Prior associations with the APC/C complex during prometaphase makes cyclin B1 a better substrate for the cell cycle–regulating ubiquitin ligase in metaphase (see also a related paper by Di Fiore et al. in this issue).
Abstract
The ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) is activated at prometaphase by mitotic phosphorylation and binding of its activator, Cdc20. This initiates cyclin A degradation, whereas cyclin B1 is stabilized by the spindle checkpoint. Upon checkpoint release, the RXXL destruction box (D box) was proposed to direct cyclin B1 to core APC/C or Cdc20. In this study, we report that endogenous cyclin B1–Cdk1 is recruited to checkpoint-inhibited, phosphorylated APC/C in prometaphase independently of Cdc20 or the cyclin B1 D box. Like cyclin A, cyclin B1 binds the APC/C by the Cdk cofactor Cks and the APC3 subunit. Prior binding to APC/CCdc20 makes cyclin B1 a better APC/C substrate in metaphase, driving mitotic exit and cytokinesis. We conclude that in prometaphase, the phosphorylated APC/C can recruit both cyclin A and cyclin B1 in a Cks-dependent manner. This suggests that the spindle checkpoint blocks D box recognition of APC/C-bound cyclin B1, whereas distinctive complexes between the N terminus of cyclin A and Cdc20 evade checkpoint control.
Introduction
Ubiquitin-dependent destruction of proteins that inhibit an upcoming event in the cell cycle provides a mechanism to govern unidirectional cell cycle progression. Like in lower organisms, in mammalian cells, cyclin B1–Cdk1 is the principal kinase catalyzing entry of G2 phase cells into mitosis (Lindqvist et al., 2009), but cyclin B1 degradation and Cdk1 inactivation drive mitotic exit and cytokinesis (Clute and Pines, 1999; Hagting et al., 2002; Wolf et al., 2006; Sullivan et al., 2008). Destruction of separase inhibitor securin at metaphase is essential for sister chromatid separation (Hagting et al., 2002; Yanagida, 2005). In mammalian cells, synchronized loss of cyclin B1 and securin thus coordinates cell division with nuclear division (Pines, 2006).
By the action of spindle checkpoint proteins (e.g., Mad1, Mad2, Bub1, BubR1, and Mps1), cyclin B1 and securin are stabilized until all chromosomes are bipolarly attached to the mitotic spindle at metaphase (Kops, 2008; Sczaniecka and Hardwick, 2008; Kulukian et al., 2009). The spindle checkpoint proteins cooperate to inhibit the function of Cdc20, a WD40 repeat–containing protein (Yu, 2007) that binds to and activates the anaphase-promoting complex/cyclosome (APC/C). APC/CCdc20 forms a multisubunit E3 ubiquitin ligase that directs proteasomal destruction of cyclin B1 and securin upon release of the spindle checkpoint (Pines, 2006; Yu, 2007; van Leuken et al., 2008).
The spindle checkpoint does not preclude binding of Cdc20 to the APC/C (Nilsson et al., 2008; Sczaniecka and Hardwick, 2008; Herzog et al., 2009; Kulukian et al., 2009). By their destruction region (involving the RXXL destruction box [D box]), APC/C substrates may interact with the WD40 domains of Cdc20 (Ohtoshi et al., 2000; Hilioti et al., 2001; Kraft et al., 2005; Passmore and Barford, 2005). On their turn, checkpoint proteins could block substrate binding to Cdc20 as shown in vitro, suggesting that the spindle checkpoint prevents recruitment of substrates to APC/CCdc20 (Herzog et al., 2009). Spindle checkpoint proteins can also induce conformational changes in the APC/C itself, thus repositioning Cdc20 (Herzog et al., 2009). This could mean that release of the spindle checkpoint helps to functionally activate Cdc20. Furthermore, the checkpoint either promotes polyubiquitination and destabilization of Cdc20 (Nilsson et al., 2008; Ge et al., 2009) or inhibits Cdc20 polyubiquitination to prevent its activation (Stegmeier et al., 2007). In conflict with the view that the checkpoint must be released before substrates can be recognized or Cdc20 can be activated is the long-standing observation that APC/CCdc20 is already active in prometaphase, targeting cyclin A for destruction, whereas cyclin B1 remains stable (Stewart et al., 1994; den Elzen and Pines, 2001; Geley et al., 2001; Wolthuis et al., 2008).
This paradox is not explained by inferring that cyclin A is an extremely efficient APC/C substrate, requiring minimal amounts of Cdc20 for its destruction, because partial depletion of Cdc20 by RNAi delays cyclin A destruction at least as well as cyclin B1 destruction (Wolthuis et al., 2008). The C-terminal dipeptide of the prometaphase APC/CCdc20 substrate Nek2A acts as a direct APC/C-binding motif (Hayes et al., 2006), suggesting that prometaphase APC/CCdc20 substrates may be recruited to the APC/C independently of Cdc20 to escape checkpoint control. However, Cdc20 is a rate-limiting factor for both cyclin A and Nek2A destruction (Hayes et al., 2006; Wolthuis et al., 2008; Kulukian et al., 2009). This indicates that, rather, an uninhibited pool of Cdc20 may specifically direct the destruction of prometaphase APC/C substrates.
To be degraded in prometaphase, cyclin A depends on the conserved Cdk cofactors called Cks (the mammalian p9 proteins Cks1 and Cks2, orthologous to fission yeast Suc1 and Xenopus laevis Xe-p9, collectively referred to as Cks). Cks can bind phosphorylated cyclin–Cdk substrates such as the APC/C by its anion-binding pocket (Pines, 1996; Sudakin et al., 1997; Patra et al., 1999; Shteinberg and Hershko, 1999). This suggests that Cks functions to stabilize complexes between cyclin–Cdks and their phosphorylated ligands. It could be acting as a processivity factor to facilitate multiphosphorylation events by cyclin–Cdks that are retained to their prephosphorylated substrates (Pines, 1996). Patra and Dunphy (1998) previously showed that Cks-dependent phosphorylation by cyclin B1–Cdk1 is required to activate the Xenopus APC/C in mitotic extracts. However, it is also possible that Cks acts as a targeting subunit, recruiting cyclin A to the APC/C when the APC/C is phosphorylated in mitosis (Wolthuis et al., 2008).
Apart from cyclin A, cyclin B1 also binds to Cks. In this study, we investigated interplay between cyclin B1–Cdk1–Cks and the mitotic APC/C. We found that although the subcellular localization of cyclin B1–Cdk1 was independent of Cks, Cks promoted mitotic APC/C phosphorylation and activation, as measured by securin destruction in cells. Remarkably, Cks also retained cyclin B1–Cdk1 to spindle checkpoint–inhibited APC/CCdc20 as a substrate, secondary to enhancing APC/C phosphorylation. This required APC3, but not Cdc20 or the cyclin B1 D box, yet enhanced cyclin B1 turnover in metaphase to protect cells against cytokinesis failure.
We conclude that Cks plays a dual role in cyclin B1 destruction in human cells: to activate the APC/C by promoting cyclin B1–Cdk1-dependent phosphorylation and to facilitate binding of cyclin B1 to phosphorylated APC/CCdc20 to make it a better substrate. Our data imply that cyclin B1 is recruited to APC/CCdc20 well before recognition of the D box by Cdc20, as triggered by release of the spindle checkpoint (Kraft et al., 2005; Herzog et al., 2009). In prometaphase, cyclin B1, like cyclin A, is directed to phosphorylated APC/C by its Cdk and Cks partners, but cyclin A differs from cyclin B1 in the way its N-terminal destruction region binds to Cdc20 in G2 phase. This may facilitate competition between cyclin A and spindle checkpoint proteins in mitosis. We conclude that processive mitotic cyclin ubiquitination requires their Cks-dependent retention at the APC/C in the spindle checkpoint, a binding step that precedes D box–dependent ubiquitination.
Results
Cks proteins facilitate cyclin B1–Cdk1-dependent APC/C phosphorylation and activation
We aimed to study the roles of both Cks1 and Cks2 downstream of cyclin B1–Cdk1. Therefore, we used a non-Cks–binding mutant of human Cdk1, Cdk1-P242L. We call this mutant Cdk1–1N, as it is orthologous to budding yeast Cdc28-1N that has a normal G1 and S function but confers a mitotic defect (Surana et al., 1991). To follow how mitotic progression depends on the ability of Cdk1 to bind Cks, we aimed to complement cells depleted of endogenous Cdk1 by fluorescent Cdk1 or Cdk1–1N constructs.
We had found that Cdk1–EYFP, when overexpressed in control cells, does not localize like cyclin B1 in mitotic cells (Fig. 1 A and Fig. S1 A). However, this was different in cells from which endogenous Cdk1 was depleted, and RNAi-resistant Cdk1–EYFP (Cdk1*–EFYP) was used to restore expression (transient cotransfections in U2OS cells; Fig. 1 C). In these cells, fluorescent Cdk1 colocalized with cyclin B1 on centrosomes in G2 phase, entered the nucleus in prophase, and stained mitotic spindle, chromosomes, and kinetochores in prometaphase (Fig. 1 C, Fig. S1 B, and Video 1). At metaphase, Cdk1 localization became dispersed as cyclin B1 was degraded (Fig. 1 C, M). Cdk1 remarkably escaped protein destruction, probably by dissociating from cyclin B1 at the proteasome (Nishiyama et al., 2000; Chesnel et al., 2007). Although Cdk1-depleted cells show mitotic defects, restoring expression by RNAi-resistant, fluorescent Cdk1 completely restored normal mitotic progression (Fig. 1, C and E; Lindqvist et al., 2007). We conclude that fluorescently tagged Cdk1 is functional and that the intracellular localization pattern of fluorescent, RNAi-resistant Cdk1 acts as a single-cell readout for efficient depletion of endogenous Cdk1 by our shCdk1 plasmid.
Figure 1.
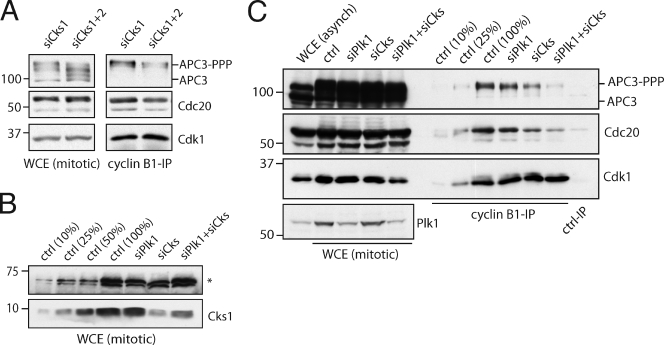
Cdk1-bound Cks proteins enhance APC/C phosphorylation and mitotic progression. (A) Cdk1–EYFP shows no distinct intracellular localization. M denotes metaphase alignment and onset of cyclin B1 destruction in U2OS cells. (B) Complementation of Cdk1 RNAi by RNAi-resistant Cdk1*–EYFP. (C) Reconstitution of Cdk1 depletion by Cdk1*–EYFP reveals cyclin B1 binding (Fig. S1 B and Video 1). Cdk1 localization gets dispersed upon cyclin B1 degradation at metaphase. (D) Complementation of Cdk1 depletion by non-Cks–binding Cdk1*–1N delays cells in mitosis. (E) Reconstituted expression of fluorescent wild-type Cdk1 rescues Cdk1 RNAi (Lindqvist et al., 2007); NEB to anaphase (NEB-A) is normal at ∼24 min (n = 8). Cdk1*–1N–EYFP-complemented cells are delayed in mitosis, NEB to anaphase is 57 min (n = 7). (right) Graph shows that Cdk1*–1N–EYFP-complemented cells delay independently of the spindle checkpoint. Knockdown of BubR1 (shBubR1; Lens et al., 2003) was verified by staining imaged cells with anti-BubR1 after fixation (not depicted). (F) Mitotic cells arrested in the spindle checkpoint were collected. Extracts were blotted with the indicated antibodies. (G) Intensities of the most phosphorylated forms of APC3 (ppp) and the unphosphorylated APC3 (u) from four different experiments as in F were quantified. A detailed description is shown in Fig. S1 E. WCE, whole cell extract. Error bars indicate standard deviations. Markers are given in kilodaltons. Bars: (A) 5 µM; (C and D) 10 µM.
Cyclin B1 binding directs Cdk1 localization (Pines and Hunter, 1994; Draviam et al., 2001; Bentley et al., 2007). Normally, Cdk1 is present in surplus over cyclin B1 (Arooz et al., 2000), but in cells from which endogenous Cdk1 is depleted, increased numbers of cyclin B1–Cdk1–EYFP complexes can be formed. This strongly indicates that the distinct cyclin B1–like localization pattern of Cdk1–*EFYP as shown in Fig. 1 C is also a marker for binding of Cdk1–*EYFP to cyclin B1.
To investigate the function of Cks binding to Cdk1, we used our Cdk1 complementation assay and followed G2 and mitosis in fluorescent cells. Cdk1*–1N–EYFP expressed in Cdk1-depleted cells revealed a similar intracellular localization as wild-type fluorescent Cdk1, with only modest reduction of spindle pole staining in some cells (Fig. 1 D). A potential role for Cks in controlling the G2/M transition was found in Xenopus (Patra and Dunphy, 1996), but as far as we could detect, Cdk1*–1N U2OS cells entered mitosis with normal kinetics. However, Cks-depleted cells delayed in mitosis, most likely in metaphase (Fig. 1, D and E [left]; and Fig. S1 D; Wolthuis et al., 2008), whereas Cks binding did not affect cyclin-associated histone 1 kinase activity in vitro (Wolthuis et al., 2008).
A Cks-dependent delay in mitotic exit was not rescued by inactivation of the spindle checkpoint (Fig. 1 E, right). This shows that non-Cks–binding Cdk1 delays mitotic exit independently or downstream of the checkpoint. Analysis of APC3 mobility shifts on blots confirmed that the Cdk1–Cks connection supports maximal APC/C phosphorylation in human cells (Fig. 1, F and G; and Fig. S1, C and E; Wolthuis et al., 2008). Both Cdk1* and Cdk1*–1N were equally stable in metaphase and anaphase (Fig. S1 D). We conclude from these experiments that in human cells, Cks does not control cyclin B1–Cdk1 localization but Cdk1-dependent APC/C phosphorylation and metaphase progression.
Cks proteins promote metaphase APC/C activity, with a further role in cyclin B1 destruction
Cks1 and Cks2 fulfill overlapping roles in mitosis (Donovan and Reed, 2003; Wolthuis et al., 2008). Depletion of 75–85% of both Cks1 and Cks2 by RNAi (further referred to as Cks depletion) did not reduce protein levels of Cdc20, cyclin A, or cyclin B1 in U2OS cells (Wolthuis et al., 2008), which is in contrast to more severe effects of knocking out Cks1 and Cks2 (Martinsson-Ahlzén et al., 2008). To investigate whether Cks depletion affected metaphase APC/C activity in human cells, like in Xenopus extracts (Patra and Dunphy, 1998), we quantified destruction of a fluorescent version of the APC/CCdc20 substrate securin (securin-Venus). Cells were treated either with siRNA oligos targeting Cks1 alone (as a negative control) or by a combination of siRNAs targeting Cks1 and Cks2. Depleting a single type of Cks had no effect on securin destruction or cyclin B1 destruction, starting ∼20 min after nuclear envelope breakdown (NEB; Fig. 2, A and B, siCks1; for further siRNA controls see Wolthuis et al., 2008). In cells depleted of both Cks1 and Cks2, metaphase onset was delayed, probably because of cyclin A stabilization, but securin destruction started upon chromosome alignment at metaphase (Fig. 2 A, siCks1 and Cks2). However, the time required to destroy similar levels of fluorescent securin was increased nearly twofold (Fig. 2, A and C [quantitation], black and white triangles; and Fig. S2 A).
Figure 2.
Cks promotes APC/C–dependent destruction of cyclin B1 and securin. (A and B) Cells were treated with siRNA pools targeting Cks1 (siCks1; control) or both Cks1 and Cks2 (siCks1 and 2). Total amounts of siRNA were kept equal. Cells transfected with either securin-Venus (A) or cyclin B1–Venus (B) and expressing similar fluorescent protein levels were followed during mitosis (see Fig. S2 A for quantified fluorescence levels and total destruction times). (C) Fluorescence levels plotted over time starting 3 min before NEB are shown. Data are representative of three independent experiments for each condition. Numbers indicate minutes. Bars, 10 µM.
Normally, degradation of cyclin B1 and securin always occurs simultaneously (see Fig. 7 A, middle; Hagting et al., 2002). Remarkably, in Cks-depleted cells, destruction of cyclin B1 was delayed as compared with securin destruction (Fig. 2, B and C [quantitation]; and Fig. S2 A). In up to 50% of the cells expressing fluorescent cyclin B1, this resulted in delayed or impaired cleavage furrow ingression or a full cytokinesis defect (Fig. 2 B, right; bottom image shows accumulation of undivided cells). Although this phenotype was seen in cells expressing ectopic cyclin B1, control cells degraded at least fivefold higher amounts of ectopic cyclin B1 and successfully completed cytokinesis (Fig. S2 A, middle). This suggested that Cks proteins do not just control general metaphase activity of the APC/C but, at least in human cells, may have an additional, specific role in cyclin B1 destruction. This helps to protect against cytokinesis failure.
Figure 7.
APC/CCdc20 binding to cyclin B1 correlates with a substrate retention step, preceding a D box–dependent ubiquitination step. (A) Cells were cotransfected with vectors coding for cyclin B1–Venus and ΔN–cyclin B1–ECFP (left), securin-cerulean and cyclin B1–Venus (middle), or securin-cerulean and cyclin B1–R202A–Venus (right). Cells expressing both proteins were followed through mitosis, and fluorescence levels were plotted. Metaphase (M) and anaphase (A) initiation judged from differential interference contrast images are shown. Graphs are representative of at least two independent experiments. (middle and right) The cells analyzed expressed cyclin B1 or securin at low levels, allowing onset of their destruction at chromosome alignment. With wild-type constructs, sister chromatid separation and cytokinesis were normal. The other fluorescent constructs were expressed to similar levels. At least 20 cells expressing single fusion proteins were analyzed with similar results. (B and C) Wild-type and nondegradable mutants of cyclin B1 bind equally well to APC/CCdc20 in mitosis, whereas non-Cdk–binding mutants of cyclin B1 do not bind. (B) Cells expressing Cdk-binding mutant cyclin B1–R202A–Venus (lane 1), nondegradable ΔN–cyclin B1–ECFP (lane 2), or control cyclin B1–Venus (lane 3) were synchronized in the spindle checkpoint and collected by mitotic shake off. Successful expression of the constructs was validated by fluorescence microscopy (not depicted). (C) Cells expressing control cyclin B1–Venus (lane 1), Cdk-binding mutant cyclin B1–R202A–Venus (lane 2), the non-Cdk–binding N-terminal destruction region of cyclin B1 fused to GFP (lane 3), or nondegradable R42A D box mutants (lanes 4 and 5) were synchronized in the spindle checkpoint and collected by mitotic shake off. (B and C) Fusion proteins were immunoprecipitated by anti-GFP antibodies, equally recognizing GFP or spectral variants. IPs were blotted and probed with antibodies against the indicated proteins. (D) Cells were treated with nocodazole, and cyclin B1 IPs were performed on extracts of the collected mitotic cells (lane 1) or on extracts from an asynchronous population (lane 2). Cells expressing the first 97 amino acids of cyclin B1 fused to GFP either containing an intact D box (lane 3) or an R42A-mutated D box (lane 4) were collected in mitosis. Anti-GFP antibodies were used for IP of the fusion proteins from extracts (lanes 3 and 4). IPs were probed for cyclin B1 and APC3. (lane 1) Binding of APC/C to endogenous cyclin B1 in mitosis as reference is shown. In prometaphase, binding is not detectable to the N-terminal fragments of cyclin B1. (E and F) Mitotic cells were collected by shake off after taxol treatment and released from the spindle checkpoint by addition of aurora B inhibitor ZM447439 but kept in mitosis by the addition of proteasome inhibitor MG132. After 1 h, cells were lysed and extracts used for IP of fusion proteins of GFP and the cyclin B1 N terminus (1–86), (cycB1-N (1X)-GFP), or tandem or sixfold repeats of the same region (cycB1-N (2X)-GFP and cycB1-N (6X)-GFP, respectively). IPs were blotted for APC3, Cdc20, or Cdk1 (as a negative control [ctrl]; E) or the cyclin B1 fragments (F). APC3 long exposure (APC3 le) is an overexposed blot to reveal minor APC/C binding to the single- or tandem-repeat cyclin B1 fragment. Markers are given in kilodaltons. WCE, whole cell extract.
Mitotic APC/C phosphorylation was similarly impaired after Cks depletion (Fig. 3, A and C) as upon reconstituting Cdk1 by non-Cks–binding Cdk1 (Fig. 1, F and G; and Fig. S1 E). Interestingly, partial Cks depletion by RNAi clearly reduced formation of stabilized interactions between APC/CCdc20 and endogenous cyclin B1–Cdk1 in mitosis (Fig. 3, A–C). Binding was reduced further when mitotic Cks RNAi cells were also depleted of the APC/C kinase Plk1, which clearly suggests that the APC/C binds cyclin B1 in a way that depends on APC/C phosphorylation (Fig. 3, B and C).
Figure 3.
Cks proteins direct cyclin B1 binding to the phosphorylated APC/C. (A) Cells were treated with siRNA pools targeting Cks1 (siCks1; control, or both Cks1 and Cks2; siCks1 + 2). The cells were thymidine released, blocked in nocodazole, and collected by mitotic shake off. Extracts were blotted with the indicated antibodies. IPs with anti–cyclin B1 antibodies were performed and blotted to examine coprecipitation of the indicated proteins. APC3 binding in cyclin B1 IPs correlates with the hyper-phosphorylated APC3 (APC3-PPP) present in extracts. (B) Cells were treated with siRNA pools targeting Plk1 (siPlk1), both Cks1 and Cks2 (siCks), or Plk1, Cks1, and Cks2 (siPlk1 + siCks). Mitotic cells were collected as in A, and extracts were Western blotted and probed with anti-Cks1 antibody. (top) A background band detected by the Cks antiserum (asterisk) is shown. Serial dilutions of control (ctrl) mitotic cells show that Cks1 knockdown is ∼75% after siCks or between 50 and 75% after siPlk1 + siCks. (C) The same lysates shown in B were used for IP of cyclin B1 and blotted to examine APC/C and Cdc20 binding. Serial dilutions of control cyclin B1 IPs are shown as a reference. Markers are given in kilodaltons. WCE, whole cell extract.
Cks retains cyclin B1 at the APC/C as an APC/C substrate
Could Cks-mediated binding of cyclin B1–Cdk1 to the APC/C make cyclin B1 a better substrate for the APC/C? To test this in cells, we aimed to measure destruction of cyclin B1 molecules bound to non-Cks–binding Cdk1–1N. In this assay, we would be able to address whether fluorescent cyclin B1 would become a less-efficient APC/CCdc20 substrate simply by no longer being able to bind Cks. Any effect found would depend exclusively on a role for Cks in targeting cyclin B1 to the APC/C because endogenous Cks levels, and thus APC/C phosphorylation, should remain unchanged by the expression of these reporters.
When we coexpressed cyan fluorescent cyclin B1 with yellow fluorescent Cdk1, both proteins colocalized up to metaphase even in the presence of endogenous Cdk1. This shows that fluorescent Cdk1, which alone is dispersed in the cytoplasm (Fig. 1 A), is often complexed to fluorescent cyclin B1 when these proteins are coexpressed at the right levels (Fig. 1 and Fig. 4 A). Thus, by analyzing cells in which fluorescent cyclin B1 colocalized with fluorescent Cdk1, indicative of their complex formation, we could address whether binding of cyclin B1 to Cks could make it a better APC/CCdc20 substrate in cells. To ensure that ectopic expression did not affect normal cell division, only the cells that showed correct onset of cyclin B1 destruction at chromosome alignment, or Cdk1 mutant cells showing highly comparable cyclin B1 fluorescence levels as control, were analyzed.
Figure 4.
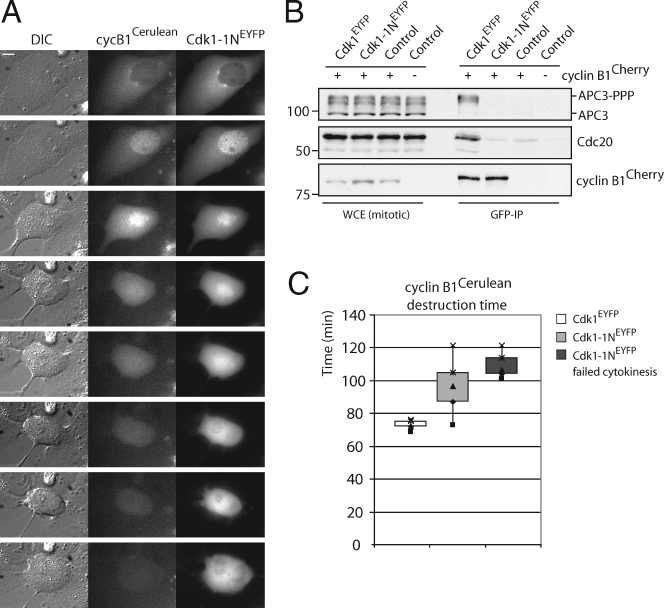
Cks promotes recruitment of cyclin B1 as an APC/C substrate. (A) Cells were cotransfected with cyclin B1–cerulean and either Cdk1*–EYFP or Cdk1*–1N–EYFP. A cell undergoing mitotic exit without cytokinesis is shown. DIC, differential interference contrast. Bar, 5 µM. (B) Reduced APC/CCdc20 binding of cyclin B1–Cdk1–1N complexes in cells, whereas total APC/C phosphorylation is normal. Checkpoint-arrested mitotic cells selected for expression of indicated constructs were collected. GFP IPs (pulling down Cdk1–EYFP) on equalized extracts were blotted for coprecipitation of the indicated proteins. Cyclin B1–Cherry is not recognized by the anti-GFP antibodies. WCE, whole cell extract. (C) Quantitative comparison of destruction times of cyclin B1 bound to Cdk1 (white) or Cdk1–1N (light gray). The destruction times of the cells expressing Cdk1–1N, which failed cytokinesis, are presented in the dark gray box. The plots contain data from three independent experiments (see Fig. S2 B for destruction curve). Markers are given in kilodaltons.
Interestingly, metaphase degradation of ectopic, fluorescent cyclin B1 bound to fluorescent Cdk1–1N was again significantly delayed as compared with normal fluorescent Cdk1 control (Fig. 4 C and Fig. S2 B). Cytokinesis also failed in 50% of these cells (Fig. 4, A and C). Crucially, total mitotic APC/C phosphorylation was now unchanged, but cyclin B1–Cdk1–1N complexes still failed to bind phosphorylated APC/C (Fig. 4 B). A model emerges from these findings in which cyclin B1–Cdk1 needs to bind Cks to stay bound to or to flux onto phosphorylated APC/C in prometaphase. This would direct efficient processing of cyclin B1 as an APC/C substrate in metaphase, control mitotic exit, and safeguard cytokinesis.
Complex formation between cyclin B1 and phosphorylated APC/CCdc20 in mitosis
To study when endogenous cyclin B1 binds to the APC/C and Cdc20, we analyzed their cell cycle–dependent interactions. Cyclin B1 immunoprecipitated from extracts of mitotic cells, arrested in nocodazole, and collected by mitotic shake off (95% 4N; >90% positive for MPM2 as analyzed by FACS; see Materials and methods) was bound to APC/CCdc20. This interaction was not found in extracts of G2 phase cells (Fig. 5, A and B; and Fig. S3, A and D). In contrast, we found few complexes between APC/C or Cdc20 and securin in G2 phase or mitosis (either with endogenous or ectopically expressed, tagged versions; Fig. S3, B and C).
Figure 5.
Binding of phosphorylated APC/CCdc20 to cyclin B1 starts in mitosis. (A) Cyclin B1 IPs from synchronized G2 phase and nocodazole-arrested mitotic cells (mitosis) are shown. See Materials and methods for synchronization details. IPs were analyzed for coprecipitating proteins by Western blotting probed for anti-APC3 (top; showing only phosphorylated APC3) or anti–cyclin B1 (middle). (bottom) The presence of unphosphorylated (lane 1) and phosphorylated (lane 2) APC3 in whole cell extracts (WCE) used for the IPs (10% of input) is shown. (B) APC/C IPs using anti-APC6 antibodies on extracts of G2 phase cells and mitotic cells. (C) Cyclin B1 IPs on extracts from G2 or mitotic cells were used in in vitro ubiquitination assays. Mitotic cells arrested in nocodazole were released but kept in mitosis by the proteasome inhibitor MG132 (NR, MG132). Control, GFP IPs. Purified E1 enzyme, the APC/C-specific E2 enzyme UbcH10, ATP, and HA-ubiquitin (HA-Ub) were added to start the reactions. HA-ubiquitin–conjugated reaction products were probed with anti-HA antibody (for further controls see Fig. S3). Asterisks indicate an aspecific background band in the control GFP IPs. (D) Comparison of mitotic cells arrested in the spindle checkpoint (Noco), released from the spindle checkpoint arrest but kept in mitosis by MG132, or allowed to enter mitosis in the presence of MG132; the latter contain high cyclin A levels (top). Cyclin B1 IPs are shown; negative control, securin (Sec) IP. Western blots were analyzed for coprecipitating proteins with the indicated antibodies. (top) Phosphorylated forms of APC3 in cyclin B1 IPs are shown. (E) Cells were transfected and selected for expression of the indicated cyclin–GFP fusion proteins. Mitotic cells that had entered mitosis in the presence of the proteasome inhibitor MG132 were collected by shake off. GFP IPs were probed for coprecipitation of Cdc20. (F) Cells were synchronized in G2 and mitosis as indicated. Anti–cyclin A IPs, anti–cyclin B1 IPs, and mitotic extracts were blotted for APC3, Cdc20, and Mad2. Comparable polyclonal antibodies were used detecting the N-terminal 430 amino acids of the respective cyclins. Markers are given in kilodaltons.
Cyclin B1 bound exclusively to the maximally phosphorylated APC/C fraction (APC3-PPP; Fig. 5 A, compare APC3 input [bottom] with APC3 captured in the cyclin B1 immunoprecipitation [IP; top]). Furthermore, the APC/C retained in cyclin B1 IPs (kept in mitosis by proteasome inhibition after inactivation of the spindle checkpoint) was functionally active in vitro (Fig. 5 C) in a way dependent on ATP, E1, and APC/C-specific E2 enzyme (Fig. S3, E–G; and unpublished data). Mitosis-specific interactions between APC/CCdc20 and cyclin B1 were further found in APC2, APC3, APC4, APC6, Cdc20, cyclin B1, and Cdk1 IPs (Fig. S3 D; and unpublished data). We estimated that >25% of endogenous cyclin B1 was bound to the mitotic APC/C in nocodazole-arrested cells (see Fig. 8 B and Materials and methods). Similar complexes were found in extracts of cells collected by mitotic shake off 12 h after thymidine release in the absence of any spindle drugs (Fig. S3 A), in mitotic cells arrested by nocodazole or taxol (Fig. 5 D and not depicted), or in cells released from the spindle checkpoint but kept in mitosis by proteasome inhibition (Fig. 5 D, NR, MG132). In conclusion, a significant fraction of endogenous cyclin B1 is recruited to proper APC/C and Cdc20 in prometaphase, regardless of the cyclin B1–stabilizing effect of the mitotic spindle checkpoint.
Figure 8.
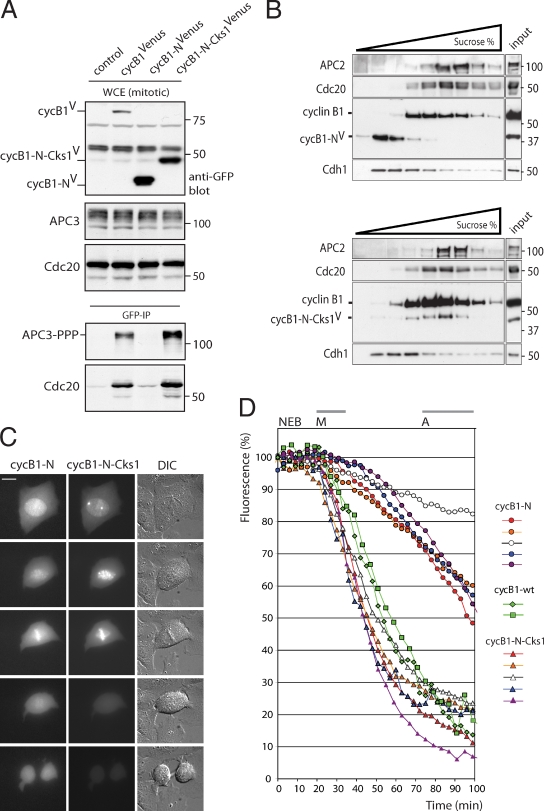
Cks enhances destruction of the N-terminal cyclin B1 fragment. (A) Cells expressing cyclin B1–Venus (lane 2), the first 86 amino acids of cyclin B1 fused to Venus (cycB1-N-Venus; lane 3), or the same N-terminal cyclin B1 fragment fused to Cks1 and Venus (cycB1-N-Cks1-Venus; lane 4) was synchronized in the spindle checkpoint and mitotic cells collected by mitotic shake off. GFP IPs on equal amounts of protein precipitated the Venus fusion proteins, which were blotted to examine coprecipitation of the indicated proteins (bottom). (B) Spindle checkpoint–arrested mitotic cells expressing cyclin B1–N–Venus or cyclin B1–N–Cks–Venus were collected, and lysates were subjected to sucrose gradient centrifugation. Fractions were blotted and probed with antibodies against the indicated proteins. (C) U2OS cells were cotransfected with vectors encoding cyclin B1–N–cerulean (cycB1-N) and cyclin B1–N–Cks–Venus (cycB1-N-Cks1) and followed as they progressed through mitosis. DIC, differential interference contrast. Bar, 10 µM. (D) Venus- and cerulean-tagged versions of cyclin B1–N or cyclin B1–N–Cks1 were cotransfected in U2OS cells. Cells either expressing cyclin B1–N or cyclin B1–N–Cks1 were followed during mitosis, and fluorescence levels were measured at NEB and plotted over time. For comparison, cells were also simultaneously transfected with three differently colored constructs, cyclin B1–N–Venus, cyclin B1–N–Cks1–cerulean, and a full-length red fluorescent cyclin B1 construct, cyclin B1–Cherry. Fluorescence levels of two triple-colored cells passing mitosis and performing a normal anaphase were included in the graph as a reference. Results of two independent experiments are shown. M, metaphase onset; A, anaphase onset. Comparable differences in destruction of the cyclin B1 reporters were found in >30 cells in four independent experiments. We found no significant effect of the different fluorescent tags on the destruction rates of the cyclin B1 fragments (Fig. S2 C). Markers are given in kilodaltons. WCE, whole cell extract.
Cyclin A and cyclin B1 bind to different pools of Cdc20
Our previous data indicated a key role for Cks in directing cyclin A–Cdk complexes to the APC/C to promote cyclin A destruction, which evades inhibition by the spindle checkpoint (Wolthuis et al., 2008). In this study, we find that Cks recruits cyclin B1 to the phosphorylated APC/C, irrespective of the spindle checkpoint and before onset of cyclin B1 destruction. This shows that cyclin A destruction cannot escape spindle checkpoint control simply because it is directed to the APC/C by Cks. Which differences in the way cyclin A and cyclin B1 bind APC/C or Cdc20 may explain their different destruction patterns?
By collecting mitotic cells in the presence of the proteasome inhibitor MG132, we found that binding of cyclin B1 to Cdc20 and phosphorylated APC/C was unaffected by the presence of stabilized cyclin A (Fig. 5 D), indicating that Cdc20 levels are sufficiently high to bind both cyclins effectively. However, we found a remarkably different timing of complex formation: a significant fraction of cyclin A interacted with Cdc20 in G2 phase, when cyclin B1 bound little or no Cdc20 (Fig. S3 D, compare input with IP; Wolthuis et al., 2008). Importantly, these cyclin A–Cdc20 complexes did not bind to the APC/C (Wolthuis et al., 2008). To compare how cyclin A and cyclin B1 may differ in the way they bind to Cdc20, we expressed GFP-tagged versions of cyclin B1, cyclin A, or an N-terminal cyclin A deletion mutant, arrested transfected cells in mitosis by proteasome inhibition, and determined Cdc20 binding to GFP IPs. Fig. 5 E shows that cyclin A binds much more efficiently to Cdc20 as compared with cyclin B1. Importantly, this difference relates to a feature within the N terminus of cyclin A that is also required for the spindle checkpoint–independent destruction of cyclin A (den Elzen and Pines, 2001; Geley et al., 2001).
How could this variation explain the different times at which cyclin A and cyclin B1 are degraded? Cyclin A binds directly to Cdc20 by its N terminus (Ohtoshi et al., 2000) even without binding to the APC/C (Wolthuis et al., 2008). This suggests a model in which Cdc20 that is bound to cyclin A escapes inhibition by spindle checkpoint proteins. Recruitment of cyclin A–Cdc20 complexes to the APC/C would depend on Cks and APC/C phosphorylation, which occurs at the same time as cyclin A destruction starts in prometaphase. In a comparison of cyclin A and cyclin B1 IPs that were normalized for the amount of Cdc20 coimmunoprecipitated, the amount of Mad2 that was detected in cyclin A IPs was indeed much lower as compared with the amount of Mad2 detected in cyclin B1 IPs (Fig. 5 F). We suggest from these experiments that cyclin A can bind Cdc20 in a way that prevents efficient inhibition of Cdc20 by the checkpoint.
Altogether, we propose that Cks binding does not influence responsiveness to the checkpoint but only the ability of mitotic cyclins to bind the APC/C. This is in agreement with our results that recruitment of cyclin B1 to the phosphorylated APC/C depends on Cks and is not blocked by the spindle checkpoint.
Complexes between cyclin B1 and APC/CCdc20 depend on APC3, not Cdc20
Our model predicts that, in contrast to cyclin A, cyclin B1 does not bind directly to Cdc20, or much less efficiently. Detection of Cdc20 in cyclin B1 IPs would thus predominantly reflect binding of cyclin B1 to mitotic APC/CCdc20 complexes. Because we found that binding of cyclin B1 to the APC/C depends on Cks and Cks can bind to phosphorylated APC/C in vitro, we tested whether the heavily phosphorylated APC subunit APC3 (Cdc27) could be involved in recruiting cyclin B1 to the APC/C. Therefore, we depleted APC3 to <5% of its endogenous levels, leading to a robust mitotic arrest (Fig. S4, A and B). Cells that arrested in mitosis by APC3 depletion, purified by mitotic shake off, expressed high levels of cyclin A and cyclin B1, whereas securin was stabilized partially (Fig. 6 A and Fig. S5 A).
Figure 6.
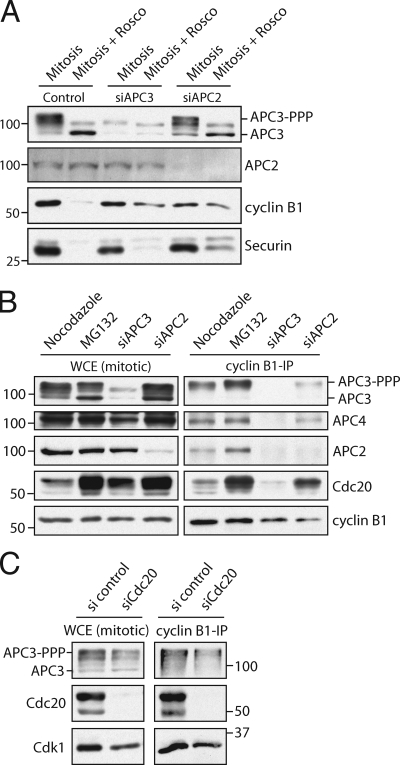
Retention and destruction of cyclin B1 critically depends on APC3. (A) Cells were arrested in mitosis by nocodazole after treatment with control siRNA (lanes 1 and 2) or arrested by treatment of siRNA pools targeting APC3 (lanes 3 and 4) or APC2 (lanes 5 and 6). 50% of these mitotic cells were released out of mitosis by the Cdk1 inhibitor roscovitine (Rosco; lanes 2, 4, and 6). Extracts were blotted and probed with antibodies against the indicated proteins. (B) IPs with anti–cyclin B1 antibodies on extracts of mitotic cells arrested in the spindle checkpoint (nocodazole) by allowing the cells to enter mitosis in the presence of MG132 or by treatment of siRNA pools targeting APC3 or APC2. Extracts and IPs were probed with antibodies against the indicated proteins. (C) IPs with cyclin B1 antibodies on extracts of mitotic cells arrested by the spindle checkpoint and treated with control siRNA or an siRNA pool targeting Cdc20. IPs were blotted and probed with antibodies against the indicated proteins. Identical results were obtained by using shRNA to target APC3 (Fig. S5). Markers are given in kilodaltons. WCE, whole cell extract.
First, we analyzed why securin was stabilized only partially in APC3-depleted cells. In yeast, overexpression of the Cdk inhibitor Sic1 induces mitotic exit independently of the APC3 subunit (Thornton and Toczyski, 2003). In this study, we added the Cdk inhibitor roscovitine to mitotic cells, either arrested by spindle drugs or by depletion of either APC3 or APC2, to test first whether cyclin B1 stabilization depended on the mitotic arrest. These cells initiated cleavage furrow formation and exited mitosis (Fig. S4, C and D). After inducing mitotic exit in cells arrested by APC3 depletion, cyclin B1 remained largely stable, whereas securin was partially degraded (Fig. 6 A; and Fig. S5, B and C). However, securin was more stable after treatment of these cells with proteasome inhibitor MG132 (Fig. S5 B) or after depletion of APC2, a cullin-like subunit essential for APC/C ubiquitin ligase activity (Fig. 6 A and Fig. S5 C). This suggests that the APC/C can partially process securin in the absence of APC3. In contrast, cyclin B1 was equally stabilized after inducing mitotic exit in cells depleted of APC3 or APC2 (Fig. 6 A and Fig. S5 C). Specific securin loss in APC3-depleted mitotic cells may depend on residual APC/C activity, processing securin more effectively as compared with cyclin B1, which is in line with securin being a better APC/C substrate in vitro (Rape et al., 2006). Alternatively, destruction of mitotic cyclins depends more critically on APC3 than destruction of securin does, like in yeast (Thornton and Toczyski, 2003). Regardless of the mechanism of securin loss, we conclude that APC3 is critical for cyclin B1 destruction independently of the mitotic arrest.
Next, we investigated the role of APC3 in complex formation between cyclin B1 and APC/CCdc20. In cells depleted of 90–95% APC3, Cdc20-binding APC/C complexes remained, as in budding yeast and in agreement with the distinct positions of APC3 and Cdc20 in the modeled APC/C structure (Fig. S5, E and F; Herzog et al., 2009). However, in the absence of APC3, the mitotic APC/C no longer bound cyclin B1 (Fig. 6 B and Fig. S5 D). Depletion of APC2 did not abrogate binding of cyclin B1 to the remaining APC/CCdc20, indicating that complex formation between cyclin B1 and APC/CCdc20 requires APC3 but not APC2 or APC/C activity (Fig. 6 B, right, compare lane 3 with lane 4). Interestingly, however, the mitotic APC/C bound normally to cyclin B1 after Cdc20 was completely depleted from cells (Fig. 6 C, note that the slightly reduced APC3 signal in lane 4 correlates with lower input of cyclin B1 in the IP). Altogether, our findings suggest that cyclin B1 recruitment to APC/CCdc20 depends on phosphorylated APC3, whereas Cdc20 is not involved in this step.
APC/CCdc20 binding to cyclin B1 correlates with a substrate retention step
How does Cks-dependent binding of cyclin B1–Cdk1 to the APC/C contribute to D box–dependent cyclin B1 destruction? Deletion of the N-terminal destruction region or the D box renders cyclin B1 almost completely stable and blocks cells in mitosis, whereas coexpressed fluorescent cyclin B1 is degraded because these cells arrest downstream of the spindle checkpoint (Fig. 7 A, left). This confirms that the D box is essential for cyclin B1 destruction and cytokinesis. However, a cyclin B1 mutant with a normal N terminus but defective in Cdk1–Cks binding (cyclin B1–R202A–Venus) also showed an impaired destruction pattern in metaphase, whereas in control cells, securin and cyclin B1 are degraded with similar efficiencies (Fig. 7 A, compare middle and right). The cyclin B1 R202A mutant was defective for APC/CCdc20 binding in mitosis (Fig. 7, B and C) in line with a critical role for Cks in recruiting cyclin B1 to APC/CCdc20, whereas wild-type and nondegradable cyclin B1 mutants, with intact Cdk–Cks binding, both bound comparably with APC/CCdc20 (Fig. 7, B and C).
The N terminus of cyclin B1, containing the D box, responds to the spindle checkpoint and starts to be degraded at metaphase but does not bind Cdk or Cks (Stewart et al., 1994; Clute and Pines, 1999). However, the N terminus of cyclin B1, overexpressed in mitotic cells, failed to bind detectably to the APC/C (Fig. 7 D). APC/C binding was also not found when cells were released from the mitotic checkpoint and kept in mitosis by proteasome inhibition (Fig. 7 E, cycB1-N (1X)-GFP), showing that the interaction between the cyclin B1 D box region and phospho-APC/C is of low affinity. Indeed, in extracts of postcheckpoint-arrested mitotic cells, we found that it required a sixfold repeat of the cyclin B1 N-terminal region to detect interactions with APC/CCdc20 (Fig. 7 E). Apparently, although upon silencing of the spindle checkpoint the D box is required for APC/CCdc20 to recognize cyclin B1 as a substrate, the D box region in itself is insufficient to promote stable interactions between cyclin B1 and mitotic APC/CCdc20. Therefore, we propose that Cdk1- and Cks-dependent binding of cyclin B1 to the APC/CCdc20 in prometaphase is required to ensure that D box–dependent processing of cyclin B1 is highly efficient after the checkpoint becomes satisfied in metaphase. In other words, we propose that binding to Cks by Cdk1 makes cyclin B1 a better APC/C substrate in cells.
Fusion proteins of the cyclin B1 N terminus and a reporter protein lacking the Cdk1 interaction region are widely used to detect metaphase APC/C activity in cells. However, we noticed that these reporter proteins are degraded very inefficiently, with >50% of fluorescence remaining at anaphase, whereas fluorescent versions of full-length cyclin B1 are almost completely degraded before anaphase. We aimed to validate that Cks is critical for efficient cyclin B1 destruction in metaphase independent of Cdk1 kinase activity. Therefore, we fused the Cks1 protein to a fluorescent fusion protein of the N terminus of cyclin B1 and tested its behavior in mitotic cells. The N terminus of cyclin B1 does not bind to APC/C or Cdc20 (Fig. 8 A, input and total APC/C phosphorylation [middle] and binding [bottom]), but fusion of the N terminus of cyclin B1 to Cks1 restored robust APC/CCdc20 binding in prometaphase to levels comparable with those found for wild-type cyclin B1 (Fig. 8 A, bottom). Furthermore, when we analyzed complex formation between APC/CCdc20 in sucrose gradients of prometaphase cell extracts, we found that ≤40% of endogenous cyclin B1 comigrated with APC/CCdc20, whereas the N-terminal cyclin B1 fragment migrated closer to Cdh1, the G1 phase APC/C activator that does not bind to the APC/C in prometaphase (Fig. 8 B, top). Interestingly, fusing Cks1 to the N-terminal cyclin B1 fragment shifted this protein in the gradient so it now comigrated with APC/CCdc20 complexes and endogenous cyclin B1, whereas Cdh1, of similar molecular mass as the cyclin B1–N–Cks fusion, remained in the fractions lacking APC/CCdc20 (Fig. 8 B, bottom).
Finally, fusing Cks1 to the N-terminal cyclin B1 fragment reporter protein resulted in an intracellular localization that was similar to that observed for wild-type cyclin B1 in mitosis (Fig. 8 C) with a slight enrichment on spindle poles. This reporter was degraded with high efficiency in metaphase at kinetics identical to those of full-length wild-type cyclin B1, whereas the N-terminal cyclin B1 fragment alone was degraded too slowly (Fig. 8, C and D; and Fig. S2 C). This shows a direct role for Cks in controlling the binding of cyclin B1–Cdk1 to the APC/C, independent of associated Cdk1 activity, to ensure efficient processing of cyclin B1 as an APC/C substrate in metaphase.
Discussion
In this study, we examined how cyclin B1 is recruited to the APC/C in mitotic cells. We found that a significant fraction of cyclin B1 is bound to the phosphorylated APC/C in an APC3-dependent but D box–independent manner, even when the spindle checkpoint stabilizes cyclin B1.
The APC/C from Xenopus mitotic extracts binds an N-terminal, D box–containing fragment of fission yeast cyclin B (Yamano et al., 2004). In contrast, we did not detect binding of an ectopically expressed N-terminal region of cyclin B1 and the phosphorylated APC/C in human mitotic cell extracts, arrested by proteasomal inhibition, unless we overexpressed a sixfold repeat of the D box–containing cyclin B1 N terminus. Although specific for an intact D box, published in vitro interactions of the fission yeast cyclin B D box fragment and Xenopus APC/C were also reported to be of low affinity, requiring high concentrations of the D box region (Yamano et al., 2004; Eytan et al., 2006).
Figs. 2, 4, 7, and 8 show that cyclin B1 depends on Cdk1–Cks for recruitment to the APC/C not just as a kinase but also as an APC/C substrate. Cks-mediated prerecruitment of cyclin B1 becomes effective after the spindle checkpoint is satisfied in metaphase and supports efficient cyclin B1 destruction. The pool of cyclin B1–Cdk1–Cks that was not prebound to the mitotic APC/C in prometaphase may flux more efficiently onto the APC/C in metaphase. Although we show that cyclin B1–Cdk1–Cks also plays a role in activating overall APC/CCdc20 activity, we thus demonstrate an additional, APC/C-docking function of Cks, facilitating cyclin B1 destruction. In Xenopus extracts, Cks was reported to predominantly direct total APC/C activity (Patra and Dunphy, 1998). Apart from evolutionary differences, possibly higher levels of APC/C activity in extracts arrested by nondegradable cyclin B1, differences in processivity of recombinant cyclin B1 as compared with endogenous cyclin B1, or reduced time resolution in comparison with live cell microscopy experiments may explain why an APC/C-docking role for Cks was not found in Xenopus extracts.
Proteasome-dependent protein destruction depends on a ubiquitin chain of significant length, so productive polyubiquitination either requires sequential rounds of encounters between ubiquitin ligase and substrate or the ability of the ubiquitination enzyme to retain its substrate while ubiquitin chains are formed (Rape et al., 2006). Cks-dependent retention of cyclin B1 to the APC/C may therefore enhance processivity of cyclin B1 ubiquitination in human cells, facilitating switch-like turnover in metaphase. This is physiologically relevant, as it can protect against a cytokinesis defect and mitotic delay (Wolf et al., 2006; for review see Sullivan and Morgan, 2007). Recently, we observed that slow, APC/CCdc20-dependent loss of cyclin B1, which permits cells to slip out of a spindle drug–induced mitotic arrest (Brito and Rieder, 2006; Huang et al., 2009), may also depend on the substrate-capturing mechanism we describe in this study (unpublished data). This provides additional evidence that cyclin B1 binds to spindle checkpoint–inhibited APC/CCdc20 as a substrate. It also suggests further implications of our findings because mitotic slippage could contribute to survival of cancer cells after treatment with antimitotic cancer drugs (Huang and Mitchison, 2009). In this respect, it is also interesting that Cks1 and Cks2 are often up-regulated in cancer (van ’t Veer et al., 2002; Inui et al., 2003; Masuda et al., 2003; Kitajima et al., 2004; Shapira et al., 2005; Keller et al., 2007).
Apparently, an APC/C targeting mechanism by Cks is shared between cyclin A and cyclin B1. This sheds light on the different timings of their destruction. We found that spindle checkpoint–independent destruction of cyclin A is preceded by strong binding of Cdc20 to the N-terminal destruction motif of A-type cyclins in G2 phase. Such binding is probably direct and was not found for cyclin B1 (Ohtoshi et al., 2000; Wolthuis et al., 2008; this study). This indicates that the N terminus of cyclin A is unique in that it effectively competes with spindle checkpoint proteins for Cdc20 binding. Interestingly, Mad2 coimmunoprecipitates with the cyclin B1–APC/CCdc20 complexes formed in mitosis, whereas cyclin A–Cdc20 complexes bind poorly to Mad2 (Wolthuis et al., 2008; this study). In conclusion, both mitotic cyclins are recruited to the prometaphase APC/CCdc20 in a manner dependent on Cks, yet only cyclin A is immediately processed and critically requires Cks for timely onset of destruction. Cdc20 bound to the N terminus of cyclin A evades checkpoint control (van Zon and Wolthuis, 2010). Cyclin B1 is also recruited to phosphorylated APC/CCdc20 in a Cks-dependent and spindle checkpoint–independent manner, but D box–dependent processing of the APC/C-bound cyclin B1 awaits inactivation of the checkpoint.
As the spindle checkpoint does not prevent formation of stable complexes between cyclin B1, the phosphorylated APC/C and Cdc20, our results imply that release of the checkpoint triggers APC/CCdc20 to bind or recognize the cyclin B1 D box as a step secondary to cyclin B1 recruitment. APC/C complexes isolated from checkpoint-arrested cells indeed interacted better with recombinant N-terminal fragments of fission yeast cyclin B1 upon checkpoint silencing (Herzog et al., 2009). In such a successive substrate-binding mechanism, D box binding to APC/CCdc20 may control correct orientation of the substrate toward the APC/C catalytic site, as initially suggested by Burton and Solomon (2001), or direct a further, Cdc20-dependent APC/C activation (Burton et al., 2005; Kimata et al., 2008; Matyskiela and Morgan, 2009). However, exactly how recognition of the cyclin B1 D box stimulates cyclin B1 ubiquitination, as a result of inactivation of the mitotic spindle checkpoint, is still unknown.
Materials and methods
Plasmids
Vectors expressing cyclin B1–Venus or cerulean, ΔN–cyclin B1–ECFP, and cyclin B1–R202A–Venus provided by J. Pines and T. Matsumoto (University of Cambridge, Cambridge, England, UK). Vectors expressing the N-terminal cyclin B1 fragments used in Fig. 7 D were provided by M. Brandeis (Hebrew University of Jerusalem, Jerusalem, Israel). The other N-terminal cyclin B1 constructs were made by replacing full-length cyclin B1 from the cyclin B1–Venus or cerulean vectors with a PCR fragment coding for the first 86 amino acids of cyclin B1 to generate cyclin B1–N–Venus or the cerulean version. Two- and sixfold repeats of this region (Fig. 7) were generated by PCR. Insertion of another PCR fragment coding for full-length Cks1 (from Cks1-YFP; Wolthuis et al., 2008) resulted in cyclin B1–N–Cks1–Venus or a cerulean version. The generation of vectors expressing wild type–securin-Venus, Cdk1*–EYFP, and Cdk1*–1N–EYFP was described previously (Wolthuis et al., 2008). GFP-INCENP was provided by G. Vader (The Whitehead Institute for Biomedical Research, Cambridge, MA). To generate the cyclin B1–Cherry construct, a fragment coding cyclin B1 from cyclin B1–Venus was ligated in a Cherry expression vector (provided by L. Janssen, Nederlands Kanker Instituut, Amsterdam, Netherlands). All generated constructs were sequence verified.
Cell culture, transfection, selection, and synchronization
The human osteosarcoma cell line U2OS was cultured in DME in the presence of 8% FCS with 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were transfected with plasmids using a standard calcium phosphate transfection protocol. For the transfections of siRNA pools, we used lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Cells were seeded in 100-mm dishes and cotransfected with 10 or 20 µg of the indicated pSuper-shRNAi constructs and/or 1 µg of indicated expression constructs and cotransfected with 1 µg pBabePuro for selection of transfected cells with puromycin, which was used in a 2 µg/ml end concentration during 24 h. Mitotic cells were collected by a gentle mitotic shake off, either arrested by APC3 RNAi, APC2 RNAi, or arrested with an active spindle checkpoint by adding nocodazole to the culture medium at a final concentration of 250 ng/ml for 15 or 12 h after thymidine arrest (the latter also for Fig. S3 A, but without any drugs, leaving the mitotic cells unperturbed). To collect a G2 population, cells were first blocked in the G1/S transition by thymidine in a final concentration of 2.5 mM for 24 h. Cells were released from the G1/S arrest in the presence of nocodazole. After 12 h, mitotic cells were removed, after which plates were thoroughly washed and remaining G2 cells were collected. To obtain spindle checkpoint–inactivated mitotic cells, cells were released from a nocodazole arrest in the presence of 5 µM of the proteasome inhibitor MG132. Mitotic cells that had entered mitosis in the presence of MG132, added 10 h after release from thymidine, were collected 15 h after release. Roscovitine was used to force the mitotic cells into G1 at a final concentration of 50 µM for 45 min. In the experiment shown in Fig. 7 E, cells collected by mitotic shake off after taxol treatment were released from spindle checkpoint arrest by addition of 1 µM of the aurora A inhibitor ZM447439 and kept in mitosis by simultaneous addition of MG132. In this case, complete checkpoint release was confirmed by monitoring formation of a 4N G1 population in a fraction of cells to which no MG132 was added. Flow cytometry was used to confirm synchrony: cells indicated as G2 were usually >90% 4N as detected by PI staining, and <10% MPM2 positive, whereas mitotic cells collected by careful mitotic shake off were 90–95% 4N and stained >90% positive for the mitotic marker MPM2. When nocodazole-arrested cells were collected by mitotic shake off and the drug was washed out, >90% of the cells divided within 2 h as detected by a near complete shift to 2N G1 cells. For flow cytometry, cells were fixed in ice-cold 70% ethanol and stained with mouse anti-MPM2 antibody (United Biomedical, Inc.) and CY-5–coupled anti–mouse (Dako). Cell cycle distribution and MPM2 positivity of transfected cells were determined as described previously (van Vugt et al., 2004).
RNAi
pSuper vectors were used for expression of shRNAi targeting human APC3 mRNA. Hybridized oligos containing specific 19-mer target sequences were ligated into pSuper (5′-TAGCCGAGAGGTAACTCCA-3′). For pSuper APC2, see Fig. S5 C. The pSuper vectors targeting mRNA of Cdk1 or BubR1 were previously described (Lens et al., 2003; Lindqvist et al., 2007). Annealed siRNA pools to target mRNA of APC3, APC2, Cdc20, Cks1, Cks2, and Plk1 were purchased from Thermo Fisher Scientific as ON-TARGETplus SMART pools.
IPs and Western blots
Cells were lysed in ELB+ (150 mM NaCl, 50 mM Hepes, pH 7.5, 5 mM EDTA, 0.3% NP-40, 10 mM β-glycerophosphate, 6% glycerol, 5 mM NaF, 1 mM Na2VO3, and protease inhibitor cocktail [Roche]). Lysates were cleared by centrifugation at 13,000 g for 8 min at 4°C. Protein levels were measured using standard Bradford analysis. For each IP, we precoupled 2 µg antibodies to 20 µl protein G Sepharose (GE Healthcare) overnight. Washed precoupled beads and cleared extracts were incubated 3–12 h at 4°C. Immunocomplexes were washed four times with ELB and collected in 50 µl protein sample buffer or directly used in ubiquitination assays. 25 µl was separated on polyacrylamide gels and blotted to nitrocellulose. For IPs, the following antibodies were used: goat anti-Cdc16/APC6 (Santa Cruz Biotechnology, Inc.), goat anti-APC4 (C18; Santa Cruz Biotechnology, Inc.), rabbit anti-Cdc20 (H-175; Santa Cruz Biotechnology, Inc.), rabbit anti–cyclin A (H-432; Santa Cruz Biotechnology, Inc.), rabbit anti–cyclin B1 (H-433; Santa Cruz Biotechnology, Inc.), rabbit anti-securin (Invitrogen), goat anti-Cdk4 (C-2; Santa Cruz Biotechnology, Inc.), and rabbit anti-GFP (provided by Neefjes laboratory, Nederlands Kanker Instituut, Amsterdam, Netherlands). For detecting proteins, the following antibodies were used: rabbit anti-APC2 (provided by H. Yu, University of Texas Southwestern Medical Center, Dallas, TX), mouse anti-APC3/Cdc27 (Transduction Laboratories), goat anti-APC4 (C18S; Santa Cruz Biotechnology, Inc.), rabbit anti-GFP (26), mouse anti-HA (HA-7; Sigma-Aldrich), mouse anti-Mad2 (MBL International), mouse anti–cyclin A (Thermo Fisher Scientific), mouse anti–cyclin B1 (GNS1), mouse anti-securin (Abcam), rabbit anti-Plk1 (Invitrogen), mouse anti-Cdc20 (E7; Santa Cruz Biotechnology, Inc.), mouse anti-Cdh1 (Thermo Fisher Scientific), mouse anti-Cdk1 (Transduction Laboratories), and rabbit anti-Cdk2 (M2; Santa Cruz Biotechnology, Inc.). Secondary PO-conjugated antibodies were obtained from Dako. Quantitation of Western blots was performed by comparing the different exposure times of autoradiograms to blots, avoiding saturation of the film. Estimation of the amount of cyclin B1 associated with APC/C was on the basis of the fold enrichment of Cdk1 signal in cyclin B1 IPs (in relation to the Cdk1 signals in whole cell extracts, run on the same blots), as compared with the fold enrichment of APC/C subunits or Cdc20 in the same cyclin B1 IPs. Top regions of the blots were probed for APC/C subunits and bottom regions for Cdk1. Typically, in our (nonsaturating) cyclin B1 IP conditions, we collected no detectable cyclin A or securin, and ∼5% of the cellular pool of APC2 or APC3 and 10–20% of the cellular pool of Cdk1 (Fig. 5 D, bottom; IPs are loaded next to 10% of the whole cell extracts on the same blots). Calculations of the concentration of mitotic cyclin B1 in U2OS cells are in the order of magnitude of ≤10 nM (Arooz et al., 2000), whereas Cdk1 and APC3 are in the range of 100 nM (Arooz et al., 2000; Kulukian et al., 2009). This suggests that at least 25–50% of endogenous cyclin B1 is in complex with mitotic APC/C. This fits well with our findings in an alternative approach: in sucrose gradients shown in Fig. 8, we found ∼40% of endogenous cyclin B1 or Cks1-tagged cyclin B1 N-terminal fragment comigrated with the peak of APC/CCdc20.
Sucrose gradient centrifugation
Spindle checkpoint–arrested mitotic cells expressing cyclin B1–N–Venus or cyclin B1–N–Cks–Venus were collected, and lysates were subjected to sucrose gradient centrifugation. Gradients were made using 15–40% sucrose solutions. Mitotic lysates (collected from four 9-cm dishes of nocodazole-treated, transfected cells) were loaded on 10 ml of gradient and centrifuged in a swinging-bucket type rotor (Ti SW 41; Beckman Coulter) at 4°C for 20 h at 40,000 rpm. 1-ml fractions were collected. 20 µl of the indicated fractions were blotted and probed with antibodies to detect comigration of expressed cyclin B1 fragments with endogenous cyclin B1, APC/C, Cdc20, or Cdh1.
Ubiquitination assay
As a source of APC/C, cyclin B1 IPs were performed as described in IPs and Western blots using equal amounts of protein from cells synchronized in G2, 8 h after thymidine release (G2) or from mitotic cells collected by mitotic shake-off (after thymidine release plus nocodazole treatment). The latter cells were released from nocodazole but kept in mitosis by addition of the proteasome inhibitor MG132 (NR, MG132). Control GFP IPs were performed on nocodazole-arrested mitotic cells. IPs were washed twice with ELB+ and twice with protein buffer (20 mM Hepes, pH 7.8, 2.5% glycerol, 6 mM MgCl2, 30 mM KCl, and 2 mM β-ME). Ubiquitination reactions were performed in protein buffer containing 1 µg HA-tagged ubiquitin, 0.1 µg His-tagged E1, 0.5 µg His-tagged E2 (UbcH10), and 5 mM ATP. Human HA-ubiquitin and human His-E2 (UbcH10) were obtained from Boston Biochemicals. Human His-E1 and E2-25K were provided by T. Sixma (Nederlands Kanker Instituut, Amsterdam, Netherlands). Reactions were started by addition of the IPs, incubated for 30 min at 30°C. Samples were separated on 10% SDS-PAGE gel, blotted to nitrocellulose, and probed with mouse anti-HA antibodies. For the assays in Fig. S3, cyclin B1 was precipitated from asynchronous cells and NR, MG132 cells (Fig. S3, E and F) or G2, M, and G1 cells (Fig. S3 G). Purified securin-His was added to the E1, E2, ubiquitin, and ATP mix as an in vitro APC/C substrate of the ubiquitination assay. The securin-His vector for protein expression in bacteria was provided by J. Pines (Nilsson et al., 2008). The in vitro reactions were blotted and probed with anti-securin antibodies. Further controls are shown in Fig. S3.
Time-lapse fluorescence microscopy
Cells plated on 35-mm glass-bottom culture dishes (WillCo Wells) were transfected the following day with 0.1 µg of the indicated expression constructs except for 0.015 µg securin-Venus and 0.025 µg N-terminal cyclin B1 constructs. For shRNAi, 1 µg pSuper-Cdk1 was coexpressed with the indicated constructs (together with 1 µg pSuper-BubR1; Fig. 1 E, right). Dishes were transferred to a heated culture chamber (5% CO2 at 37°C) on a microscope (Axiovert 200M; Carl Zeiss, Inc.) equipped with an NA 0.55 condenser and a 40× NA 1.3 Plan Neo differential interference contrast objective using a charge-coupled device camera (CoolSNAP HQ; Photometrics) with a GFP/DsRed or CFP/YFP dual-band pass filter set (Chroma Technology Corp.) to select specific fluorescence. Images were taken after 100-ms exposure time at 2× binning for the cyclin B1 and securin experiments, and quantitated and processed using MetaMorph software (Universal Imaging) and Excel (Microsoft).
Online supplemental material
Fig. S1 shows localization of cyclin B1 (EYFP) and Cdk1*–EYFP in cells, stability of the fluorescent Cdk1 constructs during mitosis, and phosphorylation of APC3 under different conditions. Fig. S2 shows destruction times of fluorescent cyclin B1 and securin constructs under different conditions. Fig. S3 shows that cyclin B1 is in complex with APC/CCdc20 in mitosis, which we do not detect for securin. Ubiquitination assays with securin as a substrate are also shown. Fig. S4 shows that APC3 RNAi causes a mitotic arrest, which can be overridden by adding the Cdk1 inhibitor roscovitine. Fig. S5 shows the stability of APC/C substrates in APC3 RNAi cells and the loss of the cyclin B1 interaction with remaining APC/CCdc20 complexes in these cells, whereas Cdc20 is still bound to remaining APC/C complexes. Video 1 shows mitotic progression of a cell in which Cdk1 RNAi was complemented by Cdk1*–EYFP expression. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200912084/DC1.
Acknowledgments
We thank Barbara di Fiore and Jon Pines for sharing data before publication. This work is funded by a Vidi grant from The Netherlands Organization for Fundamental Research (NWO) and by the KWF (Dutch Cancer Society; grants NKI 2003-2967, NKI 2007-3789, and NKI 2008-4135).
Note added in proof. Recently, we learned that di Fiore and Pines (2010), in a study published in this issue, show that Cks supports APC/C-mediated ubiquitination of cyclin A. Their work also reveals that cyclin A binds Cdc20 and competes with checkpoint proteins. Our combined results support a model in which Cks directs recruitment of both mitotic cyclins to the phosphorylated APC/C, but complexes between the N terminus of cyclin A and Cdc20 evade checkpoint control. This would explain why cyclin A destruction starts in prometaphase, when the APC/C is phosphorylated. Recognition of the destruction region of cyclin B1 by APC/CCdc20 awaits release of the spindle checkpoint.
Footnotes
Abbreviations used in this paper:
- APC/C
- anaphase-promoting complex/cyclosome
- D box
- destruction box
- IP
- immunoprecipitation
- NEB
- nuclear envelope breakdown
References
- Arooz T., Yam C.H., Siu W.Y., Lau A., Li K.K., Poon R.Y. 2000. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry. 39:9494–9501 10.1021/bi0009643 [DOI] [PubMed] [Google Scholar]
- Bentley A.M., Normand G., Hoyt J., King R.W. 2007. Distinct sequence elements of cyclin B1 promote localization to chromatin, centrosomes, and kinetochores during mitosis. Mol. Biol. Cell. 18:4847–4858 10.1091/mbc.E06-06-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.L., Solomon M.J. 2001. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 15:2381–2395 10.1101/gad.917901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.L., Tsakraklides V., Solomon M.J. 2005. Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol. Cell. 18:533–542 10.1016/j.molcel.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Brito D.A., Rieder C.L. 2006. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 16:1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnel F., Bazile F., Pascal A., Kubiak J.Z. 2007. Cyclin B2/cyclin-dependent kinase1 dissociation precedes CDK1 Thr-161 dephosphorylation upon M-phase promoting factor inactivation in Xenopus laevis cell-free extract. Int. J. Dev. Biol. 51:297–305 10.1387/ijdb.072292fc [DOI] [PubMed] [Google Scholar]
- Clute P., Pines J. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1:82–87 10.1038/10049 [DOI] [PubMed] [Google Scholar]
- den Elzen N., Pines J. 2001. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153:121–136 10.1083/jcb.153.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan P.J., Reed S.I. 2003. Germline exclusion of Cks1 in the mouse reveals a metaphase I role for Cks proteins in male and female meiosis. Cell Cycle. 2:275–276 [PubMed] [Google Scholar]
- Draviam V.M., Orrechia S., Lowe M., Pardi R., Pines J. 2001. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 152:945–958 10.1083/jcb.152.5.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eytan E., Moshe Y., Braunstein I., Hershko A. 2006. Roles of the anaphase-promoting complex/cyclosome and of its activator Cdc20 in functional substrate binding. Proc. Natl. Acad. Sci. USA. 103:2081–2086 10.1073/pnas.0510695103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S., Skaar J.R., Pagano M. 2009. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 8:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S., Kramer E., Gieffers C., Gannon J., Peters J.M., Hunt T. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137–148 10.1083/jcb.153.1.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., Den Elzen N., Vodermaier H.C., Waizenegger I.C., Peters J.M., Pines J. 2002. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157:1125–1137 10.1083/jcb.200111001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M.J., Kimata Y., Wattam S.L., Lindon C., Mao G., Yamano H., Fry A.M. 2006. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 8:607–614 10.1038/ncb1410 [DOI] [PubMed] [Google Scholar]
- Herzog F., Primorac I., Dube P., Lenart P., Sander B., Mechtler K., Stark H., Peters J.M. 2009. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 323:1477–1481 10.1126/science.1163300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilioti Z., Chung Y.S., Mochizuki Y., Hardy C.F., Cohen-Fix O. 2001. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 11:1347–1352 10.1016/S0960-9822(01)00399-2 [DOI] [PubMed] [Google Scholar]
- Huang H.C., Shi J., Orth J.D., Mitchison T.J. 2009. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 16:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui N., Kitagawa K., Miwa S., Hattori T., Chida K., Nakamura H., Kitagawa M. 2003. High expression of Cks1 in human non-small cell lung carcinomas. Biochem. Biophys. Res. Commun. 303:978–984 10.1016/S0006-291X(03)00469-8 [DOI] [PubMed] [Google Scholar]
- Keller U.B., Old J.B., Dorsey F.C., Nilsson J.A., Nilsson L., MacLean K.H., Chung L., Yang C., Spruck C., Boyd K., et al. 2007. Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J. 26:2562–2574 10.1038/sj.emboj.7601691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y., Baxter J.E., Fry A.M., Yamano H. 2008. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol. Cell. 32:576–583 10.1016/j.molcel.2008.09.023 [DOI] [PubMed] [Google Scholar]
- Kitajima S., Kudo Y., Ogawa I., Bashir T., Kitagawa M., Miyauchi M., Pagano M., Takata T. 2004. Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am. J. Pathol. 165:2147–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G.J. 2008. The kinetochore and spindle checkpoint in mammals. Front. Biosci. 13:3606–3620 10.2741/2953 [DOI] [PubMed] [Google Scholar]
- Kraft C., Vodermaier H.C., Maurer-Stroh S., Eisenhaber F., Peters J.M. 2005. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell. 18:543–553 10.1016/j.molcel.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Kulukian A., Han J.S., Cleveland D.W. 2009. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell. 16:105–117 10.1016/j.devcel.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens S.M., Wolthuis R.M.F., Klompmaker R., Kauw J., Agami R., Brummelkamp T., Kops G., Medema R.H. 2003. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. EMBO J. 22:2934–2947 10.1093/emboj/cdg307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., van Zon W., Karlsson Rosenthal C., Wolthuis R.M.F. 2007. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 5:e123 10.1371/journal.pbio.0050123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A., Rodríguez-Bravo V., Medema R.H. 2009. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185:193–202 10.1083/jcb.200812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson-Ahlzén H.S., Liberal V., Grünenfelder B., Chaves S.R., Spruck C.H., Reed S.I. 2008. Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol. Cell. Biol. 28:5698–5709 10.1128/MCB.01833-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T.A., Inoue H., Nishida K., Sonoda H., Yoshikawa Y., Kakeji Y., Utsunomiya T., Mori M. 2003. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin. Cancer Res. 9:5693–5698 [PubMed] [Google Scholar]
- Matyskiela M.E., Morgan D.O. 2009. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol. Cell. 34:68–80 10.1016/j.molcel.2009.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J., Yekezare M., Minshull J., Pines J. 2008. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10:1411–1420 10.1038/ncb1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Tachibana K., Igarashi Y., Yasuda H., Tanahashi N., Tanaka K., Ohsumi K., Kishimoto T. 2000. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 14:2344–2357 10.1101/gad.823200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtoshi A., Maeda T., Higashi H., Ashizawa S., Hatakeyama M. 2000. Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex. Biochem. Biophys. Res. Commun. 268:530–534 10.1006/bbrc.2000.2167 [DOI] [PubMed] [Google Scholar]
- Passmore L.A., Barford D. 2005. Coactivator functions in a stoichiometric complex with anaphase-promoting complex/cyclosome to mediate substrate recognition. EMBO Rep. 6:873–878 10.1038/sj.embor.7400482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D., Dunphy W.G. 1996. Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes Dev. 10:1503–1515 10.1101/gad.10.12.1503 [DOI] [PubMed] [Google Scholar]
- Patra D., Dunphy W.G. 1998. Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase- promoting complex at mitosis. Genes Dev. 12:2549–2559 10.1101/gad.12.16.2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D., Wang S.X., Kumagai A., Dunphy W.G. 1999. The xenopus Suc1/Cks protein promotes the phosphorylation of G(2)/M regulators. J. Biol. Chem. 274:36839–36842 10.1074/jbc.274.52.36839 [DOI] [PubMed] [Google Scholar]
- Pines J. 1996. Cell cycle: reaching for a role for the Cks proteins. Curr. Biol. 6:1399–1402 10.1016/S0960-9822(96)00741-5 [DOI] [PubMed] [Google Scholar]
- Pines J. 2006. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16:55–63 10.1016/j.tcb.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. 1994. The differential localization of human cyclins A and B is due to a cytoplasmic retention signal in cyclin B. EMBO J. 13:3772–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape M., Reddy S.K., Kirschner M.W. 2006. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 124:89–103 10.1016/j.cell.2005.10.032 [DOI] [PubMed] [Google Scholar]
- Sczaniecka M.M., Hardwick K.G. 2008. The spindle checkpoint: how do cells delay anaphase onset? SEB Exp Biol Ser. 59:243–256 [PubMed] [Google Scholar]
- Shapira M., Ben-Izhak O., Linn S., Futerman B., Minkov I., Hershko D.D. 2005. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 103:1336–1346 10.1002/cncr.20917 [DOI] [PubMed] [Google Scholar]
- Shteinberg M., Hershko A. 1999. Role of Suc1 in the activation of the cyclosome by protein kinase Cdk1/cyclin B. Biochem. Biophys. Res. Commun. 257:12–18 10.1006/bbrc.1999.0409 [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Rape M., Draviam V.M., Nalepa G., Sowa M.E., Ang X.L., McDonald E.R., III, Li M.Z., Hannon G.J., Sorger P.K., et al. 2007. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 446:876–881 10.1038/nature05694 [DOI] [PubMed] [Google Scholar]
- Stewart E., Kobayashi H., Harrison D., Hunt T. 1994. Destruction of Xenopus cyclins A and B2, but not B1, requires binding to p34cdc2. EMBO J. 13:584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V., Shteinberg M., Ganoth D., Hershko J., Hershko A. 1997. Binding of activated cyclosome to p13(suc1). Use for affinity purification. J. Biol. Chem. 272:18051–18059 10.1074/jbc.272.29.18051 [DOI] [PubMed] [Google Scholar]
- Sullivan M., Morgan D.O. 2007. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 8:894–903 10.1038/nrm2276 [DOI] [PubMed] [Google Scholar]
- Sullivan M., Holt L., Morgan D.O. 2008. Cyclin-specific control of ribosomal DNA segregation. Mol. Cell. Biol. 28:5328–5336 10.1128/MCB.00235-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U., Robitsch H., Price C., Schuster T., Fitch I., Futcher A.B., Nasmyth K. 1991. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 65:145–161 10.1016/0092-8674(91)90416-V [DOI] [PubMed] [Google Scholar]
- Thornton B.R., Toczyski D.P. 2003. Securin and B-cyclin/CDK are the only essential targets of the APC. Nat. Cell Biol. 5:1090–1094 10.1038/ncb1066 [DOI] [PubMed] [Google Scholar]
- van ’t Veer L.J., Dai H., van de Vijver M.J., He Y.D., Hart A.A., Mao M., Peterse H.L., van der Kooy K., Marton M.J., Witteveen A.T., et al. 2002. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 415:530–536 10.1038/415530a [DOI] [PubMed] [Google Scholar]
- van Leuken R., Clijsters L., Wolthuis R. 2008. To cell cycle, swing the APC/C. Biochim. Biophys. Acta. 1786:49–59 [DOI] [PubMed] [Google Scholar]
- van Vugt M.A., van de Weerdt B.C., Vader G., Janssen H., Calafat J., Klompmaker R., Wolthuis R.M., Medema R.H. 2004. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J. Biol. Chem. 279:36841–36854 10.1074/jbc.M313681200 [DOI] [PubMed] [Google Scholar]
- van Zon W., Wolthuis R.M.F. 2010. Cyclin A and Nek2A: APC/C-Cdc20 substrates invisible to the mitotic spindle checkpoint. Biochem. Soc. Trans. 38:72–77 10.1042/BST0380072 [DOI] [PubMed] [Google Scholar]
- Wolf F., Wandke C., Isenberg N., Geley S. 2006. Dose-dependent effects of stable cyclin B1 on progression through mitosis in human cells. EMBO J. 25:2802–2813 10.1038/sj.emboj.7601163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthuis R., Clay-Farrace L., van Zon W., Yekezare M., Koop L., Ogink J., Medema R., Pines J. 2008. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell. 30:290–302 10.1016/j.molcel.2008.02.027 [DOI] [PubMed] [Google Scholar]
- Yamano H., Gannon J., Mahbubani H., Hunt T. 2004. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell. 13:137–147 10.1016/S1097-2765(03)00480-5 [DOI] [PubMed] [Google Scholar]
- Yanagida M. 2005. Basic mechanism of eukaryotic chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:609–621 10.1098/rstb.2004.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. 2007. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell. 27:3–16 10.1016/j.molcel.2007.06.009 [DOI] [PubMed] [Google Scholar]