Dementia is common in advanced Parkinson's disease (PD), especially in the elderly [2]. Cerebral white matter lesions (CWMLs) have been described in normal aging, vascular dementia (VD), and Alzheimer's disease (AD) [4, 5]. CWMLs may be associated with increased risk of dementia, disability, and death. Whether CWML burden in AD correlates with cognitive decline remains uncertain. Reports are scant on the influence of CWMLs in PD dementia (PDD).
We reviewed medical records and autopsy findings of PD patients between 1990 and 2008 from the Sun Health Research Institute Brain and Body Donation Program. All cases reviewed had a complete neuropathological examination. Diagnosis of PD was based on UK brain bank criteria. DSM IV criteria for dementia were used. Previous studies have largely utilized ante-mortem neuroimaging quantification of white matter burden. Our study is the first to quantify CWMLs pathologically in PDD.
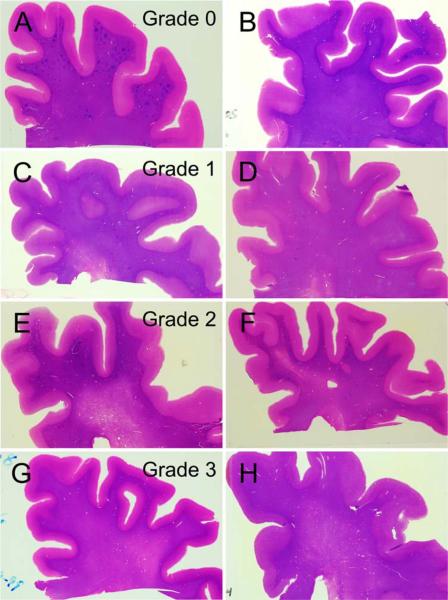
Pathologically, gross and microscopic neuropathologic assessments were made by a single observer (TGB) without knowledge of the history or diagnosis. The clinical history was reviewed subsequently in order to make an appropriate clinicopathologic diagnosis. Diagnostic histological methods were performed on standard blocks of tissue fixed in 4% buffered formaldehyde and then either dehydrated and embedded in paraffin or cryoprotected and cut on a freezing, sliding microtome. Details of the methods have been previously published [1]. Neuropathological diagnoses were based on published consensus criteria. For AD, this required an NIA-Reagan “intermediate” or “high” probability [7]. For VD, the NINDS-AIREN criteria were used [6]. For progressive supranuclear palsy (PSP) and other relevant conditions, the histopathological methodologies described by Dickson [3] were employed. CWMLs were rated in the frontal, parietal, temporal, and occipital lobes as follows: grade 1 is restriction to the immediate periventricular area, occupying less than one-third of the centrum semiovale; grade 2 is involvement of one-third to two-thirds; while grade 3 is involvement of more than two-thirds of the centrum semiovale (Fig. 1).
Fig. 1.
H&E-stained thick (40 μ) formalin-fixed, frozen sections of the frontal lobe from eight different subjects with a clinicopathologic diagnosis of Parkinson's disease, illustrating the grading system used for cerebral white matter rarefaction. Each row shows two subjects with the same grade. Grade 1 is usually restricted to the immediate periventricular area, occupying less than one-third of the centrum semiovale; grade 2 is involvement of one-third to two-thirds while grade 3 in involvement of more than two-thirds of the centrum semiovale
All subjects were confirmed to have the typical PD histopathology. Cases with concurrent pathology meeting criteria for AD, hippocampal sclerosis (HS), VD, and PSP were excluded. White matter scores were compared between PDD and PD cognitively normal (PD-CogNL) subjects. Mean levels were compared by using the paired t test. Prevalence was compared by using the McNemar test. Adjusted odds ratios were calculated by using multiple logistic regression modeling. Logistic regression was used because the distribution of white matter scores was skewed.
Of 108 subjects who were diagnosed as PD, 54 patients had no concurrent HS, PSP, AD, or VD on pathology. Three were excluded because of a history of a brain tumor or severe head injury, yielding 26 PDD and 25 PD-CogNL (Table 1). The crude mean white matter score and the prevalence of positive white matter scores were not higher in the PDD group. However, the crude comparisons were confounded by age at death, age at the onset of PD, and duration of PD. After adjusting for these factors, the odds ratio for dementia versus positive white matter score was 2.6 (95% CI = 0.43–16). Given the large CI, further analysis was done. The sample indicates 73% probability (Bayes posterior with a non-informative prior) that the OR is greater than 1.5 and 85% probability that the OR is greater than 1.0. A sample of 75 subjects per group would have 80% power to detect an OR of 2.6 (α = 0.05, π0 = 0.50).
Table 1.
Comparison of 26 subjects with Parkinson's disease dementia (PDD) versus 25 non-demented individuals with Parkinson's disease (PD-CogNL)
| PDD | PD-CogNL | Δ | P | 95% CI | |
|---|---|---|---|---|---|
| Female, n (%) | 8 (31) | 11 (44) | −0.13 | 0.39 | −0.39 to 0.14 |
| Age at death (years), mean (SD) | 77.4 (6.6) | 82.7 (5.6) | −5.3 | 0.004 | −8.7 to −1.8 |
| Age at onset of PD (years), mean (SD) | 59 (11) | 70 (12) | −11 | 0.001 | −17 to −5 |
| Duration of PD (years), mean (SD) | 17.7 (6.6) | 12.5 (7.7) | 5.2 | 0.01 | 1.2–9.3 |
| ApoE e4 carrier, n/N (%) | 4/23 (17) | 4/20 (20) | −0.03 | >0.99 | −0.30 to 0.21 |
| White matter total score, mean (SD) | 1.2 (1.6) | 1.9 (3.0) | −0.7 | 0.31 | −2.0 to 0.6 |
| White matter total score >0, n (%) | 13 (50) | 13 (52) | −0.02 | >0.99 | −0.30 to 0.26 |
Our results tentatively support the hypothesis that the cumulative burden of CWMLs correlates with dementia in PD patients with no concurrent AD, HS, VD, or PSP. A sample three times larger would be needed in order to validate this finding with adequate power.
Acknowledgments
This study was supported by the National Institute of Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, and 05-901 to the Arizona Parkinson's Disease Consortium), and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson's Research.
Footnotes
Conflict of interest statement The authors declare that they have no conflict of interest.
References
- 1.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. see comment. [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 4.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113:349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 5.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–436. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- 6.Roman GC, Tatemich TK, Erkinuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology. 1993;43:250–256. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 7.The National Institute on Aging and Reagan Institute Working Group Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(Suppl 4):S1–S2. [PubMed] [Google Scholar]