Abstract
Glatiramer acetate (GA), a synthetic random amino acid copolymer, poly(Y, E, A, K)n, is widely used for treatment of multiple sclerosis. It inhibits experimental autoimmune encephalomyelitis (EAE) in mice by competition with the antigen and by induction of regulatory T cells. A novel copolymer, poly (F, Y, A, K)n, designated FYAK, was more effective than GA in its immunomodulatory activity in EAE. Here, FYAK and GA were compared in the amelioration of another disease model in mice, experimental autoimmune uveoretinitis (EAU). When tested by co-immunization with a uveitogenic antigen, FYAK was superior to GA in its capacity to inhibit EAU induction, as well as immune processes related to this condition. Further, regulatory T cell lines specific to FYAK were more immunosuppressive than GA-specific lines in the EAU model. The superiority of FYAK-specific lines was accompanied by higher production of Th2 cytokines. These data thus demonstrate that FYAK, a novel copolymer, is superior to GA in its capacity to inhibit immunopathogenic processes in a non-central nervous system tissue.
Keywords: Autoimmunity, Copolymers, Cytokines, EAU, glatiramer acetate
1. Introduction
Glatiramer acetate [GA, poly(Y,E,A,K)n, Copolymer 1, Copaxone] is a synthetic random basic amino acid copolymer that is widely used for treatment of relapsing-remitting multiple sclerosis (MS). The efficacy of GA was first demonstrated in rodents developing experimental autoimmune encephalomyelitis (EAE), the animal model for MS and much of our knowledge about GA has been accumulated by use of this rodent model for MS (Arnon and Aharoni, 2004; Farina et al., 2005). The mode of action of GA is complex and may include both competitive binding to MHC molecules on antigen presenting cells (APC) (Arnon and Aharoni, 2004; Fridkis-Hareli et al., 1999; Fridkis-Hareli and Strominger, 1998; Fridkis-Hareli et al., 1994) and the induction of immunosuppressive cytokine-secreting copolymer-specific T-regulatory cells (Aharoni et al., 2000; Arnon and Aharoni, 2004; Duda et al., 2000; Farina et al., 2005; Stern et al., 2008). Recent studies have suggested also the involvement of dendritic cells and type II monocytes in the process (Hussien et al., 2001; Kim et al., 2004; Vieira et al., 2003; Weber et al., 2007).
Since binding to MHC molecules plays a role in the suppressive activity, additional copolymers with higher affinity for HLA-DR2, the MHC molecule strongly associated with MS, were synthesized (Fridkis-Hareli et al., 2002). Two of these novel copolymers, designated FYAK [poly(F,Y,A,K)n, CO-14, P1-2301] and VWAK [poly(V,W,A,K)n] were superior to GA in several aspects, including their affinity for HLA-DR2, their capacity to inhibit EAE and their induction of immunosuppressive cytokine-secreting T regulatory cells (Fridkis-Hareli et al., 2002; Illes et al., 2004; Stern et al., 2004; Stern et al., 2008). The immunosuppressive activity of GA, as well as that of FYAK, is not restricted to immune-mediated central nervous system (CNS) conditions (Arnon and Aharoni, 2004; Stern et al., 2008) and we have previously reported that treatment with this copolymer also inhibits development of an ocular inflammatory disease, experimental autoimmune uveoretinitis (EAU) (Zhang et al., 2000). EAU is induced in mice by immunization with a retinal-specific antigen, interphotoreceptor retinoid-binding protein (IRBP), and closely resembles EAE in various aspects (Caspi, 2002).
In the present study, GA and FYAK, one of the two novel copolymers, have been compared in their capacity to inhibit induction of EAU, as well as several parameters related to the immunopathogenic process of this ocular disease. FYAK was more effective than GA in all tested parameters. Of particular interest is the observation that regulatory cell lines specific for FYAK produced immunosuppressive cytokines at levels higher than those made by GA-specific lines.
2. Materials and Methods
2.1 Mice
Female B10.A (H-2a), B10.RIII (H-2r) and B10.BR (H-2k) mice, 6-8 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were housed in a pathogen-free facility and all procedures involving animals were performed in compliance with the NIH Guidelines on Use of Animals in Research.
2.2 Reagents
FYAK (also designated CO-14 or PI-2301) was provided by Peptimmune, Inc. (Cambridge, MA), while GA was purchased from TEVA Neuroscience Inc. (Kansas City, MO). Bovine IRBP was prepared as described by Pepperberg et al. (1991) and human IRBP peptide 161-180 was purchased from AnaSpec Inc. (San Jose, CA). Pertussis toxin (PTx) was obtained from Sigma (St. Louis, MO) and purified protein derivative (PPD) was from Parke-Davis (Morris Plains, NJ). Complete Freund's adjuvant (CFA) was prepared by adding Mycobacterium tuberculosis H37RA (Difco, Detroit, MI) to incomplete Freund's adjuvant (Difco), to a concentration of 2.5 mg/ml.
2.3. Induction of EAU and Copolymer administration
EAU was induced as described by Silver et al. (Silver et al., 1999) with minor modifications. Mice were immunized with 50μg IRBP (B10.A or B10.BR), or 10μg IRBP peptide 161-180 (B10.RIII) emulsified in CFA. The emulsion was injected into the base of the tail and two thighs in a total volume of 0.2 ml, and the B10.A or B10.BR mice were also concurrently injected with 0.5μg of PTx, intraperitoneally. GA or FYAK were incorporated in the aqueous portion of the emulsion (co-immunization) at the indicated doses. Eyes were collected 14 days post injection (p.i). and ocular sections were prepared as described elsewhere (Takase et al., 2005). Severity of disease, on a scale of 0 to 4 in half-point increments, was scored as detailed elsewhere (Takase et al., 2005).
2.4. Cytokine production
Draining lymph node cells of immunized mice were cultured in 24-well plates at 5×106 cells in 1 ml of RPMI-1640 medium, containing HL-1 serum replacement (Cambrex Bioscience, Walkersville, MD), with or without stimulants. Supernatants were collected after incubation for 48 hours (hr). Production of cytokines by copolymer-specific cell lines was measured in culture supernatants of day 2 of the 3rd or 4th stimulation cycle (see below) with the corresponding copolymer. Cytokine levels were determined by Multiplex SearchLight Technology (Pierce Biotechnology, Woburn, MA) and major differences between samples were verified by using ELISA kits from R&D Systems (Minneapolis, MN).
2.5. Generation of copolymer-specific CD4 - cell lines
Copolymer-specific CD4 T-Cell Lines were generated as described (Stern et al., 2008), with minor modifications, as follows. Spleen and draining lymph node cells from mice immunized with 200 μg of copolymer were collected 10 days p.i. and CD4 T cells were isolated as described in detail elsewhere (de Vos et al., 2000; Foxman et al., 2002). CD4 cells were then stimulated with the corresponding copolymer (10 μg/ml) at 2.5×106/ml in 24-well plates, for 3 days, in the presence of APC (irradiated syngeneic naïve splenocytes) at the ratio of 1:1. Activated CD4 T-cells were then put into a resting stage by culturing with complete DMEM medium containing 10% fetal bovine serum and IL-2 (20 ng/ml) for 5-7 days. The stimulation and resting procedures were repeated for 2 or 3 additional times.
The line cells were also tested for their responsiveness toward the corresponding copolymer. The cells were obtained from cultures of cell lines, on day 2 after re-stimulation, washed and incubated in 96 well plates, along with irradiated syngeneic APC, at the ratio of 1:1, with the corresponding copolymer at different concentrations. The proliferation response was measured by 3H-thymidine incorporation, with a pulse of 0.5 μCi/well, during the last 16 hr of 4 day total incubation.
2.6. Testing the immunosuppressive activity of copolymer-specific cell lines in vivo
Copolymer-specific cell lines specific to GA or FYAK, established as described above, using cells from B10.RIII, B10.A, or B10.BR, were injected into naïve mice of the corresponding strain, at 5 × 106 or 107, and the recipient mice were immunized one day later with IRBP peptide 161-180 (B10.RIII), or the IRBP protein (B10.A and B10.BR), as detailed above. EAU development was measured 14 days later, as described above.
2.7. Testing the immunosuppressive activity of copolymer-specific cell lines in vitro
Copolymer-specific T line cells of B10.A origin were added, at 5×104, along with the corresponding copolymer, at 10 μg/ml, to cultures made of 5×104 CD4 T cells from syngeneic mice, immunized 10 days earlier with IRBP, and 20×104 irradiated APC. 5×104 naïve CD4 T cells from syngeneic untreated mice were added to control cultures. IRBP was added to all cultures except control, at 10 or 30 μg/ml, and the proliferation level was determined after 90 hr incubation, with a 3H-thymidine pulse, at 0.5 μCi per well, given for the last 16 hr.
2.8. Flow cytometric identification of Foxp3+CD25+ Treg cells
One million copolymer-specific cell lines were first stained with anti-mouse CD4 and CD25 (BD Pharmingen, San Diego, CA), for 30 min, on ice, in phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin (BSA), then were stained for intracellular Foxp3 using allophycocyanin-conjugated anti-mouse/rat Foxp3 Staining Set (BD Pharmingen), following the producer's protocol. Cells were fixed using Fix/Perm buffer for 0.5 hr, then incubated with the anti- Foxp3 antibody (FJK-16) for 30 min at 4°C. Samples were re-suspended in PBS containing 0.2% BSA and data were acquired on a FACSCalibur (BD Biosciences, San Diego, CA), and analyzed by Flowjo software (Tree Star Inc., Ashland, OR).
2.9. Statistical analysis
Data were analyzed using statistical software Prism 5. Mann-Whitney U test was used for comparisons among groups. 2-way ANOVA was used for comparisons of cytokine production induced by the copolymers in EAU. Probability values less than 0.05 were considered statistically significant.
3. Results
3.1 Comparison of FYAK and GA in suppression of EAU development
To compare FYAK and GA for their capacity to inhibit the development of EAU we used the co-immunization procedure, in which the copolymer is incorporated into the uveitogenic antigen/CFA emulsion. Mice co-immunized with PBS and antigen/CFA were used as controls. Data from repeated experiments, with two strains of mice, B10.A (H-2a) and B10.RIII (H-2r), are summarized in Fig. 1. B10.A mice were immunized with whole IRBP and were also treated with PTx. However, B10.RIII were immunized with peptide 161-180 derived from the IRBP sequence; no treatment with PTx is necessary for disease development in these latter mice (Silver et al., 1999).
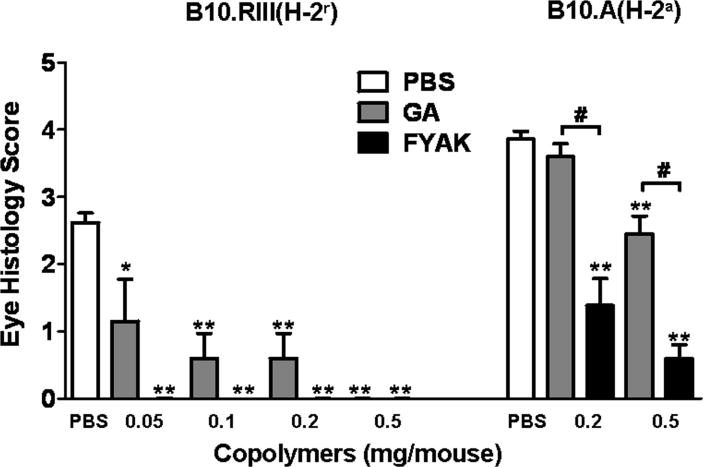
Fig. 1.
Comparison of GA and FYAK in inhibiting EAU development. The immunizing antigens and copolymers were administered, at the indicated doses, by the co-immunization route as described in Materials and Methods. EAU was induced in B10.A mice with whole IRBP and in B10.RIII mice with peptide 161-180. Severity of eye disease was determined on day 14 p.i., by histological examination and the data are means ± SEM of three experiments with each mouse strain. *p<0.01, **, p<0.0001 when the response is compared to PBS controls; #, p<0.0001 when the indicated columns are compared.
Both copolymers inhibited EAU induction in the two mouse strains. FYAK was remarkably more suppressive than GA in both strains. The inhibitory effects of the copolymers were particularly apparent in mice of the B10.RIII strain in which FYAK fully inhibited disease development even at the low dose of 0.05 mg per mouse, whereas GA was fully inhibitory in these mice only at a ten times higher dose, 0.5 mg. In B10.A mice the disease was only partially suppressed by both copolymers, but FYAK was again more inhibitory than GA at all tested doses.
3.2. Reduction of pro-inflammatory cytokine production induced by the copolymers
Inflammatory processes such as EAU are mediated by cytokines produced by lymphoid cells. Of particular importance are interferon (IFN)-γ and IL-17, produced by Th1 and Th17 cells, respectively (Bettelli et al., 2007; Steinman, 2007). We compared, therefore, the effects of copolymer treatment on the secretion of these two pro-inflammatory cytokines by draining lymph node cells when stimulated in culture with IRBP. Treatment with GA had little or no effect on the release of the tested cytokines. In contrast, treatment with FYAK inhibited the release of both IFN-γ and IL-17 (Fig. 2). The culture supernatants were also examined for Th2 cytokines, IL-4, IL-5 and IL-10, but the levels of these cytokines were marginal and no differences could be detected between control and copolymer-treated cultures (data not shown).
Fig. 2.
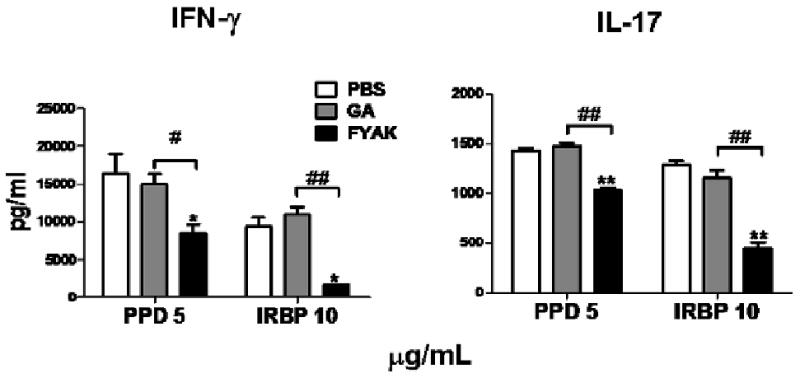
Comparison of the suppressive effects of FYAK and GA on cytokine release by T cells from treated mice. Draining lymph node cells from B10.A mice, detailed in the legend for Fig. 1, were tested for the release of Th1 and Th17 specific cytokines (IFN-γ and IL-17, respectively), following 48 hr incubation with the indicated antigens. Data are means ± SEM of duplicated assays from representative experiments. Similar patterns of cytokine release inhibition were observed in two other independent experiments. *p<0.05, **, p<0.01 when the response is compared to PBS controls; #, p<0.05, ##, p<0.01 when the indicated columns are compared.
3.3. Inhibition of EAU development by copolymer-specific T-cell lines
A major mechanism of action of GA and FYAK is induction of immunosuppressive cytokine-secreting T-regulatory cells (Arnon and Aharoni, 2004; Farina et al., 2005; Stern et al., 2008). To compare the two copolymers for their capacity to generate regulatory cells in our experimental system we established cell lines specific to GA or FYAK and tested their effect on EAU development by adoptively transferring the line cells into syngeneic recipients one day prior to induction of EAU. The line cells were tested at 5 × 106 or 107 cells per mouse and data collected with mice of three strains are recorded in Fig. 3. FYAK-specific line cells inhibited EAU development in all three mouse strains, whereas treatment with GA-specific lines was effective only with the B10.RIII strain and only at the high cell number of 107 per mouse (Fig. 3). Control syngeneic line cells, specific to an unrelated protein, hen egg lysozyme, or of cells stimulated by concanavalin A, were tested in control mice similarly immunized by IRBP. These control line cells did not exert any effect on EAU development (data not shown).
Fig. 3.
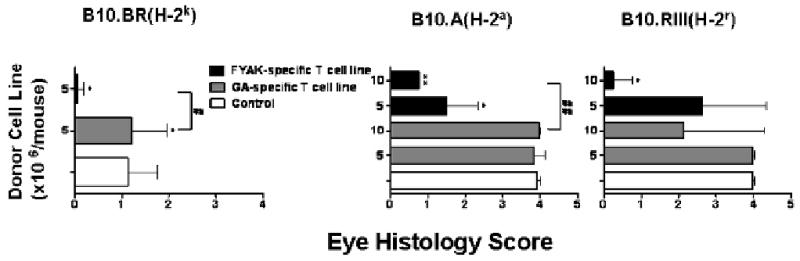
T cell lines specific to FYAK or GA inhibit EAU development. T cells of lines specific to FYAK or GA, established as detailed in Materials and Methods, were adoptively transferred intravenously at the indicated numbers to recipients of the corresponding strain. One day later recipients were immunized with IRBP, (B10.A and B10.BR), or peptide 161-180 (B10.RIII), as detailed in Materials and Methods. Eyes were collected on day 14 p.i. and analyzed for histological changes. The recorded data are means ± SEM of 3-5 mice of each group. *, P≤0.05, **, P≤0.0001 when compared to the PBS group; #, P≤0.05, ##, P≤0.0001 when the indicated two groups are compared.
3.4. FYAK-specific T-cell lines inhibit T-cell proliferation more efficiently than GA-specific lines
In addition to testing their immunosuppressive activity in vivo, we also examined the FYAK- and GA-specific cell lines for their ability to inhibit proliferation in vitro of IRBP-specific T cells in culture (Fig. 4). Copolymer-specific T cell lines were mixed with the IRBP-specific T-cells at a 1:1 ratio. The response of control cultures to which naïve CD4 T cells were added was measured for comparison. Both FYAK-specific and GA-specific T cell lines suppressed the response at the two tested concentrations of IRBP and again, the FYAK line was more inhibitory than the GA line (Fig. 4).
Fig. 4.
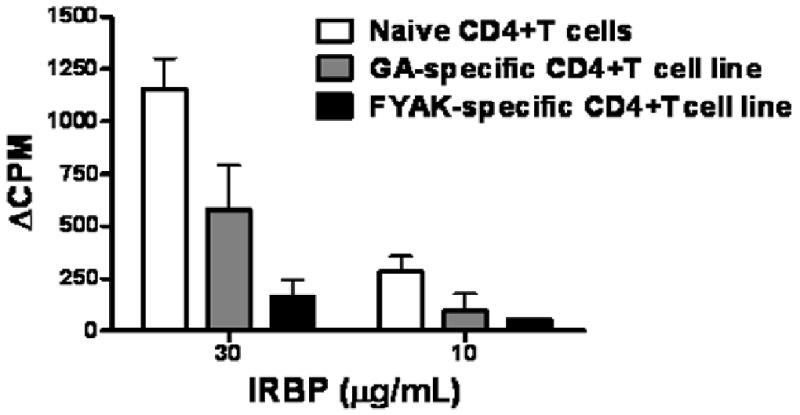
FYAK-specific lines are superior to GA-specific lines in their capacity to inhibit lymphocyte proliferation in vitro. B10.A line cells specific to FYAK or GA, or naïve CD4 cells, were added to cultures of syngeneic lymphocytes sensitized against IRBP, as detailed in Materials and Methods. The 3H-thymidine incorporation was measured after 90 hr of incubation and recorded as delta CPM. The recorded data are means ± SEM of replicates of 4 wells for each culture from a representative experiment; similar data were collected in another independent experiment. Control cultures containing FYAK-specific line cells incorporated 97+/-14 CPM when incubated with no IRBP or copolymer and 1891+/-204 CPM when incubated with only the copolymer. Control cultures containing GA-specific line cells incorporated 113+/- 31 with no addition and 1791+/-208 CPM in the presence of the copolymer. These control CPM values were subtracted from the values recorded by the bars in the Figure, that show, therefore, the net CPM stimulated by the added IRBP in these cultures.
In contrast to the difference in their inhibitory capacity on the IRBP-specific T-cells, the copolymer-specific line cells proliferated remarkably similarly to the corresponding copolymer. Data of a representative experiment are shown in Fig. 5, in which line cells specific to FYAK or GA were cultured with the corresponding copolymer at several concentrations and their response was measured by 3H-thymidine incorporation. Similar levels of proliferation were measured for the two lines in this and in two additional experiments.
Fig. 5.
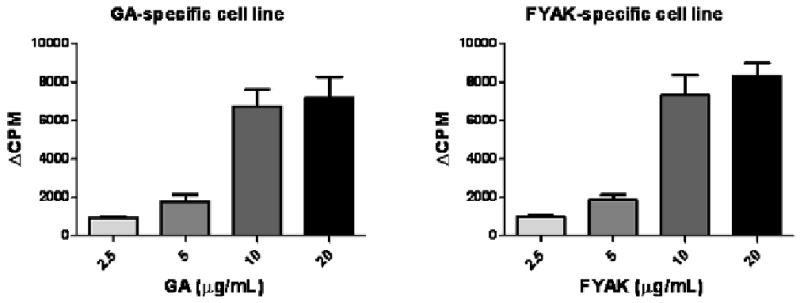
Line cells specific toward FYAK or GA responded similarly to their corresponding copolymer. Copolymer-specific line cells were collected on day 2 of re-stimulation, washed and cultured at 1.25 × 105/well, along with the same number of irradiated syngeneic APC, and with the corresponding copolymer at different concentrations, as indicated. The proliferation level was determined by 3H-thymidine incorporation, measured after a pulse of 0.5 μCi/well during the last 16 hr of the 90 hr total incubation. The data are expressed as delta CPM+/- SEM.
3.5. Secretion of Th2 cytokines by copolymer-specific Treg cells
The suppressive capacity of GA- and FYAK-specific T-cells has been attributed to the release of Th2 cytokines, in particular IL-10 (Arnon and Aharoni, 2004; Stern et al., 2008). To investigate the Th2 cytokine production by the immunosuppressive cell lines we developed against GA or FYAK from the three tested mouse strains, we measured the levels of four of these cytokines, IL-4, IL-5, IL-10 and IL-13, in their supernatants. Representative data are summarized in Table 1; similar patterns of cytokine production were obtained in repeated experiments, using supernatants collected from the cell lines after three or four cycles of activation. Remarkably, the levels of Th2 cytokines were repeatedly higher in the FYAK-specific cultures than in the GA-specific ones. The levels of IFN-γ and IL-17 were relatively low in these cultures, <2.5 ng/ml and <0.05 ng/ml, respectively, and unlike the pattern with the Th2 cytokines, the levels of IFN-γ were similar or even higher in the GA-specific lines than in the FYAK-specific lines (data not shown).
Table 1. Production of Th2-specific cytokines by CD4 T cell lines specific against GA or FYAK.
| Cytokine production (ng/ml) | |||||
|---|---|---|---|---|---|
| Mouse Strain | Cell line specificity | IL-4 | IL-5 | IL-10 | IL-13 |
| B10.RIII | GA | 15.9 | 5.4 | 4.7 | 17.6 |
| FYAK | 17.3 | 16.3 | 9.3 | 35.5 | |
| B10.A | GA | 6.6 | 8.0 | 3.7 | 2.9 |
| FYAK | 15.1 | 17.5 | 13.3 | 4.6 | |
| B10.BR | GA | 1.8 | 9.8 | 3.3 | 1.3 |
| FYAK | 10.5 | 61.6 | 32.3 | 28.1 | |
Culture supernatants were collected following incubation with the corresponding copolymer and analyzed for levels of the indicated cytokines. The recorded data were obtained from the 3rd cycle cultures of B10.RIII and B10.A and the 4th cycle cultures of B10.BR. Similar patterns of cytokine production were observed in two or three additional experiments with each mouse strain.
3.6. Expression of immunoregulatory markers by copolymer-specific T-cell lines
Although the immunosuppressive capacity of copolymer-specific lines was attributed mainly to their release of anti-inflammatory cytokines (Arnon and Aharoni, 2004; Hong et al., 2005; Stern et al., 2008), Some data suggest that copolymers may also stimulate suppressive Foxp3+ Treg cells (Hong et al., 2005). Therefore, we examined the FYAK- and GA-specific lines for expression of the markers for these Tregs, i.e., CD25 and Foxp3. Data of a representative experiment are recorded in Fig. 6 and show that a relatively small percent of the cells of both FYAK- and GA-specific line cells express both CD25 and Foxp3, with the percentage of cells expressing these markers being higher in FYAK-specific line cells.
Fig. 6.
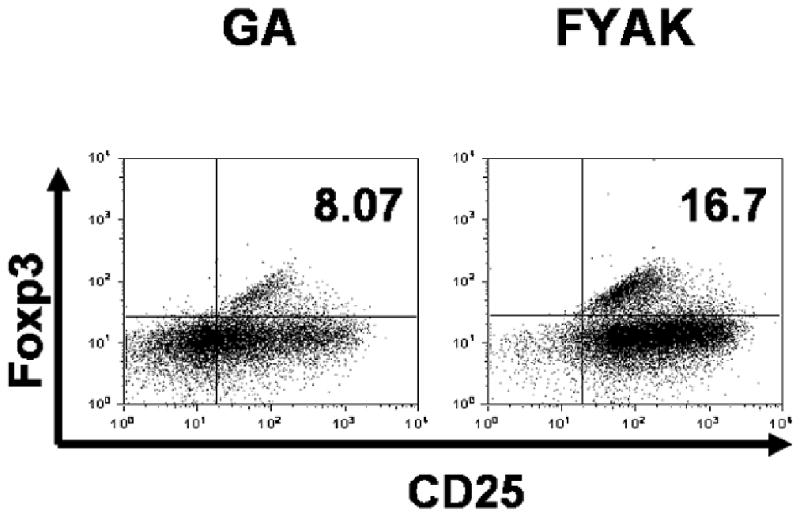
CD25+Foxp3+ Treg cells in FYAK-specific and GA-specific cell lines. Copolymer-specific cell lines of B10.A mice, collected on day 2 of the 3rd stimulation cycle, were tested by flow cytometry for the proportion of cells expressing CD25 and Foxp3. The data shown are one of two similar experiments.
4. Discussion
The present data provide new information on the use of random amino acid copolymers in a non-CNS disease model. The study also compares the immunosuppressive activities of a novel copolymer, FYAK [poly (F,Y,A,K)n] with those of GA [poly(Y,E,A,K)n], a copolymer widely used for treatment of MS (Arnon and Aharoni, 2004; Farina et al., 2005; Stern et al., 2008). The immunosuppressive capacities of the two copolymers have been previously compared in the EAE system (Illes et al., 2004; Stern et al., 2004; Stern et al., 2008). In the present study, they were compared in their immunosuppressive ability in the ocular disease model, EAU. EAU serves as a model for intraocular inflammatory eye conditions grouped under the term “uveitis”, including Behcet's disease, Vogt-Koyanagi Harada (VKH) syndrome, birdshot retinochoroidopathy and sympathetic ophthalmia (Adamus and Chan, 2002; Forrester et al., 1990; Gery et al., 2002). It is assumed that autoimmunity plays a major role in the pathogenesis of these eye diseases, a notion supported by the similarity between these human conditions and EAU, a disease induced by immunization with ocular specific antigens (Adamus and Chan, 2002; Caspi, 2002; Gery et al., 2002).
FYAK ameliorated EAU as well as it inhibits EAE and was superior to GA in all assays employed in the present study. The effect on EAU induction was tested with two mouse strains, B10.A and B10.RIII (Fig. 1). GA inhibited moderately the development of EAU in B10.A mice, as was found previously (Zhang et al., 2000), but was found here to be remarkably more inhibitory in the highly susceptible B10.RIII mice. In both strains, however, treatment with FYAK was clearly more effective than with GA, reducing the disease severity in B10.A mice to lower levels and completely inhibiting disease development in B10.RIII mice at doses at which GA achieved only a partial effect. These data and those in Fig. 3 also show that the effectiveness of FYAK and the difference between it and GA is relatively broad and not restricted to a single haplotype.
The difference between B10.A and B10.RIII mice in their response to the inhibitory effect of the copolymers on EAU induction is of note. The two copolymers were tested in this system by co-immunization with the uveitogenic antigen. It is conceivable, therefore, that the 20 amino acid long 161-180 peptide used for induction of disease in the B10.RIII mice was an easier competition target for binding to mouse MHC proteins than the large IRBP protein (∼140 kDa) used in the B10.A mice. A major effect of both FYAK and GA appears to be due to the generation of regulatory T cells that secrete immunosuppressive cytokines (Aharoni et al., 2000; Arnon and Aharoni, 2004; Duda et al., 2000; Farina et al., 2005; Stern et al., 2008). However, at least a part of the copolymers' activity in this model is attributable to competition with the uveitogenic antigen when administered by co-immunization in CFA.
Treatment with GA induces a shift toward Th2 in both animals and humans (Aharoni et al., 2000; Arnon and Aharoni, 2004; Dhib-Jalbut et al., 2003; Duda et al., 2000; Farina et al., 2005; Neuhaus et al., 2000) and a similar observation was made in mice treated with FYAK (Stern et al., 2008). Data recorded in the present study are in line with these observations and, remarkably, we found that FYAK-specific cell lines were superior to GA-specific lines in their production levels of Th2 type cytokines, IL-4, IL-5, IL-10 and IL-13.
Acknowledgments
This study was supported by the Intramural Research Program of the National Eye Institute, NIH, and by grants NMSS RG3796A3 and NIH RO1 AI49524 to JLS. We thank Peptimmune Inc. for generously providing the FYAK copolymer. We are also grateful to Drs. G. Shi, C.E. Egwuagu and C.-R. Yu for helpful advice.
Footnotes
Disclosures: J.L.S. declares a conflict of interest. He is a member of the Scientific Advisory Board of Peptimmune, Inc., who are developing FYAK (PI2301) for clinical trial. Other co-authors have no financial conflict of interests with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamus G, Chan CC. Experimental autoimmune uveitides: multiple antigens, diverse diseases. Int Rev Immunol. 2002;21:209–229. doi: 10.1080/08830180212068. [DOI] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci U S A. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci U S A. 2004;101 2:14593–14598. doi: 10.1073/pnas.0404887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Caspi RR. Th1 and Th2 responses in pathogenesis and regulation of experimental autoimmune uveoretinitis. Int Rev Immunol. 2002;21:197–208. doi: 10.1080/08830180212063. [DOI] [PubMed] [Google Scholar]
- de Vos AF, Fukushima A, Lobanoff MC, Vistica BP, Lai JC, Grivel JC, Wawrousek EF, Whitcup SM, Gery I. Breakdown of tolerance to a neo-self antigen in double transgenic mice in which B cells present the antigen. J Immunol. 2000;164:4594–4600. doi: 10.4049/jimmunol.164.9.4594. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Chen M, Said A, Zhan M, Johnson KP, Martin R. Glatiramer acetate-reactive peripheral blood mononuclear cells respond to multiple myelin antigens with a Th2-biased phenotype. J Neuroimmunol. 2003;140:163–171. doi: 10.1016/s0165-5728(03)00170-x. [DOI] [PubMed] [Google Scholar]
- Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J Clin Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Weber MS, Meinl E, Wekerle H, Hohlfeld R. Glatiramer acetate in multiple sclerosis: update on potential mechanisms of action. Lancet Neurol. 2005;4:567–575. doi: 10.1016/S1474-4422(05)70167-8. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Liversidge J, Dua HS, Towler H, McMenamin PG. Comparison of clinical and experimental uveitis. Curr Eye Res. 1990;9(Suppl):75–84. doi: 10.3109/02713689008999424. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Zhang M, Hurst SD, Muchamuel T, Shen D, Wawrousek EF, Chan CC, Gery I. Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1- versus Th2-mediated ocular inflammation. J Immunol. 2002;168:2483–2492. doi: 10.4049/jimmunol.168.5.2483. [DOI] [PubMed] [Google Scholar]
- Fridkis-Hareli M, Neveu JM, Robinson RA, Lane WS, Gauthier L, Wucherpfennig KW, Sela M, Strominger JL. Binding motifs of copolymer 1 to multiple sclerosis- and rheumatoid arthritis-associated HLA-DR molecules. J Immunol. 1999;162:4697–4704. [PubMed] [Google Scholar]
- Fridkis-Hareli M, Santambrogio L, Stern JN, Fugger L, Brosnan C, Strominger JL. Novel synthetic amino acid copolymers that inhibit autoantigen-specific T cell responses and suppress experimental autoimmune encephalomyelitis. J Clin Invest. 2002;109:1635–1643. doi: 10.1172/JCI15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkis-Hareli M, Strominger JL. Promiscuous binding of synthetic copolymer 1 to purified HLA-DR molecules. J Immunol. 1998;160:4386–4397. [PubMed] [Google Scholar]
- Fridkis-Hareli M, Teitelbaum D, Gurevich E, Pecht I, Brautbar C, Kwon OJ, Brenner T, Arnon R, Sela M. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells--specificity and promiscuity. Proc Natl Acad Sci U S A. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I, Nussenblatt RB, Chan CC, Caspi RR. Autoimmune disease of the eye. In: Theofilopoulos AN, Bona CA, editors. The molecular pathology of autoimmune diseases. Taylor & Francis; New York, NY: 2002. pp. 978–998. [Google Scholar]
- Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien Y, Sanna A, Soderstrom M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–110. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- Illes Z, Stern JN, Reddy J, Waldner H, Mycko MP, Brosnan CF, Ellmerich S, Altmann DM, Santambrogio L, Strominger JL, Kuchroo VK. Modified amino acid copolymers suppress myelin basic protein 85-99-induced encephalomyelitis in humanized mice through different effects on T cells. Proc Natl Acad Sci U S A. 2004;101:11749–11754. doi: 10.1073/pnas.0403833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ifergan I, Antel JP, Seguin R, Duddy M, Lapierre Y, Jalili F, Bar-Or A. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol. 2004;172:7144–7153. doi: 10.4049/jimmunol.172.11.7144. [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Farina C, Yassouridis A, Wiendl H, Then Bergh F, Dose T, Wekerle H, Hohlfeld R. Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci U S A. 2000;97:7452–7457. doi: 10.1073/pnas.97.13.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54:1057–1060. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Silver PB, Chan CC, Wiggert B, Caspi RR. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest Ophthalmol Vis Sci. 1999;40:2898–2905. [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Stern JN, Illes Z, Reddy J, Keskin DB, Sheu E, Fridkis-Hareli M, Nishimura H, Brosnan CF, Santambrogio L, Kuchroo VK, Strominger JL. Amelioration of proteolipid protein 139-151-induced encephalomyelitis in SJL mice by modified amino acid copolymers and their mechanisms. Proc Natl Acad Sci U S A. 2004;101:11743–11748. doi: 10.1073/pnas.0403832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JN, Keskin DB, Zhang H, Lv H, Kato Z, Strominger JL. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc Natl Acad Sci U S A. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–127. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–4488. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chan CC, Vistica B, Hung V, Wiggert B, Gery I. Copolymer 1 inhibits experimental autoimmune uveoretinitis. J Neuroimmunol. 2000;103:189–194. doi: 10.1016/s0165-5728(99)00239-8. [DOI] [PubMed] [Google Scholar]