Abstract
The 70kDa ribosomal S6 kinase 1 (p70S6K1), a downstream target of phosphoinositide 3-kinase (PI3K) and ERK mitogen-activated protein kinase (MAPK), is an important regulator of cell cycle progression, and cell proliferation. Recent studies indicated an important role of p70S6K1 in PTEN negative and AKT-overexpressing tumors. However, the mechanism of p70S6K1 in tumor angiogenesis remains to be elucidated. In this study, we specifically inhibited p70S6K1 activity in ovarian cancer cells using vector-based small interfering RNA (siRNA) against p70S6K1. We found that knockdown of p70S6K1 significantly decreased VEGF protein expression and VEGF transcriptional activation through the HIF-1α binding site at its enhancer region. The expression of p70S6K1 siRNA specifically inhibited HIF-1α, but not HIF-1β protein expression. We also found that p70S6K1 down-regulation inhibited ovarian tumor growth and angiogenesis, and decreased cell proliferation and levels of VEGF and HIF-1α expression in tumor tissues. Our results suggest that p70S6K1 is required for tumor growth and angiogenesis through HIF-1α and VEGF expression, providing a molecular mechanism of human ovarian cancer mediated by p70S6K1 signaling.
Keywords: p70S6K1, VEGF, HIF-1, tumor angiogenesis, siRNA
Introduction
P70S6K1 is activated through PI3K [1] and MAPK pathways [2]. P70S6K1 is known to regulate cell growth by inducing protein synthesis. Activated PI3K and AKT can induce cell transformation through the phosphorylation and activation of p70S6K1 [3]. P70S6K1 activation is found in various human cancers such as thyroid cancer [4], breast cancer [5], and ovarian cancer [6]. P70S6K1 plays an important role in phosphatase and tensin homologue (PTEN) negative and in AKT-overexpressing tumors. PTEN is a molecular inhibitor of PI3K. PTEN+/− mice showed increased incidence of uterine and adrenal medullary tumors with decreased PTEN levels and p70S6K1 activation. Treatment of mice with the rapamycin analog CCI-779 led to a reduction in p70S6K1 activation and inhibition of tumor growth [7]. Tumor formation by PTEN-negative or by AKT-overexpressing human cancer cells in SCID mice was inhibited by CCI-779 [8]. These findings suggest that p70S6K1 phosphorylation is associated with PI3K/PTEN/AKT activation during tumorigenesis. Although p70S6K1 is well known to play an important role in cell proliferation and cell cycle progression, little is known about its role in angiogenesis.
Angiogenesis is the stimulation of growth of new vascular endothelial cells, and the development of new blood vessels. Tumor expansion cannot proceed beyond 1–2 mm without angiogenesis [9]. Angiogenesis is a crucial factor in the progression of solid tumors and metastasis [10–12]. VEGF is a potent angiogenesis inducer which is elevated by many human solid tumors; is especially high in ovarian tumor tissue, cystic and ascitic fluids, and in the serum of patients with epithelial ovarian cancer; and is a predictor of outcome in ovarian cancer patient survival [13–16]. VEGF expression is regulated at transcriptional level by hypoxia-inducible factor 1 (HIF-1) in response to hypoxia stress and growth factor stimulation.
HIF-1 consists of HIF-1α and HIF-1β subunits [17]. HIF-1α is degraded during normoxia while HIF-1β is constitutively expressed in the cells. Under normal conditions, HIF-1α protein synthesis is regulated by activation of PI3K [18], and MAPK pathways [19]. Recent studies suggest that mTOR, the downstream target of PI3K, increases HIF-1-dependent gene expression in certain cell types [20, 21]. MTOR is an evolutionarily conserved kinase which integrates signals from nutrients and growth factors to regulate cell growth and cell cycle progression [22]. In mammals, mTOR is best known to regulate translation through p70S6K1 and the eukaryotic translation initiation factor 4E-binding proteins (4E-BP1) [23]. The mTOR inhibitor rapamycin is effective against tumors resulting from aberrantly high PI3K signaling [24,25]. Rapamycin also reduced VEGF protein expression [24] and inhibited HIF-1 activity in hypoxia cells [25]. These results suggest that p70S6K1 may play a role in tumor angiogenesis. To test the role of p70S6K1 in regulating angiogenesis, p70S6K1 siRNA is used to specifically knockdown the expression of p70S6K1. In this study, we will determine: 1) whether p70S6K1 regulates VEGF expression at protein expression and transcriptional levels in ovarian cancer cells; 2) whether p70S6K1 mediates VEGF expression by regulating HIF-1α expression; 3) the roles of p70S6K1 in tumor-inducing angiogenesis and tumor growth in vivo.
Materials and Methods
Reagents and Cell Culture
The antibodies against p70S6K1 and phospho-p70S6K1 (Thr421/Ser424) were from Cell Signaling Technology (Beverly, MA). Matrigel and antibodies against HIF-1α and HIF-1β were from BD Biosciences (Franklin Lakes, NJ). The β-actin antibody was from Sigma (St. Louis, MO). The horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and anti-mouse IgG were from Perkin Elmer Life Sciences (Boston, MA). The human ovarian cancer cell lines OVCAR-3 and A2780 were from American Type Culture Collection (Manassas, VA). The cells were cultured in RPMI 1640 (GIBCO BRL, Grand Island, NY) supplemented with 10% FBS at 37°C in 5% CO2 incubator. Trypsin (0.25%)/EDTA solution was used to detach the cells from the culture plate for passing the cells.
SiRNA and Plasmid Constructs
The oligonucleotides encoding 19-mer hairpin sequences specific against p70S6K1 were designed using siRNA converter software. Small interfering RNA constructs were: sense strand siRNA: GUUCAAGCUCAUCCAUUCUuu, and antisense strand siRNA: AGAAUGGAUGAGCUUGAACuu. A negative scrambled control siRNA has the same nucleotide composition as p70S6K1 siRNA, but lacks significant sequence homology to any gene. The oligonucleotides are synthesized, annealed, and ligated into the linearized pSilencer 2.0-U6 vector (Ambion, Austin, TX). The plasmid that expresses siRNA against p70S6K1 was named as sip70S6K1. The negative control plasmid was named as scrambled control (scrambled ctrl). VEGF promoter reporter pGL-Stu1, pMAP11wt, and pMAP11mut were described previously [26]. Plasmid encoding human HIF-1α was inserted into pCEP4 vector.
Selecting a Population of Cells that Stably Express SiRNA
A2780 and OVCAR-3 cells were plated into 6-well plates. Sip70S6K or scrambled control plasmid at 2 µg was transfected into A2780 or OVCAR-3 cells using Lipofectamine (Invitrogen), and cultured for 24 h without antibiotics. Then the cells were cultured in medium containing 500 µg/ml G418 (Sigma) until all of non-transfected cells were death. The antibiotic-resistant cells were pooled together, and cultured.
Transient Transfection and Luciferase Assay
OVCAR-3 cells were seeded in 12-well plates, and cultured to 60–70% confluence. To determine the effects of p70S6K1 on transcriptional activation of VEGF, cells were transiently transfected with sip70S6K1 or scrambled control plasmid with VEGF reporter and pCMV-β-galactosidase (β-gal) plasmids using Lipofectamine according to the manufacturer’s instruction. The transfected cells were cultured for 16 h, washed once with phosphate-buffered saline (PBS), and lysed with 1× Reporter Lysis Buffer from Promega (Madison, WI, USA). Luciferase (Luc) activities of the cell extracts were determined using the Luciferase Assay System (Promega). β-gal activities were measured using assay buffer (100 mM phosphate, pH 7.5, 2 mM MgCl2, 100 mM β-mercaptoethanol, 1.33 mg/mL o-nitrophenyl β-D-galactopyranoside). Relative Luc activity (defined as VEGF reporter activity) was calculated as the ratio of Luc/β-gal activity.
Immunoblotting Analysis
Cells were cultured in a 60 mm dish at a density of 5× 105 cells/dish in RPMI 1640 medium with 10% FBS for 24 h, then harvested, and lysed on ice for 30 min in RIPA buffer [150 mM NaCl, 100 mM Tris (pH 8.0), 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5 mM EDTA, and 10 mM NaF], supplemented with 1 mM sodium vanadate, 2 mM leupeptin, 2 mM aprotinin, 1 mM phenylmethylsulfonylfluoride, 1 mM dithiothreitol, and 2 mM pepstatin A. After centrifugation at 12,000 rpm for 15 min, the supernatant was harvested as the total cellular protein extracts. The protein concentrations were determined using Bio-Rad protein assay reagent (Richmond, CA). The total cellular protein extracts were separated by SDS-PAGE, and transferred to nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk in PBS containing 0.05% Tween 20, and incubated with antibodies against HIF-1α, HIF-1β, p70S6K1, phospho-p70S6K1 (Thr421/Ser424), and β-actin.
Quantification of VEGF Protein Levels
VEGF protein was measured using the Quantikine human VEGF ELISA kit from R&D Systems (Minneapolis, MN, USA). The cells were seeded in 12-well plates, and cultured to 90–100% confluence. Cells were switched to fresh medium. After 16 h, the supernatants were collected, and the cell number of each well was counted. VEGF levels in the supernatant (100 µl) were determined and normalized to the cell numbers. A serial dilution of human recombinant VEGF was included in each assay to obtain a standard curve.
Chicken Chorioallantoic Membrane (CAM) Angiogenesis Assay
White Leghorn chicken eggs were fertilized, and incubated at 37°C with 70% humidity. An artificial air sac was created over a region containing small blood vessels in the CAM as described previously [27]. To investigate the effect of p70S6K1 on tumor-induced angiogenesis, OVCAR-3 cells expressing sip70S6K1 or scrambled control were trypsinized, washed, and resuspended at 1× 108 cells/ml in serum-free RPMI 1640, which contained 5% Matrigel (Collaborative Biomedical Products, Bedford, MA). Aliquots (20 µl) of the mixture (two million cells) were placed on the CAM of 9-day old chicken embryos. Tumors on the CAM were photographed 5 days after implantation. The experiments were repeated twice with ten embryos for each group, and representative fields were photographed. The relative blood vessel density was determined by measuring the number of blood vessels in a unit area on the CAM. Results are obtained from replicate experiments that are normalized to those of Matrigel alone control.
Immunohistochemistry
Tissues harvested from the CAM were fixed in 10% formaldehyde, and embedded in paraffin. Sections were deparaffinized in xyline and in a serial of alcohol, and rinsed three times in deionized water. The slides were boiled with antigen retrieval buffer (10 mM citrate, pH 6.0) for 10 min, cooled for 20 min, then rinsed three times in deionized water. The slides were incubated with 5% goat serum at room temperature for 1 h, and incubated with antibodies against VEGF (1:100), and HIF-1α (1:100) in a humid chamber at 4°C overnight. After three washes in 1× PBS, the slides were incubated for 1 h with goat anti-rabbit secondary IgG, and detected by the incubation with ABC reagent for 30 min at room temperature. Sections were rinsed several times in 1× PBS. The slides were added with 1 ml DAB reagent for 5–10 min, and washed with water once. The slides were counterstained with hemotoxylin and washed for several times with water.
Statistical Analysis
The data was analyzed using SPSS statistics software package (SPSS, Chicago, IL). All of the results were expressed as mean ± SD, and the difference was considered significant at P< 0.05.
Results
Expression of siRNA against p70S6K1 (sip70S6K1) inhibited VEGF protein expression and transcriptional activation
VEGF plays an important role in human tumorigenesis and angiogenesis. However, the mechanism of its elevation in human ovarian cancer cells still remains to be elucidated. To determine the role of p70S6K1 in regulating VEGF protein level, we establish the OVCAR-3 and A2780 stable cell lines that express sip70S6K1 or scrambled control. The VEGF protein levels in the medium of cells expressing sip70S6K1 or scrambled control were determined by ELISA. To exclude the effect of cell proliferation on the expression of VEGF protein, we normalized the VEGF protein concentrations to the total cell number. Sip70S6K1 expression significantly decreased VEGF protein levels in both OVCAR-3 (Fig.1A) and A2780 (data not showed) cells.
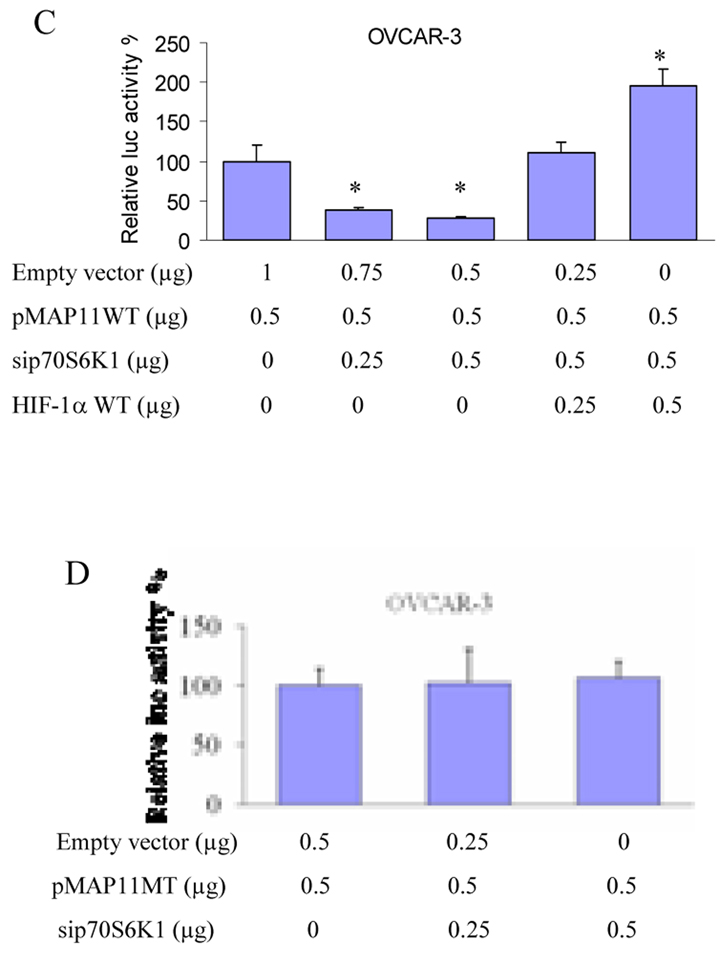
Fig. 1.
Knockdown of p70S6K1 inhibited VEGF protein expression and VEGF transcriptional activity. (A) OVCAR-3 cells stably expressing p70S6K1 siRNA (sip70S6K1) or scrambled control (scrambled ctrl) were cultured to 100% confluence. The cells were changed to fresh complete medium. Aliquots of cell supernatant were analyzed for VEGF protein levels by ELISA. (B) Cells were seeded in 12 well plates and cultured to 40–50% confluence, then transfected with VEGF promoter reporter pGL-Stu1, pCMV-β-galactosidase plasmid (β-gal), sip70S6K1 and wild-type HIF-1α plasmids at the concentrations as indicated. The empty vector was added to make total transfected DNA to 1.15 µg. Relative Luc activity was calculated as the ratio of Luc/β-gal activity. (C) The similar experiment was performed using pMAP11wt reporter as described for pGL-Stu1 reporter. (D) The pMAP11 mutant reporter was used to perform the similar experiment. *, indicates the significant difference (p<0.05).
To further determine the mechanism of p70S6K1 in regulating VEGF expression, the cells were transfected with sip70S6K1, a VEGF promoter reporter containing 2.6-kb human VEGF promoter, and β-gal plasmids. Sip70S6K1 expression inhibited VEGF transcriptional activation (Fig. 1B). To confirm the above results, we also analyzed the effect of sip70S6K1 expression on a short form of VEGF promoter reporter, pMAP11wt which contains a functional promoter fragment with the HIF-1 binding site. The OVCAR-3 cells and A2780 cells were co-transfected with pMAP11wt reporter and sip70S6K1 construct. The cells co-transfected with pMAP11wt reporter and scrambled control were used as a control. Sip70S6K1 expression significantly decreased pMAP11wt reporter activity (Fig. 1C). Overexpression of HIF-1α reversed VEGF reporter activity inhibited by expression of sip70S6K1 (Fig. 1C), suggesting that sip70S6K1 inhibits VEGF transcriptional activation via HIF-1α expression. To test whether the inhibitory effect of sip70S6K1 on VEGF transcriptional activity requires the HIF-1 binding site, we employed pMAP11mut VEGF promoter reporter which has 3-bp mutation at the HIF-1 binding site and Luc activity was performed as above. We found that sip70S6K1 expression did not inhibit the pMAP11mut VEGF reporter activity (Fig.1D). This result further demonstrated that p70S6K1 regulates VEGF transcriptional activation through the HIF-1 DNA binding site.
Sip70S6K1 expression inhibited HIF-1α expression
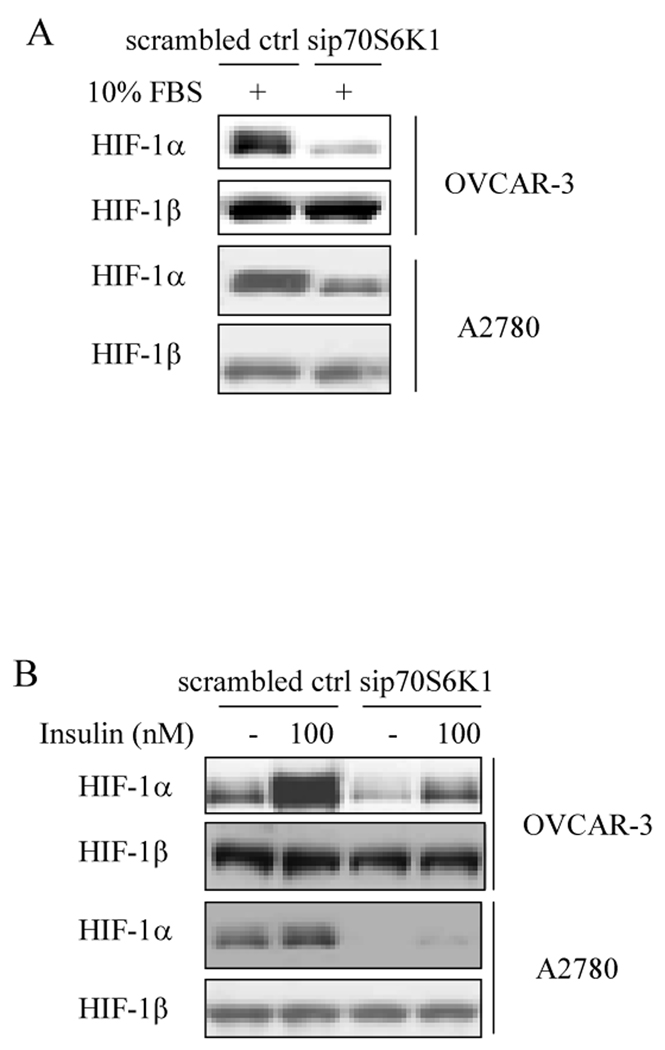
Next, to determine the effect of p70S6K1 knockdown on HIF-1α protein expression, the ovarian cancer cells that stably express sip70S6K1 or scrambled control were cultured in complete medium for 24 h or in serum-free medium for 24 h, then treated with insulin. Total proteins were analyzed by immunoblotting using antibodies against HIF-1α and HIF-1β proteins. Sip70S6K1 expression decreased HIF-1α protein level in complete medium compared to that in scrambled control cells (Fig. 2A). Insulin induced HIF-1α, but not HIF-1β expression in the control cells, while sip70S6K1 expression abolished insulin-inducing HIF-1α protein expression (Fig. 2B). These results indicate that sip70S6K1 inhibits HIF-1α protein expression.
Fig. 2. Sip70S6K1 expression inhibited HIF-1α protein expression in ovarian cancer cells.
(A) OVCAR-3 cells and A2780 cells stably expressing sip70S6K1 or scrambled control were seeded in RPMI 1640 medium with 10% FBS for 24 h. The total cellular protein extracts were prepared, and subjected to immunoblotting analysis using specific antibodies against HIF-1α and HIF-1β. (B) The cells were cultured in a 60 mm dish at a density of 5×105 cells/dish for 24 h, followed by the incubation with serum-free medium for 24 h. Cells were switched to the medium in the presence or absence of 100 nM insulin for 6 h as indicated. The total cellular proteins were prepared and subjected to immunoblotting analysis using antibodies against HIF-1α and HIF-1β.
Sip70S6K1 expression inhibited tumor-inducing angiogenesis and tumor growth
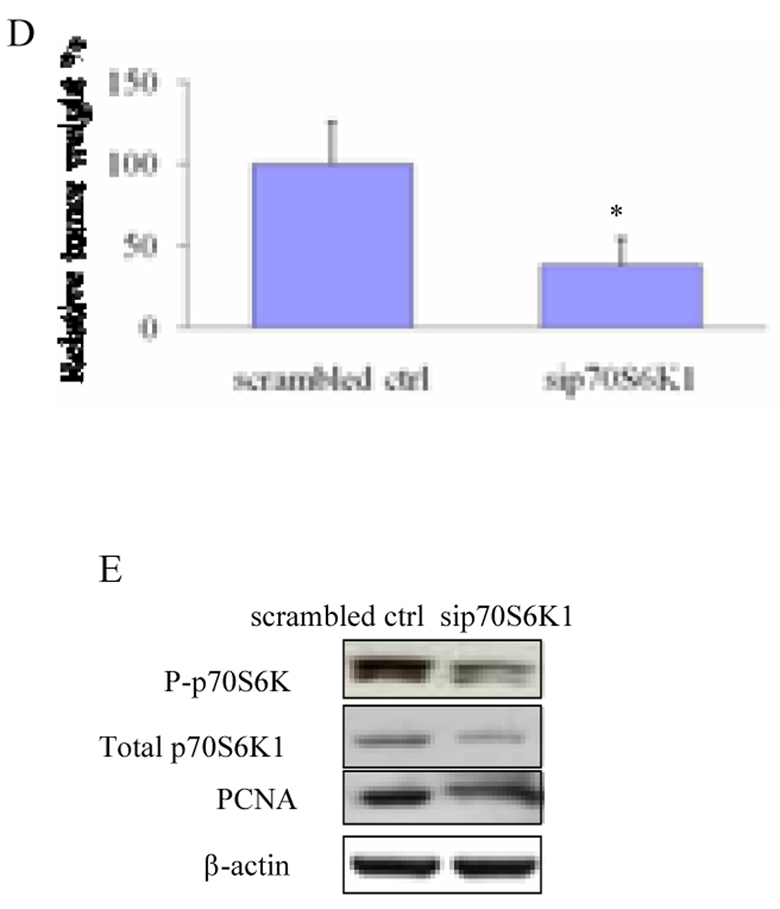
Then, to test whether specific inhibition of p70S6K1 affects tumor-inducing angiogenesis in vivo, OVCAR-3 cells stably expressing sip70S6K1 or scrambled control were mixed with Matrigel, and implanted onto the CAM. OVCAR-3 cells greatly induced angiogenesis when compared to the negative alone on the CAM, while sip70S6K1 expression inhibited angiogenesis by 60% reduction (Figs. 3A and 3B). To test the effect of sip70S6K1 expression on tumor growth, OVCAR-3 cells stably expressing sip70S6K1 or scrambled control were used to study tumor growth and angiogenesis. Tumor weight generated from sip70S6K1-expressing group was only 25% of that in the control (Fig. 3D). The effect of sip70S6K1 in vivo was confirmed by testing the expression of phospho-p70S6K1 and total p70S6K1 in the tumor tissues, showing that sip70S6K1 significantly inhibited phospho-p70S6K1 and total p70S6K1 expression (Fig.3E). PCNA is a nuclear cell proliferation marker. To study whether sip70S6K1 expression inhibited cell proliferation in the tumor tissues, PCNA levels were determined by immunoblotting in tumor tissues. A high amount of PCNA was observed in the control tumors while the knockdown of p70S6K1 greatly decreased the PCNA expression, indicating that p70S6K1 knockdown inhibited cell proliferation in vivo (Fig. 3E). Sip70S6K1 expression decreased VEGF and HIF-1α expression in tumors (data not showed), suggesting that sip70S6K1 also specifically inhibits HIF-1α and VEGF expression in vivo. These results demonstrate that sip70S6K1 expression inhibits ovarian cancer cell-inducing angiogenesis and tumor growth through HIF-1α and VEGF expression.
Fig. 3. Sip70S6K1 expression inhibited ovarian tumor angiogenesis and tumor growth in CAM model.
(A) Two million of OVCAR-3 cells expressing sip70S6K1 or scrambled control were collected in serum-free medium and mixed with the equal volume of Matrigel. The mixture or the Matrigel alone was placed on the CAM of 9 days old chicken embryos. Angiogenesis of tumors in the CAM was photographed 5 days after implantation. The experiments were repeated twice with five embryos for each group and representative fields were photographed. (B) The relative blood vessel density was determined by measuring the number of blood vessels in a unit area on Day 5. Results are presented as mean ± SD from replicate experiments, and normalized to results obtained with the CAMs implanted by Matrigel alone. (C) The cells were treated and implanted onto CAM as above. The tumors were harvested after incubation for 9 days. The tumors were photographed and weighed. (D) Tumor weight was obtained from ten tumors in different embryos in each treatment. (E) Parts of tumor tissues from chicken embryos were frozen in liquid nitrogen, and total proteins were extracted and analyzed. Immunoblotting analysis was performed using antibodies against PCNA, phospho-p70S6K1 (Thr421/Ser424), total p70S6K, and β-actin. *, indicates the significant difference (p<0.05).
Discussion
P70S6K1 is known to play an important role in cell proliferation and cell cycle progression. Growth factors activate p70S6K1 through the activation of PI3K and AKT in response to mitogenic stimulation [28]. Inhibition of mTOR activity by rapamycin resulted in G1 arrest in ovarian cancer cells and inhibition of tumor growth [29, 30]. Rapamycin also showed antiangiogenic effects in mouse model in vivo [30]. However, there is no direct evidence to show the role of p70S6K1 in tumor growth and angiogenesis. VEGF is overexpressed in most human tumors including ovarian cancer for inducing angiogenesis and tumor growth. In this study, we showed that knockdown of p70S6K1 by siRNA inhibited VEGF protein level in human ovarian cancer cells. VEGF expression is regulated through at least three mechanisms including gene transcription, translational activation, and mRNA stabilization. To investigate the mechanism of p70S6K1-mediated VEGF expression, we used VEGF promoter-reporter constructs to prove that p70S6K1 regulates VEGF expression through increasing its transcriptional activation, indicating that p70S6K1 may be involved in angiogenesis.
The transcriptional regulation of VEGF may be mediated by HIF-1 in response to hypoxia [26]. Recently, growth factors have been shown to increase expression of HIF-1α through PI3K signaling pathway [31–34]. To further determine the mechanism of p70S6K1 knockdown in regulating VEGF expression, we demonstrated that p70S6K1 regulates VEGF transcriptional activation through its HIF-1α binding site and HIF-1 protein expression.
Consistent with the results in vitro, we also found that p70S6K1 is required for ovarian tumor growth and angiogenesis in vivo by suppressing VEGF and HIF-1α expression. Taken together, this study demonstrates that p70S6K1 is required for tumor growth and angiogenesis through VEGF and HIF-1α expression both in vitro and in vivo. This novel finding provides a potential mechanism by targeting p70S6K1 for human ovarian cancer therapy in the future.
Research Highlights.
P70S6K1 regulates VEGF expression;
P70S6K1 induces transcriptional activation through HIF-1α binding site;
P70S6K1 regulates HIF-1α, but not HIF-1β protein expression;
P70S6K1 mediates tumor growth and angiogenesis through HIF-1α and VEGF expression.
Acknowledgment
This work was supported by the National Major Fundamental Research Program of China Grant 2007CB947002; by Grants 30871296 and 30570962 from National Natural Science Foundation of China; and by Grant R01CA109460 from NCI, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv. Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 3.Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. U.S.A. 2001;98:136–141. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr. J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- 5.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 2010;5:29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 6.Trinh XB, Tjalma WA, Vermeulen PB, Van den EG, I Van dA, Van Laere SJ, Helleman J, Berns EM, Dirix LY, van Dam PA. The VEGF pathway and the AKT/mTOR/p70S6K1 signalling pathway in human epithelial ovarian cancer. Br. J. Cancer. 2009;100:971–978. doi: 10.1038/sj.bjc.6604921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, Frost P, Dreisbach V, Blenis J, Gaciong Z, Fisher P, Sawyers C, Hedrick-Ellenson L, Parsons R. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkman J. What is the evidence that tumors are angiogenesis dependent? J.Natl.Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 11.Sharma RA, Harris AL, Dalgleish AG, Steward WP, O'Byrne KJ. Angiogenesis as a biomarker and target in cancer chemoprevention. Lancet Oncol. 2001;2:726–732. doi: 10.1016/S1470-2045(01)00586-1. [DOI] [PubMed] [Google Scholar]
- 12.Granci V, Dupertuis YM, Pichard C. Angiogenesis as a potential target of pharmaconutrients in cancer therapy. Curr.Opin.Clin.Nutr.Metab Care. 2010 doi: 10.1097/MCO.0b013e3283392656. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Wang L, Zhang W, Tang B, Zhang J, Song H, Yao D, Tang Y, Chen X, Yang Z, Wang G, Li X, Zhao J, Ding H, Reed E, Li QQ. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004;24:1973–1979. [PubMed] [Google Scholar]
- 14.Chen CA, Cheng WF, Lee CN, Chen TM, Kung CC, Hsieh FJ, Hsieh CY. Serum vascular endothelial growth factor in epithelial ovarian neoplasms: correlation with patient survival. Gynecol.Oncol. 1999;74:235–240. doi: 10.1006/gyno.1999.5418. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Maity P. Isolation and purification of vascular endothelial growth factor (VEGF) from ascitic fluid of ovarian cancer patients. Pathol.Oncol.Res. 2004;10:104–108. doi: 10.1007/BF02893464. [DOI] [PubMed] [Google Scholar]
- 16.Diniz Bizzo SM, Meira DD, Lima JM, Mororo JS, Casali-da-Rocha JC, Ornellas MH. Peritoneal VEGF burden as a predictor of cytoreductive surgery outcome in women with epithelial ovarian cancer. Int.J.Gynaecol.Obstet. 2010;109:113–117. doi: 10.1016/j.ijgo.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, Yancopoulos GD, Jaffe RB. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin.Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- 18.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 19.Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol.Biol.Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 21.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol.Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 23.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 24.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 25.Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, Frost P, Gibbons J, Lichtenstein A. A. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol.Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc.Natl.Acad.Sci.U.S.A. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumont FJ, Su Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996;58:373–395. doi: 10.1016/0024-3205(95)02233-3. [DOI] [PubMed] [Google Scholar]
- 30.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, runs BCJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat.Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 31.Eliceiri BP, Klemke R, tromblad SS, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J.Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 33.Burroughs KD, Oh J, Barrett JC, DiAugustine RP. Phosphatidylinositol 3-kinase and mek1/2 are necessary for insulin-like growth factor-I-induced vascular endothelial growth factor synthesis in prostate epithelial cells: a role for hypoxia-inducible factor-1? Mol.Cancer Res. 2003;1:312–322. [PubMed] [Google Scholar]
- 34.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J.Biol.Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]