Abstract
The longitudinal heterologous neutralization response against two HIV-1 subtype C isolates was studied in 33 ART-naive individuals recently infected with HIV-1 subtype C from India. Seven of 33 (21%) seroconverters demonstrated a consistent response against both isolates (65–100% neutralization), whereas the remaining 26 (79%) were nonresponders. Four of the seven responders demonstrated a neutralization response (>75% neutralization) within 2–3 months of infection and in the remaining three, the response was demonstrated between 22 and 38 months after infection. In the past, HIV vaccines targeted the V3 region for the development of neutralizing antibodies. However, recent studies have shown that anti-V3 antibodies are generated after HIV-1 infection, but are not effective in neutralizing virus. In this study, the V3 sequences of HIV-1 from seven responders were analyzed and compared with those from nonresponders. The V3 region sequences from early and late responders did show certain mutations that were not found in the nonresponders; however none of these mutations could explain the neutralization responses. This suggested that HIV-1 envelope regions other than the V3 domain may be involved in generating a neutralization response. This is the first report that describes the pattern of emergence and persistence of the heterologous neutralization response in recently HIV-1 subtype C-infected individuals from India and studies its association with sequence variation in the V3 region.
Early reports using envelope glycoprotein gp120 have reported success in preventing HIV infection in nonhuman primate models.1,2 Soon it became apparent that the envelope proteins did not induce neutralizing antibodies (NAbs) that could neutralize primary isolates. However, the isolation of monoclonal antibodies (MAbs) that target conserved epitopes of the HIV envelope protein and show a broad neutralization ability, passive immunization, and subsequent protection of macaques with cocktails of these MAbs against challenge with pathogenic HIV-1/SIV chimeric virus (SHIVs) has recently focused attention on neutralizing antibodies.3,4 This is also supported by a recent study reporting an inverse correlation between HIV-1 incidence and peak neutralizing antibody levels conducted during a randomized trial of rgp120 vaccine.5
Considering the encouraging results obtained in the recent past, a number of strategies are being actively pursued in an effort to identify and generate new immunogens that aim to overcome limitations of the early prototypes. Many of these new approaches are focusing on recently transmitted CCR5 tropic, non-syncytium-inducing (NSI) isolates, since the immune response generated against recently transmitted, immunologically naive virus is responsible for efficient control of virus multiplication leading to a better prognosis.6 Various studies have shown the emergence of HIV-1-specific neutralization activity in seroconverters.7–13 However, the time at which they emerge and their specific role during primary HIV infection as well as during disease progression are still unclear.
The majority of the observations about the neutralization response reported so far have been made in HIV-1 subtype B infections and data on NAb responses in non-subtype B infections are limited. HIV-1 subtype C is the predominant subtype in India with occasional reports of subtypes A and B.14,15 Available data on Indian HIV-1 gp120 subtype C sequences suggest that they tend to segregate away from subtype C strains from other regions of the world.16,17 Recognizing the existence of biological and behavioral vulnerability in many at-risk subpopulations and challenges in sustaining a behavioral change, a number of HIV vaccine trials are being contemplated in India. In addition to the basic knowledge about circulating HIV subtypes, the identification of immune correlates associated with subtype C infection is essential in implementing strategies for vaccine development in India. The cytotoxic T-lymphocyte (CTL) responses in Indian subtype C-infected individuals have been studied18; however, data on NAb responses are scarce.
The V3 region, an exposed accessible loop of gp120, is the main target of NAbs as it plays a central role in viral tropism and entry and induces production of anti-V3 antibodies, either after natural infection or specific immunization, which block either the attachment of the virus to the cell or subsequent postbinding events. Earlier reports demonstrate that amino acid (AA) substitution in the V3 region affects viral sensitivity to neutralization and is selected by NAbs.19 Since the neutralizing activity may be highly type specific, understanding of structural motifs of the HIV env protein, responsible for generating NAb responses in primary infection, may help in the development of an immunogen capable of neutralizing recently transmitted viruses. While studying the dynamics of the neutralization response in a cohort of seroconverters, an attempt was made to explore the association between absolute CD4 count, plasma viral load, NAb response, and sequence variation in the V3 domain of the env gene. This is the first report that describes the pattern of emergence and persistence of the NAb response in subtype C-infected individuals recently infected in India, which might influence future vaccine studies (oral presentation: AIDS Vaccine 2005 International Conference, Montreal, Canada).
Between May 1995 and December 1999, 87 blood samples were collected (in EDTA vacutainer tubes) from 33 antiretroviral treatment (ART)-naive HIV-1 seroconverters after obtaining informed consent. Out of 33, 25 seroconverters were males and eight were females with a mean age of 28 years (range 17–47 years) and 26 years (range 18–35 years), respectively. These seroconverters were part of a cohort of patients “at risk” for HIV infection attending sexually transmitted diseases (STD) clinics in Pune, India, who were screened for anti-HIV antibodies at 3-month intervals. The HIV diagnosis was carried out using HIV-1 and HIV-2 Combi ELISA (Recombigen HIV-1/HIV-2, Cambridge Biotech, Galway, Ireland, and Genetic Systems, Genelabs Diagnostics, Singapore). Reactive samples were tested further by a rapid test (Recombigen HIV-1/HIV-2 Rapid Test Device, Cambridge Biotech, Galway, Ireland). ELISA reactive samples were subjected to HIV-1 Western blot (Cambridge Biotech, Galway, Ireland).
The recent infection was confirmed by antibody seroconversion. Those who developed anti-HIV antibody in the subsequent visit were classified as recent seroconverters. The date of primary HIV infection was estimated as the mid point between the last negative antibody test and the first positive antibody test. The mean time from seroconversion to sample collection for neutralization was 3.3 months (range 1–9 months). The plasma was collected at two time points from 22 seroconverters and more than three time points from the remaining 11 subjects. The samples were collected up to 4–55 months after the estimated date of HIV infection.
During follow-up, CD4 cell counts were estimated by two-color analysis using anti-CD4 antibodies conjugated with phycoerythrin (Becton Dickinson, San Jose, CA) on the whole blood sample. The HIV-1 RNA was quantitated by reverse transcriptase polymerase chain reaction (RT-PCR) using the Amplicor HIV-1 Monitor test, version 1.5 (Roche Molecular Systems, Branchburg, NJ) on plasma stored at − 70°C. The HMA was performed using reagents provided by the NIH AIDS Research and Reference Reagent Program and the HMA technique as previously described.14 The mean absolute CD4 count and log plasma HIV-1 RNA level of these seroconverters at the first available visit were 645.8 cells/mm3 (range 28–1498 cells/mm3) and 4.1 log HIV-1 RNA copies/ml (range 2.37–5.6 log HIV-1 RNA copies/ml), respectively. Genotyping analysis using env HMA revealed that all seroconverters were infected with HIV-1 subtype C.
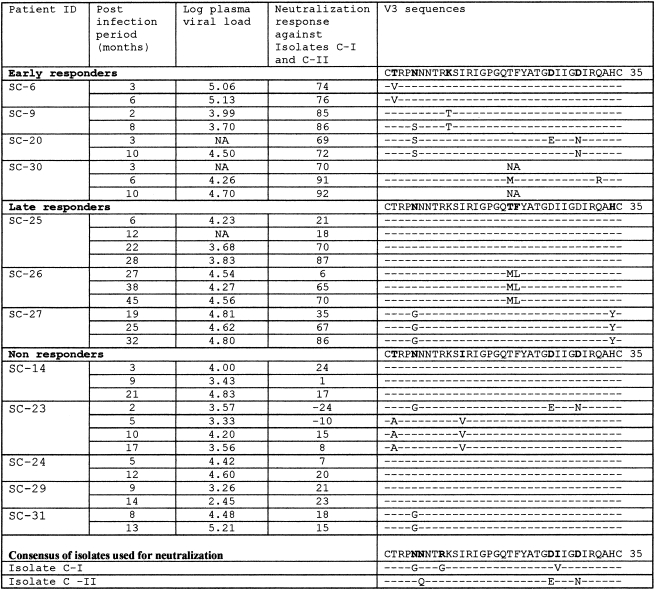
The HIV-1 isolates (isolate C-I and isolate C-II) used for neutralization were obtained using the cocultivation method from HIV-positive heterosexual males. Isolate C-I was obtained in March 1999 and isolate C-II in March 1995. Both isolates were subtype C, CCR5 tropic, NSI and were considered to be closely related to the strain, which is predominant in India, on the basis of homology of the sequences obtained (91% homology). The V3 sequences of the two isolates are given in Table 1. Comparison of the V3 sequences of isolates C-I and C-II with the consensus derived from three clones of the two isolates showed minor variations.
Table 1.
Env V3 Sequences, Plasma Viral Load, and Neutalization Response of Early Responders, Late Responders, and Nonrespondersa
Association of postinfection period, plasma viral load, mean neutralization response (against isolate C-I and C-II), and AA substitution in the V3 region in early responders (n = 4), late responders (n = 3), and nonresponders (n = 5) was studied. The V3 region of three groups appeared to be relatively conserved with minor variations in the sequences.
The viruses were propagated only once in phytohemagglutinin-P (Sigma, USA)-activated peripheral blood mononuclear cells (PBMCs) obtained from an HIV-negative donor and titrated using GHOST-CCR5 cells (a gift from Dr. Zolla-Pazner, New York Veterans Affairs Medical Center, New York, NY). The neutralization response in plasma samples was assessed against two subtype C Indian primary isolates (isolate C-I and isolate C-II) using GHOST-CCR5 cells as described previously.20 Briefly, the cells seeded at a concentration of 6 × 104 cells/well/0.5 ml of growth medium in the 24-well tissue culture plates were allowed to grow for 24 h, when at least 70% confluence was seen. A fixed dilution of the pretitrated virus stock yielding around 8–12% infected cells as determined by green fluorescent protein (GFP) was used in the neutralization assay. Equal volumes of virus and heat-inactivated 1:10 diluted plasma were mixed and incubated at 37°C for 1 h. The GHOST-CCR5 cells were infected in duplicate with the virus–antibody mixture in the presence of DEAE-Dextran (8 μg/ml). After overnight infection, the cells were washed, fed with the growth medium, and incubated for 3–4 days. The monolayers were washed, resuspended in 10 mM EDTA, fixed in 2% formaldehyde, and analyzed by FACSort flow cytometer (Becton Dickinson, San Jose, CA).
The fixed cells were gated based on forward scatter and side scatter. The number of infected cells was determined using a scattergram of fluorescence versus forward scatter after setting the gate with uninfected cells. A total of 15,000 events was scored.
The percent neutralization was calculated as
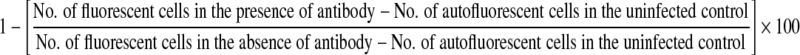 |
The percent neutralization used for analysis was based on a total of 15 assays using 15 HIV-negative plasma samples and two isolates. A panel containing four HIV-negative plasma samples was included in every assay. The mean percent neutralization (isolate C-I = 17.3%, SD = 8.3, and isolate C-II = − 5.3%, SD = 0.03) obtained with four HIV-negative plasma samples was subtracted from the percent neutralization of the respective test sample. For every sample, the percent neutralization was represented as a mean of at least two or more assays. The neutralization response above 50% (obtained after subtracting the background reading) that has been used as an end point in many previous studies11,20,21 was considered as positive neutralization. Seroconverters showing positive neutralization (neutralization > 50%) on all visits were classified as responders and those demonstrating an inconsistent positive neutralization response between the two visits or less than 50% neutralization were classified as nonresponders.
Of the 33 seroconverters, seven (21%) demonstrated a positive heterologous neutralization response against isolate C-I and nine (27%) against isolate C-II (Table 2). When the percent neutralization obtained against both isolates (isolates C-I and C-II) was combined and the mean percent neutralization was calculated, seven (21%) seroconverters demonstrated a positive neutralization response. The remaining 26 (79%) seroconverters did not reveal a significant neutralization response at any of the longitudinally collected specimens. It could be because the viruses used in this study were primary isolates and not the laboratory-adapted strains and hence would have exhibited more resistance toward neutralization.
Table 2.
Neutralization Response Demonstrated by Seroconvertersa
| |
Neutralization response against |
|
|
|---|---|---|---|
| Categorization of seroconverters (n = 33) | Isolate C-I | Isolate C-II | Neutralization response against isolates C-I and C-II |
| Responders | 7 (21%)b | 9 (27%) | 7 (21%)c |
| Nonresponders | 26 (79%) | 24 (73%) | 26 (79%) |
This table shows % neutralization response demonstrated individually against isolates C-I and C-II (column 2) as well as the mean % neutralization against both isolates (column 3). The neutralization > 50% was considered as positive neutralization. Seroconverters showing < 50% neutralization and those demonstrating inconsistent positive neutralization responses between the two visits were considered as nonresponders. Seroconverters showing positive neutralization on all visits were classified as responders.
Number (%).
Of the seven responders showing neutralization against both isolates, four were early responders (responses observed within 6 months postinfection) and three were late responders (responses observed > 6 months postinfection).
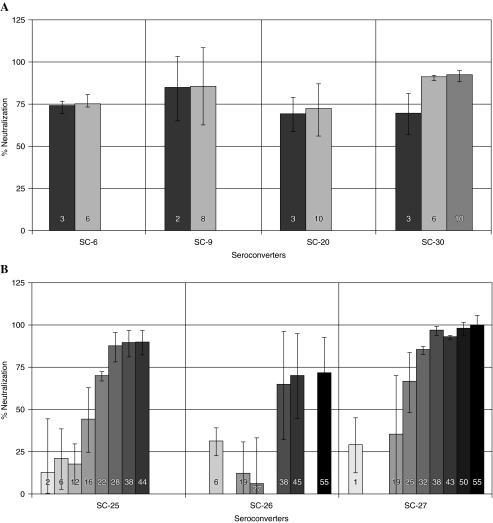
To ascertain the temporality of the emerging neutralizing antibody response in early HIV infection, the seroconverters showing a neutralization response within the first 6 months of infection were categorized as “early responders” and those showing a neutralization response after 6 months were categorized as “late responders.” Four of seven (57%) seroconverters demonstrated an early neutralization response against isolate C-I (one at 2 months and the remaining three at 3 months) and six of nine (67%) seroconverters demonstrated an early neutralization response against isolate C-II (two at 2 months and the remaining four at 3 months). When the percent neutralization against both isolates (isolate C-I and isolate C-II) was combined and the mean percent neutralization response was calculated, four of seven (57%) seroconverters demonstrated an early response. Figure 1A shows the mean neutralization response demonstrated by these four early responders. The neutralization response was demonstrated at 2 months in one seroconverter and at 3 months in the remaining three, which remained consistent up to 6–10 months. However, subsequent samples could not be tested for NAb response due to the lack of an adequate quantity of plasma samples.
FIG. 1.
Neutralization response demonstrated by early and late responders. The numbers on the bars are postinfection months. (A) The mean neutralization response (against isolates C-I and C-II) demonstrated by four early responders. SC-9 demonstrated a neutralization response at 2 months and SC-6, SC-20, and SC-30 at 3 months of infection. The neutralization response remained consistent up to 6–10 months; however, the neutralization response could not be studied subsequently due to the nonavailability of samples. (B) The mean neutralization response (against isolates C-I and C-II) demonstrated by three late responders. SC-25 demonstrated a neutralization response at 22 months, which remained consistent up to 44 months. SC-26 and SC-27 demonstrated a neutralization response at 38 and 25 months postinfection, which remained consistent up to 55 months.
Studies have reported that the NAbs emerge at different time points after HIV infection. Recent studies have shown the presence of type-specific NAbs between 3 and 12 months of infection in 14 individuals diagnosed in an acute phase of HIV-1 infection.22 Antibodies that neutralize infecting/autologous viruses appear earlier7,11,12 than those neutralizing heterologous viruses.8 An effective neutralization response against heterologous viruses and against different HIV-1 subtypes is critical in developing HIV vaccines. In the present study, we focused on the heterologous response due to the nonavailability of autologous viruses. A study group of recent seroconverters was selected because the available data suggest that primary viremia is restricted by the host immune response, characterized by the appearance of virus-specific antibodies and the CTLs.23 So far, the emergence and the role of neutralizing antibodies in controlling early viremia are controversial, as antibodies are generally detected late after infection. In a study of an HIV seroconverter treated with HAART during early infection, a rapid autologous neutralizing antibody response, which remained sustained for at least 8 months, was detected following treatment cessation after 1.5 years. The CTL responses were minimal despite treatment discontinuation and rebound viremia was downregulated, suggesting that neutralizing antibodies may play an important role in the control of viremia.9
Among 18 Dutch seroconverters, the emergence of heterologous neutralization activity has been reported within 0–10 months (mean = 3.9 months) after seroconversion, with the majority (n = 15) developing a response within 3 months and the remaining (n = 3) between 6 and 10 months.13 However, in the present study, one of the four early responders demonstrated a neutralization response against both subtype C isolates as early as 2 months after infection and in the remaining three responders, the response was seen 3 months after infection. A similar study in subtype C-infected South African female sex workers has reported an extensive cross clade neutralization response against subtype B and subtype C from South Africa and Malawi at 5 months postseroconversion.24
In the second group of three seroconverters who did not show a neutralization response during the first year (tested on two to four occasions), the emergence of an antibody response was demonstrated late, between 22 and 38 months after infection. Figure 1B shows the mean neutralization response (against isolate C-I and isolate C-II) demonstrated by three late responders. One of the three seroconverters (SC-25) demonstrated a neutralization response against both isolates at 22 months, which remained consistent up to 44 months. The other two seroconverters, SC-26 and SC-27, demonstrated a neutralization response at 38 and 25 months postinfection, which remained consistent up to 55 months. A similar late onset of heterologous neutralization response in seroconverters, usually after 2 years of seroconversion, has been reported by Moog et al.8
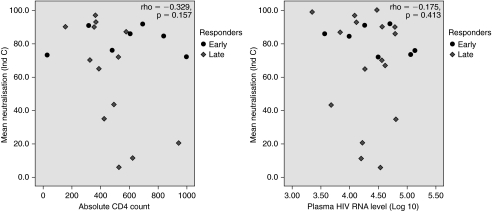
In all responders (early and late), irrespective of time of emergence, the NAbs did not show any association with absolute CD4 count (p = 0.157) and plasma viral load (p = 0.413) when Spearman's rank correlation coefficient method was applied. The scatter plots of mean NAb response (against isolates C-I and C-II) with absolute CD4 count and plasma viral load are shown in Fig. 2. This is in agreement with some previously reported studies10,25; however, other studies have reported a significant association between the increase in the neutralizing antibody response and the decreased plasma viral load.7,11 The data were also analyzed to determine the relationship between the absolute CD4 count and the plasma viral load within the postinfection period, which did not indicate any temporal relationship (data not shown).
FIG. 2.
Neutralizing antibody response against absolute CD4 count and plasma viral load in responders. The scatter plots show Spearman's rank correlation coefficient between the NAb response (against isolates C-I and C-II) and the absolute CD4 count and plasma viral load in responders.
The HIV-1 envelope is the only protein found on the surface of the virion, and hence is the only realistic target for development of neutralizing antibodies. The V3 loop of gp120 is of particular interest because it plays a key role in viral tropism and entry, and has been reported to elicit potent antibodies,26,27 which either block binding or postbinding events. Some studies have detected the presence of V3-specific antibodies prior to the development of autologous and heterologous NAbs8,26; however, a recent report suggests that they were not found to mediate autologous neutralization.28 Studies using antibodies specific for linear determinants of the V3 antibodies demonstrated that neutralization mediated by these antibodies was subtype specific, mediating neutralization of T cell line-adapted (TCLA) viruses.13 Since the V3 antibodies neutralized many TCLA and some primary strains, and as their presence could be correlated with protection of chimpanzees,29 the V3 loop has been elaborately studied as a probable vaccine strategy. Recent data show that conformational determinants of V3 were able to mediate broader neutralization of primary isolates.30 In view of the hypothesis that the neutralization response generated in our seroconverters could be due to polymorphism in the V3 region owing to selective immunological pressure31,32 or is related to exposure of epitopes in the V3 loop,33 the C2-V3 region of the selected sequential samples of seroconverters demonstrating an early neutralization response (n = 4), late neutralization response (n = 3), and no neutralization response (n = 5) was sequenced. In addition, as reported earlier,34 the presence of less potential N-linked glycosylation (PNLG) on the V3 loop of subtype C viruses must have enhanced the epitope exposure, thereby resulting in a stronger V3-directed NAb response, encouraged us to sequence the V3 region. To verify this, the DNA was extracted from selected concomitantly collected PBMC samples (as described in Table 1) using the Qiagen Blood DNA Extraction kit (Qiagen, Hilden, Germany) and was used to amplify the 500-bp fragment of the env gene of HIV-1 using nested PCR. The amplified env gene PCR product was sequenced using primers covering the C2-V3 region. The primers used for amplification as well as sequencing were ED31 and ED33. The generated sequences were analyzed using DNASTAR software and subsequent alignment was done for the V3 region using Clustal X software.
The percent homology between the consensus sequences of isolates C-I and C-II with the consensus sequences of the V3 region of early responders (91%), late responders (93%), and nonresponders (94%) did not show much sequence variation. As reported earlier,16 26 of 35 amino acids in the V3 sequences of seroconverters were well conserved, which is a characteristic feature of subtype C viruses, with minor variations in the sequences of early/late responders and nonresponders. Table 1 demonstrates the mean neutralization response shown by three groups—early, late, and nonresponders—against isolate C-I and isolate C-II and the AA substitution in the V3 region. Minor mutations were seen resulting in AA changes in the following positions in the V3 region of early and late responders: T2V, N5S, K10T, T19N/M, F20L/A, D29N, and H34Y. The PNLG, which is known to mask V3 epitopes of subtype B viruses, was well conserved at position 301 (conventional HXB2 numbering) in all the V3 sequences of early, late, and nonresponders as well as of isolate C-I and isolate C-II.
The high degree of conservation in the V3 region supports the data suggesting that this region may not be the target of the potent neutralizing responses in these individuals and perhaps anti-V3 antibodies in subtype C infection may not be of great biological relevance in driving the viral escape from the neutralization response. The neutralization response obtained in our study was not associated with any particular mutation in the V3 region, which may be an indication that other variable regions of the HIV-1 envelope might be involved in generating a neutralization response,27,35 which needs to be studied in greater detail. However, an antibody response could be generated by the interaction of the V3 loop with other regions of gp120, which may play a role in neutralization. This is supported by a recent finding suggesting that antibodies directed at the V1V2, V4, and V5 regions contribute to autologous neutralization, with V1V2 playing a more substantial role. These data also suggest that the C3-V4 regions form important structural motifs and epitopes in this region are major targets of the early autologous neutralization response in HIV-1 subtype C infection.28 Another possibility is the role played by the host genetic factors in the generation of NAbs against the virus; however, in the work done so far these factors could not be studied.
We compared the alignment of gp160 sequences of Indian subtype C and non-Indian subtype C viruses using Clustal X software and identified 35 conserved, predicted neutralization epitopes, 12 of which are reported in the HIV immunology database and 23 of which are not reported. Out of 23 predicted epitopes, 18 are found in non-Indian subtype C sequences while 5 are conserved only in Indian subtype C sequences. A single epitope (GPGQ), present in the V3 region that is recognized by NAbs, was also found in responders as well as nonresponders studied for V3 sequence variation; however, none of the responders had any unique V3 sequence that could explain the responses.
HIV-1 subtype C is widely prevalent in India and South Africa, which carry the heaviest burden of HIV infections. HIV-1 subtype C from South Africa is the most widely studied C subtype. We compared neutralization properties and V3 sequences of HIV-1 from these two countries. This comparison revealed that the V3 region of viruses from both countries is highly conserved and did not differ in length. The percentage homology between the V3 regions of Indian and South African HIV-1 subtype C strains from seroconverters was found to range from 73 to 100% (mean = 85%, SD = 5.8). The comparison of neutralization properties of viruses from recent seroconverters from India and South Africa34 revealed that subtype C from India is more resistant to neutralization than subtype C from South Africa. The most striking similarity between the clade C reference strains from both places is their uniform resistance to MAb 2G12 and MAb 2F5. However, the relative resistance of Indian subtype C viruses to neutralization by MAb IgG1b12 distinguished them from South African subtype C viruses. There was no difference in the overall sensitivity to sCD4 and MAb 4E10. This comparison suggested that some neutralization epitopes are partially shared between subtype C from India and subtype C from South Africa and also that there is a need to study genetic and neutralization characteristics of recently transmitted Indian subtype C in greater detail.
We would like to emphasize that in the present study, the neutralization response was assessed more cautiously. The heterologous neutralization response was studied for primary isolates with a known passage history in a large number of plasma samples from seroconverters with a documented date of seroconversion. The HIV-seronegative plasma samples were used to draw a baseline response for stringent classification of a positive neutralization response based on a robust threshold. The neutralization index of 50% more than the mean index in the panel of normal plasma was considered as a positive neutralizing antibody response, as most of the plasma from HIV-infected individuals causes 50% neutralization and was used as an end point in many previous studies.11,20,21
To summarize, the results of our preliminary study demonstrated the presence of heterologous neutralization activity in early HIV infection. Although V3 is hypothesized to be the main epitope for eliciting neutralizing antibody, the neutralization response obtained in our study was not found to be associated with the V3 region and indicated that other regions of the HIV-1 envelope might be involved in generating a neutralization response.
Acknowledgments
We would like to thank Dr. Sanjay Mehendale, Deputy Director (SG), and the entire project staff of “HIVNET” for their valuable contribution in counseling, specimen collection, clinical care, and data management of the study participants. We also gratefully acknowledge the help of Dr. Arun Risbud, Dr. Madhuri Thakar, Mrs. Bharati Mahajan, Mrs. Sangeeta Kulkarni, Mrs. Varsha Kale, Mr. Prasad Tondare, and Mrs. Kavita Dhande for assistance in laboratory investigations and Mrs. Radhika Brahme and Mr. Kishore Kumar for help in data analysis. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, NIAID (AI 33879-02), NIH-NCRR OPD-GCRC (5M01RR00722), the NIH-Fogarty International Center (D43TW0000), and intramural research grants from the Indian Council of Medical Research.
References
- 1.Arther LO. Bess JW., Jr Waters DJ. Pyle SW. Kelliher JC. Nara PL, et al. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989;63:5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman PW. Gregory TJ. Riddle L. Nakamura GR. Champe MA. Porter JP, et al. Protection of chimpanzee from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not with gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 3.Ferrantelli F. Rasmussen RA. Buckley KA. Li PL. Xu W. Wang T, et al. Complete protection of neonatal macaques against oral challenge with pathogenic SHIV by human anti-HIV monoclonal antibodies. J Infect Dis. 2004;189:2167–2173. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 4.Mascola JR. Stiegler G. VanCott TC. Katinger H. Carpenter CB. Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert PB. Peterson ML. Follmann D. Hudgens MG. Francis DP. Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 6.Lyles RH. Munoz A. Yamashita TE. Bazmi H. Detels R. Rinaldo CR, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 7.Lathey JL. Pratt RD. Spector SA. Appearance of autologous neutralizing antibody correlates with reduction in viral load and phenotypic switch during primary infection with human immunodeficiency virus type-1. J Infect Dis. 1997;175:231–232. doi: 10.1093/infdis/175.1.231. [DOI] [PubMed] [Google Scholar]
- 8.Moog C. Fleury HJA. Pellegrin I. Kirn A. Aubertin AM. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type-1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montefiori DC. Altfeld M. Lee PK. Bilska M. Zhou J. Johnston MN, et al. Viremia control despite escape from a rapid and potent autologous neutralizing antibody response after therapy cessation in an HIV-1-infected individual. J Immunol. 2003;170:3906–3914. doi: 10.4049/jimmunol.170.7.3906. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrin I. Legrand E. Neau D. Bonot P. Masquelier B. Pellegrin J, et al. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquir Immune Defic Syndr Hum Retroviral. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Ruppach H. Nara P. Raudonat I. Elanjikal Z. Rubsamen-Waigmann H. Dietrich U. Human immunodeficiency virus (HIV)-positive sera obtained shortly after seroconversion neutralizes autologous HIV type 1 isolates on primary macrophages but not on lymphocytes. J Virol. 2000;74:5403–5411. doi: 10.1128/jvi.74.12.5403-5411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X. Decker JM. Wang S. Hui H. Kappes JC. Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 13.Zwart G. Back NKT. Ramautarsingh C. Valk M. Van der Hock L. Goudsmit J. Frequent and early HIV-1 MN neutralizing capacity in sera from Dutch HIV-1 seroconverters is related to antibody reactivity to peptides from the gp 120 V3 domain. AIDS Res Hum Retroviruses. 1994;10:245–250. doi: 10.1089/aid.1994.10.245. [DOI] [PubMed] [Google Scholar]
- 14.Gadkari DA. Moore D. Sheppard HW. Kulkarni SS. Mehendale SM. Bollinger RC. Transmission of genetically diverse strains of HIV-1 in Pune, India. Indian J Med Res. 1998;107:1–9. [PubMed] [Google Scholar]
- 15.Jameel S. Zaffrullah M. Ahmad M. Kapoor GS. Sehgal S. A genetic analysis of HIV-1 from Punjab, India reveals the presence of multiple variants. AIDS. 1995;9:685–690. doi: 10.1097/00002030-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Agnihotri K. Tripathy S. Jere A. Jadhav S. Kurle S. Paranjape R. gp120 sequences from HIV type 1 subtype C early seroconverters in India. AIDS Res Hum Retroviruses. 2004;20:889–894. doi: 10.1089/0889222041725217. [DOI] [PubMed] [Google Scholar]
- 17.Shankarappa R. Chatterjee R. Learn GH. Neogi D. Ding M. Roy P. Ghosh A. Kingsley L. Harrison L. Mullins JI. Gupta P. Human immunodeficiency virus type 1 env sequences from Calcutta in eastern India: Identification of features that distinguish subtype C sequences in India from other subtype C sequences. J Virol. 2001;75:10479–10487. doi: 10.1128/JVI.75.21.10479-10487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakar MR. Bhonge LS. Lakhashe SK. Shankarkumar U. Sane SS. Kulkarni SS. Mahajan BA. Paranjape RS. Cytolytic T lymphocytes (CTLs) from HIV-1 subtype C-infected Indian patients recognize CTL epitopes from a conserved immunodominant region of HIV-1 Gag and Nef. J Infect Dis. 2005;192:749–759. doi: 10.1086/432547. [DOI] [PubMed] [Google Scholar]
- 19.da Silva J. The evolutionary adaptation of HIV-1 to specific immunity. Curr HIV Res. 2002;1:363–371. doi: 10.2174/1570162033485249. [DOI] [PubMed] [Google Scholar]
- 20.Cecilia D. Kewalramani VN. O'Leary J. Volsky B. Nyambi P. Burda S, et al. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore JP. Burton DR. HIV-1 neutralizing antibodies: How full is the bottle? Nat Med. 1999;5:142–144. doi: 10.1038/5502. [DOI] [PubMed] [Google Scholar]
- 22.Gray ES. Moore PL. Choge IA. Decker JM. Bibollet-Ruche F. Li H, et al. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connick E. Marr DG. Zhang X. Clark SJ. Saag MS. Schooley RT, et al. HIVspecific cellular and humoral immune responses in primary HIV infection. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 24.Bures R. Morris L. Williamson C. Ramjee G. Deers M. Fiscus SA, et al. Regional clustering of shared neutralization determinants on primary isolates of clade C human immunodeficiency virus type 1 from South Africa. J Virol. 2002;76:2233–2244. doi: 10.1128/jvi.76.5.2233-2244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aasa-Chapman MMI. Hayman A. Newton P. Cornforth D. Williams I. Borrow P, et al. Development of the antibody response in acute HIV-1 infection. AIDS. 2004;18:371–381. doi: 10.1097/00002030-200402200-00002. [DOI] [PubMed] [Google Scholar]
- 26.Nyambi PN. Mbah HA. Burda S. Williams C. Gorny MK. Nadas A, et al. Conserved and exposed epitopes on intact and native human immunodeficiency virus type 1 virions of group M. J Virol. 2000;749:7096–7107. doi: 10.1128/jvi.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spenlehauer C. Saragosti S. Sarasgosti S. Harvey HJA. Kirn A. Aubertin AM, et al. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell line adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore PL. Gray ES. Choge IA. Ranchobe N. Mlisana K. Abdool Karim SS, et al. The C3-V4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J Virol. 2008;82:1860–1869. doi: 10.1128/JVI.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emini EA. Schleif WA. Nunberg JH. Conley AJ. Eda Y. Tokiyoshi S, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;20:355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 30.Gorny MK. Williams C. Volsky B. Revesz K. Cohen S. Polonis VR, et al. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize HIV-1 primary isolates from various clades. J Virol. 2004;76:9035–9045. doi: 10.1128/JVI.76.18.9035-9045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis J. Balfe P. Arnold C. Kaye S. Tedder RS. McKeating JA. Development of a neutralizing antibody response during acute primary human immunodeficiency virus type 1 infection and the emergence of antigenic variants. J Virol. 1998;72:8943–8951. doi: 10.1128/jvi.72.11.8943-8951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Means RE. Matthews T. Hoxie JA. Malim MH. Kodama T. Desrosiers RC. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: Correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol. 2001;75:3903–3915. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bou-Habib DC. Roderiquez G. Oravecz T. Berman PW. Lusso P. Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B. Decker JM. Johnson RW. Bibollet-Ruche F. Wei X. Mulenga J, et al. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J Virol. 2006;80:5211–5218. doi: 10.1128/JVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyatt R. Moore JP. Accola M. Desjardin E. Robinson J. Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp 120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]