Abstract
High-dose (28-30mg/kg/day) ursodeoxycholic acid (UDCA) treatment improves serum liver tests in patients with primary sclerosing cholangitis (PSC) but does not improve survival and is associated with increased rates of serious adverse events. The mechanism for the latter undesired effect remains unclear. High-dose UDCA could result in the production of hepatotoxic bile acids, such as lithocholic acid (LCA), due to limited small bowel absorption of UDCA and conversion of UDCA by bacteria in the colon. We determined the serum bile acid composition in 56 patients with PSC previously enrolled in a randomized, double-blind controlled trial of high dose UDCA versus placebo. Samples for analysis were obtained at baseline and at the end of treatment. The mean changes in UDCA (16.86 vs 0.05 μmol/L) and total bile acid (17.21 vs −0.55 μmol/L) levels were significantly higher in the UDCA group (n=29) compared to placebo (n=27) when pretreatment levels were compared (p<0.0001). LCA was also markedly increased (0.22 vs 0.01 μmol/L) in the UDCA group compared to placebo (p=0.001). No significant changes were detected for cholic acid (CA), deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA). Patients (n=9) in the UDCA group who reached clinical endpoints of disease progression (development of cirrhosis, varices, liver transplantation or death) tend to have greater increase in their post-treatment total bile acid levels (34.99 vs 9.21 μmol/L) (p<0.08) compared to those who did not.
Conclusion
High-dose UDCA treatment in PSC patients results in marked UDCA enrichment and significant expansion of the total serum bile acid pool including lithocholic acid.
Keywords: sclerosing cholangitis, bile acids, lithocholic acid, treatment, outcome
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by inflammation and destruction of the extrahepatic and/or intrahepatic bile ducts, resulting in biliary cirrhosis, need for liver transplantation and reduced life expectancy (1). Up to now there are no reports of a medical therapy able to halt disease progression. Ursodeoxycholic acid (UDCA), initially tested at a dose of 13-15mg/kg/day, has shown some beneficial effects in patients with PSC as measured by liver biochemistry (2). Subsequent studies with higher drug doses showed even more favorable outcomes (3-6). However, a recently published randomized, double-blind controlled trial of high dose UDCA (28-30mg/kg/day) versus placebo failed to demonstrate improvement in survival and was associated with increased rates of serious adverse events (7).
The mechanism for this unexpected detrimental effect remains unclear. It has been postulated that high-dose UDCA treatment allows unabsorbed drug to enter the colon and be modified into hydrophobic, hepatotoxic bile acids, such as lithocholic acid (LCA) (8-10). LCA is hepatotoxic in animal models, leading to segmental bile duct obstruction, destructive cholangitis and periductal fibrosis (11, 12). Nonetheless, a recent study testing the effect of various, escalating UDCA doses on biliary composition, showed only minimal changes of all bile acids, except for UDCA which was proportionally enriched (13).
The aim of our study was to determine the serum bile acid composition after high-dose UDCA treatment during a randomized, double-blind controlled trial and to correlate the changes in bile acid levels with clinical outcomes.
Materials and Methods
Patients were entered in the present study based on criteria followed for our high-dose UDCA versus placebo randomized, double-blind controlled trial (7). Difficulties related to the multicenter nature of the study and the long enrollment period did not allow all of the initial study patients to be analyzed for bile acid composition. The study was approved by the Institutional Review Boards at each site.
Inclusion Criteria
PSC diagnosis was based upon the following criteria: 1) chronic cholestatic disease of at least 6 months’ duration; 2) serum alkaline phosphatase at least 1½ times the upper limits of normal; 3) retrograde, operative, percutaneous, or magnetic resonance cholangiography findings consistent with PSC within 1 year of the study entry and 4) liver biopsy in the previous 1 year that was compatible with the diagnosis of PSC and available for review.
Exclusion Criteria
Patients were excluded if they had any of the following: 1) coexisting conditions that would limit their life expectancy to less than 2 years; 2) inability to provide consent; 3) treatment with UDCA, corticosteroids or immunosuppressives in the 3 months prior to study entry; 4) end stage liver disease as determined by clinical and laboratory parameters; 5) previous intraductal stones or operations on the biliary tree, other than cholecystectomy and 6) findings of other liver disease such as hepatitis B or C, hemochromatosis, Wilson disease or primary biliary cirrhosis.
Drug administration
Patients received UDCA at a dose of 28-30 mg/kg/day (Axcan Pharma, Mont-St. Hiliare, Canada) in divided doses given with meals or an identical placebo.
Monitoring
Serum samples were obtained at entry into the study and at the end of treatment. All available paired samples were retrieved and used for analysis of bile acid composition. Disease progressions to cirrhosis, development of varices, cholangiocarcinoma, liver transplantation or death during the trial were considered as clinical endpoints.
Analytical Methods
Serum bile acids were analyzed qualitatively by conventional and quantitatively by isotope-dilution gas chromatography-mass spectrometry (GCMS), respectively, as described, with the modification that alkaline hydrolysis with 2M sodium hydroxide in 90% ethanol (v/v; 1h at 67 °C) instead of enzymatic hydrolysis with cholylglycine hydrolase was performed (14). Deuterium labeled lithocholic, deoxycholic, chenodeoxycholic, cholic and ursodeoxycholic acids as internal standards were obtained from CDN Isotopes, Quebec, Canada.
Statistical Analyses
Baseline characteristics were calculated as the median (range) for continuous variables. The number and percent in each group were tabulated for categorical variables. Bile acid concentrations were calculated as the mean±standard deviation. The chi-square test of independence was used to determine statistical significance for categorical data. For the continuous variables, the Wilcoxon rank sum test was used. Baseline characteristics with a p≤0.2 significance were entered in a multivariate model of multiple linear regression analysis to explore possible correlations per bile acid.
Results
From 2001 to 2005 150 patients with PSC were entered in the study. Serum for bile acid analysis was available at baseline and at the end of study (mean treatment duration 2.38±0.56 years) in 56 of these patients. The baseline characteristics of this subset of patients compared to the remaining patients (n=94) is shown in Table 1. Patients analyzed for bile acid composition were younger (41.8 vs 49.3 years), more likely to have concomitant inflammatory bowel disease (93% vs 68%) and had lower Mayo risk score (0.015 vs 0.58). The baseline characteristics of this cohort of patients per treatment were comparable, as well (Table 2).
Table 1. Clinical and Laboratory Characteristics of the Study Patients at Entry.
| Characteristic | Bile Acid Analysis (n= 56 ) | No Bile Acid Analysis (n= 94 ) | p value |
|---|---|---|---|
| Age, years | 41.8 (17.8-75.5) | 49.3 (20.3-73.6) | 0.0017 |
| Duration of disease, years | 0.69 (0.04-7.5) | 1.62 (0.005-49.5) | 0.09 |
| Female sex, n (%) | 23 (41) | 41 (44) | 0.76 |
| Colitis, n (%) | 52 (93) | 63 (68) | 0.0004 |
| Varices, n (%) | 8 (14) | 18 (19) | 0.36 |
| Histologic stage, n (%) | |||
| I | 15 (26) | 35 (38) | 0.09 |
| II | 20 (35) | 20 (22) | 0.07 |
| III | 13 (23) | 24 (26) | 0.54 |
| IV | 10 (18) | 13 (14) | 0.47 |
| Alkaline phosphatase* | 4.0 (1.2-12.5) | 3.3 (0.52-26.9) | 0.25 |
| Aspartate aminotransferase* | 1.6 (0.6-4) | 1.9 (0.5-8.0) | 0.12 |
| Bilirubin, mg/dL* | 0.9 (0.3-4.5) | 1 (0.3-7.2) | 0.18 |
| Mayo risk score | 0.015 [(−1.05)-1.8] | 0.58 [(−1.4)-2.4] | 0.0015 |
Data are presented as the median (range) unless otherwise indicated.
Values represent multiples of the upper limits of normal.
Table 2. Baseline Characteristics of Patients at Entry.
| Characteristic | UDCA (n= 29 ) | Placebo (n= 27 ) | p value |
|---|---|---|---|
| Age, years | 43.4 (20.4-75.5) | 38.3 (17.8-63.1) | 0.09 |
| Duration of disease, years | 0.78 (0.06-7.5) | 0.69 (0.04-6.3) | 1 |
| Female sex, n (%) | 14 (48%) | 9 (33%) | 0.25 |
| Colitis, n (%) | 27 (93%) | 25 (93%) | 0.94 |
| Colectomy, n (%) | 7 (24%) | 5 (19%) | 0.60 |
| Alkaline phosphatase* | 3.8 (1.2-12.5) | 4.4 (1.2-8.1) | 0.84 |
| Aspartate aminotransferase* | 1.8 (0.7-4) | 1.5 (0.6-4) | 0.64 |
| Bilirubin, mg/dL* | 0.7 (0.3-2) | 1 (0.3-4.5) | 0.09 |
| Mayo risk score | 0.03 [(−1.05)-1.8)] | −0.05 [(−1.04)-1.2] | 0.50 |
Data are presented as the median (range) unless otherwise indicated.
Values represent multiples of the upper limits of normal.
Baseline bile acid composition
At baseline the bile acid pool consisted of cholic acid (CA) (32%), LCA (13%), deoxycholic acid (DCA) (21%), UDCA (11%) and chenodeoxycholic acid (CDCA) (23%). GCMS spectra indicating the prevalence of significant amounts (>0.05 μmol/L) of uncommon bile acids were not observed. Only traces of hydroxylation products of UDCA were occasionally seen (15). The GCMS systems used did not separate UDCA from isoUDCA.
Patients with colectomy (n=12) had significantly lower levels of DCA (p<0.0001), whereas patients with baseline alkaline phosphatase ≥4xULN (upper limit of normal) (n=28) had lower levels of CA (p=0.03) (Table 3). Disease severity at entry as assessed by total bilirubin, Mayo risk score and histological stage did not seem to considerably affect the baseline bile acid composition, although patients with baseline total bilirubin ≥0.9mg/dL had higher values of CA (p=0.05). In a multivariate analysis model the only significant relationship revealed was between colectomy (p=0.001), baseline alkaline phosphatase ≥4xULN (p=0.05) and low levels of DCA.
Table 3. Effect of Baseline Characteristics on Bile Acid Composition.
| Colectomy | |||||
|---|---|---|---|---|---|
| (μmol/L) | DCA |
CA |
CDCA |
UDCA |
LCA |
| Colectomy (n=12) |
0.07±0.05 | 0.54±0.55 | 0.32±0.25 | 0.07±0.09 | 0.08±0.07 |
| No colectomy (n=44) |
0.27±0.22 | 0.76±2.44 | 0.40±0.88 | 0.13±0.14 | 0.11±0.07 |
| p value | <0.0001 | NS | NS | NS | NS |
|
| |||||
| Alkaline phosphatase | |||||
|
| |||||
| Alkaline phosphatase< 4xULN (n=28) |
0.27±0.25 | 0.95±3.06 | 0.46±1.09 | 0.10±0.10 | 0.12±0.06 |
| Alkaline phosphatase≥ 4xULN (n=28) |
0.18±0.16 | 0.47±0.42 | 0.30±0.22 | 0.14±0.15 | 0.09±0.08 |
| p value | NS | 0.03 | NS | NS | NS |
|
| |||||
| Total Bilirubin | |||||
|
| |||||
| Total bilirubin< 0.9 (n=27) |
0.25±0.22 | 0.36±0.46 | 0.26±0.24 | 0.08±0.06 | 0.10±0.08 |
| Total bilirubin≥ 0.9 (n=29) |
0.20±0.21 | 1.03±2.98 | 0.49±1.06 | 0.15±0.16 | 0.11±0.06 |
| p value | NS | 0.05 | NS | NS | NS |
Data are presented as the mean ± standard deviation.
ULN=upper limit of normal
LCA=lithocholic acid, DCA=deoxycholic acid, UDCA=ursodeoxycholic acid, CDCA=chenodeoxycholic acid, CA=cholic acid
Changes in bile acid levels after treatment
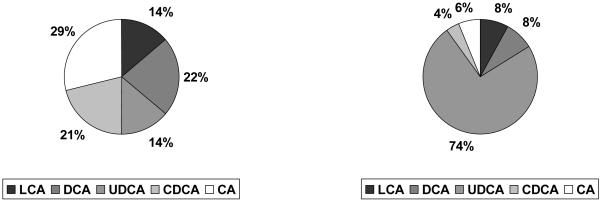
Figure 1 shows the post treatment percentage of each bile acid per treatment group. No significant changes between treatment groups were detected for CA, DCA and CDCA. UDCA was significantly increased (16.86 vs 0.05 μmol/L, p<0.0001) and the total bile acid pool was significantly expanded (17.21 vs −0.55 μmol/L, p<0.0001) in the UDCA group compared to placebo. LCA was also markedly increased in the UDCA group compared to placebo (0.22 vs 0.01 μmol/L, p=0.001). The change in LCA levels after UDCA treatment seemed to positively correlate with the change in UDCA levels (p=0.19).
Fig. 1.
Mean post treatment percentage of bile acids per treatment group (left=placebo, right=UDCA).
LCA=lithocholic acid, DCA=deoxycholic acid, UDCA=ursodeoxycholic acid, CDCA=chenodeoxycholic acid, CA=cholic acid
Correlation with changes in liver tests and clinical outcome
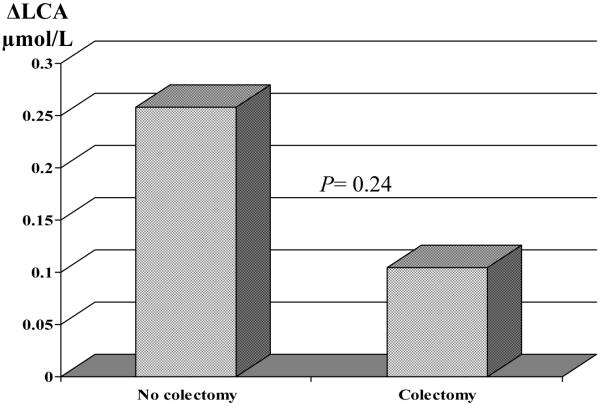
The UDCA and LCA enrichment did not show any significant relationship with the changes in values of liver tests (levels of alkaline phosphatase, aspartate aminotransferase, bilirubin, Mayo risk score) (data not shown). However, female and older patients were more likely to have a greater increase in their LCA value after UDCA treatment (Table 4). Patients with colectomy (n=7) tended to have less LCA increase after treatment than those without colectomy (Fig. 2). However, patients with colectomy did not have worse outcome irrespective of the treatment group.
Table 4. Change in LCA levels by Baseline Characteristics.
| Characteristic | Minimal ΔLCA | Marked ΔLCA | ||
|---|---|---|---|---|
| 0.002 [(−0.14)-0.07] μmol/L (n= 14) |
0.32 (0.13-1.28) μmol/L (n= 15) |
|||
| Age, years | 42.4 (20.4-49.8) | 48.7 (30.7-75.5) | ||
| p-value | 0.05 | |||
| Female sex, n (%) | 4 (29) | 10 (67) | ||
| p-value | 0.04 | |||
| BMI | 27.4 (23.8-33.1) | 26.5 (20.6-38.8) | ||
| p-value | 0.71 | |||
| Colectomy, n (%) | 4 (29) | 3 (20) | ||
| p-value | 0.58 | |||
| Histologic stage≥2, n (%) | 11 (79) | 10 (67) | ||
| p-value | 0.66 | |||
| Alkaline phosphatase* | 3.5 (1.2-12.1) | 4.7 (1.41-12.5) | ||
| p-value | 0.34 | |||
| Aspartate aminotransferase* | 1.6 (0.8-3.8) | 2.2 (0.7-4) | ||
| p-value | 0.94 | |||
| Bilirubin, mg/dL* | 0.7 (0.3-2) | 0.8 (0.4-2) | ||
| p-value | 0.74 | |||
| Mayo risk score | −0.24 [(−0.67)-1.8] | 0.36 [(−1.05)-1.5] | ||
| p-value | 0.31 | |||
Data are presented as the median (range) unless otherwise indicated. ΔLCA= lithocholic acid after treatment-lithocholic acid at entry BMI= Body Mass Index
Values represent multiples of the upper limits of normal.
Fig. 2.
Effect of colectomy on LCA change (UDCA group).
ΔLCA= lithocholic acid after treatment-lithocholic acid at entry
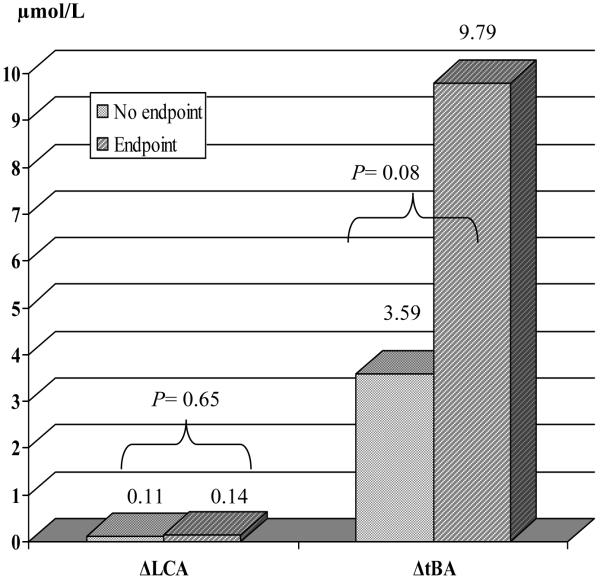
Patients in the UDCA group who reached clinical endpoints during therapy (n=9) tended to have higher increase in their LCA and total bile acid levels compared to those who did not (Fig. 3). The increase in total bile acids was almost entirely due to enrichment with UDCA. Table 5 summarizes the range of bile acid changes in these patients. The changes were similar in all patients, apart from one (patient 5), possibly indicating non-compliance.
Fig. 3.
Change in LCA and total bile acids by outcome (UDCA group).
ΔLCA= lithocholic acid after treatment-lithocholic acid at entry, ΔtBA= total bile acids after treatment-total bile acids at entry
Table 5. Changes in bile acids in patients with a clinical endpoint.
| ΔDCA |
ΔCA |
ΔCDCA |
ΔUDCA |
ΔLCA |
ΔtBA |
|
|---|---|---|---|---|---|---|
| Patient 1 | −0.05 | −0.05 | 0.52 | 4.58 | −0.13 | 4.87 |
| Patient 2 | 0.01 | −0.02 | 0.34 | 5.25 | 0.32 | 5.73 |
| Patient 3 | −0.1 | −0.21 | −0.17 | 13.92 | 0.20 | 13.62 |
| Patient 4 | 0.08 | 0.62 | 2.80 | 80.05 | 0.14 | 83.72 |
| Patient 5 | 0.08 | −0.44 | −0.24 | −0.07 | 0.05 | −0.64 |
| Patient 6 | 0.27 | 1.07 | 1.61 | 74.11 | 1.28 | 78.33 |
| Patient 7 | 0.11 | −0.54 | −0.23 | 3.65 | 0.83 | 3.77 |
| Patient 8 | −0.01 | −0.18 | 0.01 | 9.99 | −0.01 | 9.79 |
| Patient 9 | −0.01 | −0.17 | 0.22 | 115.72 | 0.03 | 115.79 |
Data are presented in μmol/L.
ΔDCA= deoxycholic acid after treatment-deoxycholic acid at entry, ΔCA= cholic acid after treatment-cholic acid at entry, ΔCDCA= chenodeoxycholic acid after treatment-chenodeoxycholic acid at entry ΔUDCA= ursodeoxycholic acid after treatment-ursodeoxycholic acid at entry ΔLCA= lithocholic acid after treatment-lithocholic acid at entry, ΔtBA= total bile acids after treatment-total bile acids at entry
Discussion
UDCA has shown some beneficial effects in patients with PSC (2). The inability to demonstrate slowing of disease progression has resulted in several studies to explore the effectiveness of different UDCA doses over the last decade (3-6). In the most recent study, high dose UDCA (28-30mg/kg/day) treatment was associated with increased rates of serious adverse events without any obvious explanation (7). Modification of bile acid composition has been speculated to potentially underlie the effects of the drug.
In our present study we investigated the serum bile acid composition in PSC patients under high-dose UDCA treatment. At baseline the primary bile acids CA and CDCA predominated. Disease severity as assessed with biochemical markers did not seem to considerably affect the bile acid composition. At the end of treatment we found a significant expansion of the total serum bile acid pool and marked UDCA and LCA enrichment in the UDCA-treated patients compared to placebo when pretreatment levels were compared. Additionally we found that the increase in serum bile acid levels seemed to correlate with worse outcome, since the subset of patients that reached the clinical endpoints of disease progression during UDCA therapy tend to have higher bile acid levels.
The proposed UDCA mechanisms of action in hepatobiliary disorders include expansion of hydrophilic bile acid pool and hypercholeresis (16). Early studies in gallstone patients had shown that UDCA administration can modify the composition of circulating bile acids, leading to UDCA predominance (17, 18). Multiple subsequent studies, most of them in patients with primary biliary cirrhosis, have verified this modification, generally revealing an overall expansion of the total bile acid pool (8, 9, 13, 14, 19-22). Rost et al in a study of biliary bile acid composition in patients with PSC have specifically indicated that UDCA enrichment is augmented (43.1%±0.3% to 58.6%±2.3% of total bile acids) parallel to an escalating dose of UDCA (10-13mg/kg/d to 22-25mg/kg/d), reaching a plateau at the highest dose. This enrichment did not further increase beyond that dose (13).
In our study, the mean changes in UDCA and total bile acid levels post treatment were significantly higher in the UDCA group compared to placebo (p<0.0001). The mean post-treatment UDCA percentage in the bile acid pool was 74%, far higher than any other study ever reported, implying that enrichment is increased proportionally to the dose. Nonetheless this high enrichment did not correspond with better outcome. Therefore further investigation on biliary enrichment has to be performed, especially in regard to clinical outcome.
Changes in the levels of other bile acids under UDCA treatment have been generally considered to follow an inverse relationship between the increase of UDCA levels and the decrease in levels of CA, CDCA, DCA and LCA. Results however are not homogeneous and the changes are not significant in the majority of cases (13, 21, 22). In PSC patients it has been suggested in one study that LCA levels are not increased, even after high-dose UDCA treatment (13). Nonetheless the antibiotics administrated in that study during the endoscopic retrograde cholangiography procedure used to obtain samples for bile acid analysis might have interfered with the results.
Non significant changes between the two treatment groups for CDCA, CA and DCA levels were observed in our study after treatment. Levels of CDCA, CA and DCA were slightly decreased in the placebo group, whereas in the UDCA group CA showed a tendency to decrease and DCA and CDCA were slightly increased. However we found that LCA is clearly increased after treatment in the UDCA group compared to placebo (p=0.001).
An increase in the level of LCA could possibly represent a consequence of high-dose UDCA treatment, since LCA is mainly produced by bacterial 7-dehydroxylation of unabsorbed bile acid that pass into the colon (8-10, 23). UDCA absorption has been generally shown to be slow and incomplete (24-26). Moreover it is inversely related with the severity of cholestasis (25). PSC patients are expected to experience various levels of cholestasis; in our study though total bilirubin, as a marker of cholestasis, was not significantly elevated. However the dose of UDCA given was among the highest ever tried in PSC patients. LCA levels could have been influenced by the surgical removal of the colon (27); nevertheless, in our subset of patients with colectomy no significant differences from patients with intact colon were demonstrated, which may be due to the limited number of patients. In addition, 5 of 7 patients with colectomy in the UDCA group had an ileal pouch procedure, which may have potentially interfered with the amount of LCA production.
Under normal conditions bile is already relatively “toxic”, but actual liver toxicity is prevented by various mechanisms including maintenance of appropriate bile composition and normal bile flow (28). High levels of LCA disrupt this equilibrium since this bile acid is toxic per se and highly hydrophobic. In addition to that, LCA has been proven to promote bile duct injury in animal models through obstruction by LCA crystals, finally resulting in destructive cholangitis (12). In our study LCA levels tended to be higher in patients in the UDCA group who reached clinical endpoints of disease progression compared to those who did not. This relationship did not reach statistical significance, but this may be due to the small number of patients that reached a clinical endpoint. However, we think that our data are currently not solid enough to support the hypothesis that the worse outcome seen in this subset of patients can be explained solely by an increase in LCA levels.
A link of LCA action to cholangitis that is implied by our findings would certainly be exciting. However, we suggest that other potential explanations for the paradoxical effect that UDCA had in some patients treated with high doses have to be also investigated before drawing final conclusions on UDCA mechanisms of action in PSC patients. Bile infarct aggravation due to increased bile flow and biliary pressure in the setting of biliary obstruction and modulation of apoptosis due to activated stellate cell life prolongation could present alternate mechanisms (29, 30). In our study bile acid levels tended to be higher in patients in the UDCA group who reached clinical endpoints of disease progression compared to those who did not. These changes could be useful as a way to assess disease severity and to follow disease course. Further studies focused on the relationships between bile acid levels and markers of disease progression are warranted to explore this possibility.
Finally we would like to comment on some limitations of our study. First, we have to point out that serum samples for bile acid analysis were available in only 56 of the 150 initial patients. Given the “sub-analysis” nature of the current study it would be ideal to maintain the same number of patients as the initial study. Unfortunately administrative issues, as earlier outlined, did not allow this to happen. Lastly, we would like to cite the significant difference in the percentage of patients having concomitant inflammatory bowel disease (IBD) between the group of patients that were analyzed for bile acid composition or not (93% vs 68%). In theory, inflammation in the colon can influence the intestinal absorption and bacterial degradation of UDCA. However, the currently available data show that no significant differences in the bile acid composition between PSC patients under UDCA treatment who have colitis or not were detected (27). The small number of patients without IBD included in our study (n=4) did not allow to perform a comprehensive statistical analysis in order to check this hypothesis.
In summary, we suggest that high-dose UDCA treatment results, as expected, in total bile acid expansion and significant UDCA enrichment in patients with PSC. LCA is markedly increased as well. Further studies are needed in order to fully understand UDCA induced liver damage in patients with PSC.
Acknowledgments
Financial support: This research was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK56924)
List of abbreviations
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- GCMS
gas chromatography-mass spectrometry
- LCA
lithocholic acid
- PSC
primary sclerosing cholangitis
- UDCA
ursodeoxycholic acid
- ULN
upper limit of normal
- IBD
inflammatory bowel disease
REFERENCES
- 1.Silveira M, Lindor K. Clinical features and management of primary sclerosing cholangitis. World J Gastroenterol. 2008;14(21):3338–3349. doi: 10.3748/wjg.14.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindor KD, Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group Ursodiol for primary sclerosing cholangitis. N Engl J Med. 1997;336(10):691–5. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 3.Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48(5):792–800. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96(5):1558–62. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SA, Bansi DS, Hunt N, Von Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121(4):900–7. doi: 10.1053/gast.2001.27965. [DOI] [PubMed] [Google Scholar]
- 6.Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129(5):1464–72. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batta AK, Salen G, Arora R, Shefer S, Tint GS, Abroon J, et al. Effect of ursodeoxycholic acid on bile acid metabolism in primary biliary cirrhosis. Hepatology. 1989;10(4):414–9. doi: 10.1002/hep.1840100404. [DOI] [PubMed] [Google Scholar]
- 9.Crosignani A, Podda M, Battezzati PM, Bertolini E, Zuin M, Watson D, et al. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991;14(6):1000–7. [PubMed] [Google Scholar]
- 10.Fedorowski T, Salen G, Tint GS, Mosbach E. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 1979;77:1068–1073. [PubMed] [Google Scholar]
- 11.Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La Rosa T, et al. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology. 1997;26(1):9–21. doi: 10.1002/hep.510260102. [DOI] [PubMed] [Google Scholar]
- 12.Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, et al. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168(2):410–22. doi: 10.2353/ajpath.2006.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rost D, Rudolph G, Kloeters-Plachky P, Stiehl A. Effect of high-dose ursodeoxycholic acid on its biliary enrichment in primary sclerosing cholangitis. Hepatology. 2004;40(3):693–8. doi: 10.1002/hep.20370. [DOI] [PubMed] [Google Scholar]
- 14.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129(2):476–85. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Marschall HU, Griffiths WJ, Götze U, Zhang J, Wietholtz H, Busch N, et al. The major metabolites of ursodeoxycholic acid in human urine are conjugated with N-acetylglucosamine. Hepatology. 1994;20:845–853. doi: 10.1002/hep.1840200412. [DOI] [PubMed] [Google Scholar]
- 16.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001;35(1):134–46. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 17.Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. Part II. Dig Dis Sci. 1982;27(9):833–56. doi: 10.1007/BF01391378. [DOI] [PubMed] [Google Scholar]
- 18.Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. part I. Dig Dis Sci. 1982;27(8):737–61. doi: 10.1007/BF01393771. [DOI] [PubMed] [Google Scholar]
- 19.Poupon RE, Chrétien Y, Poupon R, Paumgartner G. Serum bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid therapy. Hepatology. 1993;17(4):599–604. doi: 10.1002/hep.1840170412. [DOI] [PubMed] [Google Scholar]
- 20.Combes B, Carithers RL, Maddrey WC, Munoz S, Garcia-Tsao G, Bonner GF, et al. Biliary bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1999;29(6):1649–54. doi: 10.1002/hep.510290618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindor KD, Lacerda MA, Jorgensen RA, DeSotel CK, Batta AK, Salen G, et al. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93(9):1498–504. doi: 10.1111/j.1572-0241.1998.00470.x. [DOI] [PubMed] [Google Scholar]
- 22.van de Meeberg PC, Wolfhagen FH, Van Berge-Henegouwen GP, Salemans JM, Tangerman A, van Buuren HR, et al. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. J Hepatol. 1996;25(6):887–94. doi: 10.1016/s0168-8278(96)80293-5. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1–15. doi: 10.3109/00365529409103618. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph G, Kloeters-Plachky P, Sauer P, Stiehl A. Intestinal absorption and biliary secretion of ursodeoxycholic acid and its taurine conjugate. Eur J Clin Invest. 2002;32(8):575–80. doi: 10.1046/j.1365-2362.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- 25.Sauer P, Benz C, Rudolph G, Kloeters-Plachky P, Stremmel W, Stiehl A, et al. Influence of cholestasis on absorption of ursodeoxycholic acid. Dig Dis Sci. 1999;44(4):817–22. doi: 10.1023/a:1026686530785. [DOI] [PubMed] [Google Scholar]
- 26.Walker S, Rudolph G, Raedsch R, Stiehl A. Intestinal absorption of ursodeoxycholic acid in patients with extrahepatic biliary obstruction and bile drainage. Gastroenterology. 1992;102(3):810–5. doi: 10.1016/0016-5085(92)90162-r. [DOI] [PubMed] [Google Scholar]
- 27.Rost D, Rudolph G, Kloeters-Plachky P, Stiehl A. Effect of colitis and ileoanal pouch on biliary enrichment of ursodeoxycholic acid in primary sclerosing cholangitis. Dig Dis Sci. 2006;51(3):618–22. doi: 10.1007/s10620-006-3180-5. [DOI] [PubMed] [Google Scholar]
- 28.Trauner M, Fickert P, Halilbasic E, Moustafa T. Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases. Wien Med Wochenschr. 2008;158(19-20):542–8. doi: 10.1007/s10354-008-0592-1. [DOI] [PubMed] [Google Scholar]
- 29.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lambert F, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123(4):1238–51. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101(12):2790–9. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]