after surgery, the mice were tested in the Morris water maze and object recognition tasks. (b), Lowering corticosterone levels restores hippocampal learning in insulin resistant mice, while a high replacement dose of corticosterone was associated with learning impairments comparable to sham–operated diabetic mice. Differences in performance on the first day of training in adrenalectomized db/db mice receiving low–dose corticosterone replacement were due to improvements over successive trials, as no differences were observed during the first trial (see Results). (c), db/db mice that had been adrenalectomized and given a low replacement dose of corticosterone spent more time exploring the novel object, relative to sham–operated db/db mice, and to db/db mice administered a high dose of corticosterone. Asterisk (*) reflects significance at p<0.05 following one–way repeated measures ANOVA. Error bars = s.e.m.
Higher doses of corticosterone also reinstated deficits in object recognition memory. db/db mice that had been adrenalectomized and administered a low dose of corticosterone spent more time exploring the novel object than sham–operated db/db mice. In contrast, in adrenalectomized db/db mice that received a higher dose of corticosterone the reduction in novel object preference was identical to that seen in sham–operated db/db mice (F2,8 = 11.05, p = 0.03; Fig. 7c). Again, db/db mice that had been adrenalectomized and administered a low dose of corticosterone showed a preference for the novel object that was similar to non–diabetic animals.
Administration of a high dose of corticosterone had complex effects on the endocrine parameters of adrenalectomized db/db mice. These animals exhibit hyperglycemia, at a level similar to sham–operated db/db mice (mg dL–1; mean ± s.e.m., fasting = 339.30 ± 34.10; fed = 450.59 ± 46.04). Serum insulin levels were also elevated (ng ml–1; mean ± s.e.m., 1.98 ± 0.57). db/db mice receiving a high dose of corticosterone show increased water intake, similar to sham–operated db/db mice (ml d–1, mean ± s.e.m., 52.73 ± 7.24). However, their food intake and body weights were similar to those of wildtype animals (food intake, g d–1; 5.97 ± 0.65; body weight, g; 41.07 ± 4.34). These results suggest that elevated corticosterone levels contribute centrally to learning deficits, and peripherally to the endocrine characteristics of diabetes.
Discussion
Diabetes is associated with multiple adverse effects on the brain, some of which may result primarily from direct consequences of chronic hyperglycemia. However, our findings demonstrate a pivotal role for the adrenal steroid corticosterone as a mediator of diabetes–induced impairments in hippocampal synaptic plasticity and neurogenesis, and associated cognitive deficits. Lowering corticosterone levels prevented the diabetes–induced impairment of learning and memory in insulin deficient rats and insulin resistant mice. Maintaining normal physiological levels of corticosterone also restored LTP at perforant path–dentate gyrus synapses and prevented the impairment of adult neurogenesis in the dentate gyrus. The restorative effect of lowering corticosterone levels was observed when recordings were made under conditions that either permit or exclude the contribution of newly generated neurons. Enhancement of hippocampal function by normalizing corticosterone levels in diabetic animals was completely reversed by administration of high levels of corticosterone, demonstrating that corticosterone (rather than some other adrenal–derived factor) was responsible for the adverse effects of diabetes on hippocampal plasticity. These findings strongly support a role for elevated corticosterone levels in impaired hippocampal plasticity and cognition induced by diabetes.
It is well–established that chronic exposure to high levels of corticosterone is detrimental for learning and synaptic plasticity in non–diabetic animals24-29. The corticosterone–mediated adverse effects of diabetes were not determined by changes in insulin production, because they occurred in db/db mice with elevated insulin levels, and in insulin–deficient rats. We also observed no change in hippocampal insulin levels under baseline conditions in diabetic animals. However, this does not rule out the possibility that insulin signaling pathways might be impaired in diabetes. The effects of insulin on learning and memory oppose those of glucocorticoids at multiple levels. Specifically, intrahippocampal insulin37 or activation of insulin signaling pathways38 can block the effects of stress on learning and memory. Exposure to elevated corticosterone levels reduces insulin receptor signaling in multiple somatic tissues, including the brain39. Therefore, it is possible that the negative effect of diabetes on hippocampal plasticity may be attributable to an interaction between elevated glucocorticoids and insulin receptor signaling.
Local cerebral glucose utilization is tightly linked with neural activity and cognition. In contrast, glucocorticoids inhibit glucose utilization in neurons40. In normal (i.e. non–diabetic) animals, hippocampus–dependent learning is correlated with a decrease in extracellular glucose levels, and intrahippocampal injection of glucose improves performance41. No studies to date have reported an effect of diabetes on learning–induced changes in hippocampal glucose metabolism, but alterations in basal hippocampal glucose transporter expression have been demonstrated in diabetic animals42. While we observed no difference in glucose levels in whole hippocampal homogenates from insulin resistant mice or insulin deficient rats, our results do not preclude a role for corticosterone in modulating the diabetes–induced alterations in hippocampal glucose metabolism.
Lowering corticosterone levels in diabetes can restore behavioral function on tasks that recruit both new and mature neurons. While the Morris water maze task is not influenced by antimitotic treatment35, 43, newly generated neurons are activated following this task at a higher rate, relative to mature neurons44. Similar distinctions have been reported with respect to the role of adult–generated neurons in recognition memory; enhancement of performance on the novel object preference task following environmental enrichment was reversed by systemic treatment with an antimitotic45, but spontaneous alternation in the Y–maze, which also involves recognition memory, was unaffected following focal cranial irradiation35. Although it remains to be determined whether adult–generated granule neurons make a meaningful contribution to performance on these tasks under baseline conditions, the therapeutically relevant question is whether new neurons can enhance performance following neurodegeneration or injury.
The fact that lowering corticosterone levels had no effect on postprandial glucose levels in serum from db/db mice is in line with previous studies. Adrenalectomy and corticosterone replacement failed to normalize fed glucose values in the ob/ob mouse18. Similar results were observed following treatment with the glucocorticoid receptor antagonist RU486 in the Zucker (fa/fa) rat, with no effect of antiglucocorticoid treatment on fed glucose levels19. In contrast, treatment with antisense oligonucleotides directed against the glucocorticoid receptor restored normal fasting glucose levels in Zucker diabetic rats20. Taken together, these results suggest that inhibiting the actions of corticosterone by various methods will influence fasting, but not fed glucose levels in animal models of type 2 diabetes.
Studies of human subjects have provided evidence that diabetes adversely affects learning and memory, but also suggest that not all cognitive domains are equally affected. Diabetic humans exhibit accelerated decline on tasks that require episodic memory and rapid information processing, while attention and language abilities are unaffected2. Because episodic memory places a greater demand on temporal lobe structures, and language and attention primarily recruit other cortical and prefrontal regions, these data have been interpreted to suggest that the hippocampus is particularly susceptible to the negative consequences of diabetes. Other studies have begun to explore the role of cortisol in diabetes–induced cognitive deficits in humans. For example, inhibition of the enzyme 11–β–hydroxysteroid dehydrogenase 1 (11βHSD1), which locally modulates the actions of glucocorticoids in the brain by reactivating cortisol from its inactive form, was shown to ameliorate cognitive deficits in humans with Type 2 diabetes46. Overall, the task–specific cognitive impairments induced by diabetes, and the demonstration of improved cognitive performance in diabetic humans following treatments that alter the availability of cortisol, suggests that elevated cortisol levels in human diabetics may also be contributing to deficits in hippocampal function.
Supplementary Material
Note that we do NOT copy edit or otherwise change supplementary information, and minor (nonfactual) errors in these documents cannot be corrected after publication. Please submit document(s) exactly as you want them to appear, with all text, images, legends and references in the desired order, and check carefully for errors.
Acknowledgements
This research was supported by NIH NRSA Predoctoral fellowship F31AG024690–03 to A.M.S. through Princeton University, and by the Intramural Research Program of the National Institute on Aging. We thank Dan L. Longo for helpful suggestions, and Tamikia Lamb, Olga Carlson, Julissa S. Villareal, and Richard Telljohann for technical assistance. We are also grateful to Elizabeth Gould and Henriette van Praag for comments on the manuscript.
This work was supported by a NRSA predoctoral fellowship to A.S. and by the National Institute on Aging Intramural Research Program.
Appendix
Materials and Methods
Animals and Surgery
Animal care and experimental procedures followed NIH guidelines and were approved by the National Institute on Aging Animal Care and Use Committee. Adult male Sprague–Dawley rats were purchased from Charles River Laboratories and housed individually for a minimum of 2 weeks before the start of experiments. Streptozocin was administered via the femoral vein at a dose of 70 mg kg–1 as described16. In order to be included in the study, STZ–treated rats were required to exhibit serum glucose levels ≥ 200 mg dL–1. Male leptin receptor mutant (db/db) mice, bred on a C57BL/6 background, were purchased from Jackson Laboratories. Age–matched male C57BL/6 mice were used as controls. To determine whether glucocorticoids are involved in the neurological consequences of diabetes, rats and mice were subjected to bilateral adrenalectomy or sham operation. Adrenalectomized animals received corticosterone replacement (25 μg ml–1 or 250 μg ml–1 in 0.9% saline; Sigma) in the drinking water to manipulate glucocorticoid levels30. Corticosterone replacement was available to the animals immediately following surgery. Mice were adrenalectomized at postnatal day 30; rats were adrenalectomized at postnatal day 90. All rats and mice were administered a single injection of the DNA synthetic marker bromodeoxyuridine (300 mg kg–1; n=6–8 animals per group). This dose was based on previous studies47 demonstrating maximal labeling at 300 mg kg–1. Animals were euthanized 2 hours or 3 weeks post–BrdU. In a separate experiment, two–month–old wildtype mice were implanted with Alzet minipumps to deliver the antimitotic drug AraC into the right lateral ventricle (2.2 mg ml–1, 0.25 μl hour–1, pump model 1002; bregma coordinates AP –0.3 mm, ML –1.0mm). These animals were injected once with BrdU (300 mg kg–1; n=6–8 animals per group) and euthanized 24 hours later. All animals had ad libitum access to food and water, and the room was maintained on a 12 hour light–dark schedule (lights on at 06:00). For some experiments, the animals were weighed once weekly, and their food and water were weighed on two successive days per week for 4 weeks. Food consumption was measured in grams per day, and water bottle weights were converted to volumes. The techniques for quantifying levels of glucose, insulin, and corticosterone are described in Supplementary Methods.
Electrophysiology and Behavioral testing
The procedures used for slice preparation and recording are available in Supplementary Methods. Procedures for water maze training and novel object preference testing are also included in Supplementary Methods.
Immunohistochemistry and Microscopy
Immunolabeling for BrdU and Ki67 was carried out as described15. Full description of the methods for brightfield and fluorescence tissue labeling are available in Supplementary Methods. We quantified single- and double-labeled cells using standard protocols15. Detailed cell counting criteria are available in Supplementary Methods. We also quantified the optical intensity of fluorescence staining for synaptophysin48; full description available in Supplementary Methods.
Statistics
Statistical analyses were made using SPSS version 11.0 (Chicago, Illinois, USA), with significance set at (p < 0.05); graphs were generated using Graphpad Prism 4 software (San Diego, CA). Cell counts, hormone profiles, feeding, drinking, animal weights, and the amount of LTP were compared using separate 2 × 2 ANOVA designs (diabetes × surgery). Behavioral data from the Morris water maze and novel object preference task were analyzed using 2 × 2 repeated measures ANOVA. The number of BrdU–labeled cells at 3 weeks post–injection was compared across diabetic and non–diabetic animals using bidirectional, unpaired t–tests. Percentages of cells co–expressing BrdU and a cell type–specific marker were also analyzed using t–tests. In experiments where we administered a high dose of corticosterone to adrenalectomized db/db mice, behavioral data from the Morris water maze and novel object preference tasks were analyzed using one–way repeated measures ANOVA with Tukey's post hoc.
Footnotes
Supplementary Information accompanies this paper.
Competing interests statement. The authors declare that they have no competing financial interests.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 2.Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobol Aging. 2005;26(Suppl 1):S26–S30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood CE, Winocur G. High–fat diets, insulin resistance and declining cognitive function. Neurobiol. Aging. 2005;26(Suppl 1):42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child. Neuropsychol. 2004;10:36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, et al. Place learning and hippocampal synaptic plasticity in streptozotocin–induced diabetic rats. Diabetes. 1996;45:1259–1266. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, et al. Water maze learning and hippocampal synaptic plasticity in streptozotocin–diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 7.Li XL, et al. Impairment of long–term potentiation and spatial memory in leptin receptor–deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 8.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1996;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 9.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 10.Kamal A, Biessels GJ, Urban IJ, Gispen WH. Hippocampal synaptic plasticity in streptozotocin–diabetic rats: impairment of long–term potentiation and facilitation of long–term depression. Neuroscience. 1999;90:737–745. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin–treated diabetic rats. Acta Neurol Scand. 2007 doi: 10.1111/j.1600-0404.2007.00928.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontan-Lozano A, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Duan W, Mattson MP. Evidence that brain–derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 15.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan O, et al. Hyperglycemia does not increase basal hypothalamo–pituitary–adrenal activity in diabetes but it does impair the HPA response to insulin–induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2005;289:R235–246. doi: 10.1152/ajpregu.00674.2004. [DOI] [PubMed] [Google Scholar]
- 18.Tokuyama K, Himms–Hagen J. Increased sensitivity of the genetically obese mouse to corticosterone. Am J Physiol. 1987;252:202–208. doi: 10.1152/ajpendo.1987.252.2.E202. [DOI] [PubMed] [Google Scholar]
- 19.Langley SC, York DA. Effects of antiglucocorticoid RU486 on development of obesity in obese fa/fa Zucker rats. Am J Physiol. 1990;259:539–544. doi: 10.1152/ajpregu.1990.259.3.R539. [DOI] [PubMed] [Google Scholar]
- 20.Watts LM, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54:1846–1853. doi: 10.2337/diabetes.54.6.1846. [DOI] [PubMed] [Google Scholar]
- 21.Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- 22.MacLullich AM, et al. Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology. 2005;30:505–515. doi: 10.1016/j.psyneuen.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Elgh E, et al. Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer's disease. Biol. Psychiatry. 2006;59:155–161. doi: 10.1016/j.biopsych.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur. J. Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 25.Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress–induced impairments in spatial memory. Eur. J. Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long–term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur. J. Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerr DS, Campbell LW, Hao SY, Landfield PW. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989;245:1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- 28.Korz V, Frey JU. Stress–related modulation of hippocampal long–term potentiation in rats: Involvement of adrenal steroid receptors. J. Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long–term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 30.Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montaron MF, et al. Lifelong corticosterone level determines age–related decline in neurogenesis and memory. Neurobiol. Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 33.Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- 34.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 37.Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. Insulin protects against stress–induced impairments in water maze performance. Behav. Brain Res. 2007;176:230–236. doi: 10.1016/j.bbr.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Revest JM, et al. The MAPK pathway and Egr–1 mediate stress–related behavioral effects of glucocorticoids. Nat. Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 39.Piroli GG, et al. Corticosterone impairs insulin–stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- 40.Sapolsky RM. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J. Neurosci. 1986;6:2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reagan LP, et al. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2820–2825. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal–dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult–generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 45.Bruel–Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long–term memory following environmental enrichment. Eur. J. Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 46.Sandeep, et al. 11Beta–hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc Natl Acad Sci U S A. 2005;101:6734–9. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 48.Kozorovitskiy Y, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note that we do NOT copy edit or otherwise change supplementary information, and minor (nonfactual) errors in these documents cannot be corrected after publication. Please submit document(s) exactly as you want them to appear, with all text, images, legends and references in the desired order, and check carefully for errors.