Abstract
Tissue engineering offers immense promise for bone regeneration. Human umbilical cord mesenchymal stem cells (hUCMSCs) can be collected without invasive procedures required for bone marrow MSCs. The objective of this study was to investigate the physical properties and the differentiation capacity of hUCMSCs on calcium phosphate cement (CPC) scaffolds with improved dissolution/resorption rates. CPC consisted of tetracalcium phosphate and dicalcium phosphate anhydrous, with various tetracalcium phosphate/dicalcium phosphate anhydrous ratios. At 1/3 ratio, CPC had a dissolution rate 40% faster than CPC control at 1/1. The faster-resorbable CPC had strength and modulus similar to CPC control. Their strength and modulus exceeded the reported values for cancellous bone, and were much higher than those of hydrogels and injectable polymers for cell delivery. hUCMSCs attached to the nano-apatitic CPC and proliferated rapidly. hUCMSCs differentiated into the osteogenic lineage, with significant increases in alkaline phosphatase activity, osteocalcin, collagen I, and osterix gene expression. In conclusion, in this study we reported that hUCMSCs attaching to CPC with high dissolution/resorption rate showed excellent proliferation and osteogenic differentiation. hUCMSCs delivered via high-strength CPC have the potential to be an inexhaustible and low-cost alternative to the gold-standard human bone marrow mesenchymal stem cells. These results may broadly impact stem-cell-based tissue engineering.
Introduction
Stem-cell-based tissue engineering offers immense promise for bone repair and regeneration, and for treating other debilitating diseases and conditions.1–5 Human bone marrow mesenchymal stem cells (hBMSCs) can differentiate into bone tissue, neural tissue, cartilage, muscle, and fat.6–9 hBMSCs can be harvested from the patient's bone marrow, expanded in culture, induced to differentiate, and combined with a scaffold to repair bone defects.10–15 These new approaches are highly important as 6 million bone fractures occur each year in the United States.16 Musculoskeletal conditions cost $215 billion annually,16,17 and these numbers are increasing as the population ages.18
The harvest of hBMSCs is an invasive procedure, and the proliferation and differentiation potential declines with aging. Recently, human umbilical cord mesenchymal stem cells (hUCMSCs) were derived and shown to differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells.19–23 hUCMSCs have several advantages: (1) umbilical cords can be collected at a low cost; (2) hUCMSCs are an inexhaustible stem cell source; (3) they can be collected without an invasive procedure required for BMSCs, and without the controversies of human embryonic stem cells; (4) hUCMSCs are a primitive MSC population with high plasticity and developmental flexibility; (5) hUCMSCs appear to cause no immunorejection and are not tumorigenic.20 In recent studies, hUCMSCs were cultured on tissue culture plastic21 and polymer scaffold for osteogenic differentiation.23
While bio-inert implants can induce an undesirable fibrous capsule in vivo, bioactive implants with bone-like calcium phosphate (CaP) minerals beneficially bond to neighboring bone.24–26 This is because CaP minerals provide a preferred substrate for cell attachment and support the proliferation and expression of osteoblast phenotype.24,25 Hence, hydroxyapatite (HA) and other bioactive CaP scaffolds are important for bone repair.26–28 However, for sintered bioceramics to fit into a bone cavity, the surgeon needs to machine the graft or carve the surgical site, leading to increases in bone loss, trauma, and surgical time.18 In contrast, calcium phosphate cements (CPCs) can be molded and set in situ to provide intimate adaptation to complex bone defects.29–34 One such cement is comprised of tetracalcium phosphate [TTCP: Ca4(PO4)2O] and dicalcium phosphate anhydrous (DCPA: CaHPO4), and is referred to as CPC.35–38 CPC self-hardens to form a resorbable HA implant. Due to its excellent bioactivity and ability to be replaced by new bone, CPC was approved in 1996 by the Food and Drug Administration for repairing craniofacial defects, thus becoming the first CPC for clinical use.36 Previous studies have cultured osteoblastic cells, rat BMSCs, and hUCMSCs on CPC.39–42 However, there has been no report on hUCMSC seeding on CPCs with different TTCP/DCPA ratios.
In this study, we described for the first time the seeding of hUCMSCs on CPC scaffolds with different TTCP/DCPA ratios. The purpose of varying the TTCP/DCPA ratio was to increase the CPC dissolution/resorption rate. hUCMSC proliferation and osteo-differentiation were investigated. It was hypothesized that (1) the CPC dissolution/resorption rate can be increased by tailoring the TTCP/DCPA ratio, without compromising mechanical and setting properties, and that (2) hUCMSCs will have excellent proliferation and osteo-differentiation on CPC, and changing the TTCP/DCPA ratio will not adversely affect the function of the attached hUCMSCs.
Materials and Methods
Fabrication of CPC with different TTCP/DCPA ratios
TTCP was synthesized from a solid-state reaction at 1500°C between DCPA and CaCO3 (J.T. Baker).36–38 The mixture was ground to obtain TTCP particles with sizes of ∼1–80 μm (median = 17 μm). DCPA was ground to obtain particle sizes of 0.4–3.0 μm (median = 1.0 μm). TTCP and DCPA were mixed to form the CPC powder. The following TTCP/DCPA molar ratios were used: 1/3, 1/2.5, 1/2, 1/1.5, 1/1, 2/1, and 3/1. The purpose of varying this ratio was to increase the CPC dissolution/resorption rate. Previous studies usually used a single TTCP/DCPA molar ratio of 1/1,36–38 and hence CPC with this ratio is termed “Traditional CPC control.”
CPC was rendered fast-setting and washout-resistant via a liquid containing 0.2 mol/L sodium phosphate (Na2HPO4) in water.38 CPC powder was mixed with the liquid at a powder-to-liquid mass ratio of 3.5 to 1. The paste was placed into a mold of 3 × 4 × 25 mm3 and set at 100% relative humidity for 4 h at 37°C. Then, the specimens were demolded and immersed in water at 37°C for 20 h before mechanical testing.
Mechanical properties, density, and porosity
A three-point flexural test43 with a span of 20 mm was used to fracture the specimens on a Universal Testing Machine (5500R; MTS). Flexural strength S = 3FmaxL/(2bh2), where Fmax is the maximum load on the load–displacement curve, L is span, b is specimen width, and h is thickness. Elastic modulus E = (F/c)(L3/[4bh3]), where load F divided by displacement c is the slope of the curve in the linear elastic region.
CPC specimens were dried in a vacuum oven at 60°C for 24 h. The density, d, was measured using the specimen weight divided by its volume. The volume was calculated by the specimen dimensions.44 Six specimens were thus measured for each material. Following a previous study,44 the CPC porosity, P, was obtained by the following: P = (dHA − d)/dHA, where dHA is the density of fully dense HA (3.14 g/cm3).
CPC setting time and conversion to HA
The CPC paste was filled into a mold of 6 mm diameter and 3 mm deep. The assembly was incubated in the humidor at 37°C. Setting time was measured using the Gilmore needle method with a flat tip of 1.06 mm diameter under a load of 454 g.45 CPC was considered set when the needle loaded onto the surface failed to leave a perceptible indentation.
CPC conversion to HA was measured using powder X-ray diffraction. CPC specimens were ground to fine powders and the X-ray diffraction patterns were recorded (Rigaku DMAX 2200) using graphite-monochromatized Cu Kα radiation (λ = 0.154 nm) generated at 40 kV and 40 mA. The sample was scanned from 20° to 40° (2θ) in a continuous mode (2° 2θ min−1, time constant 2 s), and peak intensities were recorded on a computer. The relative peak intensities of the 013 (29.2°) and 040 (29.8°) reflections for TTCP, the 110 (26.6°) reflection for DCPA, and the 002 (25.9°) reflection for HA were measured three times. The averaged values were used to calculate the percentage conversion of CPC to HA.46
CPC dissolution rate
As an initial assessment of in vivo resorption, the CPC dissolution rates were measured in a solution that simulates the acidic physiological fluids produced by osteoclasts. Specimens were immersed in a solution with ionic composition similar to that of serum ([Ca] = 1.15 mmol/L; [P] = 1.2 mmol/L; [KCl] = 133 mmol/L) except for the pH, which was adjusted to 3.0 with HCl.47,48 Dissolution of CPC was measured following a previously described procedure.47 Briefly, the measurement was conducted in a jacketed, 100 mL glass vessel connected to a circulating bath at 37°C. A combination pH electrode and a calcium-ion-selective electrode were used as the sensors for triggering delivery of the P- and Ca-titrant solutions, respectively, by two independent automatic titrators (Dosimat 665; Brinkmann). The demineralizing solution (40 mL) was placed in the vessel. A CPC disk of 12 mm diameter and 2 mm thickness was placed in the demineralizing solution. Dissolution of CPC increased the calcium and phosphate concentrations and the pH, which triggered the addition of the titrants. This in turn maintained the solution composition to be essentially constant.47,48 The dissolution rate was expressed as the amount of CPC dissolved per specimen surface area per hour (mg/[cm2 · h]). Although there may be inhomogeneities in the CPC composition, previous studies showed that CPC dissolved at a relatively constant rate.47,48 Using a dual-constant composition method, the rates of Ca and P dissolution were measured separately and independently, and the rates of both Ca and P dissolution were found to be nearly constant.47 Another study measured the dissolution rates of new CPC samples and of samples that had been predissolved by 50%. No difference was found in the dissolution rates, suggesting that there were no measurable changes in the dissolution rate of CPC over time in the acidic solution.48
hUCMSC seeding on CPC scaffolds and live/dead staining
The harvest of hUCMSCs was approved by the University of Kansas (HSCL 15402, 7/17/2009). The length of the umbilical cords was about 20 cm, the pregnancy was at least 37 weeks, the birth weight of the baby was at least 6 pounds, and the cells were harvested within 24 h after the delivery. hUCMSCs were harvested as described previously.19,22 Briefly, the cords obtained from an obstetrician was incubated in hyaluronidase (MP Biomedical) and collagenase type I (Sigma) for 30 min at 37°C. Then, the vascular tissue was removed, and the cords were minced and plated in a modified Dulbecco's modified Eagle's medium for 1 week. The cord remnants were then removed and the attached cells were harvested. To ensure a homogeneous population of stem cells, passage-4 cells were characterized by flow cytometry to analyze specific surface antigens of MSC lineage including CD29, CD49e, CD73, CD90, and CD105, as described in a separate study.49 Approximately 0.5 × 106 cells per vial were used for each antigen. Nonspecific binding was first blocked with a staining buffer containing 2% fetal bovine serum for 15 min, and the cells were then incubated with single label antigen for 20 min on ice. Mouse isotype antigens served as a negative control. Flow cytometry analysis was conducted using a FACscan flow cytometer (BD Biosciences). As described in a previous work,49 the flow cytometry results confirmed that the hUCMSCs had a relatively high purity of above 95%. For example, 98% of the cells were positive to CD29.
The use of hUCMSCs was approved by the University of Maryland (HP-42131, 5/29/2009). The hUCMSCs were cultured in a low-glucose Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) (control media). Passage-4 hUCMSCs were used. The osteogenic medium contained 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid, and 10 nM 1α,25-Dihydroxyvitamin (Sigma).21,23 Following a previous study,50 150,000 cells were diluted into 2 mL of osteogenic medium and added to each well containing a CPC disk of 2 mm thickness and 12 mm diameter. After 1, 4, and 8 days,50 the medium was removed and the cells were washed in Tyrode's Hepes buffer. Cells were stained and viewed by epifluorescence microscopy (TE300; Nikon). Staining was done for 1 h with 2 mL of Tyrode's Hepes buffer containing 2 μmol/L calcein-AM and 2 μmol/L ethidium homodimer-1 (Molecular Probes). Calcein accumulates inside live cells having intact membranes causing them to fluoresce green. Ethidium-homodimer-1 enters dead cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to their DNA, causing the nuclei of dead cells to fluoresce red.39
The percentage of live cells was measured. Three randomly chosen fields of view were photographed from each disk (five disks yielded 15 photos per material). The cells were counted. NLive is the number of live cells, and NDead is the number of dead cells. The percentage of live cells, PLive = NLive/(NLive + NDead).41 The attached cell density, DLive, was measured.41 DLive is the number of live cells attached to the specimen divided by the area, A: DLive = NLive/A. Both PLive and DLive were measured, because a high value of PLive only means no dead cells; it does not necessarily mean a large number of live cells that attach to the specimens.
Water-soluble tetrazolium salts-1 viability assay of hUCMSCs
hUCMSC viability was assessed using the water-soluble tetrazolium salts-1 (Wst-1) colorimetric assay, which measures the cellular mitochondrial dehydrogenase activity (Dojindo).39 At 8 days in the osteogenic medium, CPC with cells was transferred to wells in a 24-well plate and rinsed with 1 mL of Tyrode's Hepes buffer. One milliliter of Tyrode's Hepes buffer and 0.1 mL of Wst-1 solution (5 mmol/L Wst-1 and 0.2 mmol/L 1-methoxy-5-methylphenazinium methylsulfate in water) were added to each well and incubated at 37°C for 2 h. The absorbance at 450 nm was measured with a microplate reader (Wallac 1420 Victor2; PerkinElmer).39
Osteogenic differentiation of hUCMSCs on CPC
Each well containing a CPC disk with the osteogenic medium were seeded with 150,000 cells and cultured for 1, 4, and 8 days50 for quantitative real-time reverse transcription polymerase chain reaction measurement (7900HT; Applied Biosystems). The total cellular RNA on the scaffolds was extracted with TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA. TaqMan gene expression kits were used to measure the transcript levels of the proposed genes on human alkaline phosphatase (ALP; Hs00758162_m1), osteocalcin (OC; Hs00609452_g1), collagen type I (Coll I; Hs00164004), Osterix (Hs00541729), and glyceraldehyde 3-phosphate dehydrogenase (Hs99999905). Relative expression for each target gene was evaluated using the 2-ΔΔCt method.50 The Ct values of target genes were normalized by the Ct of the TaqMan human housekeeping gene glyceraldehyde 3-phosphate dehydrogenase to obtain the ΔCt values. These values were then subtracted by the Ct value of the hUCMSCs cultured on tissue culture polystyrene in the control medium for 1 day (the calibrator) to obtain the ΔΔCt values.
Scanning electron microscopy and statistical analysis
A scanning electron microscope (SEM, JEOL 5300) was used. Cells cultured for 4 days on specimens were rinsed with saline, fixed with 1% glutaraldehyde, subjected to graded alcohol dehydrations, rinsed with hexamethyldisilazane, sputter coated with gold, and examined in SEM.39
One-way and two-way analyses of variance were performed to detect significant effects of the variables. Tukey's multiple comparison tests were used at p-value of 0.05.
Results
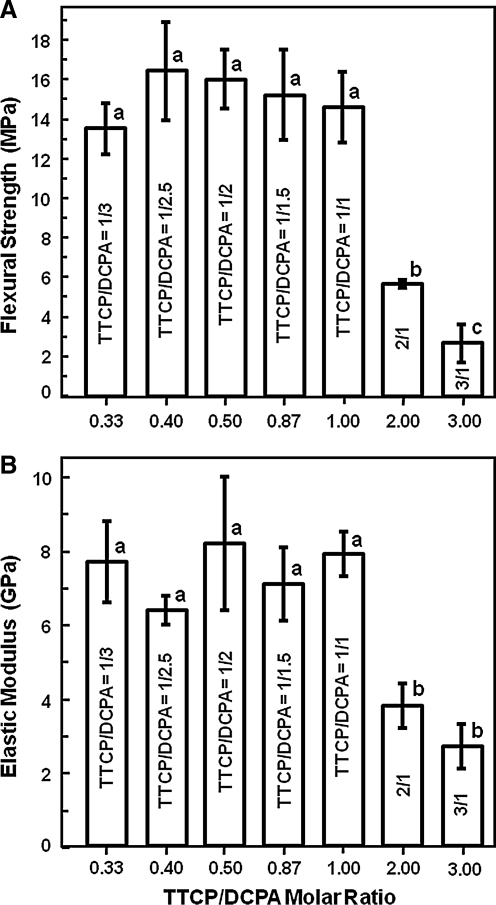
The TTCP/DCPA ratio was systematically varied to investigate its effect on the mechanical properties of CPC. Figure 1 plots the flexure strength and elastic modulus of CPC with different TTCP/DCPA ratios. In Figure 1A, when the TTCP/DCPA ratio was increased from 0.33 to 1, the strengths were between 14–16 MPa, similar to each other (p > 0.1). However, when the TTCP/DCPA ratio was further increased to 2 and 3, the strength decreased (p < 0.05). The modulus in Figure 1B showed a similar trend.
FIG. 1.
(A) Flexure strength and (B) elastic modulus of CPC with different TTCP/DCPA molar ratios. The x-axis shows the molar ratio (e.g., TTCP/DCPA = 1/3 = 0.33). Each value is the mean of six measurements, with the error bar showing 1 SD (mean ± SD; n = 6). In each plot, bars with dissimilar letters indicate values that are significantly different (p < 0.05). TTCP, tetracalcium phosphate; DCPA, dicalcium phosphate anhydrous; SD, standard deviation.
Therefore, three CPCs were selected for subsequent experiments: TTCP/DCPA = 1/3 = 0.33 (referred to as CPC0.33); TTCP/DCPA = 1/2 = 0.5 (CPC0.5); and TTCP/DCPA = 1/1 (CPC1, the control). The purpose was to increase the CPC dissolution rate, without compromising its strength, compared to CPC control.
The specimen density (mean ± standard deviation; n = 6) was measured to be 1.72 ± 0.03 g/cm3 for CPC0.33, 1.74 ± 0.03 g/cm3 for CPC0.5, and 1.79 ± 0.06 g/cm3 for CPC1, not significantly different (p > 0.1). The pore volume fraction was 45.2% ± 0.8%, 44.5% ± 0.4%, and 43.0% ± 2.0%, for CPC0.33, CPC0.5, and CPC1, respectively (p > 0.1).
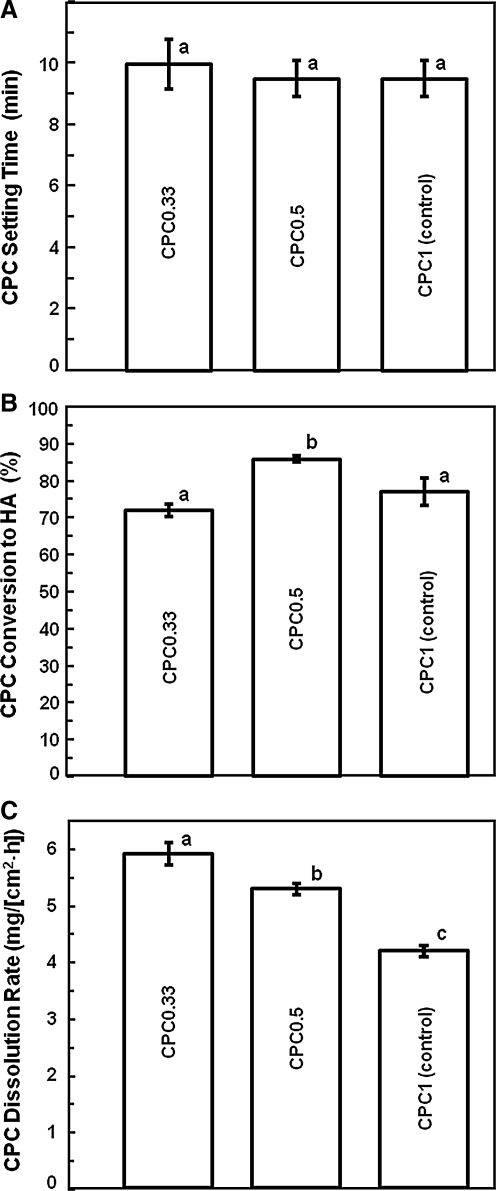
CPC setting time, conversion to HA, and dissolution rate were measured to understand their dependence on the TTCP/DCPA ratio. Figure 2 shows that varying the TTCP/DCPA ratio from 1/3 to 1/1 did not change the setting time (p > 0.1). Conversion to HA was 86.0% ± 1.3% for CPC0.5, higher than 71.7% ± 1.8% for CPC0.33 and 77.2% ± 3.7% for CPC1 (p < 0.05).
FIG. 2.
Physical properties of CPC scaffolds with various TTCP/DCPA ratios. (A) CPC setting time, (B) conversion to HA, and (C) dissolution rate. Each value is mean ± SD; n = 3. In each plot, values with dissimilar letters are significantly different (p < 0.05). CPC, calcium phosphate cement; HA, hydroxyapatite.
Decreasing the TTCP amount and increasing the DCPA amount increased the CPC dissolution rate (Fig. 2C). The CPC0.33 dissolution rate (mg/[cm2 · h]) was 5.9 ± 0.1, faster than 5.3 ± 0.1 for CPC0.5, and 4.2 ± 0.2 for CPC1 (p < 0.05). Compared to CPC1, CPC0.33 had a 40% faster dissolution rate.
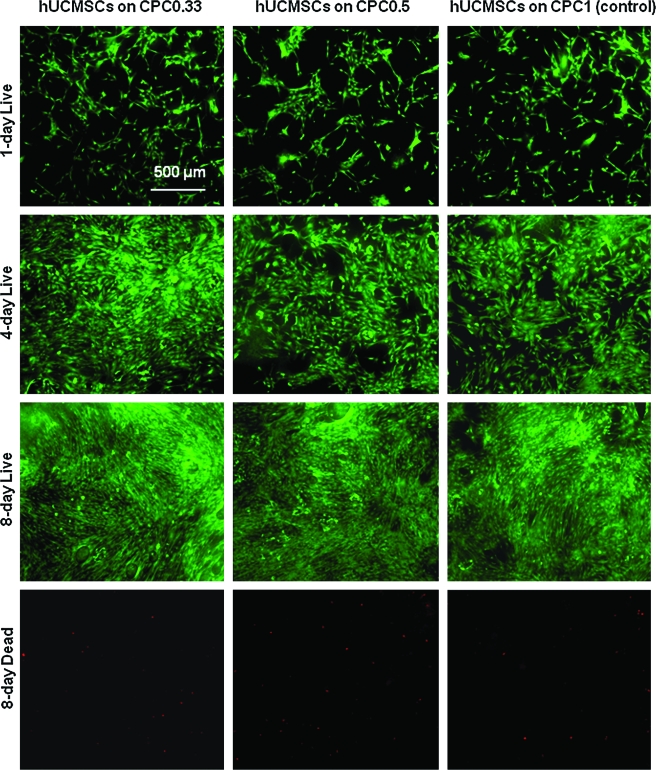
hUCMSCs were seeded on CPC to investigate whether increasing the dissolution rate of CPC would have an adverse effect on cell viability. Figure 3 shows the live/dead photos of hUCMSCs on CPCs. hUCMSCs proliferated from 1 to 8 days. The density of live cells adherent to each material was similar at the same time point. Dead cells (stained red) were very few on all materials.
FIG. 3.
Representative live/dead staining photos of hUCMSCs seeded on the three CPC scaffolds. Live cells (stained green) adhered and attained a normal, polygonal morphology. hUCMSCs proliferated on the scaffolds and increased in numbers from 1 day, to 4 days and 8 days. Dead cells (stained red, shown for day 8) were very few. hUCMSC, human umbilical cord mesenchymal stem cells. Color images available online at www.liebertonline.com/ten.
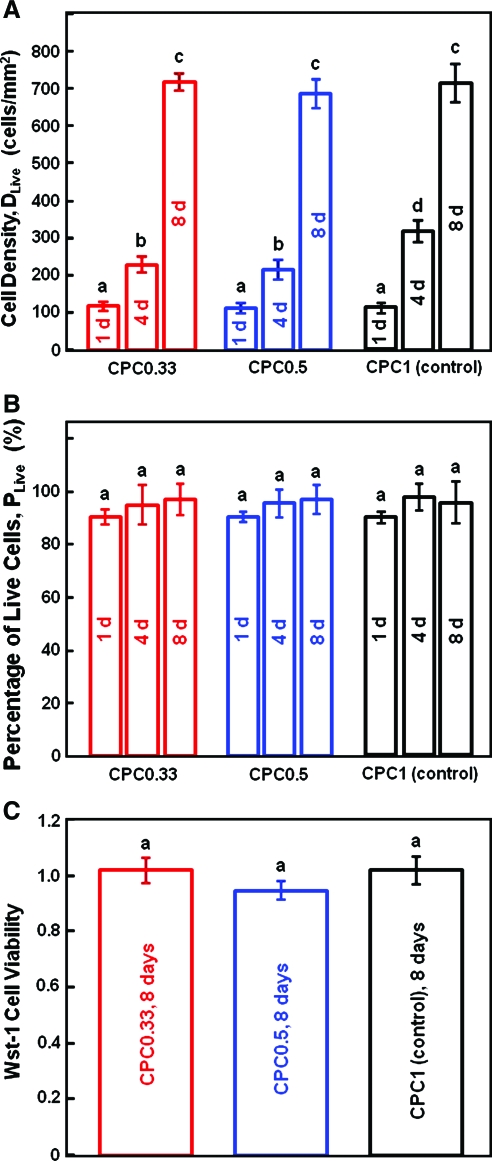
For quantification, Figure 4A shows that the cell density DLive was ∼120 cells/mm2 on all CPCs at 1 day. By 8 day, DLive increased to 720 cells/mm2. Hence, the cell density increased by sixfold in a week. Comparing CPC0.33 with the CPC1 control, the dissolution rate was increased by 40% without adversely affecting the hUCMSC proliferation. Figure 4B plots the percentage of live cells, PLive. For CPC0.33, PLive was 90% ± 3%, 94% ± 7%, and 96% ± 6%, at 1, 4, and 8 days, respectively (p > 0.1). In Figure 4C, the Wst-1 assay quantified the metabolic activity of the hUCMSCs. The cell viability was proportional to the amount of dehydrogenase activity in the cells. The absorbance (mean ± standard deviation; n = 5) was 1.0 ± 0.1 for the CPC1 control, 0.9 ± 0.1 for CPC0.5, and 1.0 ± 0.1 for CPC0.33 (p > 0.1). Hence, increasing the CPC dissolution did not compromise the hUCMSC viability.
FIG. 4.
hUCMSC proliferation and viability on the three CPC scaffolds. (A) Live cell density (number of attaching live cells per specimen area). (B) Percentage of live cells. (C) Cell viability measured by the water-soluble tetrazolium salts-1 (Wst-1) assay. Each value is mean ± SD; n = 5. In each plot, values with dissimilar letters are significantly different (p < 0.05). The cell density increased by sixfold in a week due to proliferation. Reducing the TTCP/DCPA ratio from 1/1 to 1/3, which increased the CPC dissolution rate by 40%, did not adversely affect the hUCMSC viability and proliferation. Color images available online at www.liebertonline.com/ten.
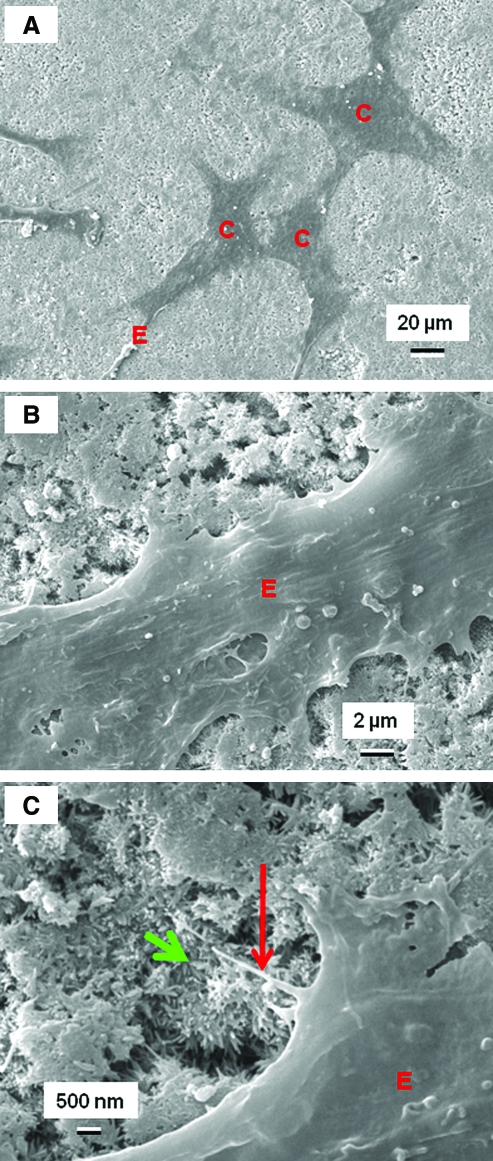
SEM was used to examine hUCMSC attachment to CPCs of different TTCP/DCPA ratios. In Figure 5A, the hUCMSCs (designated as “C”) attached to CPC1 control. In Figure 5B, hUCMSCs developed long cytoplasmic extensions “E,” attaching to the nano-apatite crystals that make up CPC. In Figure 5C, the primary extension E had sprouted secondary extension (long arrow), anchoring to the nano-apatite (short arrow). Scanning electron microscopy showed that the hUCMSC body had a size of 20–40 μm, and the extensions had lengths of about 30–50 μm.
FIG. 5.
Scanning electron micrographs of hUCMSCs. In (A), hUCMSCs (designated as “C”) that were attached on the CPC1/1 control showed a good spread with healthy polygonal shapes. Similar features were observed on the other samples. In (B), hUCMSCs had cytoplasmic extensions “E,” attaching to the nano-apatite crystals that make up CPC. In (C), the primary extension E had sprouted secondary extension, indicated by the long arrow. They anchored to the apatite nano-crystals, which are indicated by the short arrow. Color images available online at www.liebertonline.com/ten.
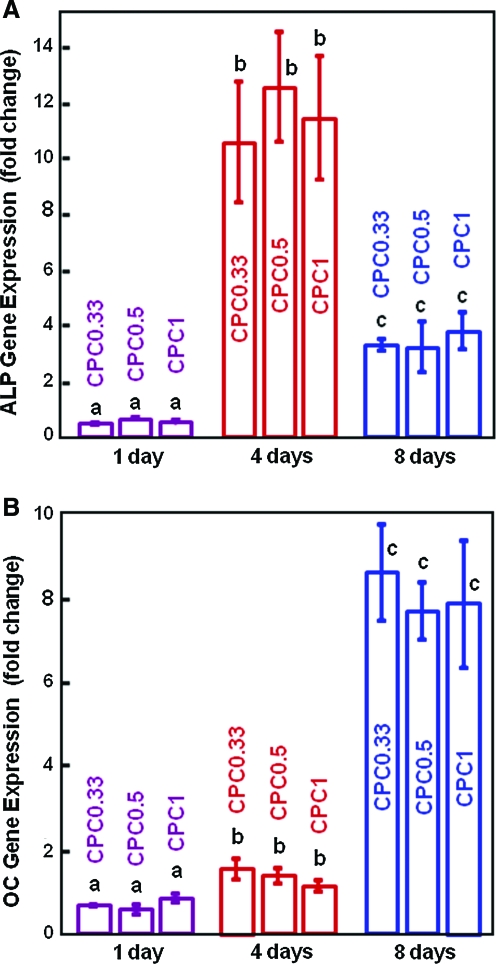
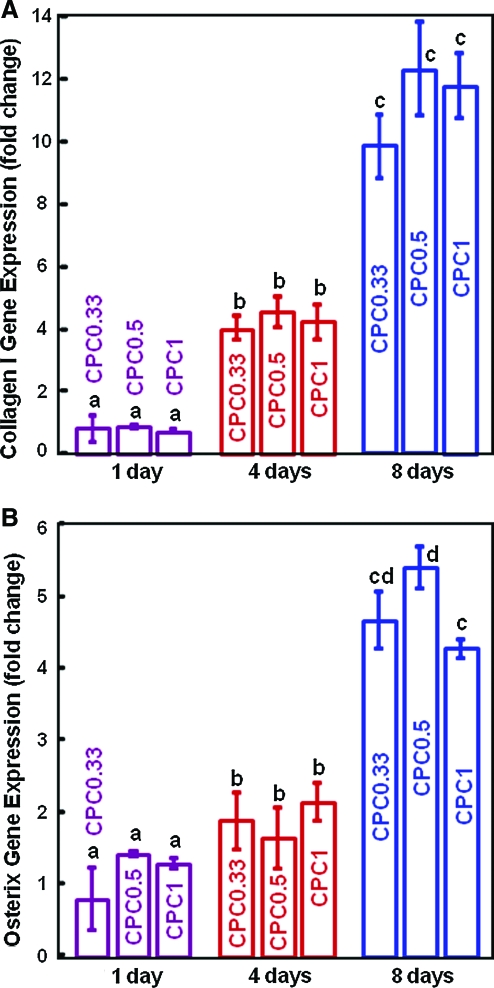
The osteogenic differentiation of hUCMSCs on CPCs was investigated to determine if the fast-dissolution CPC0.33 would compromise the differentiation compared to the traditional CPC1, and whether CPC can support osteodifferentiation and hence serve as carriers for hUCMSC delivery. Figure 6A, B plots ALP and OC gene expression. ALP was increased by 20-fold and peaked at day 4, and then decreased at day 8. At day 8, OC was increased by 12-fold compared to day 1 (p < 0.05). Figure 7 plots gene expression of two other osteogenic markers: collagen type I and osterix. Compared to day 1, these markers were slightly increased at day 4, and greatly increased at day 8 (p < 0.05). Further, at each time point, there is no significant difference between the fast-dissolving CPC0.33 and the traditional CPC1 (p > 0.1). These data demonstrate that the hUCMSCs successfully differentiated down the osteogenic lineage on all CPCs. Reducing the TTCP/DCPA ratio from 1/1 to 1/3, which increased the CPC dissolution rate by 40%, did not compromise the osteodifferentiation of hUCMSCs.
FIG. 6.
hUCMSC osteodifferentiation on CPC scaffolds: (A) ALP activity gene expression, and (B) OC gene expression (mean ± SD; n = 5). In each plot, bars with dissimilar letters indicate values that are different (p < 0.05). ALP was increased by 20-fold and peaked at day 4. At day 8, OC was increased by 12-fold compared to that at day 1 (p < 0.05). ALP, alkaline phosphatase; OC, osteocalcin. Color images available online at www.liebertonline.com/ten.
FIG. 7.
hUCMSC osteodifferentiation on CPC scaffolds: (A) Collagen type I gene expression, and (B) osterix gene expression (mean ± SD; n = 5). In each plot, bars with dissimilar letters indicate values that are different (p < 0.05). At day 8, collagen I was increased by ∼15-fold compared to that at day 1. Osterix was increased by fourfold compared to that at day 1 (p < 0.05). Color images available online at www.liebertonline.com/ten.
Discussion
The present study described for the first time the seeding of hUCMSCs on CPC scaffolds with different TTCP/DCPA ratios and different dissolution rates. CPCs have several merits, including the ability to be injected or molded to the desired shape, which is especially important for craniofacial repairs to achieve esthetics. The paste can set in situ to form a scaffold, which can be gradually resorbed and replaced by new bone.36 Extensive studies have been performed on CaP compositions and mechanical properties,29–31 injectability,32 growth factors delivery,33 CaP–polymer composites,34 and reinforced CaP scaffolds.37,38 A strong scaffold can help deliver stem cells to a wide range of load-bearing locations to enhance bone regeneration. For example, mandibular and maxillary ridge augmentation would be an ideal use for CPC, since CPC could be molded to the desired shape for esthetics, and then set to form a scaffold to induce bone formation. These implants, however, would be subject to early loading by provisional dentures and need to be resistant to flexure. Major reconstructions of the maxilla or mandible after trauma or tumor resection would require a moldable implant with good fracture resistance, so would the support of metal implants or augmentation of deficient implant sites. All these applications would be better served with CPC that has fracture resistance and rapid bone regeneration capability via stem cell delivery. With the TTCP/DCPA ratio ranging from 1/3 to 1/1, the CPC flexural strength was 14–16 MPa, and the elastic modulus was 6–8 GPa. These values exceeded the reported tensile strength of 3.5 MPa51 and elastic modulus of 0.3 GPa52 for natural cancellous bone.
In comparison, injectable polymeric carriers for cell delivery had a compressive strength of about 0.7 MPa and modulus of 0.008 GPa.53 Hygrogels had a tensile strength of about 0.07 MPa and a modulus of 0.0001 GPa.54,55 While these novel materials are meritorious for stem cell delivery in non-load-bearing applications, it was concluded that “Hydrogel scaffolds are used in nonload-bearing bone tissue engineering. … They do not possess the mechanical strength to be used in load bearing applications.”56 Therefore, the CPCs with different TTCP/DCPA ratios are mechanically stronger, and may be useful for stem cell delivery in a wide range of load-bearing maxillofacial and orthopedic applications.
The TTCP/DCPA ratios in this study are selected because a ratio of 1 results in stoichiometric HA (traditional CPC), and a ratio of 0.5 results in Ca-deficient HA, which has a faster dissolution rate than stoichiometric HA.57 Ratios <0.5 (e.g., CPC0.33) will further increase the CPC dissolution due to residual DCPA, which has a relatively fast dissolution.57 Previous studies showed that traditional CPC was slowly resorbed and replaced by new bone after 6–18 months.36,58 Another study showed that CPC had excellent soft tissue response and steadfast adherence to the adjacent bone, and 12%–25% by volume of the CPC was resorbed after 6 months.59 These studies appeared to show that the traditional CPC (TTCP/DCPA = 1/1) was partially resorbed and partially remaining after 6 months in vivo. It is desirable to increase the CPC resorption rate so that it can be completely resorbed and replaced by new bone within 6 months. The present study showed that compared to the traditional CPC, the CPC0.33 had a 40% faster dissolution rate. This is an initial in vitro assessment of the in vivo resorption for CPC, and animal studies are required to quantify how faster CPC0.33 can be resorbed and replaced by new bone in vivo, compared to the traditional CPC. Further, the present study showed that the faster resorption of CPC0.33 was achieved without compromising the strength and other physical properties, as well as stem cell attachment, proliferation, and osteo-differentiation.
The present study showed that after 1-day incubation, the hUCMSCs were able to adhere, spread, and remain viable on all CPC scaffolds. The spreading and development of cytoplasmic extensions are indicative of the good viability and attachment of hUCMSCs on CPCs. The nano-sized apatite crystals of CPC, which is similar to the minerals in natural bone, likely have enhanced the hUCMSC attachment and function. At day 8, the cell proliferation and viability were equivalent on all CPCs, and the cell density had a sixfold increase due to proliferation. These results demonstrate that the CPCs are noncytotoxic and compatible with hUCMSCs.
Previous studies showed that ALP, OC, Coll I, and osterix gene expression played key roles in the osteogenic differentiation of MSCs.1,10,20,21,60,61 The present study also showed that these osteogenic markers were all highly expressed in the hUCMSCs on CPCs. Stem cells offer immense promise for tissue engineering,1–6 and BMSCs are commonly studied for bone engineering.7–11 However, BMSCs for autogenous use can cause donor-site morbidity, are limited in number, and have lower self-renewal and differentiation potential with aging. Therefore, there is a strong need for alternative MSCs. Recent studies showed that hUCMSCs could be guided to osteo-differentiate with a high potential for bone regeneration.19–23 However, to date, little has been reported on hUCMSC interactions with bioactive scaffolds such as CPC and HA. Recent studies showed that rat BMSCs proliferated on CPC and osteodifferentiated with elevated ALP activity.41 However, when hBMSCs were cultured on CPC, an elevated ALP activity was not consistently measured, likely because the ion activities of CPC setting and component dissolution adversely affected hBMSC differentiation.62 This indicates that the rat BMSCs were more tolerant to CPC than hBMSCs. In the present study, hUCMSCs on CPC successfully differentiated and showed elevated ALP, OC, Coll I, and osterix. This suggests that the hUCMSCs were more tolerant to the CPC ion activities than the adult hBMSCs, and more successful in osteogenic differentiation on CPC. The reason for this may be attributed to the primitiveness and youth of the hUCMSCs, which may be similar to the fact that a child who breaks a bone usually heals much faster than an adult patient. This study showed that the CPC dissolution and resorption rate can be significantly increased, without compromising the hUCMSC proliferation and osteo-differentiation. Hence, hUCMSCs may be a superior, inexhaustible, and low-cost alternative to the gold-standard hBMSCs. These results may broadly impact the field of stem-cell-based regenerative medicine. Nonetheless, because the effects of toxicity, degradation, and osteogenic potential may be different between in vitro culture and in the living body, in vivo animal studies are needed to investigate hUCMSC delivery via CPC-based scaffolds with different TTCP/DCPA ratios for bone tissue engineering.
Conclusions
hUCMSC seeding on self-setting CPC scaffolds with different TTCP/DCPA ratios and dissolution rates was reported for the first time. Decreasing the TTCP/DCPA ratio from the traditional ratio of 1 to 0.33 increased the CPC dissolution rate by 40%, without compromising the strength and setting time. The strength and modulus of CPC0.33 exceeded those of natural cancellous bone, and were orders of magnitude higher than those for hydrogels and injectable polymeric carriers for cell delivery. While hBMSCs are commonly studied for tissue engineering, they require an invasive procedure to harvest. hUCMSCs are a relatively new stem cell source. The present study showed that hUCMSCs had excellent viability, proliferation, and high ALP, OC, Coll I, and osterix gene expression while seeded on CPC with TTCP/DCPA ratio from 1 to 0.33. Therefore, CPC with tailored dissolution/resorption rates may be promising scaffolds to deliver stem cells in load-bearing locations to enhance bone regeneration. hUCMSCs have the potential to serve as an inexhaustible and low-cost alternative to the gold-standard hBMSCs. These results may broadly impact the field of stem-cell-based regenerative medicine.
Footnotes
This work is an official contribution of the National Institute of Standards and Technology (NIST); not subject to copyright in the United States.
Acknowledgments
We thank Dr. C.G. Simon at the National Institute of Standards and Technology for discussions, and A.A. Giuseppetti for help with the SEM. We are indebted to Dr. M.D. Weir and Dr. J.P. Fisher at the University of Maryland for discussions and help. This study was supported by NIH grants R01DE14190 (HX), R01DE17974 (HX), R01DE11789 (LC), the State of Kansas (MD), Maryland Stem Cell Research Fund (HX), and the University of Maryland Dental School.
Disclosure Statement
No competing financial interests exist.
References
- 1.Datta N. Holtorf H.L. Sikavitsas V.I. Jansen J.A. Mikos A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Mao J.J. Giannobile W.V. Helms J.A. Hollister S.J. Krebsbach P.H. Longaker M.T. Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon D.M. Hawkins E.C. Francke-Carroll S. Fisher J.P. Effect of construct properties on encapsulated chondrocyte expression of insulin-like growth factor-1. Biomaterials. 2007;28:299. doi: 10.1016/j.biomaterials.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Propagation of undifferentiated human embryonic stem cells in hyaluronic acid hydrogels. Proc Natl Acad Sci. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang N. Varghese S. Lee H.J. Zhang Z. Ye Z. Bae J. Cheng L. Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci. 2008;105:20641. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill E.E. Boontheekul T. Mooney D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci. 2006;103:2494. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahaman M.N. Mao J.J. Stem cell based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y.C. Kaigler D. Rice K.G. Krebsbach P.H. Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 9.Lu H.H. Tang A. Oh S. Spalazzi J.P. Dionisio K. Compositional effects on the formation of a calcium phosphate layer and the response osteoblast-like cells on polymer-bioactive glass composites. Biomaterials. 2005;26:6323. doi: 10.1016/j.biomaterials.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Benoit D.S.W. Durney A.R. Anseth K.S. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogeneic differentiation. Biomaterials. 2007;28:66. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Mao J.J. Vunjak-Novakovic G. Mikos A.G. Atala A. Regenerative medicine: Translational Approaches and Tissue Engineering. Boston: Artech House; 2007. [Google Scholar]
- 12.Benoit D.S.W. Nuttelman C.R. Collins S.D. Anseth K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Leach J.K. Kaigler D. Wang Z. Krebsbach P.H. Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann S. Hagenmuller H. Koch A.M. Muller R. Vunjak-Novakovic G. Kaplan D.L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials. 2007;28:1152. doi: 10.1016/j.biomaterials.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Reilly G.C. Radin S. Chen A.T. Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praemer A. Furner S. Rice D.P. Musculoskeletal conditions in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. [Google Scholar]
- 17.Ambrosio A.M.A. Sahota J.S. Khan Y. Laurencin C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res B. 2001;58:295. doi: 10.1002/1097-4636(2001)58:3<295::aid-jbm1020>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Laurencin C.T. Ambrosio A.M.A. Borden M.D. Cooper J.A. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Wang H.S. Hung S.C. Peng S.T. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 20.Can A. Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 21.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 22.Bailey M.M. Wang L. Bode C.J. Mitchell K.E. Detamore M.S. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 23.Wang L. Singh M. Bonewald L.F. Detamore M.S. Signaling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 24.Ducheyne P. Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 25.Murphy W.L. Hsiong S. Richardson T.P. Simmons G.A. Mooney D.J. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Pilliar R.M. Filiaggi M.J. Wells J.D. Grynpas M.D. Kandel R.A. Porous calcium polyphosphate scaffolds for bone substitute applications—in vitro characterization. Biomaterials. 2001;22:963. doi: 10.1016/s0142-9612(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 27.Russias J. Saiz E. Deville S. Gryn K. Liu G. Nalla R.K. Tomsia A.P. Fabrication and in vitro characterization of three-dimensional organic/inorganic scaffolds by robocasting. J Biomed Mater Res A. 2007;83:434. doi: 10.1002/jbm.a.31237. [DOI] [PubMed] [Google Scholar]
- 28.Miranda P. Pajares A. Saiz E. Tomsia A.P. Guiberteau F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J Biomed Mater Res A. 2008;85:218. doi: 10.1002/jbm.a.31587. [DOI] [PubMed] [Google Scholar]
- 29.Ginebra M.P. Rilliard A. Fernández E. Elvira C. Román J.S. Planell J.A. Mechanical and rheological improvement of a calcium phosphate cement by the addition of a polymeric drug. J Biomed Mater Res. 2001;57:113. doi: 10.1002/1097-4636(200110)57:1<113::aid-jbm1149>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Barralet J.E. Gaunt T. Wright A.J. Gibson I.R. Knowles J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res B. 2002;63:1. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 31.Bohner M. Gbureck U. Barralet J.E. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials. 2005;26:6423. doi: 10.1016/j.biomaterials.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 32.Bohner M. Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Link D.P. van den Dolder J. van den Beucken J.J. Wolke J.G. Mikos A.G. Jansen J.A. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-β1 loaded gelatin microspheres. Biomaterials. 2008;29:675. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Link D.P. van den Dolder J. van den Beucken J.J. Cuijpers V.M. Wolke J.G. Mikos A.G. Jansen J.A. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A. 2008;87:760. doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 35.Brown W.E. Chow L.C. A new calcium phosphate water setting cement. In: Brown P.W., editor. Cements Research Progress. Westerville, OH: American Ceramic Society; 1986. pp. 352–379. [Google Scholar]
- 36.Friedman C.D. Costantino P.D. Takagi S. Chow L.C. Bone source hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res B. 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Xu H.H.K. Quinn J.B. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials. 2002;23:193. doi: 10.1016/s0142-9612(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 38.Xu H.H.K. Takagi S. Quinn J.B. Chow L.C. Fast-setting and anti-washout calcium phosphate scaffolds with high strength and controlled macropore formation rates. J Biomed Mater Res A. 2004;68:725. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 39.Xu H.H.K. Simon C.G., Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005;26:1337. doi: 10.1016/j.biomaterials.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Link D.P. van den Dolder D.J. Wolke J.G. Jansen J.A. The cytocompatibility and early osteogenic characteristics of an injectable calcium phosphate cement. Tissue Eng. 2007;13:493. doi: 10.1089/ten.2006.0015. [DOI] [PubMed] [Google Scholar]
- 41.Moreau J.L. Xu H.H.K. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials. 2009;30:2675. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao L. Burguera E.F. Xu H.H.K. Amin N. Ryou H. Arola D.D. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate–chitosan–biodegradable fiber scaffolds. Biomaterials. 2010;31:840. doi: 10.1016/j.biomaterials.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Society for Testing and Materials. West Conshohocken, PA: ASTM International; 2004. ASTM D 790-03: Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastic and Electrical Insulating Materials. [Google Scholar]
- 44.Xu H.H.K. Quinn J.B. Takagi S. Chow L.C. Eichmiller F.C. Strong and macroporous calcium phosphate cement: effects of porosity and fiber reinforcement. J Biomed Mater Res A. 2001;57:457. doi: 10.1002/1097-4636(20011205)57:3<457::aid-jbm1189>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Guide to Dental Materials and Devices. 7th. 1974/1975. American Dental Association; Chicago: ADA Specification No. 9 for Dental Silicate Cement; pp. 194–202. [Google Scholar]
- 46.Ishikawa K. Takagi S. Chow L.C. Ishikawa Y. Properties and mechanisms of fast-setting calcium phosphate cements. J Mater Sci Mater Med. 1995;6:528. [Google Scholar]
- 47.Chow L.C. Markovic M. Takagi S. A dual constantcomposition titration system as an in vitro resorption model for comparing dissolution rates of calcium phosphate biomaterials. J Biomed Mater Res B. 2003;65:245. doi: 10.1002/jbm.b.10009. [DOI] [PubMed] [Google Scholar]
- 48.Hirayama S. Takagi S. Markovic M. Chow L.C. Properties of calcium phosphate cements with different tetracalcium phosphate and dicalcium phosphate anhydrous molar ratios. J Res Natl Inst Stand Technol. 2008;113:311. doi: 10.6028/jres.113.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L. Wang L. Detamore M.S. Comparative analysis of two enzymatic methods for the isolation of mesenchymal stem cells from the human umbilical cord Wharton's Jelly. Stem Cells Dev. 2010 (In review). [Google Scholar]
- 50.Kim K. Dean D. Mikos A.G. Fisher J.P. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10:1810. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damien C.J. Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 52.O'Kelly K. Tancred D. McCormack B. Carr A. A quantitative technique for comparing synthetic porous hydroxyapatite structure and cancellous bone. J Mater Sci Mater Med. 1996;7:207. [Google Scholar]
- 53.Shi X. Sitharaman B. Pham Q.P. Liang F. Wu K. Billups W.E. Wilson L.J. Mikos A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28:4078. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drury J.L. Dennis R.G. Mooney D.J. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Kuo C.K. Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part I. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 56.Drury J.L. Mooney D.J. Review. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 57.Matsuya S. Takagi S. Chow L.C. Effect of mixing ratio and pH on the reaction between Ca4(PO4)2O and CaHPO4. J Mater Sci Mater Med. 2000;11:305. doi: 10.1023/a:1008961314500. [DOI] [PubMed] [Google Scholar]
- 58.Sugawara A. Fujikawa K. Kusama K. Nishiyama M. Murai S. Takagi S. Chow L.C. Histopathologic reaction of a calcium phosphate cement for alveolar ridge augmentation. J Biomed Mater Res. 2002;61:47. doi: 10.1002/jbm.10010. [DOI] [PubMed] [Google Scholar]
- 59.Stelnicki E.J. Ousterhout D.K. Hydroxyapatite paste (Bone source) used as an onlay implant for supraorbital and malar augmentation. J Craniofac Surg. 1997;8:367. doi: 10.1097/00001665-199708050-00007. [DOI] [PubMed] [Google Scholar]
- 60.Lavery K. Hawley S. Swain P. Rooney R. Falb D. Alaoui-Ismaili M.H. New insights into BMP-7 mediated osteoblastic differentiation of primary human mesenchymal stem cells. Bone. 2009;45:27. doi: 10.1016/j.bone.2009.03.656. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z.Y. Teoh S.H. Chong M.S. Schantz J.T. Fisk N.M. Choolani M.A. Chan J. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 62.Weir M.D. Xu H.H.K. Culture human mesenchymal stem cells with calcium phosphate cement scaffolds for bone repair. J Biomed Mater Res B. 2010 doi: 10.1002/jbm.b.31563. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]