Abstract
Objectives
Adipose-derived mesenchymal stem cells (ADMSCs) are a unique population of stem cells with therapeutic potential in the treatment of connective tissue injuries. Growth differentiation factor-5 (GDF)-5 is known to play a role in tendon repair and maintenance. The aim of this study was to investigate the effects of GDF-5 on proliferation and tendonogenic gene expression of rat ADMSCs.
Methods
ADMSCs were treated in culture with different concentrations of GDF-5 (0–1000 ng/mL) for 12 days. Biochemical, temporal, and concentration kinetic studies were done. Extracellular matrix (ECM) synthesis, tendonogenic differentiation, and matrix remodeling gene and protein expression were analyzed.
Results
GDF-5 led to increased ADMSC proliferation in a dose- and time-dependent manner. ADMSCs demonstrated enhanced ECM (collagen type I, decorin, and aggrecan) and tendonogenic marker (scleraxis, tenomodulin, and tenascin-C) gene expression with 100 ng/mL of GDF-5 (p < 0.05). ECM and tendon-specific markers showed time-dependent increases at various time points (p < 0.05), although decorin decreased at day 9 (p < 0.05). GDF-5 did alter expression of matrix remodeling genes, with no specific trends observed. Western blot analysis confirmed dose- and time-dependent increases in protein expression of tenomodulin, tenascin-C, Smad-8, and matrix metalloproteinase-13.
Conclusion
In vitro GDF-5 treatment can induce cellular events leading to the tendonogenic differentiation of ADMSCs. The use of combined GDF-5 and ADMSCs tissue-engineered therapies may have a role in the future of tendon repair.
Introduction
While the incidence of tendon ruptures has increased dramatically due to an increase in athletic participation in the general population,1,2 the long-term clinical outcome for surgical treatment of such injuries remains variable. Studies have shown that healed tendon tissue possesses a higher cell density and decreased collagen fiber organization in comparison to the preinjured state. This altered cell organization serves as a foundation for poor results such as re-rupture, restrictive adhesions, and suboptimal functionality after treatment of tendon lacerations.3–5 Therefore, innovative treatment options to improve tendon healing are of great interest. Present research has focused on novel tissue engineering techniques, including the use of active mesenchymal cells and growth factors, as alternative methods for tendon repair.
Bone marrow stromal cells (BMSCs) are pluripotent progenitor cells that have the ability to differentiate into a variety of musculoskeletal tissue precursors such as osteoblasts, chondrocytes, adipocytes, and myocytes when placed under specific conditions in vitro.6,7 Further, BMSCs have been transplanted to various tissue injury sites, with enhanced tissue repair being achieved.8,9 For these reasons bone-marrow-derived mesenchymal stem cells (MSCs) are among the best characterized stem cells. There are, however, drawbacks to their use. Bone-marrow-harvesting procedures are highly invasive, painful procedures with reported complication rates as high as 30%.10 It is also well known that bone marrow isolations often yield a low number of stem cells.11
Adipose-derived mesenchymal stem cells (ADMSCs) have become a focus of research in recent years due to their similar multipotential properties, high responsiveness to distinct environmental cues, and ease of isolation.12,13 Typically, adipose tissue is abundant in both humans and animals and can be easily harvested from subcutaneous tissue through percutaneous or limited open aspiration techniques.14,15 Adipose also provides a large volume of viable pluripotent stromal cells when harvested compared to that of bone marrow.16 The ability to harvest such a high yield of stromal cells from small fractions of fat could mean that patients with low percentages of body fat, such as high performance atheletes, could serve as autogenic sources of precursor cells for their own tissue-engineered therapies. In addition, studies have shown that ADMSCs have similar characteristics with BMSCs, with potential to differentiate into lineages of multiple mesodermal tissues, such as bone, cartilage, fat, and muscle when under the appropriate conditions and provided key environmental cues.17–19 However, to our knowledge, the ability of ADMSCs to undergo tendonogenic differentiation has not been described extensively in the literature.
Growth factors are peptide signaling molecules with diverse biological roles, including the regulation of cell metabolism, cell proliferation, and differentiation, and the production of extracellular matrix (ECM) molecules.20,21 In vitro studies have shown that growth factors such as bone morphogenetic protein-2 (BMP-2), platelet-derived growth factor, fibroblast growth factor-2, transforming growth factor β1, insulin-like growth factor-1, and vascular endothelial growth factor can all promote proliferation and differentiation of MSCs into different lineages.13–17 However, little is known about the effect of growth differentiation factor-5 (GDF-5) on MSC differentiation.
GDF-5 is also known as cartilage-derived morphogenetic protein-1 and BMP-14. It has been shown to play a role in a variety of musculoskeletal processes, including joint formation, endochondral ossification, and tendon and ligament maintenance and repair.20,21 Mice deficient in GDF-5 are characterized by short limbs, abnormal joint development, and a reduction in the number of phalanges in the digits. GDF-5 mutations identified in humans result in several distinct forms of skeletal dysplasia.22 Work from our institution has previously shown that mice deficient in the gene for GDF-5 protein demonstrate impaired tendon healing, manifested by altered structural and mechanical properties of the repair tissue.23 Recent studies have demonstrated that GDF-5 induces neotendon and neoligamentous formation when implanted in ectopic sites, and recombinant GDF-5 protein implanted on collagen sponges or on suture material enhances Achilles tendon healing in rodents.24 Further, it was recently reported that GDF-5 gene therapy increased rat Achilles tendon tensile strength, without inducing bone or cartilage formation within the healed tendon.25 Recent microarray data from our laboratory showed that GDF-5, when used to treat tendon fibroblasts, plays a role in the regulation of genes critical to cell proliferation, inflammation, and ECM production.26
Other groups from within our laboratory have shown successful osteogenic differentiation of GDF-5-treated ADMSCs in vitro and on a bioengineered scaffold.27,28 Such combined tissue-engineered therapies also have significant potential in the bioengineering of tendon.29 Preliminary data from our group have been promising, showing that GDF-5 treatment leads to increases of overall proliferation, total DNA, hydroxyproline (OHP), and glycosaminoglycan (GAG) expression of rat ADMSCs.30,31 However, the effect of GDF-5 on the tendonogenic differentiation of ADMSCs has not yet been fully investigated. Thus, it remains worthwhile to study the cellular and molecular response of ADMSCs to GDF-5 treatment to devise strategies to promote tendon healing, repair, and regeneration. In this study, we examined the time- and dose-related effects of GDF-5 on ADMSCs, hypothesizing that GDF-5 induces ADMSCs to produce genes and proteins consistent with tendonogenic differentiation.
Materials and Methods
Preparation of ADMSCs
Fisher 344 rat adipose tissue was obtained from the inguinal region. Briefly, the fat was washed extensively with Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with 2% penicillin/streptomycin. Collagenase 0.01% (Sigma Chemical) was added to the washed sample and the mixture was agitated at 37°C for 1 h. The collagenase was inactivated with an equal volume of DMEM/10% fetal bovine serum (FBS) and the infranatant was centrifuged for 10 min at 1500 rpm. The pellet was resuspended in DMEM/10% FBS and transferred to a T-75 flask containing 15 mL of the control medium (DMEM, 10% FBS, and 1% penicillin/streptomycin). The cell culture medium was changed every third day. Cells were maintained at a subconfluent level and passaged using trypsin–ethylenediaminetetraacetic acid (EDTA) (GibcoBRL).
Cell proliferation of GDF-5-treated ADMSCs
The MTT [3-(4, 5-dimethylthiazol)-2, 5-diphenyltetrazolium bromide] (Sigma Chemical) assay was performed to evaluate the effect of GDF-5 on the growth of ADMSCs. Cells were incubated in 24-well microtiter plates (1.9 × 104 cells/well) with 300 μL medium. The culture medium was then changed every other day. To study the concentration kinetics of GDF-5, ADMSCs were maintained in culture media supplemented with GDF-5 protein at concentrations of 0, 1, 10, 100, and 1000 ng/mL for 4 days. During our dose-response analysis, 100 ng/mL of GDF-5 demonstrated the largest differences in response measures and therefore was the selected concentration for our time-dependent analysis. Cells were then cultured with or without GDF-5 protein (100 ng/mL) for 12 days. Over the time course of the experiment, cells were analyzed at 3, 6, 9, and 12 days. MTT (30 μL/well) was added, and the plates were incubated for 3 h. Subsequently, 0.04 N hydrochloric acid solution was added to each well. The absorbance was measured at a test wavelength of 490 nm, and a reference was measured at a wavelength of 650 nm using a microplate reader for the calculation of cell growth (relative to a standard curve of cell number vs. absorbance).
GDF-5 treatment of ADMSCs: Concentration and time kinetics
Rat ADMSCs were grown to confluence at 37°C in a humidified, 5% CO2 incubator. Cells were then passaged and plated at a density of 5.0 × 104/well in six-well plates. To study the concentration kinetics of GDF-5, ADMSCs maintained in culture media were supplemented with GDF-5 at concentrations of 0, 1, 10, 100, and 1000 ng/mL for 4 days. At the day 4 time point, cells were harvested for biochemical analysis, total RNA extraction, and real-time polymerase chain reaction (PCR) analysis. For time-dependent kinetics, cells were cultured with or without GDF-5 protein (100 ng/mL) for 12 days. Over the time course of the experiment, cells were harvested at 3, 6, 9, and 12 days for biochemical analysis, total RNA extraction, and real-time PCR analysis.
Biochemical analysis of GDF-5-treated ADMSCs
A biochemical analysis was performed to determine the amount of DNA, GAG, and collagen in GDF-5-treated and untreated ADMSCs in vitro in the time- and concentration-dependent manners described previously. After incubation, cells were washed with 5 mM phosphate-buffered EDTA and dissolved in papain buffer (125 μg/mL papain and 5 mM l-cysteine in phosphate-buffered EDTA) at 60°C for 18 h. DNA content in the harvested samples was determined using Hoechst 33258 dye (bisbenzimide) in a fluorometric assay with calf thymus DNA used as the standard. GAG content was determined with dimethylmethylene blue in a colorimetric assay, with dermatan sulfate as the standard. The GAG/DNA ratio was then calculated to normalize ratios relative to DNA content.
To determine collagen content, as indicated by OHP, papain-treated samples were hydrolyzed with 6 N HCl for 18 h at 110°C. The dimethylaminobenzaldehyde colorimetric assay was performed to determine OHP content with purified OHP as a standard. The OHP/DNA ratio was then calculated. All the samples and standards were processed in triplicate and average values were used for analysis.
Real-time PCR for gene expression
To determine gene expression, total RNA was prepared from each group of cells in the time and concentration kinetics studies. Reverse transcriptase reactions were annealed (70°C, 10 min), and then followed by first-strand cDNA synthesis (42°C, 60 min) and heat inactivation (95°C, 5 min). The resulting cDNA was stored frozen (−70°C) until assayed by real-time PCR. Dilutions of the rat ADMSC RNA standard were added to give final concentrations of 0.01–100 ng of total RNA.
Using the QuantiTect SYBR Green PCR kit (Qiagen), the real-time PCRs were performed with 25 μL of the SYBR Green kit master mix and forward and reverse primers 300 nM each (Table 1). The 3 μL of cDNA sample was added to the final reaction mixture undiluted from the reverse transcriptase reaction. The 96-well real-time PCR format included six 10-fold dilutions. The wells of the plate were sealed with optical adhesive covers (BioRad Laboratories) and centrifuged at low speed (300 g, 5 min) to ensure complete mixing. Each sample was analyzed in at least duplicate with the iCycler instrument (BioRad Laboratories). The PCR protocols used involved activation of AmpliTaq Gold DNA polymerase followed by 40 cycles of denaturation at 94°C for 30 s, respective annealing temperature for 30 s, and extension at 72°C for 30 s. The PCR threshold cycle number (CT) for each sample was calculated at the point where the fluorescence exceeded the threshold limit. The threshold limit was fixed along the linear logarithmic phase of the fluorescence curves at 10–20 standard deviations above the average background fluorescence. Standard curve equations were calculated by regression analysis of average CT versus the log10 of the rat ADMSCs cDNA expressed relative to the total RNA present. Relative expression of the target genes was normalized to 18S expression using the 18S primer (QuantumRNA 18S PCR products; Ambion).
Table 1.
Sequences, Product Size, and Annealing Temperatures of the Primers That Were Used in Real-Time–Polymerase Chain Reaction Experiments
| Gene | Primer | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| Scleraxis | Forward: CGA AGT TAG AAG GAG GAG GGT | 55 | 108 |
| Reverse: CGC TCA GAT CAG GTC CAA AG | |||
| Tenomodulin | Forward: GGA CTT TGA GGA GGA TGG | 55 | 128 |
| Reverse: CGC TTG CTT GTC TGG TGC | |||
| Tenascin-C | Forward: GCT ACT CCA GAC GGT TTC | 53 | 199 |
| Reverse: TTC CAC GGC TTA TTC CAT | |||
| Col I (α 1) type I | Forward: AGG CTT TGA TGG ACG CAA TG | 54 | 78 |
| Reverse: GCG GCT CCA GGA AGA CC | |||
| Col III (α 1) type I | Forward: AGG CTT TGA TGG ACG CAA TG | 54 | 153 |
| Reverse: GCG GCT CCA GGA AGA CC | |||
| Aggrecan | Forward: ACC CGA CAA TTT CTT TGC | 55 | 345 |
| Reverse: GGT CTC ATC GTC CGC TTC | |||
| Decorin | Forward: TGG CAG TCT GGC TAA TGT | 53 | 130 |
| Reverse: ACT CAC GGC AGT GTA GGA | |||
| MMP-3 | Forward: ATG AAC GAT GGA CAG ATG | 50 | 134 |
| Reverse: TGT GGA GGA CTT GTA GAC T | |||
| MMP-13 | Forward: TGT GAC CCA GCC CTA TCC | 53 | 246 |
| Reverse: ACC CTC CAT AAT GTC ATA CCC | |||
| TIMP-2 | Forward: CAA AGC AGT GAG CGA GAA | 52 | 259 |
| Reverse: CCA GGG CAC AAT AAA GTC |
Col, collagen; MMP, matrix metalloproteinase; TIMP-2, tissue inhibitor of matrix metalloproteinase-2.
Western blots for protein analysis
The levels of tenomodulin, Smad-8, tenascin-C, and matrix metalloproteinase-13 (MMP-13) protein were assessed by immunoblotting using sodium dodecyl sulfate–polyacrylamide gel electrophoresis to resolve proteins from ADMSCs treated with or without GDF-5. Cells were lysed with lysis buffer (25 mM Tris [pH 8.0] and 150 mM NaCl) containing 1 mM EDTA, 1% Triton X-100, and 1% sodium deoxycholate. The lysates were centrifuged for 10 min at 14,000 rpm at 4°C and protein content was estimated by Lowry method. The lysated cellular proteins (35 μg) solubilized in Laemmli sample buffer were resolved by electrophoresis on 10% or 12% sodium dodecyl sulfate–polyacrylamide gels. Electrophoresis was performed at constant voltage (150 V), and then the resolved proteins were transferred from the polyacrylamide gel to nitrocellulose membrane (Pierce) by semidry transfer (BioRad Laboratories) for 30 min at constant current (15 V) using transfer buffer (25 mM Tris, 192 mM glycine, and 20% [v/v] methanol). To eliminate nonspecific interactions of antibodies with the membrane, the nitrocellulose membrane was blocked with TTBS (10 mM Tris-HCl [pH 8.0] 150 mM NaCl, and 0.05% Tween-20) containing 5% milk (5 g/100 mL) for 4 h at room temperature. Membranes were incubated with the respective primary antibody (Santa Cruz Biotechnology) diluted 1:200 in TTBS overnight at 4°C. Actin was used as a loading control, and the antibody was diluted 1:5000 in TTBS for 1 h (Sigma Chemical). The membranes were washed three times with TTBS. The membranes were incubated with horseradish-peroxidase-linked donkey anti-goat (Santa Cruz Biotechnology) 1:60,000 in TTBS for 1 h at room temperature. The actin membranes were incubated with horseradish-peroxidase-linked goat anti-mouse (BioRad Laboratories) 1:40,000 in TTBS. After three washes with TTBS, protein bands were observed by enhanced chemiluminescence using the Amersham ECL Plus Western Blotting Detection system (GE Healthcare) and film (Lumi-Film; Roche Molecular Biochemicals).
Statistical analysis
Data are expressed as mean and standard deviation. The significance of differences was determined using one-way analysis of variance with post hoc analysis for the concentration kinetics studies and paired t-tests for the time kinetics studies. p-Values <0.05 were considered to be statistically significant.
Results
Concentration and time kinetics
For each experiment in this study, we initially performed concentration kinetics studies with GDF-5 concentrations of 0, 1, 10, 100, and 1000 ng/mL. Upon completion of those studies, we determined that 100 ng/mL was the optimal concentration and proceeded to perform the time kinetics studies with a fixed concentration of 100 ng/mL.
Treatment with GDF-5 increases ADMSC proliferation
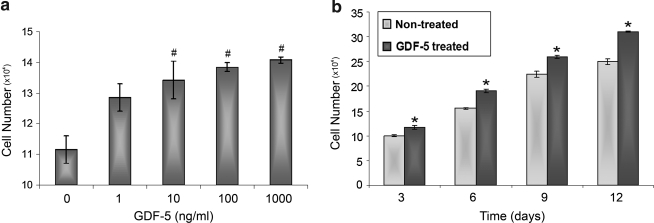
We determined the dose- and time-dependent effects of GDF-5 on the proliferation rate of ADMSCs. ADMSC proliferation increased with time and concentrations of GDF-5 compared to controls. In the concentration kinetics studies, there was a significant increase in ADMSC proliferation treated with GDF-5 concentrations of 1, 10, 100, and 1000 ng/mL (p < 0.01). In the time kinetics studies, the proliferation rate of ADMSCs treated with 100 ng/mL of GDF-5 increased significantly at all time points (p < 0.001) (Fig. 1).
FIG. 1.
The concentration-dependent (a) and time-dependent (b) effect of growth differentiation factor-5 (GDF-5) on the proliferation rate of adipose-derived mesenchymal stem cells (ADMSCs). For concentration kinetics, cells were harvested at day 4. For time kinetics, cells were treated with 100 ng/mL of GDF-5. Cell viability was measured as absorbance using MTT [3-(4, 5-dimethylthiazol)-2, 5-diphenyltetrazolium bromide] assay. The proliferation rate of ADMSCs was significantly increased with increasing time and concentrations of GDF-5 (*p < 0.001 and #p < 0.01).
Treatment with GDF-5 leads to dose- and time-dependent increases in GAG and OHP
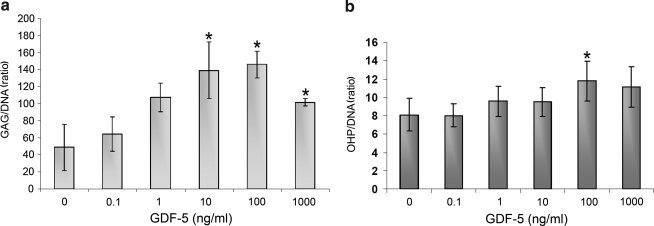
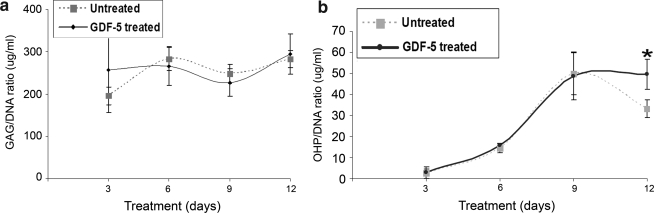
The concentration kinetics/biochemical studies showed a significant increase in the ratio of GAG/DNA at concentrations of 10 and 100 ng/mL (p < 0.05) when measured at day 4 (Fig. 2a). However, in the time kinetics studies, there was no significant difference in the GAG/DNA ratio at day 3, 6, 9, or 12 (Fig. 3a). The OHP/DNA ratio was not found to increase in a concentration-dependent manner on day 4 (Fig. 2b), but with increasing time of treatment, OHP/DNA increased relative to control at the 12 day time point (p < 0.05) in the time kinetics studies (Fig. 3b).
FIG. 2.
(a) Proteoglycan (glycosaminoglycan [GAG]/DNA) ratio and (b) hydroxyproline (OHP/DNA) content of ADMSCs treated with different concentrations of GDF-5 for 4 days. The GAG/DNA ratio was significantly increased at 10 and 100 ng/mL of GDF-5 (*p < 0.05). The OHP/DNA ratio exhibited a trend indicative of an increase in response to GDF-5 but was not statistically different at any concentration.
FIG. 3.
(a) Proteoglycan (GAG/DNA) ratio and (b) hydroxyproline (OHP/DNA) content of ADMSCs treated with or without 100 ng/mL of GDF-5 for 3, 6, 9, or 12 days. GAG/DNA was not statistically different from the control in this study. The OHP/DNA ratio was increased compared to control at day 12 (*p < 0.05).
GDF-5 treatment leads to increased expression of tendon-cell-specific genes
Real-time PCR on RNA extracted from the cells treated with and without GDF-5 was performed using primers for the following genes: collagen type I (Col I), Col III, aggrecan, decorin, tenascin-C, scleraxis, tenomodulin, MMP-3, MMP-13, and tissue inhibitor of metalloproteinase-2 (TIMP-2).
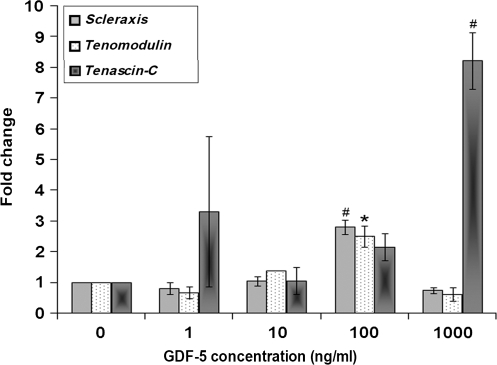
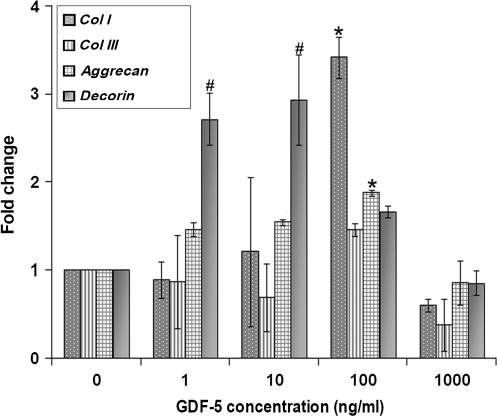
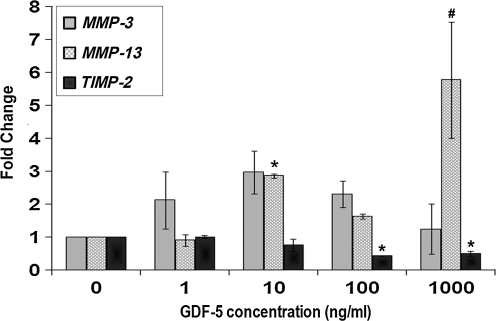
The concentration kinetics studies for gene expression of tendonogenic markers scleraxis and tenomodulin showed that each increased compared to controls with GDF-5 concentrations of 100 ng/mL (p < 0.01 and p < 0.05), while tenascin-C showed an eightfold increase with 1000 ng/mL of GDF-5 (p < 0.01) (Fig. 4). Col I expression increased in cells treated with 100 ng/mL of GDF-5 compared to control (p < 0.05). No significant difference was found for Col III in the concentration kinetics study. Decorin was increased at GDF-5 concentrations of 1 and 10 ng/mL (p < 0.01), and aggrecan was observed to be increased in cells treated with 100 ng/mL of GDF-5 compared to control cells (p < 0.05) (Fig. 5). MMP-13 demonstrated a threefold increase at 10 ng/mL (p < 0.05) and a sixfold increase at 1000 ng/mL (p < 0.01). On the other hand, TIMP-2 expression was slightly decreased at 100 and 1000 ng/mL of GDF-5 (p < 0.05) (Fig. 6).
FIG. 4.
Concentration-dependent effect of GDF-5 treatment on expression of tendonogenic markers of ADMSCs. Expression of scleraxis and tenomodulin was significantly increased at the concentration of 100 ng/mL of GDF-5. Expression of tenascin-C significantly increased at the concentration of 1000 ng/mL of GDF-5 (#p < 0.01 and *p < 0.05).
FIG. 5.
Concentration-dependent effect of GDF-5 treatment on expression of genes for macromolecular components of extracellular matrix and cell adhesion molecules. Expression of collagen I (Col I) was increased at the concentration of 100 ng/mL of GDF-5. Expression of aggrecan was increased at 100 ng/mL of GDF-5, and decorin was increased at 1 and 10 ng/mL of GDF-5 (#p < 0.01 and *p < 0.05).
FIG. 6.
Concentration-dependent effect of GDF-5 treatment on expression of matrix metalloproteinases (MMPs) and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) genes. Expression of MMP-13 was increased at 10 and 1000 ng/mL of GDF-5. In contrast, expression of TIMP-2 was decreased at 100 and 1000 ng/mL of GDF-5 (#p < 0.01 and *p < 0.05).
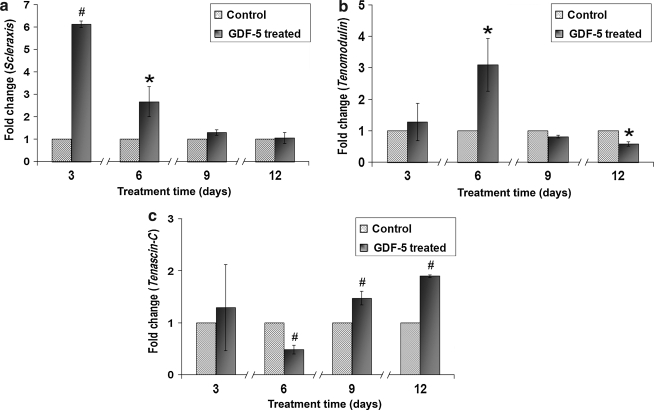
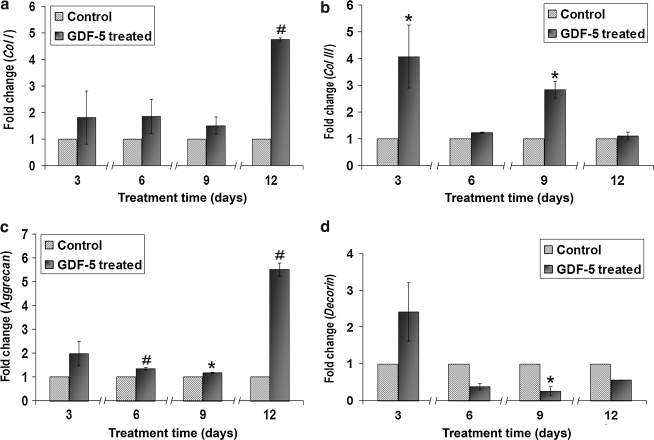
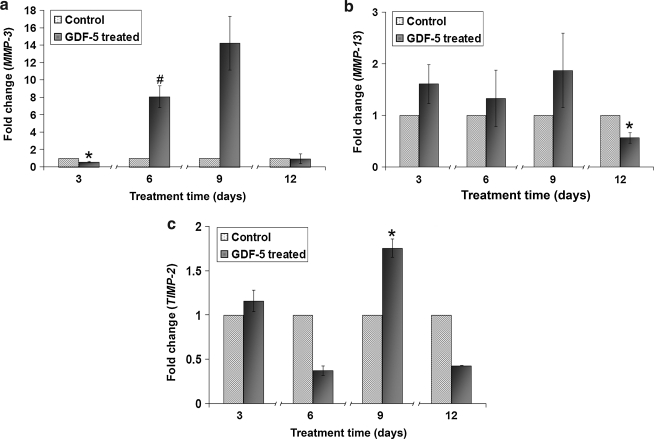
The time-dependent effects of GDF-5 on ADMSCs were analyzed at 3, 6, 9, and 12 days. The data indicated that expression of scleraxis increased at days 3 and 6 (p < 0.05). Expression of tenomodulin increased at day 6, with a mild decrease at day 12 (p < 0.05). Tenascin-C expression decreased at day 6, with increased expression at days 9 and 12 (p < 0.01) (Fig. 7). Among the ECM-related genes, Col I showed an increase at day 12 (p < 0.01), and Col III showed significant increases at days 3 and 9 (p < 0.05). Expression of aggrecan increased throughout days 6, 9, and 12 in comparison to controls (p < 0.01 and p < 0.05). In contrast, decorin expression decreased at day 9 (p < 0.05) (Fig. 8). MMP-3 expression decreased slightly at day 3 and then increased substantially at day 6 (p < 0.05 and p < 0.01), with a large upward trend at day 9 that was not statistically significant. Finally, MMP-13 decreased at day 12 (p < 0.05), while TIMP-2 expression increased at day 9 (p < 0.05) (Fig. 9).
FIG. 7.
Time-dependent kinetics of GDF-5 on ADMSCs for gene expression of (a) scleraxis, (b) tenomodulin, and (c) tenascin-C. Expression of scleraxis was significantly increased at days 3 and 6. Expression of tenomodulin was increased significantly at day 6 and slightly decreased at day 12. Expression of tenascin-C was increased at days 9 and 12 (#p < 0.01 and *p < 0.05).
FIG. 8.
Time-dependent kinetics of GDF-5 on ADMSCs for gene expression of (a) Col I, (b) Col III, (c) aggrecan, and (d) decorin. Expression of Col I was increased at day 12, while Col III increased at days 3 and 9. Expression of aggrecan significantly increased at days 6, 9, and 12. In contrast, expression of decorin decreased at day 9 (#p < 0.01 and *p < 0.05).
FIG. 9.
Time-dependent kinetics of GDF-5 on ADMSCs for gene expression of (a) MMP-3, (b) MMP-13, and (c) TIMP-2. Expression of MMP-3 was decreased at day 3 and increased at day 6. MMP-13 was decreased at day 12, and TIMP-2 was increased at day 9 (#p < 0.01 and *p < 0.05).
Western blots show increases in proteins associated with tendon cell differentiation
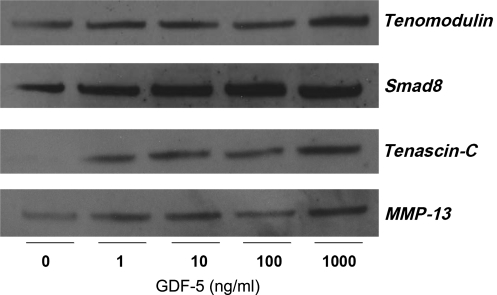
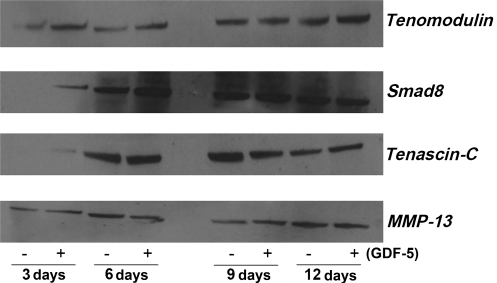
We performed Western blots to determine the time- and dose-dependent effects of GDF-5 on protein expression for tenomodulin, tenascin-C, Smad-8, and MMP-13. Results for the concentration kinetics studies showed an increase in expression of all four proteins compared to controls (Fig. 10). Additionally, time kinetics samples with and without GDF-5 (100 ng/mL) treatment were collected at 3, 6, 9, and 12 days. Tenomodulin showed increased expression at 3, 6, and 12 days compared to controls, while tenascin-C showed increased expression at days 3, 6, and 12, with a decrease at day 9. MMP-13 showed increased expression at days 3 and 9, but decreased expression at days 6 and 12. Smad-8 increased at days 3, 6, and 9 (Fig. 11).
FIG. 10.
Concentration-dependent effect of GDF-5 on the protein expression for tendonogenic markers and MMP-13. Western blot revealed that expression uniformly increased when compared to controls for the proteins in question.
FIG. 11.
Time-dependent effect of GDF-5 on protein expression of tendonogenic markers and MMP-13. Tenomodulin increased at days 3, 6, and 12; Smad-8 increased at days 3, 6, and 9; tenascin-C increased at days 3, 6, and 12 but decreased at day 9; and MMP-13 increased at days 3 and 9 but decreased at days 6 and 12.
Discussion
Tendon healing involves both cellular and extracellular processes, and only through the optimization of both can neotendon capable of withstanding compressive and tensile forces be formed. This work investigated the therapeutic potential of ADMSCs treated with GDF-5, focusing in particular on genetic and molecular evidence for tendonogenic differentiation.
With their ability to self-renew, divide, and differentiate into mesenchymal tissues as well as their ease of extraction and isolation, ADMSCs represent a unique population of cells with the potential to aid in the treatment of connective tissue injuries. However, the promise of ADMSCs in tendon repair has yet to be translated into a real-world therapy.32 Previous studies have demonstrated successful isolation, expansion, and differentiation of multipotent MSCs from adipose tissue of humans and different animal species, including mouse and rat. Earlier studies also showed that ADMSCs isolated from rats have the potential to differentiate into adipogenic, osteogenic, myogenic, and chondrogenic lineages, provided that differentiation conditions are optimized.33 However, there is little evidence that ADMSCs can differentiate into a tendonogenic lineage.34
Research is ongoing regarding the role of growth factors in tendon regeneration. Our lab previously showed that GDF-5-deficient mice exhibit delayed tendon healing, as determined histologically, biochemically, and ultrastructurally.23 These results suggested that GDF-5 plays a crucial role in the normal tendon-healing process. Other investigators showed that GDF-5-coated sutures can temporarily increase the strength and thickness of regenerate tendon in rats.35 Additionally, it was demonstrated that implanting GDF-5 into collagen sponges that bridged Achilles tendon defects in rats led to increased tensile strength.36 These studies presented physical evidence suggesting that GDF-5 promotes neotendon formation, but the mechanisms by which this occurs were not elucidated. Other studies as well as preliminary data from our laboratory have shown that other members of the BMP family, such as GDF-6, GDF-7, and transforming growth factor-β, play a role in tendon healing.37 However, as mentioned above, our evidence and the literature most strongly support the role of GDF-5 in tendon development, healing, and repair. We therefore investigated the in vitro effect of GDF-5 on ADMSCs, hypothesizing that genes and proteins consistent with tendonogenic differentiation would be upregulated.
First, we observed that GDF-5 had a mitogenic effect on ADMSCs in a concentration-dependent manner when measured at day 4 as well as a time-dependent manner when cells were treated with 100 ng/mL of GDF-5. Because the highly differentiated and minimally proliferative nature of tenocytes presents a significant limitation with regard to neotendon formation at injury sites,38 ADMSCs treated with GDF-5 may prove to be a superior cell source in this regard.
We then examined the effect of GDF-5 on four genes/proteins that have been identified as potential markers for tendon formation: scleraxis, tenomodulin, tenascin-C, and Smad-8. The transcription factor scleraxis was found to demarcate the tendon-forming syndetome during development,39 and early expression of scleraxis was reported to be a highly specific marker for tenocyte progenitor cell populations.40 Additionally, scleraxis was reported to upregulate expression of tenomodulin, a late marker of tendon generation.41 Our data showed that treatment with GDF-5 led to an early increase in scleraxis transcription that was followed by increases in both tenomodulin mRNA and protein expression. We then investigated the effect of GDF-5 on the transcription factor Smad-8 and the extracellular protein tenascin-C. Previous studies showed that Smad-8 transfection promoted the healing of rat tendon defects as well as upregulated expression of scleraxis, thereby inducing the differentiation of MSCs into tendon progenitors.42 We found that GDF-5 induced a substantial early increase in Smad-8 protein expression, indicating that GDF-5 may help to initiate the differentiation of ADMSCs into tenocytes. We also observed late increases in tenascin-C by both real-time PCR and Western blot. Tenascin-C is an ECM protein that has been implicated in tendon development, as it has been found in tendon primordia as well as all differentiated tendon.43 While it is a relatively nonspecific marker, the late increased expression of tenascin-C was consistent with the hypothesis that GDF-5 induces ADMSCs to progress down a tendonogenic lineage. Notably, 100 ng/mL of GDF-5 was the concentration at which the tendonogenic markers were most upregulated in our studies.
Essentially, a tendon fiber is composed of tenocytes, parallel collagen fibrils, and ECM components such as proteoglycans.44 GDF-5 stimulated a threefold increase in Col III transcription at day 3 that was followed by a fivefold increase in Col I at day 12. This temporal relationship is consistent with the roles of collagen in tendon formation—Col I is the primary collagen secreted by mature tenocytes, while Col III plays a role in early wound healing and in endotenon and epitenon formation.38 Additionally, OHP was increased in ADMSCs treated with GDF-5 versus control at day 12, providing biochemical evidence that GDF-5 induced increased collagen production.
Because a tendon's extracellular environment is critical to its function, we then investigated the effect of GDF-5 on various ECM components produced by ADMSCs. We focused on the proteoglycans decorin and aggrecan. Decorin is responsible for the organization of collagen fibers and has been identified in the tensional segments of tendon, while aggrecan has been localized to the compressed segments of tendon.45,46 We observed that GDF-5 treatment of ADMSCs induced a significant and persistent increase in aggrecan transcription. While we did note a transient decrease in decorin transcription at day 9, we also found that it was increased at 100 ng/mL of GDF-5 at day 4. We then performed biochemical studies that showed that GDF-5 increased overall GAG at day 4, providing further molecular evidence that GDF-5 may upregulate proteoglycan production.
Other ECM components that we studied were the matrix remodeling proteins MMP-3, MMP-13, and TIMP-2. The balance between the MMPs and their inhibitors, the TIMPs, determines the composition of the ECM and thereby helps to control tendon generation and function.33 MMP-3 can degrade a broad range of target endopeptides as well as activate other MMPs, while MMP-13 is a collagenase specific for collagens. TIMP-2 is a nonspecific inhibitor of various MMPs.34 The use of aprotonin, a broad-spectrum proteinase inhibitor that also inhibits various MMPs, for the treatment of tendinopathy has been described in the literature with variable but acceptable results.35,47 It has been suggested that MMP-3 is associated with normal tendon maintenance and repair,36 and MMP-13 expression was increased and MMP-3 and TIMP-2 expression was decreased in studies of ruptured supraspinatus and Achilles tendons.37 Of note, TIMP-1 and −2 were shown to increase expression at the edges of acutely ruptured tendons further supporting the difference in tendon response to acute versus chronic injury.33,38 The variability of these past results further confirm the theory that an important interplay exists between the matrix reassembly activity of MMPs and the inhibitory effects of TIMPs on that process. We report that GDF-5 induced a decrease in MMP-3 expression at day 3 followed by a large increase at day 6, as well as moderate variable changes in MMP-13 and TIMP-2 at varying times and concentrations. These findings suggest that GDF-5 plays a role in both normal turnover and maintenance of the tendon like cells and in the connective tissue degradation process associated with tendon healing. Further, GDF-5 likely regulates a number of MMPs and TIMPs each in an individual protein-specific manner. Further studies will help to elucidate the relationships among ECM proteins, growth factors, and the fate of stem cells.
There are limitations to our study. No consistent significant changes were observed in OHP or proteoglycan ratios expressed by ADMSCs treated with GDF-5. It is possible that expression of such markers from ADMSCs would potentially increase at later time points had they been studied. We have shown that GDF-5 increases the proliferation of ADMSCs in a concentration- and time-dependent manner. Further, expression of neotendon markers scleraxis and tenomodulin confirms that tendon-like cell differentiation does occur when ADMSCs are treated with GDF-5. However, more investigation is needed to confirm the optimal environmental cues required to facilitate this process. Another potential weakness of our study was the analysis and reporting of our concentration kinetic results at day 4, but not analyzing our time kinetic data on a time frame that would include analysis on day 4 as well. This was done in accordance with the previous procedures established in our lab. Western blot analysis was not done for all proteins studied. In the future, efforts will be made to perform such paired analysis for each protein of interest. Last, the optimal concentration for growth factor delivery and treatment is debatable. In this study we chose the concentration of 100 ng/mL of GDF-5 for our study based on preliminary data from our laboratory and the fact that this concentration showed the most consistent effects on ADMSCs. Additional investigations are needed to determine the ideal concentration of GDF-5 for research purposes and for tissue engineering and regenerative therapy.
Conclusion
In summary, suboptimal treatment modalities for tendon injuries have led to the investigation of adjunct therapies, such as growth factors and stem cells. Previous studies have provided gross evidence suggesting that ADMSCs and GDF-5 individually can promote the growth of tendon-like tissue. Here, we provide genetic and molecular evidence that ADMSCs, when cultured in vitro with GDF-5, can be induced to form intracellular and extracellular genes and proteins consistent with tendonogenic differentiation. To our knowledge this study is the first to report the potential tendonogenic differentiation promoting effects of GDF-5 on ADMSCs. Further studies may help to support a role for ADMSCs, GDF-5, and combined therapies for enhanced tendon repair.
Acknowledgments
This research was supported by grants from NIH-NIAMS to Abhinav Bobby Chhabra (ARO52891) and an Institutional NRSA to MaCalus V. Hogan (T32 AR050960).
Disclosure Statement
No competing financial interests exist.
References
- 1.Moller A. Astron M. Westlin N. Increasing incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:479. doi: 10.3109/17453679608996672. [DOI] [PubMed] [Google Scholar]
- 2.Jozsa L. Kvist M. Balint B.J. Reffy A. Jarvinen M. Lehto M. Barzo M. The role of recreational sport activity in Achilles tendon rupture. A clinical, pathoanatomical, and sociological study of 292 cases. Am J Sports Med. 1989;17:338. doi: 10.1177/036354658901700305. [DOI] [PubMed] [Google Scholar]
- 3.Kleinert H.E. Serafin D. Kutz J.E. Atasoy E. Reimplantation of amputated digits and hands. Orthop Clin North Am. 1973;4:957. [PubMed] [Google Scholar]
- 4.Morberg P. Jerre R. Sward L. Karlsson J. Long-term results after surgical management of partial Achilles tendon ruptures. Scand J Med Sci Sports. 1997;7:299. doi: 10.1111/j.1600-0838.1997.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 5.Józsa L. Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997. [Google Scholar]
- 6.Zhou S. Yates K.E. Eid K. Glowacki J. Demineralized bone promotes chondrocyte or osteoblast differentiation of human marrow stromal cells cultured in collagen sponges. Cell Tissue Bank. 2005;6:33. doi: 10.1007/s10561-005-4253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain G. Fox J. Ashton B. Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Banwart J.C. Asher M.A. Hassanein R.S. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Silber J.S. Anderson D.G. Daffner S.D. Brislin B.T. Leland J.M. Hilibrand A.S. Vaccaro A.R. Albert T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sasso R.C. LeHuec J.C. Shaffrey C. Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 11.Varma M.J. Breuls R.G. Schouten T.E. Jurgens W.J. Bontkes H.J. Schuurhuis G.J. van Ham S.M. van Milligen F.J. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007;16:91. doi: 10.1089/scd.2006.0026. [DOI] [PubMed] [Google Scholar]
- 12.Chen F.H. Rousche K.T. Tuan R.S. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 13.Merceron C. Vinatier C. Clouet J. Colliec-Jouault S. Weiss P. Guicheux J. Adipose-derived mesenchymal stem cells and biomaterials for cartilage tissue engineering. Joint Bone Spine. 2008;75:672. doi: 10.1016/j.jbspin.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Katz A.J. Tholpady A. Tholpady S.S. Shang H. Ogle R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 15.Oedayrajsingh-Varma M.J. van Ham S.M. Knippenberg M. Helder M.N. Klein-Nulend J. Schouten T.E. Ritt M.J. van Milligen F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 16.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Ugarte D.A. Morizono K. Elbarbary A. Alfonso Z. Zuk P.A. Zhu M. Dragoo J.L. Ashjian P. Thomas B. Benhaim P. Chen I. Fraser J. Hedrick M.H. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 18.Kern S. Eichler H. Stoeve J. Kluter H. Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez A.M. Elabd C. Amri E.Z. Ailhaud G. Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Oshin A.O. Caporali E. Byron C.R. Stewart A.A. Stewart M.C. Phenotypic maintenance of articular chondrocytes in vitro requires BMP activity. Vet Comp Orthop Traumatol. 2007;20:185. doi: 10.1160/vcot-06-07-0061. [DOI] [PubMed] [Google Scholar]
- 21.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis-West P.H. Abdelfattah A. Chen P. Allen C. Parish J. Ladher R. Allen S. MacPherson S. Luyten F.P. Archer C.W. Mechanisms of GDF-5 action during skeletal development. Development. 1999;126:1305. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- 23.Chhabra A. Tsou D. Clark R.T. Gaschen V. Hunziker E.B. Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 24.Merino R. Macias D. Ganan Y. Economides A.N. Wang X. Wu Q. Stahl N. Sampath K.T. Varona P. Hurle J.M. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- 25.Bolt P. Clerk A.N. Luu H.H. Kang Q. Kummer J.L. Deng Z.L. Olson K. Primus F. Montag A.G. He T.C. Haydon R.C. Toolan B.C. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J Bone Joint Surg Am. 2007;89:1315. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 26.Hogan M. Kesturu G. James R. Balian G. Hurwitz S. Chhabra A. Growth and differentiation factor-5 regulation of extracellular matrix gene expression in murine tendon fibroblasts. J Tissue Eng Regen Med. (In press). [DOI] [PubMed]
- 27.Shen F.H. Zeng Q. Lv Q. Choi L. Balian G. Li X. Laurencin C.T. Osteogenic differentiation of adipose-derived stromal cells treated with GDF-5 cultured on a novel three-dimensional sintered microsphere matrix. Spine J. 2006;6:615. doi: 10.1016/j.spinee.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q. Li X. Beck G. Balian G. Shen F.H. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Butler D.L. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Shearn J.T. Gooch C. Awad H. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 30.Starnes T. Huang D. Kesturu G. James R. Balian G. Chhabra A.B. GDF-5 induced tendon repair and regeneration. AAOS 2007 Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- 31.Kesturu G. James R. Starnes T. Balian G. Chhabra A. Huang D. Treatment with GDF-5 induces tendonogenic differentiation of adipose derived stromal cells. Proceedings of 53rd Orthopaedic Research Society Meeting; San Diego, CA. 2007. [Google Scholar]
- 32.Tapp H. Hanley E.N., Jr. Patt J.C. Gruber H.E. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med. 2009;234:1. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B. Cao B. Li G. Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 34.Uysal A.C. Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther. 2009. Nov 26, [Epub ahead of print]. [DOI] [PubMed]
- 35.Rickert M. Jung M. Adiyaman M. Richter W. Simank H.G. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- 36.Aspenberg P. Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- 37.Mehta V. Mass D. The use of growth factors on tendon injuries. J Hand Ther. 2005;18:87. doi: 10.1197/j.jht.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q. Chen Z. Piao Y. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 39.Towler D.A. Gelberman R.H. The alchemy of tendon repair: a primer for the (S)mad scientist. J Clin Invest. 2006;116:863. doi: 10.1172/JCI28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweitzer R. Chyung J.H. Murtaugh L.C. Brent A.E. Rosen V. Olson E.N. Lassar A. Tabin C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 41.Shukunami C. Takimoto A. Oro M. Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann A. Pelled G. Turgeman G. Eberle P. Zilberman Y. Shinar H. Keinan-Adamsky K. Winkel A. Shahab S. Navon G. Gross G. Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aslan H. Kimelman-Bleich N. Pelled G. Gazit D. Molecular targets for tendon neoformation. J Clin Invest. 2008;118:439. doi: 10.1172/JCI33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W. Li L. Leet A.I. Seo B. Zhang L. Shi S. Young M.F. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 45.Rees S.G. Flannery C.R. Little C.B. Hughes C.E. Caterson B. Dent C.M. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J. 2000;350(Pt 1):181. [PMC free article] [PubMed] [Google Scholar]
- 46.Carter D.R. Beaupre G. Skeletal Function and Form: Mechanobiology of Skeletal Development, Aging and Regeneration. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 47.Wolfman N.M. Hattersley G. Cox K. Celeste A.J. Nelson R. Yamaji N. Dube J.L. DiBlasio-Smith E. Nove J. Song J.J. Wozney J.M. Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]