Abstract
Smooth pursuit eye movements allow the approximate stabilization of a moving visual target on the retina. To study the dynamics of smooth pursuit, we measured eye velocity during the visual tracking of a Gabor target moving at a constant velocity plus a noisy perturbation term. The optimal linear filter linking fluctuations in target velocity to evoked fluctuations in eye velocity was computed. These filters predicted eye velocity to novel stimuli in the 0- to 15-Hz band with good accuracy, showing that pursuit maintenance is approximately linear under these conditions. The shape of the filters were indicative of fast dynamics, with pure delays of merely ∼67 ms, times-to-peak of ∼115 ms, and effective integration times of ∼45 ms. The gain of the system, reflected in the amplitude of the filters, was inversely proportional to the size of the velocity fluctuations and independent of the target mean speed. A modest slow-down of the dynamics was observed as the contrast of the target decreased. Finally, the temporal filters recovered during fixation and pursuit were similar in shape, supporting the notion that they might share a common underlying circuitry. These findings show that the visual tracking of moving objects by the human eye includes a reflexive-like pathway with high contrast sensitivity and fast dynamics.
INTRODUCTION
Smooth pursuit eye movements allow a moving visual target to be stabilized on the retina. The system is configured as a negative feedback loop: retinal slip velocity (the error signal) is the difference between the desired target velocity and actual eye velocity (Keller and Heinen 1991; Lisberger et al. 1987; Robinson et al. 1986; Thier and Ilg 2005). A useful strategy in studying smooth pursuit consists in measuring how motion signals are integrated in space and time to drive eye movements and comparing the results to the integration properties of neurons selective to visual motion in the brain (Born and Pack 2002; Born et al. 2006; Heinen and Watamaniuk 1998; Masson 2004; Masson et al. 2000; Osborne and Lisberger 2007; Osborne et al. 2005, 2007; Schoppik et al. 2008; Spering and Gegenfurtner 2007). In particular, an important feature of this system is the pure delay between the onset of retinal image motion and the onset of smooth pursuit eye movements, which plays a key role in establishing limits on stability and gain of pursuit (Goldreich et al. 1992; Krauzlis and Lisberger 1994; Lisberger et al. 1987; Ringach 1995; Robinson et al. 1986).
One way in which the delay between the onset of image motion and the onset of eye motion has been estimated in previous studies is by means of the step-ramp paradigm, where a fixated visual target jumps in one direction and immediately starts moving in the opposite direction at a constant velocity (Rashbass 1961; Robinson 1965). For an appropriate size of the initial step, this stimulus evokes smooth pursuit initiation without the occurrence of early saccades, facilitating the estimation of latency (Carl and Gellman 1987; Rashbass 1961; Robinson 1965). Using a variety of visual targets, pure delays during the initiation of smooth pursuit in human subjects are found to be broadly distributed between 100 and 300 ms (Braun et al. 2008; Knox 1998; Lindner and Ilg 2000; Rashbass 1961; Robinson 1965; Spering et al. 2005). The shortest latencies of pursuit initiation have been obtained in a “gap-paradigm,” where the fixation is extinguished 100–200 ms before the onset of the target, yielding delays of ∼90 ms (Krauzlis and Miles 1996b).
Mathematical analysis and numerical simulations have shown that, if such long and variable latencies were to apply during the maintenance phase of pursuit, they could create a grave problem, because achieving gains near one would also make the system unstable and prone to oscillations (Goldreich et al. 1992; Krauzlis and Lisberger 1994; Ringach 1995; Robinson et al. 1986). Notably, measurements of pursuit latency during its maintenance phase tend to be on the low end of the distribution of values obtained for pursuit initiation. Indeed, delays of ∼93 ms were reported in response to step increases in target velocity during pursuit (Behrens et al. 1985); around ∼110 ms in response to brief, biphasic perturbations of target velocity (a single cycle of a sinusoidal function) (Churchland and Lisberger 2002); and ∼85 ms in response to a positional error induced by a briefly flashed visual stimulus near the target (Blohm et al. 2005). Thus it seems plausible that the delays during pursuit maintenance might actually be considerably smaller than those inferred from the initiation of the movement. One aim of this study was to re-examine the measurement of latencies during pursuit initiation and maintenance in the same subjects and the same visual conditions.
Another important goal of our experiments was to measure the detailed dynamics of pursuit maintenance and assess its linearity. We approached this problem by having subjects track a small Gabor target moving at a constant velocity plus a white-noise perturbation signal (Fig. 1). From these data, we computed the optimal linear filter that linked fluctuations in the velocity of the target to the evoked fluctuations in eye velocity (Mulligan 2002; Osborne and Lisberger 2007). By analyzing the shape of the resulting temporal filters, we were able to estimate not only pure delays but also the time-to-peak of the responses and the integration time of the system. Importantly, once the optimal linear filter was recovered, we were able to test the linearity of pursuit maintenance by predicting the mean eye velocity in response to novel stimuli and assessing its goodness-of-fit to the measured responses.
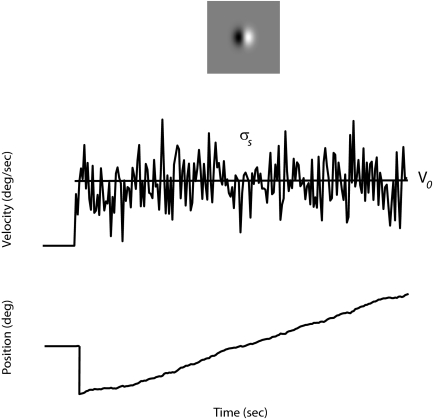
FIG. 1.
Experimental design. A small Gabor target (top), jumps horizontally to the left or right and moves in the opposite direction with a mean velocity V0. Riding on top of this mean velocity, there is a discrete-time, Gaussian white-noise signal, with an SD σs (middle). The resulting position of the target over time, obtained by integration of the velocity signal, is shown at the bottom. Eye velocity during smooth pursuit segments in response to such stochastic motion trials were isolated and analyzed. The best linear filter linking the stimulus and eye velocity fluctuations about their means estimated by cross-correlating the 2 signals.
To anticipate the results, we found that pursuit maintenance is surprisingly fast and well-described as a linear system. Pure delays estimated by white-noise analysis were noticeably smaller than those obtained for pursuit initiation in a step-ramp paradigm or in response to brief biphasic perturbations of the target velocity during pursuit. The gain of the system was largely determined by the SD of the target velocity fluctuations, decreasing as the size of the fluctuations increased. Interestingly, the linear filters obtained during pursuit and fixation were similar in shape to each other, supporting the hypothesis they might share a common mechanism of gaze stabilization (Krauzlis 2004). These findings indicate that the visual tracking of moving objects by the human eye includes a reflexive-like pathway with high contrast sensitivity and fast dynamics.
METHODS
Stimulus generation and presentation
Observers were seated in a dimly illuminated room facing a Viewsonic G225f monitor with a refresh rate of 120 Hz at a viewing distance of 100 cm. The active screen area subtended 22° of visual angle in the horizontal direction and 15° of visual angle in the vertical direction, and the screen resolution was 1,024 × 768 pixels. The screen was viewed binocularly, but only the movements of the right eye were monitored. The subject's head was stabilized by a chin rest and a support for the forehead. Visual stimuli were generated using a Cambridge Research Visage system under the control of Matlab. The visual target consisted of a Gabor patch constructed with a vertically oriented sinusoidal grating with a spatial frequency of 1 cycle/° as the carrier, multiplied by a Gaussian window with an SD of 0.2° (Gegenfurtner et al. 2003). The Gabor was antisymmetric, and the phase was randomly changed between 0 and 180° from trial to trial. Each trial started with the subject fixating a Gabor patch located at the center of the screen. After a 0.5-s delay, the target would jump to ±5° along the horizontal meridian and start moving in the opposite direction. The direction of drift was randomized from trial to trial. As our analysis depends on the collection of sufficiently large steady-state pursuit segments, we maximized the duration of each trial by using a large initial jump. The Gabor patch moved rigidly with a position given by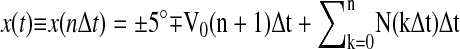 . Here, n = 0, 1,…represents the nth frame in the stimulus sequence, Δt is the frame duration (in our case, 1/120 Hz or 8.33 ms), t = nΔt represents time from the onset of the motion, V0 represents the mean velocity of the stimulus, and N(kΔt) represents a Gaussian perturbation drawn independently for each frame with an SD of σs.
. Here, n = 0, 1,…represents the nth frame in the stimulus sequence, Δt is the frame duration (in our case, 1/120 Hz or 8.33 ms), t = nΔt represents time from the onset of the motion, V0 represents the mean velocity of the stimulus, and N(kΔt) represents a Gaussian perturbation drawn independently for each frame with an SD of σs.
We collected data at baseline velocities of V0 = 2, 4, 8, and 16°/s and perturbation SD of σs = αV0, where the SD factor α = 0.125, 0.25, 0.5, and 1. We also collected data for a “fixation” condition where V0 = 0 and σs = 0.5, 1, 2, 4, 8, and 16°/s.
To compare the smooth pursuit latencies estimated during pursuit maintenance with those obtained in pursuit initiation, we ran sessions of step-ramp trials. For these trials, the initial jump was of ±0.5°, and the stimulus was moving at a constant velocity of 4°/s without any velocity noise. We estimated the latency in each individual trial as the intersection of two regression lines of the eye position trace (sampled at 1 kHz): one to the baseline before the onset of movement and another to the trace during the tracking (Carl and Gellman 1987). We also tried a method based on the velocity signal, computed by differentiation of the position signal and filtering by a Hamming window of length 81 samples. The intersection of the regression of the rising phase of the velocity signal with zero provided an estimate of the latency in each trial (Krauzlis and Miles 1996a). This second method resulted in a distribution of latencies that was not statistically different from the one based on position (data not shown).
We also measured eye velocity responses to brief temporal perturbations of the target velocity consisting of a single cycle of a sine wave during tracking (Churchland and Lisberger 2002; Schwartz and Lisberger 1994). The perturbation cycle had a frequency of 5 Hz, amplitude of 2.83°/s, and a mean velocity of 4°/s. The amplitude value was chosen to match an SD of 2°/s for the perturbation signal. In 25% of the trials, the velocity initially increased during the perturbation (peak-first condition), in another 25% of the trials, the velocity initially decreased (peak-last), and in the remaining 50% of the trials, there was no perturbation (no perturbation condition). For each subject, a total of 400 trials were measured with these conditions randomly interleaved.
Observers
The two authors [males, age 30 (A.T.) and 43 yr (D.R.)], both trained psychophysical observers with normal (A.T.) and corrected to normal vision (D.R.), participated in all the experiments. Four naïve subjects [males, age 46 (Z.L.), 20 (P.L.), 30 (J.F.), and 70 yr (D.T.)], with normal vision but no prior psychophysical experience, were tested on a subset of experiments to verify the consistency of the main findings across experienced and naïve subjects. All the experiments were conducted with the approval of the Chancellor's Office for the Protection of Research Subjects at UCLA, with subjects providing their informed consent.
Eye movement recording and processing
Eye movements were recorded by an SR-Research Eyelink-II system at a sampling frequency of 1 kHz. Each session consisted of 100 pursuit trials, with nine-point calibration stimuli interspersed every 25 trials. Each trial began with the Gabor patch centered on the screen and the subject indicating his readiness by pressing a button. Eye position data were sampled at 1 kHz and saved on disk for later processing. These traces were subsequently differentiated and subsampled at 120 Hz using Matlab's resample function. This brought both eye and target velocity signals to a common sampling frequency of 120 Hz. Saccades were automatically detected by thresholding the velocity trace at 4 SD of the average eye velocity fluctuations. Data segments from within 100 ms of detected saccades (either before or after) were excluded from analysis, as was the initiation of pursuit. Different saccade detection algorithms were tried, leading to similar results. Thus our findings were not sensitive to this preprocessing step.
The optimal linear filter was computed by the cross-correlation of the velocity perturbation signal, N(nΔt), and the eye velocity signal, Veye(nΔt), at time lags corresponding to integer number of stimulus frames. The result, normalized by the variance of the target velocity fluctuations, results in the estimated impulse response of the system, h(nΔt) (Mulligan 2002). The data points in Fig. 2 A show the estimated correlation values at these intervals.
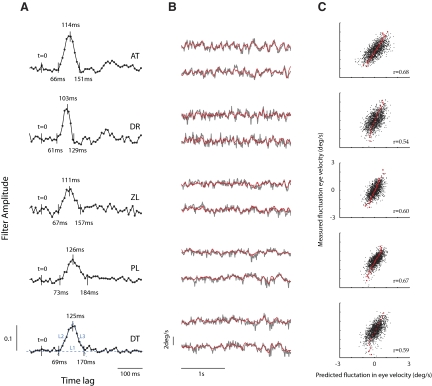
FIG. 2.
A linear model provides a reasonable description of pursuit maintenance. A: optimal temporal filters estimated in 1 stimulus condition (a baseline velocity of 4°/s and a Gaussian perturbation noise with an SD of 2°/s) for 5 subjects. The pure delay, time-to-peak, and full-width at half-height were estimated from the linear regression to the slopes of the dominant peak and the baseline (blue lines in bottom graph). B: predicted vs. measured velocity traces. Gray lines show the mean eye velocity in response to pursuit of the same perturbation noise (average of 20 trials). Red curves show the predicted response obtained by convolving the impulse response with the stimulus noise. The impulse response was measured from a different set of noise trials. C: scatterplot of measured vs. predicted eye velocity fluctuations show no obvious nonlinearity (such as saturation of response). Red points show the average eye velocity for different windows of predicted eye velocity. Correlation coefficients between predicted and measured responses are in the inset.
An important technical point must be included here regarding the stimulus and data timing. Note that as defined above, N(kΔt) represents the instantaneous velocity between the k − 1-th and kth frame in the stimulus sequence. However, our eye data collection was triggered with the vertical sync pulse of the monitor before the presentation of the first frame in the stimulus sequence. Because the stimulus is shown approximately half a frame later (because it appears in the middle of the screen), these two offsets cancel each other out, so there is no need for a correction of the timing.
Because of the surprisingly short delays observed in our data, we took extreme caution to ensure that the timing of the signals was accurate. Both the eye movement data and the TTL signal from the Visage system were sampled and time-stamped by the Eyelink-II board, thus preventing any issues arising from clock synchronization across machines. The appearance of the TTL signal at the VSYNC preceding the first stimulus frame was verified by monitoring the TTL signal and the output of a photocell pointing at the center of the screen with an oscilloscope during the flashed presentation of a bright stimulus on the first frame of a stimulus sequence.
Analysis of the shape of the linear filters
From each measured impulse response, we estimated its latency, time-to-peak, and half-width at half-height (or effective width) as follows. First, we calculated the mean baseline response at times t < 0. This provides one line in the graph we define as L1. Second, we found the sample points that were closest to the half-maximum value of the kernel on both sides of the peak. These samples, along with four of their immediate neighbors (2 on each side), were used to compute two additional regression lines: L2, fitting the rising phase of the response; L3, fitting the falling phase of the response. The intersection between L1 and L2 defined our estimate of latency. The intersection between L1 and L3 defined our estimate of decay (Fig. 2A, blue dashed lines in the bottom panel for observer D.T.). The difference between decay and latency times divided by two provides an estimate of full-width at half-height.
Linear predictions
We selected a set of five noise patterns to be used repeatedly in different trials. These five perturbation patterns were presented 20 times each, in different directions, in a 100-block trial. The mean eye velocity in response to each perturbation was computed. The impulse response estimated from a different set of noise trials was used to predict this response by convolving it with the actual perturbations. The data shown in Fig. 2 were obtained at V0 = 4°/s and σs = 2°/s. The coherence between predicted and measured signals was computed using Matlab's cohere function. For the prediction of responses to the sine-wave perturbation (Fig. 5), we simply convolved the waveform with the impulse response estimated for V0 = 4°/s and σs = 2°/s, because these have matching values of mean velocity and SD.
FIG. 5.
Responses and linear predictions to brief sinusoidal perturbations. A single-cycle sinusoidal perturbation was introduced during the tracking of a Gabor target (top), as previously done by Chruchland and Lisberger (2002). The traces show the mean response to each of the stimuli in 5 subjects. The red line represents the linear prediction of the response obtained by convolving the optimal filter for each subject (obtained independently from white-noise trials) with the sinusoidal perturbation. The correlation coefficient between the predicted and measured responses is shown in the inset.
RESULTS
Examples of the optimal linear filters recovered during pursuit of a target with mean velocity V0 = 4°/s and perturbation size of σs = 2°/s are shown in Fig. 2A. The filters had an early dominant peak followed, in two experienced subjects (subjects A.T. and D.R.), by weaker ringing at later times. Naïve subjects (Z.L., P.L., and D.T.) showed somewhat broader peaks and no obvious ringing. We concentrate here on the dominant peak, which can be characterized by its latency, time-to-peak, and full-width at half-height (or effective width). The delays and time-to-peaks were reasonably consistent across subjects, with delays of ∼67 ms and times-to-peak of ∼115 ms (Fig. 2A). Assuming linearity, the temporal filter represents the expected response to a brief velocity pulse of unit area and therefore is sometimes referred to as the impulse response of the system.
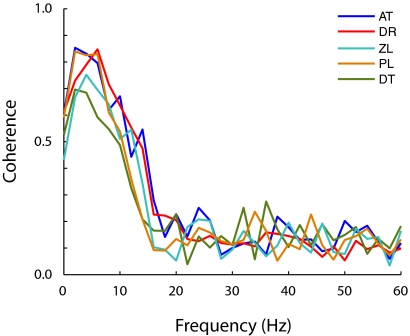
To assess the ability of these filters to account for eye velocity responses during pursuit, we measured the mean response to the repeated presentation of the same noise perturbation (total of 5 noise patterns repeated 20 times each in random order and drift direction) and compared it with the prediction generated by convolving the stimulus with the impulse response. We found that predicted and measured velocity traces matched very well (Fig. 2B, gray lines represent measured responses and red lines represent the predictions). We observed no obvious nonlinear relationships (such as compressive nonlinearities) between the predicted and measured responses (Fig. 2C), and the average correlation coefficient between the signals was 0.62 ± 0.06 (SD). Note that the predictions seem to account better for the slower temporal components in the response (Fig. 2B). This is made evident in the shape of the empirical coherence between the predicted and measured responses (Fig. 3), which peaks at ∼5 Hz, reaching values as high as 0.85, and it is significant up to frequencies of 15 Hz, confirming the ability of the pursuit system to follow high temporal frequencies (Goldreich et al. 1992).
FIG. 3.
Coherence between linear predictions and measured responses. The graph shows that linear predictions account for a substantial amount of the response variance within the 0- to 15-Hz band in 5 subjects.
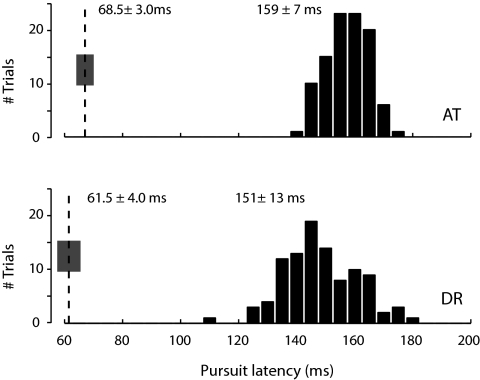
We estimated linear filters for various combinations of mean velocity and perturbation size in two subjects. Across all these conditions, pursuit dynamics were consistently fast, with a delay of ∼65 ms (68.5 ± 3.0 ms for A.T. and 61.5 ± 4.0 ms for D.R.), a peak response of ∼110 ms (110.4 ± 4.4 ms for A.T. and 99.2 ± 4.5 ms for D.R.), and a full-width at half height of ∼40 ms (42.1 ± 2.8 ms for A.T. and 37.1 ± 3.5 ms for D.R.). Notably, when latencies were measured during the initiation phase of pursuit using a step-ramp paradigm under the same experimental conditions and in the same subjects, the outcome was a distribution of significantly longer latencies, averaging 155 ms (Fig. 4; 1-sided t-test, P < 10−10). This shows that estimating pure delays during the initiation and maintenance of pursuit yields different values, even when performed on the same subjects and with identical visual targets.
FIG. 4.
Pure delays estimated during pursuit maintenance differ greatly from those obtained during the initiation of pursuit. The histograms show the distribution of pursuit latencies obtained in a step-ramp experiment using the method of Carl and Gellman (1987). The vertical dashed lines on the left show the mean latencies of the impulse response estimated for different combinations of baseline speed and noise SD during pursuit maintenance. The gray boxes indicate ±SD of the latency estimate.
A previous study estimated the latency pursuit to brief velocity perturbations during tracking at ∼110 ms (Churchland and Lisberger 2002). Because this value is higher than the ones estimated by our technique, we replicated their paradigm to gain insight into the reasons behind the discrepancy. The mean responses to one sinusoidal cycle perturbing the velocity of a target moving at a baseline speed of 4°/s is shown in Fig. 5. For each of the five subjects, we measured the average eye fluctuations evoked by a perturbation of a single sinusoidal cycle with its peak coming first or last (Fig. 5, top) and to a control condition where the perturbation was absent. The amplitude of the sinusoid was 2.83°/s, so that its effective SD was σs = 2.83/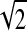 = 2 °/sec. The red lines show the predicted eye fluctuations obtained by convolving the stimulus waveform with the optimal filter estimated independently from our white-noise experiments with V0 = 4°/s and σs = 2°/s. The predictions provide a good fit to the responses, accounting for an average 60% of the variance in the traces. Two curious trends can be observed in these data that seem to indicate systematic departures from linearity. First, there is a tendency for the predictions to peak-first stimuli to be better than peak-last. Second, the initial phase of the responses appear to be better fit than the later phase. Despite these differences, the fact that one can predict >60% of the total response variance to such stimuli by linear filters obtained from completely different measurements is remarkable.
= 2 °/sec. The red lines show the predicted eye fluctuations obtained by convolving the stimulus waveform with the optimal filter estimated independently from our white-noise experiments with V0 = 4°/s and σs = 2°/s. The predictions provide a good fit to the responses, accounting for an average 60% of the variance in the traces. Two curious trends can be observed in these data that seem to indicate systematic departures from linearity. First, there is a tendency for the predictions to peak-first stimuli to be better than peak-last. Second, the initial phase of the responses appear to be better fit than the later phase. Despite these differences, the fact that one can predict >60% of the total response variance to such stimuli by linear filters obtained from completely different measurements is remarkable.
The estimated delays in these experiments (indicated by short vertical line segments in each trace) ranged between 89 and 115 ms. These values are in fact closer to those obtained by Churchland and Lisberger (2002) but are still higher than the ones obtained by the analysis of the optimal linear filters.
How could the linear prediction (which is based on the optimal linear filters) be reasonably accurate while the resulting delay estimates be clearly higher? The reason is that the sinusoidal shape of the perturbation makes the stimulus ramp-up slowly at what is considered to be the onset of the perturbation. This causes any initial response to be small and susceptible to masking by noise, leading to a consequent overestimation of the latency. The fact that our linear predictions accurately predict the responses to the sinusoidal perturbations shows a self-consistency between these two datasets and provides yet another measure of the linearity of the system.
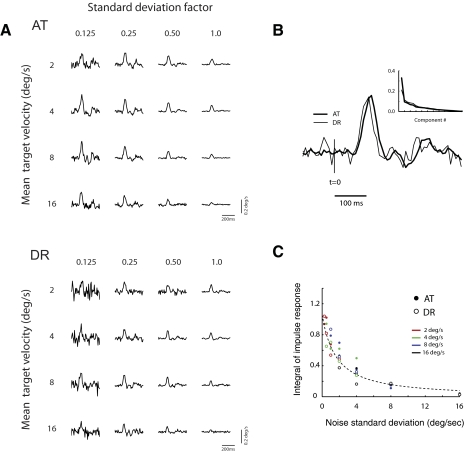
Next, we analyzed the family of filters obtained by running the experiment with different combinations of the baseline velocity, V0, and perturbation size,σs. We ran all combinations of baseline velocities of V0 = 2, 4, 8 and 16°/s and noise SD of σs = αV0, where α = 0.125, 0.25, 0.5, and 1. The resulting filters for all these conditions in two subjects are shown in Fig. 6 A. To analyze the variability in filter shapes across all conditions, we calculated the singular-value decomposition and found the spectrum to be dominated by the first eigenvalue (Fig. 6B, inset). This means that the entire set of filters can be reasonably approximated by the scaling of a single profile (as shown in Fig. 6B). When the gain of the filter was plotted against the SD of the noise, all points fell onto a single curve (Fig. 6C), meaning that gain was largely determined by the size of the velocity fluctuations. The curve shows an inverse relationship between the size of the fluctuations and the gain of the filter.
FIG. 6.
Estimates of the impulse response under different experimental conditions. A: estimates of temporal filters obtained for a number of baseline velocities and noise SD factors. B: a singular value decomposition of the family of filters shows that they can be reasonably described as the scaling of a single shape. Inset: the spectrum of a singular value decomposition of the family of impulse responses. A single dominant eigenvalue is evident. The graph shows the 1st principal component for the 2 subjects. C: the gain of the impulse response, represented here by the integral of the impulse response in each case, is largely determined by the SD of the target fluctuations. The dashed line corresponds to a fit of the empirical curve A/[1 + (σstim/σ0)α] to the data of both subjects. Estimated parameters have values σ0 = 1.92°/s, α = 1.16, and A = 1.05°/s. The value σ0 = 1.92°/s can be interpreted as an estimate of intrinsic noise at the input to visual motion detectors. Points are color coded according to the mean target velocity.
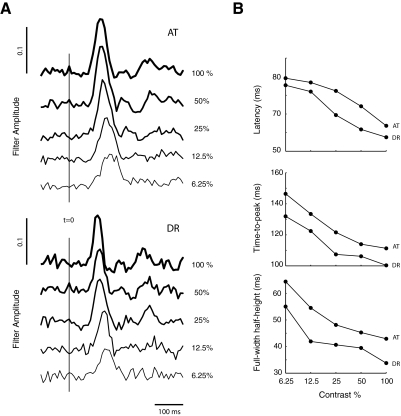
To obtain some information about the likely visual pathways involved in driving these fast responses, we asked how the optimal filter depended on the contrast of the visual target (Spering et al. 2005). Consistent with prior reports, we found an increase in the latency, time-to-peak, and effective width increase with decreasing target contrast, implying a slowing down of the dynamics as the stimulus weakened (Fig. 7A) (Mulligan 2002; Spering et al. 2005). Nevertheless, even for the smallest contrast tested, the latencies never went above 80 ms, and effective widths never exceeded 65 ms (Fig. 7B).
FIG. 7.
Dependence of the optimal linear filter on the contrast of the visual target. A: temporal filters estimated at different contrast levels for 2 subjects. The dynamics of pursuit are slowed down as the contrast of stimulus decreases. The panels show the impulse response measured at different contrast levels for a baseline velocity of 4°/s and a Gaussian perturbation noise with an SD of 2°/s. B: dependence of latency, time-to-peak, and effective width as a function of target contrast.
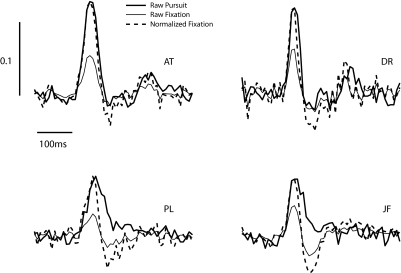
Finally, to test the hypothesis that fixation and pursuit may share similar underlying mechanisms of gaze stabilization, we compared the shape of the optimal filters obtained during steady-state pursuit and in a “fixation” condition, where the baseline velocity of the target was zero. The amplitude of the optimal temporal filters in the fixation condition were smaller than those in the pursuit condition, (Fig. 8, solid curves). To compare the shape of the filters, we normalized the fixation filter to have the same maximal amplitude as that of the pursuit filter (Fig. 8, dashed curves). It can be seen that the pure delay and time-to-peak of the filters are nearly identical. In the later phase of the response, however, the optimal filters for the pursuit condition tended to show a more biphasic response. This is particularly evident in the naïve subjects (Fig. 8, subjects P.L. and J.F.). One possible reason for the observed differences may be the effect of practice in the task, which we are now exploring. Nevertheless, the nearly perfect agreement of the filters in the initial phase of the response seems consistent with the notion that gaze stabilization during pursuit and fixation share a common underlying circuitry.
FIG. 8.
Gaze stabilization during pursuit and fixation have similar dynamics. The raw filters (solid curves) show that the gain of gaze stabilization during a fixation condition (a baseline velocity of 0) is smaller than that obtained during pursuit. However, the shapes of the filters in the 2 conditions are similar in the early phase of the response, as shown by the normalized responses (dashed lines). In the late phase, the optimal filters of some subjects show a more biphasic responses.
DISCUSSION
A psychophysical reverse correlation method was used to recover the temporal filter that best linked fluctuations in target velocity to fluctuations in eye velocity during steady-state pursuit movements (Mulligan 2002; Osborne and Lisberger 2007). The use of discrete-time Gaussian white noise as the perturbation term offers three main advantages. First, the target velocity fluctuations are unpredictable, thereby ruling out a contribution of top-down predictive mechanisms of target trajectories to our measurements (Barnes and Asselman 1991; Bennett and Barnes 2004; Churchland et al. 2003; Deno et al. 1995; Fukushima 2003; Jarrett and Barnes 2001; Kowler 1989; van den Berg 1988). Second, the method is very efficient and filters with good signal-to-noise were obtained with few (∼100) pursuit trials. This is because every segment of steady-state pursuit contributes data that goes into the computation of the filter. Third, the estimation of the linear filter can be performed by simple cross-correlation of the target and eye velocity signals.
In an earlier study, Mulligan (2002) had a subject track the two-dimensional random walk of a Gaussian luminance spot and estimated optimal filters. The resulting temporal filters were strongly biphasic and clearly different from that the ones we obtained in our pursuit condition. It is likely that the pursuit of a target performing a two-dimensional random walk is closer to our fixation condition, for which we observed biphasic filters in some of the subjects (Fig. 8). It is also possible that the shape of the filters obtained by Mulligan (2002) included the temporal filtering used to compute the eye velocity signal (which, unfortunately, is not described in detail in his study).
We assessed the linearity of the system by generating predictions to new noise patterns (Fig. 2) and to single-cycle, sinusoidal perturbations (Fig. 4). The predictions were reasonable, accounting for an average 60% of the variance in the eye velocity traces, indicating that smooth pursuit is approximately linear under the conditions tested. Departures from linearity have previously been reported for perturbations with large amplitudes (∼10–20°/s), but these differences diminished as the size of the perturbations decreased (Churchland and Lisberger 2002). Our tests of linearity were restricted to perturbations of small size, with noise SD of 2°/s, so our data are not in contradiction with those in this previous study. Despite this approximate linearity, we observed some signs of systematic deviations in our predictions to brief perturbations that deserve further study (Fig. 5): the linear predictions were consistently better for the peak-first condition and there was a tendency for a discrepancy between the predicted and measured responses to diverge toward the late phase of the perturbation. In addition, one should consider that our experiments were performed over a limited range of velocities and that linearity may be further compromised at higher pursuit speeds.
A central observation was that the dynamics of pursuit maintenance were fast, with latencies of ∼67 ms and effective widths of ∼45 ms. In contrast, latencies measured during pursuit initiation using a step-ramp paradigm averaged ∼155 ms under the same conditions in the same subjects. Even under optimal conditions in a gap-paradigm, which yields the smallest latencies for pursuit initiation, delays are on the order of ∼90 ms (Krauzlis and Miles 1996b).
The dynamics of pursuit maintenance were only moderately slowed down by luminance contrast, with latencies remaining below 80 ms for the lowest contrasts tested (Fig. 7). These findings are in general agreement with the effect of contrast reported by Mulligan (2002). In comparison, latencies in the step-ramp task measured under varying contrast levels range widely between 100 and 300 ms (Braun et al. 2008; Ilg 1997; Spering et al. 2005), pointing to further differences between the dynamics of pursuit measured during its maintenance and initiation phases.
These findings show that the dynamics of pursuit maintenance and initiation are different from one another. Independently of the possible origins of the discrepancy, a lesson from these results is that the use of measurements obtained during pursuit initiation to model and explain properties of pursuit maintenance (including its gain and stability), may not be entirely justified.
There might be several reasons for the differences seen in the latencies of pursuit initiation and maintenance. One reason is simply that pursuit initiation and maintenance might be controlled by different mechanisms. This possibility seems consistent with a recent study of individual differences in human observers, showing that pursuit initiation is dominated by first-order motion mechanisms while pursuit maintenance is driven by position tracking mechanisms (Wilmer and Nakayama 2007). However, it is unclear if position tracking mechanisms alone can be fast enough to support the rapid dynamics shown in our measurements (Blohm et al. 2005; Masson and Stone 2002; Pola and Wyatt 1980). It is also possible that delays measured in the step-ramp paradigm confound pursuit initiation with other processes, such as disengagement from fixation or the initiation of pursuit in the direction of the step. The former idea is supported by the finding that latencies measured in a step-ramp paradigm depend on whether or not a temporal gap is introduced between the disappearance of fixation and appearance of a moving target (Krauzlis and Miles 1996a,b). The latter notion is consistent with the finding of ∼20% longer latencies in the step-ramp paradigm than in a simple ramp (Carl and Gellman 1987; Robinson 1965). A further hurdle with the estimation of latency measurements during pursuit initiation is that they are susceptible to contamination by anticipatory movements (Freyberg and Ilg 2008; Krauzlis and Miles 1996a; Merrison and Carpenter 1995; van den Berg and Collewijn 1987). The use of unpredictable perturbations during steady-state pursuit in our experimental design circumvents these problems.
Our estimates of pure delays during pursuit are closer to, but still considerably smaller than, those resulting from the average responses to brief velocity perturbations during tracking (Behrens et al. 1985; Churchland and Lisberger 2001, 2002; Schwartz and Lisberger 1994). For example, delays of ∼93 ms were reported in response to step increases in target velocity during pursuit (Behrens et al. 1985) and delays of ∼110 ms in response to biphasic perturbations of target velocity (Churchland and Lisberger 2002). The exact method used to estimate latencies may differ from study to study, so we replicated the experimental design of Churchland and Lisberger (2002) and found that, the responses to brief velocity perturbations were consistent with the predictions of the optimal linear filter. The longer delays in this experimental paradigm are a simply consequence of the gradual ramping-up of the perturbation signal.
Measurements of the optimal linear filter under varying mean target velocities and perturbations sizes were well accounted for by a single change in gain. The gain decreased as the SD of the target fluctuations increased. An interesting consequence of this behavior is that, given a fixed target velocity, the SD of the eye velocity signal is independent of the size of the fluctuations in the target velocity. This behavior is expected if visual motion detectors decrease their gain as the variance of the input signal increases (Borst et al. 2005; Brenner et al. 2000).
We conjecture that the short latencies and narrow effective widths of the estimated impulse response indicates that our technique is isolating a reflexive-like pathway that contributes to gaze stabilization during pursuit and fixation. Note that the temporal filter is estimated over 200 s of steady-state pursuit data. Any variability in the dynamics of the system over time would effectively smooth the resulting temporal profile. The sharp features of the impulse response, such as the corner defining pure delay (Fig. 2A), indicate that pursuit maintenance operates without substantial temporal variability, as one would expect in a reflexive-like movement. In addition, the similarity in the shape of the optimal filters during fixation and pursuit, in particular in the early phase of the response, suggests these two systems share a common component for the stabilization of gaze, as suggested previously (Krauzlis 2004). Note that our measurements of gaze stabilization dynamics during pursuit and fixation are not in contradiction with the differences observed in the dynamics of stimulus onset and offset (Luebke and Robinson 1988). Our study concerns the responses to target velocity fluctuations about a fixed mean velocity, whereas Luebke and Robinson (1988) studied the dynamics of pursuit during jumps in mean velocity, including a condition that brought the velocity to zero (that is, a transition to fixation).
To appreciate the fast dynamics of the linear filters, one should note that their effective width, in the order of ∼45 ms, is comparable to that of individual direction selective cells in monkey V1 and middle temporal area (MT) (Bair and Movshon 2004). This leaves no opportunity for further temporal integration of motion signals in downstream areas. In addition, response latencies in MT are ∼45 ms (Bair and Movshon 2004), leaving barely ∼20 ms of transmission delay for the eyes to move. A possible circuit for fast pursuit dynamics is one mediated by the direct projection from MT/MST to the pretectal nucleus of the optic tract, which has previously been shown to exhibit short latencies of ∼55 ms during pursuit (Mustari et al. 1988).
Caution should be exercised when comparing timing from monkey electrophysiological data with human behavioral data. Future electrophysiological and behavioral research is needed to show the exact pathways involved in generating the fast pursuit dynamics observed here. An approach that might be fruitful in dissecting the underlying circuitry consists in using a similar reverse-correlation method during electrophysiological recordings. This would allow the comparison of behavioral and electrophysiological data in the same subject and allow the study of fluctuations in neuronal firing with fluctuations in target and eye velocities at different points along the smooth pursuit pathway (Schoppik et al. 2008).
GRANTS
This work was supported by National Eye Institute Grants EY-12816 and EY-18322.
REFERENCES
- Bair and Movshon 2004.Bair W, Movshon JA. Adaptive temporal integration of motion in direction-selective eurons in macaque visual cortex. J Neurosci 24: 7305–7323, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes and Asselman 1991.Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. J Physiol 439: 439–461, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens et al. 1985.Behrens F, Collewijn H, Grusser OJ. Velocity step responses of the human gaze pursuit system. Experiments with sigma-movement. Vision Res 25: 893–905, 1985. [DOI] [PubMed] [Google Scholar]
- Bennett and Barnes 2004.Bennett SJ, Barnes GR. Predictive smooth ocular pursuit during the transient disappearance of a visual target. J Neurophysiol 92: 578–590, 2004. [DOI] [PubMed] [Google Scholar]
- Blohm et al. 2005.Blohm G, Missal M, Lefevre P. Direct evidence for a position input to the smooth pursuit system. J Neurophysiol 94: 712–721, 2005. [DOI] [PubMed] [Google Scholar]
- Born and Pack 2002.Born RT, Pack CC. Integration of motion signals for smooth pursuit eye movements. Ann NY Acad Sci 956: 453–455, 2002. [DOI] [PubMed] [Google Scholar]
- Born et al. 2006.Born RT, Pack CC, Ponce CR, Yi S. Temporal evolution of 2-dimensional direction signals used to guide eye movements. J Neurophysiol 95: 284–300, 2006. [DOI] [PubMed] [Google Scholar]
- Borst et al. 2005.Borst A, Flanagin VL, Sompolinsky H. Adaptation without parameter change: dynamic gain control in motion detection. Proc Natl Acad Sci USA 102: 6172–6176, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun et al. 2008.Braun DI, Mennie N, Rasche C, Schutz AC, Hawken MJ, Gegenfurtner KR. Smooth pursuit eye movements to isoluminant targets. J Neurophysiol 100: 1287–1300, 2008. [DOI] [PubMed] [Google Scholar]
- Brenner et al. 2000.Brenner N, Bialek W, de Ruyter van Steveninck R. Adaptive rescaling maximizes information transmission. Neuron 26: 695–702, 2000. [DOI] [PubMed] [Google Scholar]
- Carl and Gellman 1987.Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. J Neurophysiol 57: 1446–1463, 1987. [DOI] [PubMed] [Google Scholar]
- Churchland and Lisberger 2002.Churchland AK, Lisberger SG. Gain control in human smooth-pursuit eye movements. J Neurophysiol 87: 2936–2945, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland et al. 2003.Churchland MM, Chou IH, Lisberger SG. Evidence for object permanence in the smooth-pursuit eye movements of monkeys. J Neurophysiol 90: 2205–2218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland and Lisberger 2001.Churchland MM, Lisberger SG. Experimental and computational analysis of monkey smooth pursuit eye movements. J Neurophysiol 86: 741–759, 2001. [DOI] [PubMed] [Google Scholar]
- Deno et al. 1995.Deno DC, Crandall WF, Sherman K, Keller EL. Characterization of prediction in the primate visual smooth pursuit system. Biosystems 34: 107–128, 1995. [DOI] [PubMed] [Google Scholar]
- Freyberg and Ilg 2008.Freyberg S, Ilg UJ. Anticipatory smooth-pursuit eye movements in man and monkey. Exp Brain Res 186: 203–214, 2008. [DOI] [PubMed] [Google Scholar]
- Fukushima 2003.Fukushima K Frontal cortical control of smooth-pursuit. Curr Opin Neurobiol 13: 647–654, 2003. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner et al. 2003.Gegenfurtner KR, Xing D, Scott BH, Hawken MJ. A comparison of pursuit eye movement and perceptual performance in speed discrimination. J Vis 3: 865–876, 2003. [DOI] [PubMed] [Google Scholar]
- Goldreich et al. 1992.Goldreich D, Krauzlis RJ, Lisberger SG. Effect of changing feedback delay on spontaneous oscillations in smooth pursuit eye movements of monkeys. J Neurophysiol 67: 625–638, 1992. [DOI] [PubMed] [Google Scholar]
- Heinen and Watamaniuk 1998.Heinen SJ, Watamaniuk SN. Spatial integration in human smooth pursuit. Vision Res 38: 3785–3794, 1998. [DOI] [PubMed] [Google Scholar]
- Ilg 1997.Ilg UJ Slow eye movements. Prog Neurobiol 53: 293–329, 1997. [DOI] [PubMed] [Google Scholar]
- Jarrett and Barnes 2001.Jarrett CB, Barnes G. Volitional selection of direction in the generation of anticipatory ocular smooth pursuit in humans. Neurosci Lett 312: 25–28, 2001. [DOI] [PubMed] [Google Scholar]
- Keller and Heinen 1991.Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res 11: 79–107, 1991. [DOI] [PubMed] [Google Scholar]
- Knox 1998.Knox PC Stimulus predictability and the gap effect on pre-saccadic smooth pursuit. Neuroreport 9: 809–812, 1998. [DOI] [PubMed] [Google Scholar]
- Kowler 1989.Kowler E Cognitive expectations, not habits, control anticipatory smooth oculomotor pursuit. Vision Res 29: 1049–1057, 1989. [DOI] [PubMed] [Google Scholar]
- Krauzlis 2004.Krauzlis RJ Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis and Lisberger 1994.Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comput Neurosci 1: 265–283, 1994. [DOI] [PubMed] [Google Scholar]
- Krauzlis and Miles 1996a.Krauzlis RJ, Miles FA. Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res 36: 1973–1985, 1996a. [DOI] [PubMed] [Google Scholar]
- Krauzlis and Miles 1996b.Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol 76: 2822–2833, 1996b. [DOI] [PubMed] [Google Scholar]
- Lindner and Ilg 2000.Lindner A, Ilg UJ. Initiation of smooth-pursuit eye movements to first-order and second-order motion stimuli. Exp Brain Res 133: 450–456, 2000. [DOI] [PubMed] [Google Scholar]
- Lisberger et al. 1987.Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10: 97–129, 1987. [DOI] [PubMed] [Google Scholar]
- Luebke and Robinson 1988.Luebke AE, Robinson DA. Transition dynamics between pursuit and fixation suggest different systems. Vision Res 28: 941–946, 1988. [DOI] [PubMed] [Google Scholar]
- Masson 2004.Masson GS From 1D to 2D via 3D: dynamics of surface motion segmentation for ocular tracking in primates. J Physiol Paris 98: 35–52, 2004. [DOI] [PubMed] [Google Scholar]
- Masson et al. 2000.Masson GS, Rybarczyk Y, Castet E, Mestre DR. Temporal dynamics of motion integration for the initiation of tracking eye movements at ultra-short latencies. Vis Neurosci 17: 753–767, 2000. [DOI] [PubMed] [Google Scholar]
- Masson and Stone 2002.Masson GS, Stone LS. From following edges to pursuing objects. J Neurophysiol 88: 2869–2873, 2002. [DOI] [PubMed] [Google Scholar]
- Merrison and Carpenter 1995.Merrison AF, Carpenter RH. “Express” smooth pursuit. Vision Res 35: 1459–1462, 1995. [DOI] [PubMed] [Google Scholar]
- Mulligan 2002.Mulligan JB Sensory processing delays measured with the eye-movement correlogram. Ann NY Acad Sci 956: 476–478, 2002. [DOI] [PubMed] [Google Scholar]
- Mustari et al. 1988.Mustari MJ, Fuchs AF, Wallman J. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol 60: 664–686, 1988. [DOI] [PubMed] [Google Scholar]
- Osborne et al. 2007.Osborne LC, Hohl SS, Bialek W, Lisberger SG. Time course of precision in smooth-pursuit eye movements of monkeys. J Neurosci 27: 2987–2998, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne and Lisberger 2007.Osborne LC, Lisberger SG. Spatial and temporal integration of stochastic motion direction signals in primate pursuit eye movements. Society for Neuroscience Annual Meeting, San Diego, CA, Nov. 3–7, 2007.
- Osborne et al. 2005.Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola and Wyatt 1980.Pola J, Wyatt HJ. Target position and velocity: the stimuli for smooth pursuit eye movements. Vision Res 20: 523–534, 1980. [DOI] [PubMed] [Google Scholar]
- Rashbass 1961.Rashbass C The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach 1995.Ringach DL A tachometer feedback model of smooth-pursuit eye-movements. Biol Cybern 73: 561–568, 1995. [DOI] [PubMed] [Google Scholar]
- Robinson 1965.Robinson DA The mechanics of human smooth pursuit eye movement. J Physiol 180: 569–591, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson et al. 1986.Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern 55: 43–57, 1986. [DOI] [PubMed] [Google Scholar]
- Schoppik et al. 2008.Schoppik D, Nagel KI, Lisberger SG. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron 58: 248–260, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz and Lisberger 1994.Schwartz JD, Lisberger SG. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis Neurosci 11: 411–424, 1994. [DOI] [PubMed] [Google Scholar]
- Spering and Gegenfurtner 2007.Spering M, Gegenfurtner KR. Contrast and assimilation in motion perception and smooth pursuit eye movements. J Neurophysiol 98: 1355–1363, 2007. [DOI] [PubMed] [Google Scholar]
- Spering et al. 2005.Spering M, Kerzel D, Braun DI, Hawken MJ, Gegenfurtner KR. Effects of contrast on smooth pursuit eye movements. J Vis 5: 455–465, 2005. [DOI] [PubMed] [Google Scholar]
- Thier and Ilg 2005.Thier P, Ilg UJ. The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol 15: 645–652, 2005. [DOI] [PubMed] [Google Scholar]
- van den Berg 1988.van den Berg AV Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Exp Brain Res 72: 95–108, 1988. [DOI] [PubMed] [Google Scholar]
- van den Berg and Collewijn 1987.van den Berg AV, Collewijn H. Voluntary smooth eye movements with foveally stabilized targets. Exp Brain Res 68: 195–204, 1987. [DOI] [PubMed] [Google Scholar]
- Wilmer and Nakayama 2007.Wilmer JB, Nakayama K. Two distinct visual motion mechanisms for smooth pursuit: evidence from individual differences. Neuron 54: 987–1000, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]