Abstract
This study describes the stability and selectivity of four-contact spiral nerve-cuff electrodes implanted bilaterally on distal branches of the femoral nerves of a human volunteer with spinal cord injury as part of a neuroprosthesis for standing and transfers. Stimulation charge threshold, the minimum charge required to elicit a visible muscle contraction, was consistent and low (mean threshold charge at 63 weeks post-implantation: 23.3 ± 8.5 nC) for all nerve-cuff electrode contacts over 63 weeks after implantation, indicating a stable interface with the peripheral nervous system. The ability of individual nerve-cuff electrode contacts to selectively stimulate separate components of the femoral nerve to activate individual heads of the quadriceps was assessed with fine-wire intramuscular electromyography while measuring isometric twitch knee extension moment. Six of eight electrode contacts could selectively activate one head of the quadriceps while selectively excluding others to produce maximum twitch responses of between 3.8 and 8.1 Nm. The relationship between isometric twitch and tetanic knee extension moment was quantified, and selective twitch muscle responses scaled to between 15 and 35 Nm in tetanic response to pulse trains with similar stimulation parameters. These results suggest that this nerve-cuff electrode can be an effective and chronically stable tool for selectively stimulating distal nerve branches in the lower extremities for neuroprosthetic applications.
1. Introduction
Stimulation of the peripheral nerves has been shown to be both an important therapeutic intervention and a means of restoring function to a damaged or pathologic nervous system [1-3]. In restoring function to individuals with spinal cord injury (SCI), functional electrical stimulation (FES) of the peripheral nervous system has been particularly effective at reducing some of the negative secondary physiological effects of the injury, as well as in returning motor function to paralyzed individuals [4-6]. Many of these FES systems have utilized muscle-based electrodes which are placed near the peripheral nerve at the location it enters the muscle. While these systems have been successful clinically, they require the placement of at least one electrode at the motor point of each target muscle, and, because of the electrode geometry and distance from the nerve, often require sizable currents (on the order of 20 mA) to produce functionally useful muscle contractions [6]. As neuroprosthetic applications become more and more complex, the time required to implant these types of systems and the power necessary to drive them may become prohibitive.
Nerve-cuff electrodes, which wrap around the nerve at more proximal locations, may provide a means of overcoming these limitations. Because of their intimate contact with the nerve, these electrodes require much lower current amplitudes to cause muscle contractions [7-9]. Furthermore, because they can be implanted around the nerve proximal to branching points, individual nerve-cuff electrodes can recruit multiple muscles [10-13]. In order for this to occur effectively for neuroprosthetic applications, however, the electrodes must either be placed in specific anatomical locations proximal to branching points for synergistic muscles and distal to branches for undesired muscles, or they must selectively stimulate the various motor neurons within the nerve to selectively stimulate desired muscles while excluding undesired ones.
The Case Western Reserve University (CWRU) self-sizing spiral nerve-cuff electrode (Figure 1) takes this second approach [14-16]. The cuff, which wraps twice around the nerve, has four contacts designed to be spaced equally around the circumference of the nerve. It has been shown in the cat sciatic nerve and in the human optic nerve that a single cuff can selectively recruit multiple independent populations of neurons to produce a variety of graded responses in both sensory and motor systems [12, 17]. Additionally, it has recently been shown that these electrodes could be implanted on nerves in the upper extremities of humans with SCI to selectively restore hand and arm motor function [18]. There has not been a report to date, however, on the ability of the CWRU nerve-cuff electrode to selectively recruit the muscles of the lower extremities for FES applications such as standing and transferring after SCI. We have recently presented results of the use of these nerve-cuff electrodes in stimulating the femoral nerves non-selectively for complete recruitment of the quadriceps for knee extension during standing and transfers [19]. In that study, we found that nerve-cuffs wrapped around the femoral nerves were better able to maintain knee extension during standing than muscle-based electrodes on vastus lateralis, but we did not investigate the ability of the cuffs to selectively stimulate the individual heads of the quadriceps. This paper reports results derived from a second, independent recipient of multi-contact spiral nerve-cuffs in whom we tested the hypothesis that any one contact of the nerve-cuff electrodes can provide a chronically stable interface to selectively recruit separate heads of the quadriceps at levels high enough to be functionally useful during standing.
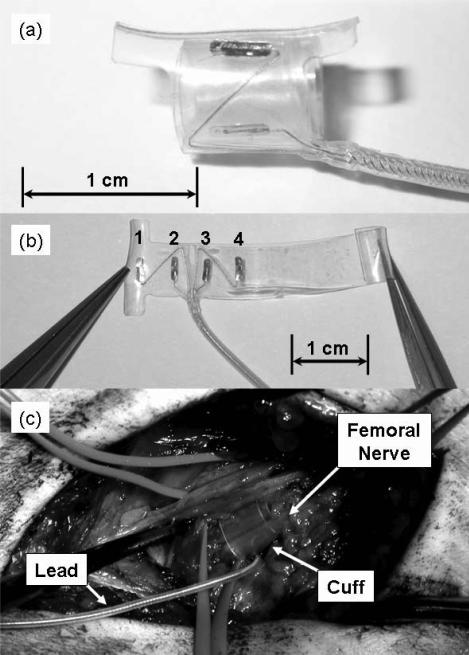
Figure 1.
(a.) The CWRU spiral nerve-cuff electrode. The electrode is designed to wrap twice around the nerve so that four contacts are equally spaced around the circumference. (b.) The unspiraled cuff with labels for contacts 1, 2, 3, and 4. (c.) A spiral nerve-cuff around the femoral nerve. The cuff is placed distal to nerve branches that innervate rectus femoris and sartorius, but proximal to branches that innervate the vasti.
2. Methods
2.1. Subject selection
Two CWRU self-sizing spiral nerve-cuff electrodes were implanted bilaterally around branches of the femoral nerves in one male volunteer with chronic SCI. The participant (age: 43, 11 years post-injury) had a motor-complete, C6 level, ASIA B injury. Informed consent was acquired prior to the subject's participation in the study and all experimental protocols were approved by the Institutional Review Board of MetroHealth Medical Center, Cleveland OH, under an active Investigational Device Exemption from the US Food and Drug Administration.
2.2. Nerve-cuff electrodes and implanted stimulator
Two four-contact self-sizing spiral nerve-cuff electrodes were implanted on bilateral femoral nerves (Figure 1,c) distal to branches for rectus femoris (RF) and sartorius (SART), both of which generate hip flexion, an undesirable muscle action for standing. During implantation, the femoral nerve dimensions (typically between 1 and 1.5 mm tall and 8 and 10 mm wide) were measured and cuffs with an appropriate spiraled diameter were chosen to fit snugly around the nerve. Intraoperative electromyography (EMG) testing was performed during implantation of the nerve-cuff electrodes to ensure, as best as possible, that the cuffs contained all branches of the femoral nerve innervating vastus lateralis (VL), vastus medialis (VM), and vastus intermedius (VI), which all extend the knee, but few if any branches to the hip flexor muscles. Each of the four electrode contacts (surface area: 0.456 mm2) in each nerve-cuff was connected to a separate and independently variable stimulation channel from a CWRU 16-channel implanted stimulator-telemeter (IST-16) capable of producing constant-current, charge-balanced biphasic stimulus pulses with amplitudes of 0.1, 0.8, 1.4 and 2.1 mA and pulse widths ranging from 1 to 255 μs in 1 μs intervals with a compliance voltage of 33 V [20, 21]. The remaining eight stimulation channels were connected to muscle-based electrodes implanted in bilateral erector spinae, gluteus maximus, semimembranosus, and the posterior portion of adductor magnus as part of a CWRU standing/transfer system [5, 6, 22]. The titanium case of the IST-16, which was sutured to the abdominal fascia at to the left of the umbilicus, served as the common return electrode. After implantation, the participant underwent a six week recovery period followed by an eight week reconditioning exercise program designed to build both strength and endurance. Details of the reconditioning program have been described elsewhere [5, 22, 23]. Custom patterns of stimulation to the nerve-cuff contacts as well as the muscle-based electrodes in the hip and trunk extensors were defined to achieve the sit-to-stand transition and provide static upright standing.
2.3. Evaluation of nerve-cuff stimulation charge threshold stability
If a peripheral nerve interface for FES is to be successfully implemented chronically, it is necessary that the response to stimulation via that interface be reliable and repeatable. To test the day-to-day reliability of the nerve-cuff interface and to determine if any undesirable electrophysiological or immune response effects such as electrochemical degradation or excessive scarring were occurring at the electrode interface, the minimum charge required to elicit a muscle response was measured at multiple time points after implantation. At each interval, a physical therapist performed a stimulated manual muscle test, in which stimulus current amplitude and pulse width were varied while quadriceps muscle response was recorded. Using these data, stimulation charge threshold was defined as:
| (1) |
where Qth is the minimum charge in nC from a nerve-cuff contact required to generate a visible muscle contraction, I is the stimulation pulse current amplitude (0.8, 1.4, or 2.1 mA), and PWth is the threshold pulse width in μs. Charge threshold was calculated at every time interval for each contact in both left and right femoral nerve-cuff electrodes, and a one-way analysis of variance (ANOVA) was performed to determine if there was a statistically significant change in stimulation threshold at any time after implantation for each contact.
2.4. Selectivity of stimulation
In order to quantify selectivity of the nerve-cuff electrodes, EMG signals were recorded from VL, VM, VI, RF, and SART, along with isometric knee extension moment while single stimulus pulses were applied to the nerve via each of the four electrode contacts. During all EMG and moment recordings, pulse width modulated twitch recruitment curves were generated by varying pulse width between 1 and 255 μs using a binary search adaptive sampling algorithm at a current amplitude of 1.4 mA. Stimulus pulses were randomly applied to one of the four electrode contacts within a nerve-cuff at a frequency of 0.25 Hz to avoid fatigue effects. Three data points were collected for each pulse width, and means and standard deviations were calculated. Selectivity of each cuff contact was quantified as the maximum twitch knee extension moment that was generated when only one of the recorded EMG signals was superthreshold. Threshold was defined as 10% of the maximal EMG signal elicited from each muscle, and a Student's t-test was used to determine if an EMG response was superthreshold. Single stimulus pulses were used rather than trains of pulses because measurements could be performed more quickly with single pulses with less chance of causing muscle fatigue.
2.4.1. EMG Recordings
Paired fine-wire intramuscular electrodes (Viasys Neurocare, Conshohocken, PA) were used to acquire EMG signals (Figure 2,a). Differential recording electrode pairs with a tip-to-tip separation of 2 mm were inserted at the beginning of each experimental session via a hypodermic needle by a neurosurgeon according to accepted anatomical descriptions, and a brief cathodic stimulus pulse train was applied between each of the recording electrodes and a distant anode to ensure proper placement in the desired muscle [24, 25]. During recordings, EMG data were blanked for 3 ms to avoid saturation of the recording apparatus by any stimulus artifact, and then amplified and band-pass filtered between 10 and 1000 Hz using isolation amplifiers (CED, Cambridge). Signals were sampled, digitized, and recorded at 2400 Hz with a National Instruments DAQ card and custom LabVIEW software (National Instruments, Austin, TX).
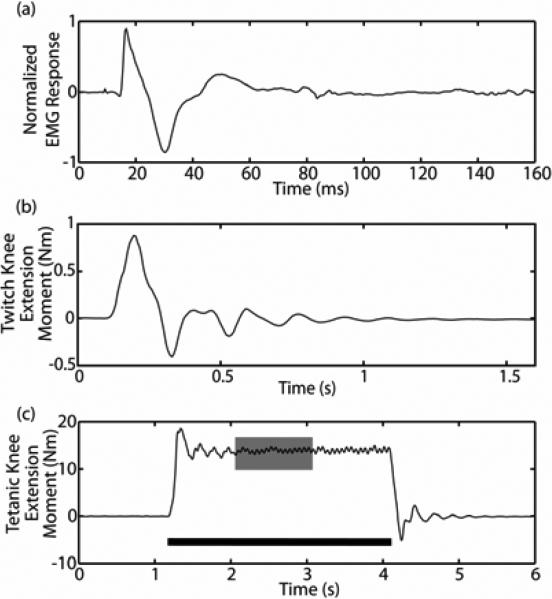
Figure 2.
(a.) An example of a typical normalized fine-wire intramuscular EMG response to a single stimulus pulse delivered via a nerve-cuff electrode contact. The triphasic M-wave response to stimulation occurs within the first 40 ms after stimulation (which occurs at t=0 s). (b.) A typical twitch knee extension moment response to a single stimulus pulse applied at t=0 s. The maximum of the response is calculated to create twitch recruitment curves. (c.) A typical tetanic knee extension moment response to a 3 second, 16 Hz train of stimuli (black bar). The middle one second of the three second response (gray box) is averaged to create tetanic recruitment curves.
The triphasic M-wave EMG response to stimulation, which generally occurs within 40 ms after stimulation, is a result of the synchronized propagation of action potentials along muscle fibers in response to a stimulus pulse [26]. M-wave data were rectified and integrated over a 35 ms window starting 5 ms after the application of a stimulus pulse because this ensured inclusion of the entire M-wave but avoided any reflex responses. EMG data for each muscle were normalized by the maximum EMG response measured from that muscle in response to a relatively high charge stimulation pulse (535 nC) from all four electrode contacts. There was a delay of approximately 1 ms between stimulus pulses from individual contacts because the implanted stimulator, which includes a single current source with a multiplexed output, cannot stimulate two channels simultaneously.
2.4.2. Isometric twitch knee extension moment
Isometric twitch knee extension moment (Figure 2,b) was recorded using a 6 DOF load cell (JR3, Woodland, CA) and software written in LabVIEW (National Instruments, Austin, TX) and Matlab (Mathworks, Natick, MA). The knee was held in 20° of flexion and one axis of the load cell was aligned with the knee joint center of rotation. Knee extension moment data were low-pass filtered at 31.25 Hz, and sampled at 150 Hz, and the maximum extension moment in response to each stimulus pulse was recorded.
2.5. Twitch/tetany relationship
While single pulse muscle twitches are useful for quickly collecting data about electrode selectivity and determining muscle recruitment properties while avoiding fatigue, they provide little functional information. Trains of stimulus pulses, rather than single pulses, are used to generate tetanic contractions during standing with a neuroprosthesis. In order to understand, in a functionally relevant sense, the degree to which nerve-cuff electrodes can selectively recruit the vasti, it is important to quantify the relationship between muscle responses to single stimulus pulses and muscle responses to trains of stimulus pulses. Durfee and MacLean previously showed that there is an approximately linear relationship between the magnitude of isometric muscle force in isolated feline muscle in response to single stimulus pulses and trains of stimulus pulses when similar stimulation parameters are used [27]. Their evidence showed that, for a given muscle, the shape of twitch and tetanic recruitment curves are essentially the same, but with a linear scaling factor accounting for the difference in magnitude between them [27]. Because we recorded isometric knee extension moment, the moment arm about the knee was constant throughout collection of recruitment curves, and this linear relationship should still hold true. To ensure that this was the case and to determine a scaling factor between isometric twitch and tetanic knee extension moment (Figure 2,c) recruitment curves, we collected both twitch and tetanic isometric recruitment curves in one experimental session. Twitch recruitment curves were collected using the procedures described above. Tetanic curves were collected with the knee in 20° of flexion with stimulus pulses applied at a frequency of 16 Hz (which is the frequency of stimulation used during standing with the neuroprosthesis), current amplitude of 1.4 mA, and pulse widths of 50, 100, 150, 200, and 250 μs. A stimulation duty cycle of 1:5 (3 seconds on: 15 seconds off) was used, and tetanic knee extension moment data were low-pass filtered at 31.25 Hz, sampled at 150 Hz, and averaged over the middle one second of stimulation. Three data points were collected at each pulse width and means and standard deviations were calculated.
3. Results
3.1. Nerve-cuff stimulation charge threshold stability
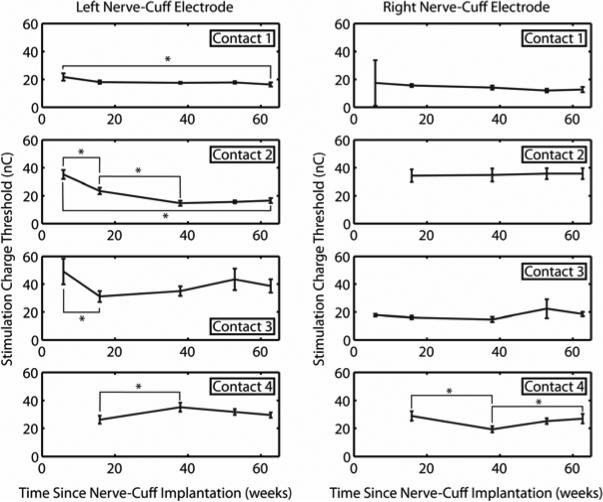
Mean stimulation charge threshold for all four contacts of both nerve-cuff electrodes are presented in Figure 3. Error bars represent standard deviation. At week 6, it was found that contacts 2 and 4 of the right electrode and contact 4 of the left electrode did not respond to stimulation, but at week 16 typical stimulation responses returned. This phenomenon commonly occurs early after implantation as a result of air bubbles covering the electrode contacts which later dissipate. Week 6 data were, therefore, not included in analyses of stimulation charge thresholds for these three contacts. Based on the results of a one-way ANOVA, stimulation charge thresholds were stable across all eight electrode contacts over the course of 63 weeks post-implantation. From week 6 to week 63, there were statistically significant decreases in threshold for contacts 1 and 2 (p<0.05) of the left electrode. There were statistically significant fluctuations in stimulation charge threshold for contacts 3 and 4 of the left electrode and contact 4 of the right electrode, but there were no statistically significant differences in the thresholds for any of these contacts between week 6 and week 63. The mean stimulation threshold across all eight contacts at the 63rd week was 23.3 ± 8.5 nC, which is similar to the range reported by Polasek, et al. (25 ± 17 nC) when CWRU self-sizing spiral nerve-cuff electrodes were acutely tested on the radial, ulnar, median, and axillary nerves of the upper extremities of human volunteers [18].
Figure 3.
Average stimulation charge threshold for bilateral nerve-cuff electrodes. Thresholds were measured three times, averages and standard deviations were calculated, and a one-way ANOVA was performed. Brackets and asterisks (*) denote statistically significant differences (p<0.05). Stimulation charge threshold decreased for left nerve-cuff contacts 1 and 2 and stayed constant or fluctuated but returned to original values for all other contacts over 63 weeks after nerve-cuff implantation. Note that week 6 data were unavailable for left contact 4, and right contacts 2 and 4. Also, data from weeks 6 and 16 for left nerve-cuff contact 2 were significantly different from all other data points for that electrode, but some brackets were omitted from the figure for clarity.
3.2. EMG and isometric twitch knee extension moment
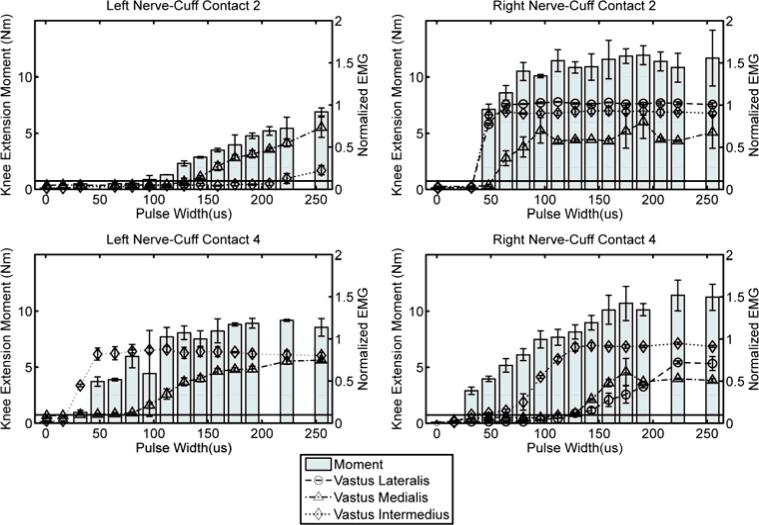
Shown in Figure 4 are four examples of pulse width modulated recruitment curves, collected from left nerve-cuff contacts 2 and 4 and right nerve-cuff contacts 2 and 4. Lines represent normalized EMG data (right axes) and bars represent isometric twitch knee extension moment data (left axes). Summary selectivity data, including the maximum pulse width that allowed selective recruitment of one muscle, as well as percent of maximal muscle activation for the selected muscle and twitch moment produced by stimulating at that pulse width, are presented for all eight nerve-cuff contacts in Table I. Data for RF and SART are omitted because neither muscle showed any response to stimulation during EMG recordings, confirming that the cuffs were located distal to branches of the femoral nerve that innervate those muscles. It should be noted that VL EMG data have been omitted for all left-nerve cuff contacts because it was found that the EMG signal was intermittently lost during experiments, possibly as a result of fine-wire intramuscular electrode movement relative to the active muscle fibers.
Figure 4.
Mean twitch EMG and isometric knee extension moment recordings when single stimulus pulses were applied three times via left nerve-cuff contacts 2 and 4 and right nerve-cuff contacts 2 and 4. Error bars are standard deviation. The horizontal black line on each plot marks 10% of normalized EMG, which is considered threshold for activation. All stimulus pulses were applied at a current amplitude of 1.4 mA.
Table 1.
Nerve-Cuff Selectivity
| Nerve-Cuff Contact | Maximum Selective Pulse Width (μs) | Muscle Selected | % Activation of Selected Muscle at Maximum Selective Pulse Width | Maximum Selective Twitch Knee Extension Moment (Nm) | Twitch/Tetany Scaling Factor | Estimated Selective Tetanic Knee Extension Moment (Nm) |
|---|---|---|---|---|---|---|
| R1 | 48 | VI | 94.2% | 8.16 | 2.53 | 20.64 |
| R2 | Not Selectivea | Not Selectivea | Not Selectivea | Not Selectivea | 3.76 | Not Selectivea |
| R3 | 80 | VI | 34.2% | 6.77 | 3.48 | 23.56 |
| R4 | 112 | VI | 76.3% | 7.65 | 4.57 | 34.96 |
| L1 | Not Selectivea | Not Selectivea | Not Selectivea | Not Selectivea | 2.61 | Not Selectivea |
| L2 | 223 | VM | 54.9% | 5.45 | 2.76 | 15.04 |
| L3 | 143 | VI | 20.8% | 4.44 | 4.33 | 19.23 |
| L4 | 64 | VI | 83.1% | 3.87 | 3.99 | 15.44 |
“Not selective” refers to contacts that did not recruit any one muscle above threshold without recruiting at least one other muscle above threshold.
Based on these data, 6 of 8 nerve-cuff contacts selectively stimulated one muscle while selectively excluding others. Specifically, three right nerve-cuff contacts selectively activated VI while avoiding other muscles, one left nerve-cuff contact selectively activated VM, and two left nerve-cuff contacts selectively activated VI. Right nerve-cuff contact 1 stimulated VI to generate over 8 Nm of knee extension moment in response to a single stimulus pulse without recruiting VL or VM. Although right nerve-cuff contact 2 did not selectively stimulate any one muscle, it did selectively exclude VM, allowing the combination of VI and VL to generate up to 7.1 Nm of knee extension moment in response to a single stimulus pulse. On the left side, contact 2 selectively activated VM without activating VI, and contact 4 selectively activated VI without activating VM.
3.3. Twitch/tetany relationship
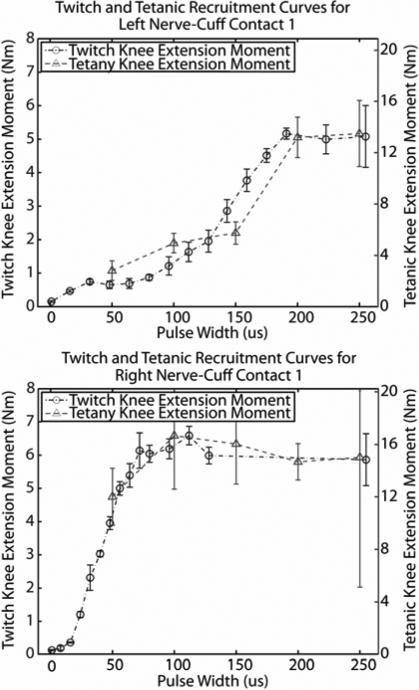
Two representative examples of pairs of twitch and tetanic knee extension moment recruitment curves are presented in Figure 5. The shapes of twitch recruitment curves were similar to the shapes of tetanic curves for each of the 8 nerve-cuff contacts. From these data, a twitch to tetany scaling factor can be extracted by calculating the ratio of the maximum moments from the two curves. Scaling factor data for all eight contacts are presented in Table I. These factors range from 2.53 up to 4.57, with a mean ± standard deviation of 3.5±.79.
Figure 5.
Isometric recruitment curves in response to single stimulus pulses (right axes) and trains of stimuli (left axes) from left and right nerve-cuff electrodes. All stimulus pulses were applied at a current amplitude of 1.4 mA.
4. Discussion
The data presented in this study suggest that, for this subject, the CWRU spiral nerve-cuff electrode provides a chronically stable interface for selectively stimulating branches of the human femoral nerve. Stability of the electrode interface is crucial to the success of a chronically implanted neuroprosthesis, because consistency of the stimulated response allows for predictable and repeatable control of the resulting limb movements. Without a stable neural interface, the use of FES to produce complex movements would likely be impossible. For the two nerve-cuff electrodes in this study, there was either no change, a slight fluctuation which returned to the starting value over time, or a slight decrease in stimulation charge threshold over the course of one year after implantation. The relatively minor changes in stimulation charge threshold suggest that any encapsulation of the electrode by surrounding tissue has not significantly affected the stimulating current in the volume conductor near the electrode, that there is little or no motion between the electrode and the nerve, and that there have not been negative electrophysiological effects on the nerve as a result of stimulation. The changes observed with some of the electrode contacts are small enough that they are unlikely to cause significant fluctuations in motor output in response to stimulation, and would therefore not affect the performance of the CWRU standing/transfer system.
Evidence from this study also suggests that the CWRU spiral nerve-cuff electrode is capable of selectively stimulating individual muscles while excluding others, although it may not be ideal for selectively activating every one of the muscles it stimulates. Six of eight nerve-cuff contacts were able to selectively stimulate one muscle while avoiding at least one other muscle, but neither nerve-cuff could selectively activate all three of the vasti. Although the cuff did not allow for selective stimulation of each of the vasti individually, three of four contacts in the right nerve-cuff electrode selectively stimulated VI to generate between 6.7 and 8.1 Nm of knee extension without activating either VL or VM. The fourth contact (contact 2), while not able to selectively activate any one muscle, was capable of selectively excluding VM while producing up to 7.1 Nm of knee extension. On the left side, two contacts were able to selectively stimulate VI while excluding VM, and a third contact selectively stimulated VM while avoiding VI. Because of recording problems with the left VL EMG electrode, it is not possible to determine whether VL was being stimulated or not when stimulation was applied via any of the four nerve-cuff contacts. However, the result that each of VM and VI can be selectively included or excluded is still an important finding. Functionally, the ability to selectively activate these two muscles could be useful in a carousel or interleaved stimulation paradigm during standing [28]. That is, one of the electrode contacts could be used to recruit VM to keep the knee locked while VI recovers from fatigue, and then, later, a second contact could be used to recruit VI while VM recovers from fatigue.
In this study, VI was selectively recruited more often than either VL or VM. This may suggest that the anatomy of the femoral nerve lends to easier recruitment of VI, or that the threshold for activation of the axons innervating VI may be lower than for either VL or VM. A lower threshold could result from a wide array of causes including larger diameter axons or physiological changes induced by the spinal cord injury. More in depth study is needed to understand this phenomenon so it can be exploited for future neural interface designs.
In order for paradigms like carousel stimulation to be effective for prolonged standing, the nerve-cuff electrodes must be able to selectively recruit their respective muscles to produce knee extension moments that are large enough to lock the knees. From the results in Table I, which are based on selectivity data and the relationships found between twitch and tetanic recruitment curves, it is estimated that any single contact from the right nerve-cuff could selectively activate VI to generate over 20 Nm of knee extension moment, with one contact generating nearly 35 Nm. Estimates of selectively generated moments for the left nerve-cuff were lower, ranging from 15 to 19 Nm. There is evidence that 35 Nm of knee extension is required for an individual with SCI using an FES system and upper extremity effort to complete a sit-to-stand transition, but after an erect stance is achieved, the knee extension moment required to keep the knees locked is likely to be substantially lower [29-35]. The knee extension moment required during standing with FES is highly variable, because it depends on a number of biomechanical parameters including body weight, height, and body position. As a result, it is not clear whether the moments produced by selectively stimulating individual heads of the quadriceps with a nerve-cuff would be sufficient to keep the knees locked during standing after the sit-to-stand transition is complete. It is clear from this single-subject pilot study, however, that the four-contact CWRU self-sizing spiral nerve-cuff electrodes on distal branches of the femoral nerve provide a stable interface for selectively recruiting individual heads of the quadriceps to produce significant knee extension moments. Further longitudinal studies should be performed with a larger subject population to confirm these findings before further definitive and generalized conclusions are drawn.
Future work with these electrodes should involve alternate methods of measuring selectivity. The use of EMG as a measure of whole muscle selectivity may tend to underestimate functional selectivity. For example, two nerve-cuff contacts could conceivably activate independent motor unit pools within a single muscle, but EMG recordings taken to be representative of the entire muscle would erroneously indicate overlapping motor unit pools. Conversely, wire or needle EMG electrodes sample only a fraction of the fibers within a muscle which may result in overestimating selectivity if overlapping motor unit pools are outside of the recording field. Yoshida, et al., describe a method that could overcome this problem, in which the sum of twitch forces elicited by stimulating the nerve with two individual electrode contacts is compared to the twitch force elicited by stimulating the nerve with those two contacts, one within the refractory period of the other [36, 37]. Under these conditions, if the two contacts are stimulating separate motor unit populations, there should be linear addition of forces and the sum of stimulating with each contact separately should equal the result of stimulating with one contact in the refractory period of the other [36]. A similar method could be performed here to estimate selectivity of the nerve-cuff electrodes.
Additional future work will include implementation of stimulation paradigms that take advantage of the selectivity of these electrodes to allow for longer stand times, such as carousel stimulation or other methods that selectively recruit only fatigue resistant fibers. Also, further work should be done to monitor the chronic stability of the selective responses reported in this paper. While the results of this study suggest that the electrode interface is stable over time, the stability of selectivity could be measured by repeating the EMG experiments at multiple time points in the future. Finally, it may be useful to explore other cuff electrode designs, such as one with a rectangular cross-section, and other stimulation paradigms, such as field steering, which might further improve selectivity and allow for selective activation of each stimulated muscle.
5. Conclusion
Based on the results of this study, we have shown that the CWRU spiral nerve-cuff electrode can provide a stable interface for stimulating branches of the human femoral nerve. Stimulation charge thresholds for two four-contact nerve-cuffs chronically implanted on the distal branches of the human femoral nerve were stable and within the range of previously reported results over one year after implantation, suggesting a stable interface between the nerve and electrode [18]. Six of eight nerve-cuff contacts could selectively activate one muscle while selectively excluding others, and could produce up to 8 Nm of isometric knee extension moment in response to a single stimulus pulse. Based on estimates of the relationship between twitch and tetanic moments, these selective responses could generate moments as large as 35 Nm in response to trains of stimulus pulses. Overall, the results of this study suggest that, for this subject, the CWRU spiral nerve-cuff electrode can provide a chronically stable and effective peripheral nerve interface for selectively activating specific muscles for neuroprosthetic applications.
Acknowledgements
This work was supported in part by the National Institutes of Health under Grant NIH 5-R01-EB001889, Grant GCRC M01 RR000080, and Grant T32-EB04314-08.
References
- 1.Chae J, Triolo R, Kilgore K, Creasey G, DiMarco A. Neuromuscular Electrical Stimulation in Spinal Cord Injury. In: Kirschblum S, et al., editors. Spinal Cord Medicine. Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 360–388. [Google Scholar]
- 2.Bertoti DB. Electrical stimulation: a reflection on current clinical practices. Assist. Technol. 2000;12:21–32. doi: 10.1080/10400435.2000.10132007. [DOI] [PubMed] [Google Scholar]
- 3.Creasey GH, Ho CH, Triolo RJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. Clinical applications of electrical stimulation after spinal cord injury. J. Spinal Cord Med. 2004;27:365–75. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- 4.Bogie KM, Triolo RJ. Effects of regular use of neuromuscular electrical stimulation on tissue health. J. Rehabil. Res. Dev. 2003;40:469–75. doi: 10.1682/jrrd.2003.11.0469. [DOI] [PubMed] [Google Scholar]
- 5.Davis JA, Triolo RJ, Uhlir J, Bieri C, Rohde L, Lissy D, Kukke S. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers--where do we stand? J. Rehabil. Res. Dev. 2001;38:609–17. [PubMed] [Google Scholar]
- 6.Uhlir JP, Triolo RJ, Davis JA, Bieri C. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12:279–87. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez FJ, Ceballos D, Schuttler M, Valero A, Valderrama E, Stieglitz T, Navarro X. Polyimide cuff electrodes for peripheral nerve stimulation. J. Neurosci. Methods. 2000;98:105–18. doi: 10.1016/s0165-0270(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 8.Tarver WB, George RE, Maschino SE, Holder LK, Wernicke JF. Clinical experience with a helical bipolar stimulating lead. Pacing Clin. Electrophysiol. 1992;15:1545–56. doi: 10.1111/j.1540-8159.1992.tb02933.x. [DOI] [PubMed] [Google Scholar]
- 9.Koole P, Holsheimer J, Struijk JJ, Verloop AJ. Recruitment characteristics of nerve fascicles stimulated by a multigroove electrode. IEEE Trans. Rehabil. Eng. 1997;5:40–50. doi: 10.1109/86.559348. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney JD, Ksienski DA, Mortimer JT. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans. Biomed. Eng. 1990;37:706–15. doi: 10.1109/10.55681. [DOI] [PubMed] [Google Scholar]
- 11.Tarler MD, Mortimer JT. Linear summation of torque produced by selective activation of two motor fascicles. IEEE Trans. Neural Syst. Rehabil. Eng. 2007;15:104–10. doi: 10.1109/TNSRE.2007.891377. [DOI] [PubMed] [Google Scholar]
- 12.Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12:251–7. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]
- 13.Veraart C, Grill WM, Mortimer JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1993;40:640–53. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- 14.Grill WM, Mortimer JT. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans. Rehabil. Eng. 1998;6:364–73. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- 15.Grill WM, Mortimer JT. Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Trans. Rehabil. Eng. 1996;4:49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- 16.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 1988;35:905–16. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 17.Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini A, Wanet-Defalque MC. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res. 1998;813:181–6. doi: 10.1016/s0006-8993(98)00977-9. [DOI] [PubMed] [Google Scholar]
- 18.Polasek KH, Hoyen HA, Keith MW, Tyler DJ. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans. Neural. Syst. Rehabil. Eng. 2007;15:76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- 19.Fisher LE, Miller ME, Bailey SN, Davis JA, Anderson JS, Rhode L, Tyler DJ, Triolo RJ. Standing after spinal cord injury with four-contact nerve-cuff electrodes for quadriceps stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008;16:473–8. doi: 10.1109/TNSRE.2008.2003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhadra N, Kilgore KL, Peckham PH. Implanted stimulators for restoration of function in spinal cord injury. Med. Eng. Phys. 2001;23:19–28. doi: 10.1016/s1350-4533(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 21.Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, Peckham PH. An externally powered, multichannel, implantable stimulatortelemeter for control of paralyzed muscle. IEEE Trans. Biomed. Eng. 1998;45:463–75. doi: 10.1109/10.664202. [DOI] [PubMed] [Google Scholar]
- 22.Davis JA, Triolo RJ, Uhlir JP, Bhadra N, Lissy DA, Nandurkar S, Marsolais EB. Surgical technique for installing an eight-channel neuroprosthesis for standing. Clin. Orthop. Relat. Res. 2001;385:237–52. doi: 10.1097/00003086-200104000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Triolo RJ, Bieri C, Uhlir J, Kobetic R, Scheiner A, Marsolais EB. Implanted Functional Neuromuscular Stimulation systems for individuals with cervical spinal cord injuries: clinical case reports. Arch. Phys. Med. Rehabil. 1996;77:1119–28. doi: 10.1016/s0003-9993(96)90133-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, DeLisa JA. Surface Anatomy for Clinical Needle Electromyography. Demos Medical; New York: 2000. [Google Scholar]
- 25.Perotto AO, Delagi EF, Iazzetti J, Morrison D. Anatomical Guide for the Electromyographer: The Limbs and Trunk. Charles C. Thomas; Springfield: 2005. [Google Scholar]
- 26.Dyck PJ. Peripheral Neuropathy. W.B. Saunders Co.; Philadelphia: 1984. [Google Scholar]
- 27.Durfee WK, MacLean KE. Methods for estimating isometric recruitment curves of electrically stimulated muscle. IEEE Trans. Biomed. Eng. 1989;36:654–67. doi: 10.1109/10.32097. [DOI] [PubMed] [Google Scholar]
- 28.McDonnall D, Clark GA, Normann RA. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12:208–15. doi: 10.1109/TNSRE.2004.828425. [DOI] [PubMed] [Google Scholar]
- 29.Bajd T, Kralj A, Turk R. Standing-up of a healthy subject and a paraplegic patient. J. Biomech. 1982;15:1–10. doi: 10.1016/0021-9290(82)90029-x. [DOI] [PubMed] [Google Scholar]
- 30.Rodosky MW, Andriacchi TP, Andersson GB. The influence of chair height on lower limb mechanics during rising. J. Orthop. Res. 1989;7:266–71. doi: 10.1002/jor.1100070215. [DOI] [PubMed] [Google Scholar]
- 31.Arborelius UP, Wretenberg P, Lindberg F. The effects of armrests and high seat heights on lower-limb joint load and muscular activity during sitting and rising. Ergonomics. 1992;35:1377–91. doi: 10.1080/00140139208967399. [DOI] [PubMed] [Google Scholar]
- 32.Kotake T, Dohi N, Kajiwara T, Sumi N, Koyama Y, Miura T. An analysis of sit-to-stand movements. Arch. Phys. Med. Rehabil. 1993;74:1095–9. doi: 10.1016/0003-9993(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 33.Kagaya H, Shimada Y, Ebata K, Sato M, Sato K, Yukawa T, Obinata G. Restoration and analysis of standing-up in complete paraplegia utilizing functional electrical stimulation. Arch. Phys. Med. Rehabil. 1995;76:876–81. doi: 10.1016/s0003-9993(95)80556-7. [DOI] [PubMed] [Google Scholar]
- 34.Shimada Y, Sato K, Matsunaga T, Tsutsumi Y, Misawa A, Ando S, Minato T, Sato M, Chida S, Hatakeyama K. Closed-loop control using a stretch sensor for restoration of standing with functional electrical stimulation in complete paraplegia. Tohoku J. Exp. Med. 2001;193:221–7. doi: 10.1620/tjem.193.221. [DOI] [PubMed] [Google Scholar]
- 35.Davis R, Houdayer T, Andrews B, Barriskill A. Paraplegia: prolonged standing using closed-loop functional electrical stimulation and Andrews ankle-foot orthosis. Artif. Organs. 1999;23:418–20. doi: 10.1046/j.1525-1594.1999.06368.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans. Biomed. Eng. 1993;40:492–4. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 37.Leventhal DK, Durand DM. Subfascicle stimulation selectivity with the flat interface nerve electrode. Ann. Biomed. Eng. 2003;31:643–52. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]