Periodontal disease presents with a wide range of clinical variability and severity. Research in the past decade has shed substantial light on both the initiating infectious agents and host immunological responses in periodontal disease, both of which have been shown to modify the progression of periodontal disease. Cullinan et al. [28] have shown that up to 46% of the general population harbor the causative organism(s) of periodontal disease, but many are able to limit the progression of periodontal disease or even clear the organism(s) if infected. Overall, Cullinan et al. [28] suggest a complex multifactorial etiology of periodontal disease between host `immune' response and environmental factors.
The characteristics of an individual's immune response to infectious agents greatly determine the severity of periodontal disease [119]. Michalowicz et al. [82] used a twin study to show that genetic hereditability appears to contribute approximately 50% to the clinical susceptibility of periodontal disease. In addition, studies have suggested dysregulation of innate immunity as playing a key role in progression of periodontal disease [110]. Specifically, the host immune response is suppressed upon low-level stimulation of critical pattern recognition receptors, leading to a muted local immune response, thus enabling periodontal disease-associated bacteria such as Porphyromonas gingivalis to evade host immune system [86, 114].
In the last decade, several epidemiological studies have also found an association between obesity and an increased incidence of periodontal disease [43, 101–104]. Recently, obesity has been shown to contribute to an individual's immune response to many pathogens such as P. gingivalis [43]. The effect of obesity on an individual's immune response was further characterized by Amar et al. [6], in showing reduced pro-inflammatory cytokine response, e.g. tumor necrosis factor-alpha, upon P. gingivalis infection in obese versus lean mice. Taken together, both obesity and chronic exposure to periodontal disease-associated bacteria, e.g. P. gingivalis, alone appear to mute the local immune response and in combination may synergize in suppressing the innate immune system, thus further exacerbating periodontal disease.
Epidemiologic evidence has further suggested that the long-term effects of periodontal disease can be linked to more serious systemic conditions such as cardiovascular disease, diabetes, and complications of pregnancy [18, 24–25, 30–31, 90]. Bahekar et al. [12] determined a statistically significant increase in prevalence of coronary heart disease in patients with periodontitis between 1.14- to 1.59-fold increase after adjusting for risk factors such as smoking, diabetes, alcohol intake, obesity, and blood pressure. Recently, Amar et al. [5] have shown that the presence of a P. gingivalis bacteremia alone, a common complication in patients with periodontal disease, is not sufficient to exacerbate atherosclerosis; rather, bacteremia coupled with intra-cellular invasion of endothelial cells by P. gingivalis is required. Upon invasion, endothelial cells activate and upregulate various adhesion molecules, thus increasing the likelihood of macrophage diapedesis and, when coupled with exposure to a high fat diet, subsequent conversion to foam cells thus furthering athroma progression [32]. In addition, mature athroma macrophages display reduced responsiveness from their pattern recognition receptors, in a fashion similar to that seen in the response to low-level stimulation of lipopolysaccharides [86, 114]. Lipopolysaccharides induces a similar state of tolerance and dysregulation in endothelial cells and circulating white blood cells [37]. Taken together, both mechanisms further exacerbate the effect of periodontal disease on the progression of atherosclerosis.
This review will focus on exploring the molecular mechanisms underlying host immune response(s) to P. gingivalis fostering periodontal disease, the exacerbating effects of obesity on periodontal disease, and the long-term accelerating effects that periodontal disease has on the progression of atherosclerosis. We suggest that innate immune tolerance is a common molecular mechanism linking periodontal disease, obesity and atherosclerosis in concert leaving an individual with a dysregulated and dysfunctional innate immune response, which is critical for enhancement of both periodontal disease and atherosclerosis.
Overall, a picture is emerging that shows tolerance in the innate immune system occurring as a result of persistent low-level exposure to P. gingivalis infection or induced by obesity paralyses the innate immune response and further aggravates periodontal disease. As a consequence of enhanced periodontal disease an individual is subjected to repeated bacteremias with P. gingivalis, which can lead to both endothelial dysfunction and repeated bouts of inflammation. Both processes affect the endothelium by increasing the likelihood for macrophages to transit across the arterial intima and under hyperlipidemia / hypercholesterolemic conditions foster the conversion of macrophages into foam cells, therefore further exacerbating atherosclerosis. Thus, understanding the mechanisms of tolerance and its effect on innate immunity may contribute valuable insights into all three conditions: periodontal disease, obesity and atherosclerosis.
Periodontal Disease's Impact on the Immune Response
Periodontal disease results from an inflammatory response caused by deposits on the teeth of bacterial biofilm (dental plaque) and its by-products. These pathogens, such as P. gingivalis, are required to invade and colonize in periodontal sites and evade the local immune response, which ultimately leads to intergradations of periodontal tissue furthering periodontal disease's progression [51, 110]. It has been shown that P. gingivalis is capable of invading both oral epithelial cells and aortic endothelial cells [33, 91]. Further, Njoroge et al. [91] have shown that the invasive properties of P. gingivalis are dependent on the fimbriae protein, as demonstrated by fimA-deficient mutant DPG3 strain of P. gingivalis fails to adhere and invade oral epithelial cells. Taken together, this demonstrates that P. gingivalis not only colonizes a periodontal site but can also be found systemically within the aortic tree and that P. gingivalis is an invasive species of bacteria with the fimbriae protein mediating its adherence and invasive properties.
Immune Response to Microbial Pathogens
The response of a mammalian host to microbial pathogens involves the activation of both innate and adaptive components of the immune system. An important cellular component of the innate immune response is the macrophage axis, since macrophages are often among the `first responders' along with polymorphonuclear leukocytes to a microbial pathogen. Macrophages are also involved in activating the adaptive arm of the immune response via antigen-presentation, thus linking the innate response to adaptive immunity. Therefore, macrophages play a critical role in proper immune function and it is important to understand how macrophages detect pathogens and their by-products (e.g. lipopolysaccharides) and the affect the detection of a pathogen has on macrophages. Macrophages detect the presence of a pathogen by a variety of repetitive motifs, referred to as pathogen associated molecular patterns, commonly found on many different pathogens. Macrophage's perform this by expressing a limited number of pattern recognition receptors found on the macrophage and endothelial cells that bind to the pathogen associated molecular patterns on the pathogen and through subsequent intracellular signaling trigger the inflammatory response [1–2, 13, 81]. Some of these receptor types include Toll-like receptors, nucleotide-binding oligomerization domains, cluster of differentiation 14, complement receptor-3, lectins, and scavenger receptors [9].
The first major class type of pattern recognition receptor that is most capable in recognition and signaling in association with P. gingivalis exposure is the Toll-like receptors family. Toll-like receptors monitor the extracellular environment and phagolysosomal compartments, and recognize pathogen associated molecular patterns that include lipopolysaccharides, flagella, CpG DNA, oxidized low density lipopeptide, endogenous proteins like heat shock protein 60 [129] and bacterial lipoprotein [80]. More specifically, activation of Toll-like receptor-2, widely known to recognize elements of gram-negative bacteria cell wall components, has been shown to lead to increase in pro-inflammatory response [17]. Thus the normal role of Toll-like receptor-2 is highly critical to the detection and clearance of gram-negative microbial pathogens, such as P. gingivalis.
The second major classes of pattern recognition receptors include soluble cytosolic nucleotide-binding oligomerization domain-like receptors. Nucleotide-binding oligomerization domain-like receptors complement this host defense by providing a second inner layer of surveillance, specifically intracellular. The nucleotide-binding oligomerization domain proteins are soluble cytosolic proteins, which recognize cell wall fragments from both gram-negative and gram-positive bacteria [21, 45, 55]. Nucleotide-binding oligomerization domain-1 recognizes a specific peptidoglycan fragment containing diaminopimelic acid, while nucleotide-binding oligomerization domain-2 recognizes a muramyl dipeptide fragment of peptidoglycan. Toll-like receptors and nucleotide-binding oligomerization domains are found on both macrophages and endothelial cells and how these cells initiate a threat-specific transcriptional response is poorly understood when these detection receptors are activated.
Activation of either Toll-like receptor-2 or nucleotide-binding oligomerization domain-1/nucleotide-binding oligomerization domain-2 receptors, common with P. gingivalis exposure, results in the activation of the nuclear factor kappa-light-chain-enhancer of activated B cell intra-cellular signaling pathway [11, 122]. This in turn triggers the increased expression of many pro-inflammatory cytokines, including tumor necrosis factor-alpha, interleukin-1 β, interleukin-6 [42, 105, 126]; chemokines including interleukin-8, monocyte chemotactic protein-1, chemokine (C-C motif) ligand 5 (RANTES) [36, 117]; C-reactive protein [29, 106] and mRNA for Toll-like receptors [99]. In periodontal disease, this signaling is most likely to occur in gingival epithelial cells, human gingival fibroblasts, and macrophages [11, 122]. However, in response to repeated bacteremias P. gingivalis has be known to trigger Toll-like receptors and nucleotide-binding oligomerization domain signaling in both aorta and cardiac endothelial cells [33].
Combined, the Toll-like receptors and nucleotide-binding oligomerization domains represent two critical layers of defense to detect pathogens and modulate the immune response differentially. Toll-like receptors detect extracellular presence of P. gingivalis whereas nucleotide-binding oligomerization domains detect intracellular presence of P. gingivalis, such that activation of nucleotide-binding oligomerization domain signaling represents a breach of the outer layers of defense for a cell and hence leads to an enhanced immune response. Park et al. [94] demonstrated that nucleotide-binding oligomerization domain signaling enhances immune response when challenging mesothelial cells, since in nucleotide-binding oligomerization domain knockout mice many common pathogen associated molecular patterns led to slightly reduced pro-inflammatory cytokines and chemokines.
Homotolerance
Lipopolysaccharides is an endotoxin found on the outer membrane of gram-negative bacteria, such as P. gingivalis, that has been shown to be a potent activator of the innate immune response, especially macrophages. Lipopolysaccharides can also be released from dying gram-negative bacteria, which in this case binds to a plasma lipopolysaccharides-binding protein. The combination lipopolysaccharides-lipopolysaccharides-binding protein complex transfers the lipopolysaccharides to first cluster of differentiation 14 and then to lymphocyte antigen 96 protein (MD-2), which ultimately activates Toll-like receptor-4 signaling and triggers an inflammatory response [26, 98] on macrophages and dendritic cells [57, 60–61]. Lipopolysaccharides has been shown to be a potent activator of the innate immune system, especially macrophages. Lipopolysaccharides binds to lipopolysaccharides-binding protein, which then binds to a receptor, cluster of differentiation 14, on macrophages and dendritic cells.
Pathogens have acquired many ways to avoid the host immune response. Unexpectedly Tanabe et al. [114] have recently shown that low-level pre-treatment stimulation of lipopolysaccharides, 0.01 to 0.1 ug/ml, has been shown to reduce tumor necrosis factor-alpha levels in response to subsequent stronger lipopolysaccharides stimulation, 1 ug/ml, within 24 hrs of the pre-treatment dosage of lipopolysaccharides. This process ultimately leads to macrophage tolerance, as demonstrated by decreased level of tumor necrosis factor-alpha [114]. In addition, Dobrovolskaia et al. [35] further characterized the reduced inflammatory response as “Homotolerance”, since cells pretreated with a specific ligand to a pattern recognition receptor, e.g. P. gingivalis lipopolysaccharides: Toll-like receptor-2 activation, and a subsequent challenge of the same pattern recognition receptors, demonstrated a reduced level of tumor necrosis factor-alpha secretion. Whereas “Heterotolerance”, i.e. the reduced immune response, was not observed when pre-treating with a ligand for a pattern recognition receptor and subsequently challenged with a ligand for a different pattern recognition receptor, e.g. pre-treatment with P. gingivalis-lipopolysaccharides and challenge with N-palmitoyl-S-[2,3-bis(palmitoyl)-(2RS)-propyl]-(R)cysteinyl-alanyl-glycine (Pam3Cys), a synthetic lipopeptide that activates different pattern recognition receptor(s) [35].
Further work by Muthukuru et al. [86] have shown that similar pre-treatment and subsequent strong challenge by P. gingivalis-lipopolysaccharides specifically demonstrated homotolerance by reduction of Toll-like receptor-2 and Toll-like receptor-4 mRNA levels, suggesting alterations in either the transcription factors present at the Toll-like receptors promoters or the Toll-like receptors genes themselves are altered. The decreased secretion of a critical inflammatory mediator, tumor necrosis factor-alpha, was shown by Tanabe et al. [114] to be the effect of lipopolysaccharides induction of a homotolerance response in macrophages. However, homotolerance was not observed across the entire spectrum of pro-inflammatory cytokine e.g. both interleukin-1 β and matrix metalloproteinase-9 showed increased secretion upon low-level pre-treatment stimulation and subsequent strong lipopolysaccharides stimulation [114].
This important finding shows the role in modulating, fine-tuning, the host inflammatory response and progression of periodontitis. We suggest that this results in muting of innate immune response by P. gingivalis, thus enabling P. gingivalis to evade the host immune defense mechanism. This appears to be a critical evolutionary feature of P. gingivalis and provides P. gingivalis a means to evade the host immune response, thus enabling it to survive and thrive in periodontal sites. Alternatively, this could also represent a host protective measure aimed at protection against endotoxin induced septic shock to prevent a systemic inflammatory response / cytokine release which could potentially be fatal [14].
Obesity and Periodontal Disease
The innate and adaptive immune responses are affected by obesity [75, 112, 115]. Obesity has been characterized as an altered systemic inflammatory state resulting from an imbalance in the cytokine network, increased level of acute-phase proteins and pro-inflammatory cytokines such as tumor necrosis factor-alpha and leukocytes [10, 43] in the plasma of obese subjects [63, 95]. Furthermore, it has been observed that macrophage infiltration of the white adipose tissue of obese mice in numbers occurs in direct proportion to adipocyte size and number [40, 124]. However, macrophage effectors' functions are impaired in obese animals, with a reduced phagocytic capacity and a defective oxidative burst [67, 73, 100]. As in humans, obese animals also display delayed wound healing that is associated with a dysregulated polymorphonuclear leukocytes and macrophages infiltration [49]. Acquired immunity is also affected by obesity, as evidenced by impaired T lymphocytes (T cell) and lymphocytes (B cell)-mediated immune responses in obese ob/ob and diabetic db/db mice [23, 74]. In addition, the levels of adipocytes secrete classical “immune” cytokines (tumor necrosis factor-alpha, interleukin-6, interleukin-1 receptor antagonist, and transforming growth factor-beta are significantly increased in obesity [41]. Whether a hyper-inflammatory state exists in diet-induced obesity remains controversial. What is known is that high fat diet can create a baseline pro-inflammatory condition in insulin-sensitive tissue such as skeletal muscle, liver, or white adipose tissue, and this inflammation may be Toll-like receptor-4-mediated. Notably, in most studies, circulating tumor necrosis factor-alpha remains undetectable, and interleukin-6 is extremely low (<10 pg/mL at baseline), questioning whether a true pro-inflammatory state actually exists [108, 111, 125].
This immune paralysis is supported in epidemiological studies of obese individuals, which found evidence of increased susceptibility to infections [39], including post-operative infectious complications, and a positive correlation between body mass index and the incidence of both community and nosocomial infections [19]. Recent evidence points to a high fat diet, which interferes with the ability of the immune system to appropriately respond to P. gingivalis infection [6]. This has also been observed in mice as greater susceptibility to infection with influenza virus as well as greater periodontal bone loss following P. gingivalis infection [111]. Dissecting the molecular mechanisms behind this dysregulation will certainly shed light on the most appropriate targets for interventional studies [107].
Obesity's Impact on the Immune Response: Homotolerance
Macrophages of obese animals are functionally impaired, which leads to reduced phagocytic capacity and defective oxidative burst [67, 73, 100]. The lack of cytokine expression in response to infections has been linked to dysfunction in macrophages and/or to a defect in maturation of monocytes [6, 84, 111]. It is increasingly recognized that obesity is characterized by a dysregulation of inflammatory pathways such as those that are activated by Toll-like receptor-2. However, the fundamental mechanisms responsible for this dysregulation are poorly understood. Recent findings by our lab have been able to shed further light on a link between obesity and innate immunity.
Recent data has demonstrated that diet-induced obesity dysregulated Toll-like receptor-2 and Toll-like receptor-4 expression, significantly reduced protein kinases B (Akt/PKB) phosphorylation in peritoneal macrophages, and reduced the immune response to P. gingivalis [127]. Toll-like receptor-2 may be a candidate for participation of the cross talk between inflammation and metabolic signals because both P. gingivalis and free fatty acids can activate it [89, 108]. Recent studies have shown that P. gingivalis-lipopolysaccharides activates phosphoinositide 3-kinases (PI3-kinase) and Akt/PKB through Toll-like receptor-2, leading to extracellular signal-regulated kinases (ERKs) or classical MAP kinases (ERK1/2) activation and tumor necrosis factor-alpha expression [109]. Pretreatment of macrophages with lipopolysaccharides from P. gingivalis, a Toll-like receptor-2 agonist, induces Toll-like receptor-2 homotolerance, see above [35], and both P. gingivalis and free fatty acids activate the innate immune response through the Toll-like receptor-2 pathway, we therefore similar hypothesize that chronic exposure to free fatty acids in obese individuals induces a similar Toll-like receptor-2 homotolerance, thus leading to reduced Toll-like receptor-2 expression, Toll-like receptor-2 signaling, and tumor necrosis factor-alpha production as we have observed in our diet-induced obesity model [127].
Zhou et al. [127] have demonstrated “homotolerance” tolerance in a diet induce obesity model upon exposure to P. gingivalis. Previous work from Muthukuru et al. [86] showed that pre-treatment of low-levels of P. gingivalis induced similar tolerance in innate immune response independently of obesity. We speculate there is an additive effect when obesity is combined with P. gingivalis-lipopolysaccharides exposure further enhancing tolerance and immune dysregulation in obese patients with periodontal disease.
Obesity Effect on Periodontal Disease: Exacerbation of Periodontal Disease
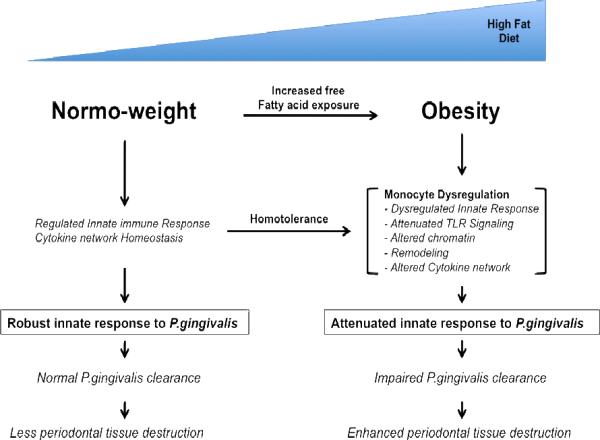
Fig. 1 illustrates how we interpret the effect of obesity on peripheral innate immune response to P. gingivalis infection. We propose that in normal mice, a homeostatic cytokine network maintains a regulated response to bacterial challenge through a cycle of transient inflammation, followed by down modulation with anti-inflammatory cytokines. As obesity develops, monocyte dysregulation develops due to the free fatty acids induced homotolerence along Toll-like receptor-2 signaling pathway. Together, these perturbations mute the homeostatic network that normally counters inflammation associated with infections. We postulate that obesity becomes associated with an altered pro- and anti-inflammatory network, an altered gene expression profile in monocytes and macrophages, an altered capacity for signaling through Toll-like receptors and other microbially induced pathways, and an altered chromatin status definable at specific gene loci. Ultimately, obesity fosters a state of attenuated innate response to P. gingivalis with an impaired P. gingivalis clearance has been established to result in enhanced tissue damage and bone loss [6].
Fig. 1.
Model for proposed effect of obesity on innate response to P. gingivalis
As exposure to free-fatty acids increases, common in obese individuals, this induces homotolerance along the Toll-like receptor-2 pathway in the innate immune system. Homotolerance alters Toll-like receptor signaling pathway by altering the expression levels of Toll-like receptor-2 and possibly chromatin remodeling at the Toll-like receptor-2 gene or other gene loci involved in the signaling pathway or cytokine release. The effect of the homotolerance leads to a dysregulated innate immune response and altered cytokine network upon exposure to P.gingivalis. As a result, the innate immune system has impaired clearance of P.gingivalis, which leads to enhancement of periodontal tissue destruction
The significance of this hypothesis is underscored by both the obesity epidemic and the fact that millions of people worldwide are affected by periodontal infection. Obesity coupled with periodontal disease appears to lead to a significantly higher level of dysregulated innate immune responses to P. gingivalis infection and increased periodontal morbidity and exacerbation of periodontal disease linked disorders, e.g. cardiovascular disease. Furthermore, this dysregulation quite possibly may be a systemic phenomena characterized by a muted innate immune response to many pathogens leaving a host potentially susceptible to the deleterious effects of these pathogens.
Role of Periodontal Disease in Atherosclerosis
Atherosclerosis is now considered to be one manifestation of a state of disordered immunity in which there is dynamic interaction between endothelial dysfunction (characterized by loss of normal endothelium-dependent vasodilatation), inflammation, and repeated cycles of `wound healing response' [83].
Impact of Periodontal Bacteria on Atherosclerosis
Coronary heart disease is a major cause of morbidity and mortality worldwide. Traditional risk factors like hypercholesterolemia, smoking and hypertension have failed to fully explain the incidence of coronary heart disease and recently epidemiological studies have pointed to links between periodontal disease and coronary heart disease [37]. A possible role of infection in atherogenesis has been postulated involving multiple pathogens, with some playing a more predominant role (e.g. P. gingivalis; Chlamydophila pneumoniae) and that coronary heart disease risk relates to the overall aggregate pathogen load, referred to as “pathogen burden”, an individual carries [37]. Specifically, Pussinen et al. [97] concluded that high levels of anti-P. gingivalis immunoglobulin G (IgG) antibodies, common in periodontal disease patients, were associated with coronary heart disease and determined an increased odds ratio (1.5) after adjusting for various common coronary heart disease risk factors/ Further, infection may not only contribute to the development of atherosclerosis [37–38]; it can also trigger plaque rupture and acute thrombotic occlusion, the major factors responsible for acute myocardial infarction and for sudden death in patients with coronary heart disease [37].
Recently it has been hypothesized that a complex relationship between human periodontal disease and increased risk for acute myocardial infarction exists [8, 34, 62, 77–79, 87, 118], mechanisms are now emerging supporting this hypothesis linking transient bacteremias, common in periodontal disease, and endothelial dysfunction [4, 116]. Periodontal infection is known to induce local inflammation, often leading to gingival ulcerations and local vascular changes, which have the potential to increase the incidence and severity of transient bacteremias. Bacteremias can occur by various methods such as dental work including flossing, surgery and cleaning. In addition, several studies have demonstrated that patients with periodontal disease have elevated levels of systemic inflammatory mediators, e.g. C-reactive protein [7]. Furthermore, Amar et al. have recently demonstrated endothelial dysfunction upon the repetitive bacteremic exposure, common in periodontal disease, and this in turn exacerbates the development of atherosclerosis and coronary heart disease [5]. Specifically, endothelial cells upregulate cell adhesion molecule, p-selectins and inter-cellular adhesion molecule-1 (ICAMs) (integrin ligands) in response to bacterial exposures, such as P. gingivalis, thus increasing the likelihood of monocyte transiting from the blood compartment to the local tissue compartment [64].
It has been suggested that periodontal disease can lead to persistent low-level bacteremia, an elevated white cell count, and systemic endotoxemias, which together could affect endothelial integrity, metabolism of plasma lipoproteins, blood coagulation, and platelet function [4, 44, 52, 65]. Recent experimental studies have elucidated how repeated bacterimic exposures of P. gingivalis lead to endothelial dysfunction. Initially insights were derived from findings that certain strains of P. gingivalis were found to have invaded human coronary artery endothelial cells and detected within atherosclerotic plaques themselves [33, 50, 96]. Specifically, P. gingivalis has been identified by polymerase chain reaction and fluorescence in situ hybridization in atheromatous plaques of patients suffering from atherosclerosis, suggesting that these microorganisms might be metabolically active within the atheroma [20].
Further, experimental studies support the concept that local inflammation within the artery wall may also contribute to the acceleration of atherosclerosis in response to endothelial infection, e.g. P. gingivalis, which would be expected to cause a spectrum of systemic effects, producing alterations in circulating cytokines, acute-phase reactants, white blood cells, and responses mediated by the immune system [37].
The validity of this concept has been demonstrated in a study by Zhu et al. [128] which showed that only the combination of cytomegalovirus infected individuals and elevated C-reactive protein levels that shows an ongoing inflammatory response which leads to an increased odds ratio of 4.3 for coronary heart disease vs. either 1.3 or 2.3 increased odd ratio for cytomegalovirus infected only or elevated C-reactive protein respectively. Further, Zhu et al. [128] suggest that infection alone is not sufficient for development of atherosclerosis, but the combination of both infection and an inflammatory response leaves the patient susceptible to pro-atherogenic effects. Several studies have demonstrated that patients with periodontal disease have elevated levels of systemic inflammatory mediators [3, 113], specifically circulating C-reactive protein a systemic marker of inflammation [4, 7]. Amar et al. [5] recently have extended this concept of infection with P. gingivalis by demonstrating that in P. gingivalis infected mice fed a high fat diet had twice as much of the aorta occupied by atherosclerotic lesions when compared to non-infected P. gingivalis mice also fed high fat diet.
Mechanistically, as shown in Fig. 2, Toll-like receptors and nucleotide-binding oligomerization domains, both pattern recognition receptors, are critical in the detection of various pathogens, e.g. P. gingivalis. Furthermore, host immune response has demonstrated increase of inflammatory mediators such as interleukin-1, tumor necrosis factor-alpha and C-reactive protein. interleukin-1 signaling seems crucial in P. gingivalis-associated atherogenesis since interleukin-1R-deficient animals failed to develop atherosclerosis [24]. Studies have also demonstrated increased vascular risk in individuals with elevated levels of a wide range of molecules such as interleukin-6, tumor necrosis factor-alpha, inter-cellular adhesion molecule-1, vascular cell adhesion molecule-1 [15] P-selectin, C-reactive protein, fibrinogen, and serum amyloid A [72].
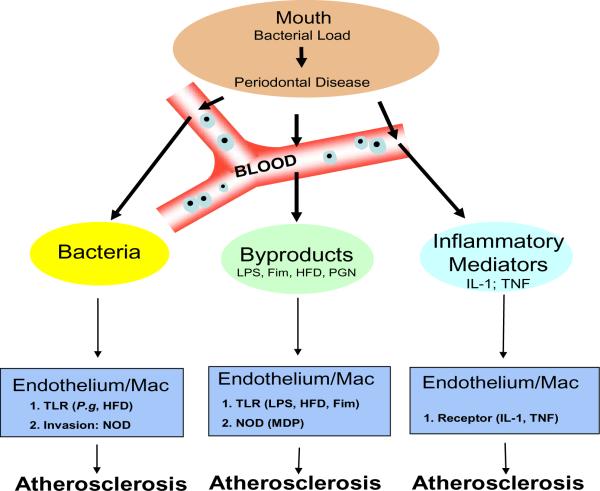
Fig. 2.
Relationship of P.gingivalis Infection and Atherosclerosis
As the bacterial load of P.gingivalis in the oral cavity increases periodontal disease becomes more severe. However, damage is not just limited to the oral cavity since local vasculature enables an entry point for bacteria, bacterial by-products, or inflammatory mediators (e.g cytokines interleukin-1 or tumor necrosis factor-α). Bacteremias, common in periodontal disease due to dental work e.g. cleaning, flossing or surgery, effect both endothelium and macrophages by inducing homotolarance along the Toll-like receptor (TLR) pathway. However, since P.gingivalis is an invasive bacterium, mediated by its fimbriae (Fim) protein, it has the ability to activate the cytosolic nucleotide oligomerization domains (NODs) through recognition of muramyl dipeptide (MDP), a fragment of peptidoglycan (PGN). In addition, various bacterial by-products, e.g. Lipopolysaccharide (LPS), can also activate both the endothelium and macrophages. Further, from the oral cavity a state of local inflammation exists and immune cells are constantly releasing inflammatory mediators into the circulation, these mediators, interleukin-1 or tumor necrosis factor-α, can activate both the endothelium and macrophages. Finally, activated endothelium and macrophages have been shown to exacerbate Atherosclerosis when coupled with a high fat diet (HFD).
In addition, the role of Toll-like receptors has recently been advocated in periodontal disease, as evidenced by reduced bone loss in periodontal disease for Toll-like receptor-2- and Toll-like receptor-4-deficient mice [27, 53]. Recently we completed studies related to the role of Toll-like receptor-2 in P. gingivalis-associated atherogenesis. Our results demonstrated an important role for Toll-like receptor-2, since Toll-like receptor-2-deficient animals are rendered less susceptible to P. gingivalis-associated atherogenesis, and application of a Toll-like receptor-2 agonist, synthetic diacyl lipopeptide (FSL-1), produced similar levels of atherogenesis as P. gingivalis in wild-type Toll-like receptor-2 mice [71].
However, not all atherosclerosis could be explained by Toll-like receptor-2 signaling, and given that P. gingivalis is an invasive pathogen, Amar et al. [5] evaluated the role of P. gingivalis invasion of endothelial cells has on atherosclerosis by hypothesizing that intracellular invasion of endothelial cells is required to elicit an immune response and subsequent development of atherosclerosis. Specifically, using an invasion-deficient strain of P. gingivalis DPG3 or an antibiotic metronidazole, which prevents P. gingivalis invasiveness, Amar et al. [5] demonstrated, that either treatment ameliorated the additional atherosclerotic lesion induced by P. gingivalis. This result suggests that endothelial invasion by P. gingivalis is required and that invasion-mediated inflammatory accounts for about half of the atherogenic process [5].
We propose the following intergraded model of the relationship of chronic P. gingivalis infection and atherosclerosis, see Fig. 2, where repeated P. gingivalis bacterimias result in repeated endothelial / macrophage invasion leading to chronic inflammatory state ultimately exacerbating atherosclerosis. Many potential mechanisms have been advanced in the attempt to explain the link between infection and atherosclerosis, including the modulation of lipid metabolism or infectious agents [120]. Chronic infection results in the chronic inflammation that results in production of pro-inflammatory cytokines that activates endothelial cells, which leads to excessive induction of adhesion molecules, cytokines, growth factors and vasoconstrictors [24].
Immune Response: Role of Nucleotide-Binding Oligomerization Domain in Infection-inflammation and Atherosclerosis
At a molecular level the implication that only invasive pathogens are sufficient to exacerbate atherosclerosis, demonstrated by Amar et al. [5], implicates intracellular recognition molecules, e.g. nucleotide-binding oligomerization domains, as a critical drivers in the inflammatory process in both endothelial cells and Macrophages.
Mammals have two closely related nucleotide-binding oligomerization domain family members: nucleotide-binding oligomerization domain-1 and nucleotide-binding oligomerization domain-2 as shown in Fig. 3. Nucleotide-binding oligomerization domain-1 appears to selectively recognize D-glutamyl-meso-diaminopimelic acid (iE-DAP) [22, 48]. Nucleotide-binding oligomerization domain-2 appears to selectively recognize, muramyl dipeptide [85] and its expression is regulated by the pro-inflammatory cytokines tumor necrosis factor, interleukin-1 β and Interferon-gamma in human endothelial cells and macrophages [70]. In addition, nucleotide-binding oligomerization domain-2 has been implicated in bacterial recognition and is frequently mutated in people with familial forms of Crohn's disease [47, 54, 56, 68, 92]. However, despite appealing similarities between nucleotide-binding oligomerization domain proteins and other pathogen recognition proteins, there is little evidence that nucleotide-binding oligomerization domain proteins directly bind to pathogens or their products and this is still a point of active research.
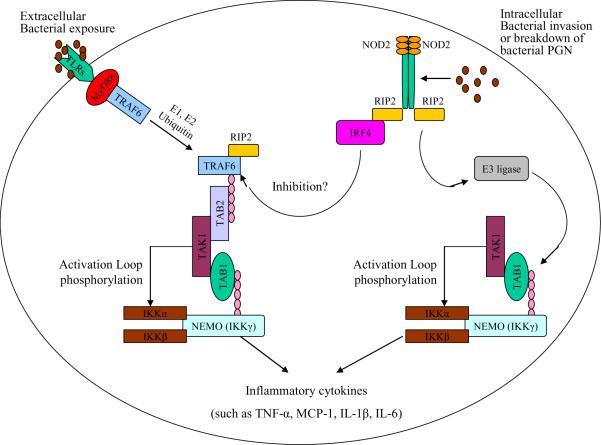
Fig. 3.
A system of pathogen recognition - Toll-Like Receptors (TLRs) and nucleotide oligomerization domain-2 (NOD2) signaling pathways
Two primary bacterial pathogen sensors involved in P.ginigivalis recognition are the extracellular Toll-like Receptors (TLRs) and the intracellular nucleotide oligomerization domain-2 NOD2 receptors. TLRs bind to P.ginigvalis Lipopolysaccharide (LPS) and upon ligand binding recruit a number of adaptor molecules, e.g. tumor necrosis factor-α Receptor Associated Factor-6 (TRAF-6) and Myeloid differentiation primary response gene-88 (MyD88). This in turn ultimately triggers the polyubiquination, via E1 and E2 ubiqination, of NF-κB Essential Modulator (NEMO), also known as Inhibitor of NF-κB kinase-γ (IKK-γ), which in turn activates IKK-α and IKK-β subunits. IKK-α and IKK-β then phosphorylates Inhibitor of NF-κB (IκB) and promotes its degradation via the proteosome thus freeing NF-κB. The intracellular NOD2 receptor is activated upon binding to fragments of peptidoglycan (PGN), muramyl dipeptide (MDP), which then recruits Receptor Interacting Protein-2 (RIP2), a serine-theronine kinase. The NOD2/RIP2 complex undergoes oligomerization itself, which enables direct binding to NEMO (IKK-γ). NOD2/RIP2/NEMO complex can then recruit E3-Ligase and promote the polyubiquination of IKK-α and, via a similar mechanism as TLR signaling, activates NF-κB. Ultimately, both TLRs and NOD2 signaling converge and result in NF-κB translocating into the nucleus and increase transcription of pro-inflammatory cytokines (e.g. tumor necrosis factor-α, MCP-1, interleukin-1β, and interleukin-6).
Data generated using nucleotide-binding oligomerization domain-2 deficient mice show that nucleotide-binding oligomerization domain-2 is involved with the response to peptidoglycan, and specifically in interleukin-12 production, pointing to an unexpected role of nucleotide-binding oligomerization domain-2 in regulating the response to bacterial products [123]. Peptidoglycan is often considered to be a Toll-like receptor-2 agonist, raising the question of how nucleotide-binding oligomerization domain-2 and Toll-like receptor-2 signaling are linked. It has been demonstrated that macrophages and dendritic cells from nucleotide-binding oligomerization domain-2-deficient mice are impaired in the production of pro-inflammatory cytokines and nitric oxide following intracellular infection with live, virulent Mycobacterium tuberculosis [58]. The peptidoglycan of M. tuberculosis stimulated the release of tumor necrosis factor-alpha and interleukin-12p40 in a partially nucleotide-binding oligomerization domain-2-dependent manner and M. tuberculosis peptidoglycan required nucleotide-binding oligomerization domain-2 for the optimal induction of tumor necrosis factor-alpha [58].
Both nucleotide-binding oligomerization domain-2 and Toll-like receptor-2 are activated by the same bacterial products (peptidoglycan derivates) [46]. Recently, the effect of nucleotide-binding oligomerization domain-2 activation on Toll-like receptor-2-mediated cytokine responses was found to be dependent on muramyl dipeptide activation dose. A biphasic role of nucleotide-binding oligomerization domain-2 was demonstrated with low ligand stimulation being synergistic to the inflammatory response while high ligand levels lead to a negative regulation of the inflammatory response with all cells remaining viable even after prolonged culture [16]. We will take advantage of this finding, that high dose muramyl dipeptide is a negative regulator of the inflammatory response, to test its effect on P. gingivalis associated atherosclerosis.
Role of Nucleotide-Binding Oligomerization Domain-2 in P. gingivalis detection within the endothelial and its association with atherosclerosis
More specifically, our data indicates a role of nucleotide-binding oligomerization domain-2 in P. gingivalis infection. Furthermore, our preliminary data, along with published reports, indicate that in macrophages and endothelial cells, nucleotide-binding oligomerization domain-2 acts as an intracellular sensor for bacterial infection. Macrophages and endothelial cells regulate molecules important for leukocyte recruitment in response to a nucleotide-binding oligomerization domain-2 ligand, implicating a link between this innate pattern recognition receptor and immunity. Finally, since vascular endothelial cells and macrophages are critical targets for the bacterial molecules (such as muramyl dipeptide), nucleotide-binding oligomerization domain-2 may play an important role in recognizing structural patterns of bacterial pathogens in the endothelium [93]. At present we can only speculate about the source or mechanism of cell entry of muramyl dipeptide or related muropeptides, which activate nucleotide-binding oligomerization domain-2. One source might be from phagocytosed bacteria, which have undergone degradation within a phagocytic vacuole. Macrophages contain intracellular hydrolases that digest peptidoglycan of intracellular and phagocytosed bacteria, generating muramyl dipeptide, among other products [59, 121]. However, it is important to recall that more than two decades ago, studies of Martin et al. [76] provided evidence for accumulation of muropeptides, which could be derived only from endogenous peptidoglycan.
Moreover, despite appealing similarities between nucleotide-binding oligomerization domain proteins and other pathogen recognition proteins with leucine-rich repeat, there is little evidence that nucleotide-binding oligomerization domain proteins directly bind to pathogens or their products. However, it can be said that nucleotide-binding oligomerization domain-2 and nucleotide-binding oligomerization domain-1 modify the responses of cells to muramyl dipeptide and iE-DAP, respectively [85]. The best chance of understanding nucleotide-binding oligomerization domain functions seems to lie in the dissection of the cellular responses to muramyl dipeptide and iE-DAP to discern how these intersect the many layers of Toll-like receptors signaling. It has been reported that macrophages or mice made insensitive to Toll-like receptors by previous exposure to microbial ligands remained responsive to nucleotide-binding oligomerization domain-1 and nucleotide-binding oligomerization domain-2 stimulation [66]. Furthermore, nucleotide-binding oligomerization domain-1 and nucleotide-binding oligomerization domain-2-mediated signaling and gene expression are enhanced in Toll-like receptors ligand-tolerant macrophages [66]. Innate immune responses induced by bacterial infection were shown to rely on nucleotide-binding oligomerization domain-1 and nucleotide-binding oligomerization domain-2 and their adaptor serine–threonine kinase RICK: kinase) [also known as receptor-interacting protein-2]. Receptor-interacting protein 2 in macrophages pretreated with Toll-like receptors ligands, but not in naïve macrophages. Thus, nucleotide-binding oligomerization domain-1 and nucleotide-binding oligomerization domain-2 are important for microbial recognition and host defense after Toll-like receptors stimulation [66] (Fig. 3).
Overall, we suggest that as endothelial cells become homotolarized by constant and/or repetitive low-level exposures of P.gingivalis bacteremias and subsequent Toll-like receptors expression levels drop thus muting Toll-like receptors signaling. However, since P.gingivalis is an invasive species of bacteria pro-inflammatory cytokines are activated through nucleotide-binding oligomerization domain-1/nucleotide-binding oligomerization domain-2 activation, which regulates the expression of the adhesion molecule inter-cellular adhesion molecule 1 and the chemokine monocyte chemotactic protein-1, two inflammatory mediators implicated in atherosclerosis, which increases the likelihood of monocyte transiting from the blood compartment to the local tissue compartment thus increasing the number of macrophages in athromas and their subsequent conversion to foam cells under high fat diet [69]. Collectively, these data warrant further studies to elucidate any potential role for nucleotide-binding oligomerization domain proteins in inflammatory pathways in atherosclerosis.
Conclusion
In this review we have strived to link exposure of P.gingivalis to exacerbations in periodontal disease through induction of homotolerance. Further, we have demonstrated the emerging understanding the role of obesity contributes to periodontal disease exacerbations, also through induction of homotolerance. Finally, we show that the long-term effects of periodontal disease exacerbate atherosclerosis through a combination of homotolerance and repeated bouts of endothelial induced inflammation due to bacterial invasion of endothelial cells at sites of athromas. Taken together, we propose an integrated model, see Fig. 4, which links periodontal disease, obesity and atherosclerosis mechanistically focusing on the dysregulation in the innate immune system induced by homotolerance as a primary driver in both periodontal disease and atherosclerosis.
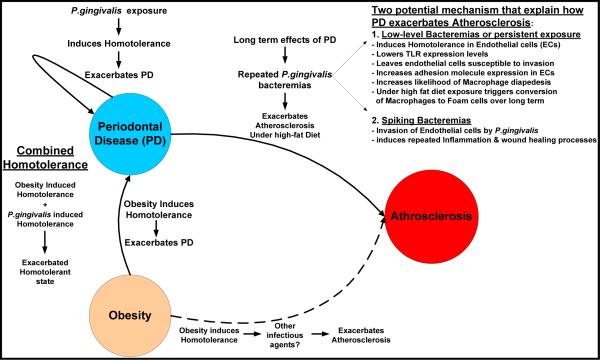
Fig. 4.
Proposed integrated model linking Periodontal Disease, Obesity, and Atherosclerosis
We propose the following integrated model linking Periodontal Disease, Obesity and Atherosclerosis with a foundation built on a foundation of dysregulated innate immunity due to the induction of homotolerance. Homotolerance can be induced either by obesity directly or by exposure to P.gingivalis. However, when obesity is combined with P.gingivalis exposure, we speculated that exacerbates the overall homotolerant state thus having an additive effect and further exacerbating Periodontal Disease. We speculate two potential mechanisms that explain how the long-term consequences of periodontal disease exacerbates Atherosclerosis. First is the induction of homotolerance through a persistent low-level bacteremia or perhaps a transient low-level bacteremia induces a tolerant state within the endothelial cells and Macrophages centered on regions of fatty-streaks/athroma development. Specifically, this results in reduced expression of Toll-like Receptors (TLR), which then leaves these cells susceptible to P. gingivalis invasion and increased adhesion receptor expression via nucleotide oligomerization domain-2 NOD2 receptor signaling. Increased adhesion receptor expression increases the likelihood of macrophages transiting from the blood compartment to the aterial intima. When coupled with a high-fat diet macrophages will uptake cholesterol from Low-desnity lipoprotein via the scavenger receptor. Over time this has been shown to foster conversion of the macrophage to a `Foam' cell and progression of Atherosclerosis. Secondly, there is a potential for a spiking bactermia during dental procedures or flossing that could actually overcome the homotolerance and result in a more traditional inflammatory response and wound healing processes which have been shown to exacerbate Atherosclerosis.
Obesity is a condition that is known to affect the periodontal complex. Although obesity is a hyper-inflammation state characterized by expanded numbers of macrophages, leukocyte and lymphocyte infiltration into adipose tissue, and an activated cytokine network, sustainability of the immune system seems paralyzed in response to various infections. Recent findings by Zhou et al. have demonstrated a form of tolerance in reduced Toll-like receptors expression levels when obese mice are repeatedly infected with P. gingivalis as compared to lean infected mice. This has the effect of reducing the tumor necrosis factor-alpha level similar to the homotolerance seen when low-levels of lipopolysaccharides pre-treatments with macrophages [65, 127]. Further, we speculate that the contribution of homotolerance induced by obesity is additive to the homotolorace induced by P. gingivalis exposure, such that a higher degree of homotolerance exists in the combined obese individual plus P.gingivalis exposure then either condition alone. Taken together, obesity mutes the immune response to P. gingivalis, which we suggest is critical in providing an immune-free environment for P. gingivalis to thrive and thus exacerbating periodontal disease.
The concept of homotolerance, i.e. a low-level stimulation of a pattern recognition receptor, e.g. Toll-like receptors or nucleotide-binding oligomerization domains , and its subsequent down regulation is emerging as a critical driver in periodontal disease progression, effects of obesity on the immune system, and ultimately on atherosclerosis progression. Endotoxin tolerance has been a known phenomenon since 1950 when Neva & Morgan [88] demonstrated tolerance in the exposure of enteric bacteria in patients with typhoid fever. However, only in the past decade have the molecular mechanisms have started to fully characterize how tolerance is achieved in the innate immune system and only very recently an understanding of it implications on various disease states, like periodontal disease or atherosclerosis.
While homotolerance appears to be a mechanism designed to fine-tune the immune system to ignore a low-level stimulation of a pattern recognition receptor, certainly ideal in areas of the body with repeated exposure to such a pathogens, e.g. in the lung or colon specifically, thus preventing repeated bouts of inflammation or perhaps even sepsis. However, in the oral cavity it appears P. gingivalis is able to take advantage of this mechanism by using it to tolarize resident and infiltrating leukocytes and effectively muting any immune response against it, thus exacerbating periodontal disease.
Further understandings of the mechanisms that generate this homotolarant state are required to fully comprehend how this state is induced, what its effects are on immune system, and what pathological conditions are affected by homotolerance. Current findings by Muthukuru et al. showed that the pattern recognition receptor expression levels drop, Toll-like receptor- 2 and Toll-like receptor-4, in response to low-level pre-treatments pointing to a reduction in mRNA. The reduced expression could possibly be caused by alterations in transcription factors at the promoter, the context of the gene at the promoter region itself, i.e. an epigenetical regulation, or even by the induction of possible siRNAs to silence generated Toll-like receptors mRNAs to name a few of the potential mechanism that can result in reduced expression levels.
In addition understand how a low-level stimulation, but not a high level stimulation induces tolerance would be critical in furthering the understanding homotolerance. Specifically, an understanding of where the `magical' threshold exists such that too little stimulation induces tolerance versus too much stimulation induces inflammation. How does the cell make this decision? What is too much stimulation and what is too little stimulation? Answers to questions such as these would also be critical in our understanding of homotolerance.
Ultimately, these understanding then can be translated into better identification of rational therapeutic designs to better focus targets on key processes of disease progression. The implications are staggering when thinking of the obesity epidemic and the prevalence of coronary heart disease in conjunction with our western diet. Further, treatments targeted to homotolerance would be more of a preventative measure instituted pre-clinical long before the overt debilitating and potentially fatal effects of coronary heart disease manifest itself in a patient.
Acknowledgments
This work is supported by grants from the National Institute of Dental and Craniofacial Research DE15989; DE015345 (SA).
References
- 1.Aderem A. Phagocytosis and the inflammatory response. J infect dis. 2003;187(Suppl 2):S340–345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Amabile N, Susini G, Pettenati-Soubayroux I, Bonello L, Gil JM, Arques S, Bonfil JJ, Paganelli F. Severity of periodontal disease correlates to inflammatory systemic status and independently predicts the presence and angiographic extent of stable coronary artery disease. J Intern Med. 2008;263:644–652. doi: 10.1111/j.1365-2796.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 4.Amar S, Han X. The impact of periodontal infection on systemic diseases. Med Sci Monit. 2003;9:RA291–299. [PubMed] [Google Scholar]
- 5.Amar S, Wu SC, Madan M. Is Porphyromonas gingivalis cell invasion required for atherogenesis? Pharmacotherapeutic implications. J Immunol. 2009;182:1584–1592. doi: 10.4049/jimmunol.182.3.1584. [DOI] [PubMed] [Google Scholar]
- 6.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- 8.Arbes SJ, Jr., Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777–1782. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 9.Areschoug T, Gordon S. Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. Contrib Microbiol. 2008;15:45–60. doi: 10.1159/000135685. [DOI] [PubMed] [Google Scholar]
- 10.Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, Brook GJ, Levy Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:674–679. doi: 10.1038/sj.ijo.0802609. [DOI] [PubMed] [Google Scholar]
- 11.Asai Y, Ohyama Y, Gen K, Ogawa T. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect Immun. 2001;69:7387–7395. doi: 10.1128/IAI.69.12.7387-7395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 14.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Borm ME, van Bodegraven AA, Mulder CJ, Kraal G, Bouma G. The effect of NOD2 activation on TLR2-mediated cytokine responses is dependent on activation dose and NOD2 genotype. Genes Immun. 2008;9:274–278. doi: 10.1038/gene.2008.9. [DOI] [PubMed] [Google Scholar]
- 17.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 18.Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: a case-control study. J Periodontol. 2005;76:418–425. doi: 10.1902/jop.2005.76.3.418. [DOI] [PubMed] [Google Scholar]
- 19.Canturk Z, Canturk NZ, Cetinarslan B, Utkan NZ, Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11:769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 20.Cavrini F, Sambri V, Moter A, Servidio D, Marangoni A, Montebugnoli L, Foschi F, Prati C, Di Bartolomeo R, Cevenini R. Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: report of two cases. J Med Microbiol. 2005;54:93–96. doi: 10.1099/jmm.0.45845-0. [DOI] [PubMed] [Google Scholar]
- 21.Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–592. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 22.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 23.Chandra RK, Au B. Spleen hemolytic plaque-forming cell response and generation of cytotoxic cells in genetically obese (C57Bl/6J ob/ob) mice. Int Arch Allergy Appl Immunol. 1980;62:94–98. doi: 10.1159/000232498. [DOI] [PubMed] [Google Scholar]
- 24.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 25.Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun. 1999;67:4231–4236. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 27.Costalonga M, Batas L, Reich BJ. Effects of Toll-like receptor 4 on Porphyromonas gingivalis-induced bone loss in mice. J Periodontal Res. 2009;44:537–542. doi: 10.1111/j.1600-0765.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 28.Cullinan MP, Hamlet SM, Westerman B, Palmer JE, Faddy MJ, Seymour GJ. Acquisition and loss of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia over a 5-year period: effect of a triclosan/copolymer dentifrice. J Clin Periodontol. 2003;30:532–541. doi: 10.1034/j.1600-051x.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 29.Dasanayake AP. C-reactive protein levels are elevated in patients with periodontitis and their CRP levels may go down after periodontal therapy. J Evid Based Dent Pract. 2009;9:21–22. doi: 10.1016/j.jebdp.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Dasanayake AP, Boyd D, Madianos PN, Offenbacher S, Hills E. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J Periodontol. 2001;72:1491–1497. doi: 10.1902/jop.2001.72.11.1491. [DOI] [PubMed] [Google Scholar]
- 31.Dasanayake AP, Russell S, Boyd D, Madianos PN, Forster T, Hill E. Preterm low birth weight and periodontal disease among African Americans. Dent Clin North Am. 2003;47:115–125. x–xi. doi: 10.1016/s0011-8532(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 32.den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 33.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Bmj. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-kappa B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- 36.Emingil G, Atilla G, Huseyinov A. Gingival crevicular fluid monocyte chemoattractant protein-1 and RANTES levels in patients with generalized aggressive periodontitis. J Clin Periodontol. 2004;31:829–834. doi: 10.1111/j.1600-051X.2004.00584.x. [DOI] [PubMed] [Google Scholar]
- 37.Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res. 2002;90:2–4. [PubMed] [Google Scholar]
- 38.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–28. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 39.Espejo B, Torres A, Valentin M, Bueno B, Andres A, Praga M, Morales JM. Obesity favors surgical and infectious complications after renal transplantation. Transplant Proc. 2003;35:1762–1763. doi: 10.1016/s0041-1345(03)00718-8. [DOI] [PubMed] [Google Scholar]
- 40.Faintuch J, Horie LM, Barbeiro HV, Barbeiro DF, Soriano FG, Ishida RK, Cecconello I. Systemic inflammation in morbidly obese subjects: response to oral supplementation with alpha-linolenic acid. Obes Surg. 2007;17:341–347. doi: 10.1007/s11695-007-9062-x. [DOI] [PubMed] [Google Scholar]
- 41.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 42.Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of interleukin-6 in gingival crevicular fluid from adults with destructive periodontal disease. J Periodontol. 1993;64:980–983. doi: 10.1902/jop.1993.64.10.980. [DOI] [PubMed] [Google Scholar]
- 43.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 44.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 45.Girardin SE, Hugot JP, Sansonetti PJ. Lessons from Nod2 studies: towards a link between Crohn's disease and bacterial sensing. Trends Immunol. 2003;24:652–658. doi: 10.1016/j.it.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 47.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 49.Goren I, Kampfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes. 2003;52:2821–2832. doi: 10.2337/diabetes.52.11.2821. [DOI] [PubMed] [Google Scholar]
- 50.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 51.Heitman J. Sexual reproduction and the evolution of microbial pathogens. Curr Biol. 2006;16:R711–725. doi: 10.1016/j.cub.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 52.Hoge M, Amar S. Role of interleukin-1 in bacterial atherogenesis. Drugs Today (Barc) 2006;42:683–688. doi: 10.1358/dot.2006.42.10.1003543. [DOI] [PubMed] [Google Scholar]
- 53.Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect Immun. 2000;68:4681–4687. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 55.Inohara Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 56.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 57.Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–363. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- 59.Johannsen L, Wecke J, Obal F, Jr., Krueger JM. Macrophages produce somnogenic and pyrogenic muramyl peptides during digestion of staphylococci. Am J Physiol. 1991;260:R126–133. doi: 10.1152/ajpregu.1991.260.1.R126. [DOI] [PubMed] [Google Scholar]
- 60.Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- 61.Johnson LL, Sayles PC. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect Immun. 2002;70:185–191. doi: 10.1128/IAI.70.1.185-191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshipura KJ, Rimm EB, Douglas CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 63.Kanbay A, Kokturk O, Ciftci TU, Tavil Y, Bukan N. Comparison of serum adiponectin and tumor necrosis factor-alpha levels between patients with and without obstructive sleep apnea syndrome. Respiration. 2008;76:324–330. doi: 10.1159/000134010. [DOI] [PubMed] [Google Scholar]
- 64.Khlgatian M, Nassar H, Chou HH, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70:257–267. doi: 10.1128/IAI.70.1.257-267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Lee FY, Li Y, Yang EK, Yang SQ, Lin HZ, Trush MA, Dannenberg AJ, Diehl AM. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Physiol. 1999;276:C386–394. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 68.Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, Tysk C, O'Morain C, Gassull M, Binder V, Finkel Y, Modigliani R, Gower-Rousseau C, Macry J, Merlin F, Chamaillard M, Jannot AS, Thomas G, Hugot JP. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–857. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loppnow H. Intited review: Vascular cells control atherosclerosis by cytokine-and innate immunity-related inflammatory mechanisms. Innate Immunity. 2008;14:63–68. doi: 10.1177/1753425908091246. [DOI] [PubMed] [Google Scholar]
- 70.Loppnow H, Werdan K, Buerke M. Vascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanisms. Innate Immun. 2008;14:63–87. doi: 10.1177/1753425908091246. [DOI] [PubMed] [Google Scholar]
- 71.Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS One. 2008;3:e3204. doi: 10.1371/journal.pone.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahmoudi M, Curzen N, Gallagher PJ. Atherogenesis: the role of inflammation and infection. Histopathology. 2007;50:535–546. doi: 10.1111/j.1365-2559.2006.02503.x. [DOI] [PubMed] [Google Scholar]
- 73.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 74.Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db) J Immunol. 1978;120:1375–1377. [PubMed] [Google Scholar]
- 75.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 76.Martin SA, Karnovsky ML, Krueger JM, Pappenheimer JR, Biemann K. Peptidoglycans as promoters of slow-wave sleep. I. Structure of the sleep-promoting factor isolated from human urine. J Biol Chem. 1984;259:12652–12658. [PubMed] [Google Scholar]
- 77.Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225:293–296. doi: 10.1111/j.1365-2796.1989.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 78.Mattila KJ. Dental infections as a risk factor for acute myocardial infarction. Eur Heart J. 1993;14(Suppl K):51–53. [PubMed] [Google Scholar]
- 79.Mattila KJ, Valtonen VV, Nieminen M, Huttunen JK. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588–92. doi: 10.1093/clinids/20.3.588. [DOI] [PubMed] [Google Scholar]
- 80.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 81.Medzhitov R, Janeway C., Jr. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 82.Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, Califano JV, Burmeister JA, Schenkein HA. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71:1699–1707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- 83.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 84.Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism. 2000;49:1295–1300. doi: 10.1053/meta.2000.9523. [DOI] [PubMed] [Google Scholar]
- 85.Murray PJ. NOD proteins: an intracellular pathogen-recognition system or signal transduction modifiers? Curr Opin Immunol. 2005;17:352–358. doi: 10.1016/j.coi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Muthukuru M, Jotwani R, Cutler CW. Oral mucosal endotoxin tolerance induction in chronic periodontitis. Infect Immun. 2005;73:687–694. doi: 10.1128/IAI.73.2.687-694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nery EB, Meister F, Jr., Ellinger RF, Eslami A, McNamara TJ. Prevalence of medical problems in periodontal patients obtained from three different populations. J Periodontol. 1987;58:564–568. doi: 10.1902/jop.1987.58.8.564. [DOI] [PubMed] [Google Scholar]
- 88.Neva FA, Morgan HR. Tolerance to the action of endotoxins of enteric bacilli in patients convalescent from typhoid and paratyphoid fevers. J Lab Clin Med. 1950;35:911–922. [PubMed] [Google Scholar]
- 89.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 90.Nishimura F, Taniguchi A, Yamaguchi-Morimoto M, Soga Y, Iwamoto Y, Kokeguchi S, Kuroe A, Fukushima M, Nakai Y, Seino Y. Periodontal infection and dyslipidemia in type 2 diabetics: association with increased HMG-CoA reductase expression. Horm Metab Res. 2006;38:530–535. doi: 10.1055/s-2006-949525. [DOI] [PubMed] [Google Scholar]
- 91.Njoroge T, Genco RJ, Sojar HT, Hamada N, Genco CA. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 93.Oh HM, Lee HJ, Seo GS, Choi EY, Kweon SH, Chun CH, Han WC, Lee KM, Lee MS, Choi SC, Jun CD. Induction and localization of NOD2 protein in human endothelial cells. Cellular immunology. 2005;237:37–44. doi: 10.1016/j.cellimm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, Inohara N, Nunez G. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–521. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Baena MJ, Marti A, Martinez JA, Moreno-Aliaga MJ. Differential inflammatory status in rats susceptible or resistant to diet-induced obesity: effects of EPA ethyl ester treatment. Eur J Nutr. 2008;47:380–386. doi: 10.1007/s00394-008-0738-3. [DOI] [PubMed] [Google Scholar]
- 96.Progulske-Fox A, Kozarov E, Dorn B, Dunn W, Jr., Burks J, Wu Y. Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system. J Periodontal Res. 1999;34:393–399. doi: 10.1111/j.1600-0765.1999.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 97.Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, Vesanen M, Mattila K, Asikainen S. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 98.Raetz CR. Biochemistry of endotoxins. Annual Review of Biochemistry. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 99.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and Toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–1959. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]
- 100.Ryan AM, Healy LA, Power DG, Byrne M, Murphy S, Byrne PJ, Kelleher D, Reynolds JV. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg. 2008;247:909–915. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 101.Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 102.Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med. 1998;339:482–483. doi: 10.1056/NEJM199808133390717. [DOI] [PubMed] [Google Scholar]
- 103.Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. J Dent Res. 2001;80:1631–1636. doi: 10.1177/00220345010800070701. [DOI] [PubMed] [Google Scholar]
- 104.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Yamashita Y. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: the Hisayama study. J Periodontal Res. 2005;40:346–353. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 105.Salvi GE, Brown CE, Fujihashi K, Kiyono H, Smith FW, Beck JD, Offenbacher S. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res. 1998;33:212–225. doi: 10.1111/j.1600-0765.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 106.Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77:933–939. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]
- 107.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. Am J Physiol Cell Physiol. 2002;282:C1205–1211. doi: 10.1152/ajpcell.00511.2001. [DOI] [PubMed] [Google Scholar]
- 110.Slots J, Genco RJ. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 111.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 112.Stallone DD. The influence of obesity and its treatment on the immune system. Nutr Rev. 1994;52:37–50. [PubMed] [Google Scholar]
- 113.Sun XJ, Meng HX, Shi D, Xu L, Zhang L, Chen ZB, Feng XH, Lu RF, Ren XY. Elevation of C-reactive protein and interleukin-6 in plasma of patients with aggressive periodontitis. J Periodontal Res. 2009;44:311–316. doi: 10.1111/j.1600-0765.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 114.Tanabe SI, Grenier D. Macrophage tolerance response to Aggregatibacter actinomycetemcomitans lipopolysaccharide induces differential regulation of tumor necrosis factor-alpha, interleukin-1 beta and matrix metalloproteinase 9 secretion. J Periodontal Res. 2008;43:372–377. doi: 10.1111/j.1600-0765.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 115.Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, Sugiyama A, Takamura Y, Okuda K. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631–636. [PubMed] [Google Scholar]
- 116.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 117.Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66:852–859. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 118.Umino M, Nagao M. Systemic diseases in elderly dental patients. Int Dent J. 1993;43:213–218. [PubMed] [Google Scholar]
- 119.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 120.Vercellotti GM. Effects of viral activation of the vessel wall on inflammation and thrombosis. Blood Coagul Fibrinolysis. 1998;9(Suppl 2):S3–6. [PubMed] [Google Scholar]
- 121.Vermeulen MW, Gray GR. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984;46:476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit Rev Oral Biol Med. 2002;13:132–142. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- 123.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 124.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weisberg SP, H.D., Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yavuzyilmaz E, Yamalik N, Bulut S, Ozen S, Ersoy F, Saatci U. The gingival crevicular fluid interleukin-1 beta and tumour necrosis factor-alpha levels in patients with rapidly progressive periodontitis. Aust Dent J. 1995;40:46–49. doi: 10.1111/j.1834-7819.1995.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A. 2009;106:10740–10745. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu J, Quyyumi AA, Norman JE, Csako G, Epstein SE. Cytomegalovirus in the pathogenesis of atherosclerosis: the role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol. 1999;34:1738–1743. doi: 10.1016/s0735-1097(99)00410-6. [DOI] [PubMed] [Google Scholar]
- 129.Zuany-Amorim C, Hastewell J, Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat Rev Drug Discov. 2002;1:797–807. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]