Abstract
Wound healing is a complex, orchestrated series of biological events that is controlled by extracellular components that communicate between cell types to re-establish lost tissue. We have found that signaling by ELR-negative CXC chemokines through their common CXCR3 receptor is critical for dermal maturation during the resolving phase. In addition there needs to be complete maturation of the epidermis and regeneration of a delineating basement membrane for proper functioning. The role of this ligand–receptor system appears confounding as one ligand, CXCL4/(PF4), is present during the initial dissolution and two others, CXCL10/(IP-10) and CXCL11/(IP-9/I-TAC), are expressed by keratinocytes in the later regenerative and resolving phases during which the basement membrane is re-established. We examined CXCR3 signaling role in healing using a mouse lacking this receptor, as all three ligands act solely via the common receptor. Reepithelialization was delayed in CXCR3-deficient mice in both full and partial-thickness excisional wounds. Even at 90 days postwounding, the epidermis of these mice appeared less mature with lower levels of E-cadherin and cytokeratin 18. The underlying basement membrane, a product of both dermal fibroblasts and epidermal keratinocytes, was not fully established with persistent diffuse expression of the matrix components laminin 5, collagen IV, and collagen VII throughout the wound bed. These results suggest that CXCR3 and its ligands play an important role in the re-establishment of the basement membrane and epidermis. These studies further establish the emerging signaling network that involves the CXCR3 chemokine receptor and its ligands as a key regulator of wound repair.
Wound healing requires orchestrated repopulation and regeneration of the lost tissue, a process that becomes deficient in normal aging, after large burns or traumatic wounds, and in numerous chronic diseases. In wounded skin, fibroblasts are recruited to recreate the dermal layer, and keratinocytes from the marginal regions migrate into the wounded area to reepithelialize the initial provisional matrix. Thus, both mesenchymal and epithelial compartments must be regenerated to accomplish proper healing; a deficiency in one results in poor to absent regain of function. This repair ensues from signals from successive waves of growth factors, chemokines, and matrix fragments that modulate the behavior of the key skin cells, predominantly fibroblasts, keratinocytes, and endothelial cells (EC). A major question remains of how the cells in the different compartments communicate to synchronize the maturation of the epidermis and dermis.
We recently found that cysteine-X amino acid-cysteine receptor 3 (CXCR3), the common receptor for the ELR-negative CXC chemokines (CXCL4/PF4, CXCL10/IP-10, CXCL9/MIG, and CXCL11/IP-9/I-TAC), regulates wound healing to channel the later stages of repair toward resolution with maturation of the dermis.1 Of interest, two of the chemokines, IP-10 from the neovasculature and epidermis, and IP-9 from redifferentiating keratinocytes, appear later in the wound healing process,1–4 suggesting that these chemokines are at least part of the key communication between the dermis and epidermis that signals an end to the regenerative phase and prompts the initiation of the remodeling phase of wound repair. In mice lacking CXCR3, fibroplasia and exuberant angiogenesis that mark a regenerative phase that persists for months with a resulting hypercellular dermis that has less collagen and fewer mature fibers resulting in a weakened dermis.1 This is likely due, in part, to the absence of CXCR3 signaling thus blocking fibroblast and endothelial cell motility in response to trophic growth factors5,6 and directly inducing apoptosis of nascent vessels (R. Bodnar & A. Wells, unpublished observations). These two cellular processes in fibroblasts and EC likely underpin the physiological role of CXCR3 in the dermis. However, the other half of the equation, reepithelialization, needs to be addressed.
In keratinocytes, CXCR3 signaling increases cell motility via reduced adhesiveness secondary to activation of calpain I (μ-calpain).7 This suggests that the same chemokines that limit dermal cellularity may accelerate reepithelialization. Furthermore, there is evidence that CXCR3 signaling alters the differentiation/maturation state of fibroblasts, channeling cells from a migratory to a contractile and synthetic phenotype.8,9 These findings compelled us to determine the role of CXCR3 signaling in epidermal repair.
Proper reepithelialization requires not only the resurfacing of the wound area with a keratinocyte layer but also the re-establishment of the basement membrane that provides for the demarcation and cohesion between the epidermal and dermal layers. This barrier undergoes maturation in components and contiguity as the provisional matrix is replaced with a mature dermis. As these components are provided by both the fibroblasts and keratinocytes, 10 it is reasonable to postulate the factors that affect the cellularity and differentation status of these cells contribute to this process. To study these events in a complex environment of wound repair, we used mice that lack CXCR3.11 By eliminating the receptor, rather than the individual ligands, we could avoid the confounding effects of overlapping ligand expression. This system tested our hypothesis that in the absence of CXCR3 signaling epidermal maturation is retarded by impaired keratinocyte migration and delayed redifferentiation, and these deficits result in a delayed formation of a mature basement membrane.
MATERIALS AND METHODS
Animals
C57BL/6J mice in which CXCR3 expression was abrogated were obtained from Dr. Wayne Hancock and Dr. Bao Lu (Millennium Pharmaceuticals Incorporated, Cambridge, MA and Perlmutter Laboratory, Children’s Hospital, Boston, MA). CXCR3 Knockout mice were engineered as previously described.11 CXCR3−/− female mice were bred with CXCR3−/− male and all the offspring were genotyped before use. All studies on these animals were performed in compliance with and after approval by the Institutional animal care and use Committees of the Veteran’s Administration and University of Pittsburgh. These animals were housed in a facility of Veteran’s Affair Medical Center, Pittsburgh, PA, accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Serological analyses did not detect blood borne pathogens or evidence of infection. Mice were housed in individual cages after wounding and maintained under a 12 hours light/dark cycle and temperature in accordance with the guidelines approved by the IACUC.
Wounding
Male and female mice (7–8 weeks of age weighing approximately 25 g) were anesthetized with an intraperitoneal injection containing ketamine (75 mg/kg) and xylazine (5 mg/kg). The backs were cleaned, shaved, and sterilized with betadine solution. For full-thickness wounds, an approximately 2 cm diameter full-thickness wound through the epidermis and dermis was made on one side of the dorsal midline, using sharp scissors, with the contralateral uninjured skin serving as a control. Partial-thickness wounds, 7 × 10 mm and 0.2–0.3 mm deep, were made with a modified Castroviejo dermatotome.12,13 The wounds were covered with liquid occlusive dressing (New-Skin® Medtech, Jackson, WY).
Primary keratinocyte isolation, culture, and characterization
Primary keratinocytes were isolated from the skin of neonatal mice by standard procedures. Skin from the torso of 2- to 3-day-old mice was exposed to 0.25% trypsin in saline overnight at 4 °C. The epidermis was removed as a sheet, minced, and then agitated at 37 °C for 45 minutes in 5 mL of keratinocyte growth media. The minced epidermis was filtered through nylon screens (Sefar Filtration Inc., Depew, NY) to remove the stratum corneum. Cells were pelleted by centrifugation and resuspended in fresh keratinocyte growth media for experiments. Keratinocyte growth media was composed of serum-free keratinocyte media without CaCl2 (Invitrogen, Carlsbad, CA) supplemented with 25 mg/mL bovine pituitary extract, 2.5 µg/mL recombinant epidermal growth factor (EGF), 5% calcium-chelated fetal bovine serum (FBS) using Chelex-100 resin (Bio-Rad Laboratories, Hercules, CA).
Cell migration assay
Cell migration was assessed by the ability of the cells to move into an acellular area in a two-dimensional wound-healing assay. At approximately 70–80% confluence, cells were detached and then replated at 1.0×106 cells/well in 24-well culture plates in complete growth media Dulbecco’s modified Eagle’s medium (DMEM) and incubated for 24 hours at 37 °C in 5%CO2. Cells were then washed with phosphate buffered saline solution (PBS), and the media were changed to DMEM containing 0.5% dialyzed FBS for 24 hours. A denuded area was generated in the middle of each well with a rubber policeman. The cells were then stimulated with EGF (10 nmol/L) in the presence or absence of IP-9 (25 ng/mL) or IP-10 (50 ng/mL) and then incubated for 24 hours. These concentrations were determined empirically to provide either maximum motility or inhibition without toxicity. Images were taken at 0 and 24 hours, and the relative distance moved into the wounded area at the acellular front was determined. All treatments were normalized to the no treatment, which equals 1.
Histological analysis
Mouse wound bed biopsies surrounded by a margin of nonwounded skin were collected at days 5, 7, 14, 21, 30, and 60 postwounding. Wound biopsies were fixed in 10% buffered formalin, processed and embedded in paraffin blocks using standard protocols. Tissue sections (5 µm) were stained with hematoxylin and eosin (H&E). H&Es were used for general tissue and cellular morphology. All wound biopsies were stained at the same time to eliminate staining variations.
Skin thickness analysis
Under the light microscope, measurements were performed using the micrometer and SPOT-Image software. Six readings were scored from each 5 µm skin section of each animal. The epidermal thickness was measured from stratum basale to stratum granulosum (excluding stratum corneum).
Epidermal maturation assessment
Histopathological examination of mouse tissues was performed blinded by a veterinary pathologist. Qualitative assessments were made concerning aspects of epidermal maturation and basement membrane remodeling. The samples were scored on a scale of 0–4 for epidermal healing (0= no migration, 1= partial migration, 2= complete migration with partial keratinization, 3= complete keratinization, 4= normal epidermis).
Sections for immunohistochemical analysis were incubated with appropriately diluted primary antibody, after antigen retrieval (BioGenex, San Ramon, CA). Antigen staining was performed using diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA), then counterstained with Mayer’s hematoxylin and coverslipped. In all cases, secondary antibody alone or serum served as a negative control, with various human tumor tissues serving as positive controls.
Murine tissue, paraffin sections of 4–5 µm were prepared for antibody staining. The following antibodies were used for immunohistochemical or immunofluorescence staining for mouse specimens: CXCR3 (1 : 250) (rabbit polyclonal, R&D Systems, Minneapolis, MN), Fibronectin (1 : 50) (rabbit polyclonal; Rockland, Gilbertsville, PA), Laminin 5 (1 : 50) (mouse polyclonal, Chemicon, Billerica, MA), Collagen IV (1 : 500) (rabbit polyclonal, Abcam, Cambridge, MA), Collagen VII (1 : 250) (rabbit polyclonal, Abcam), Cytokeratin 18 (1 : 200) (rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA), E-cadherin (prediluted) (rabbit polyclonal, Abcam), Ki67 (1 : 200) (rabbit polyclonal, Abcam), cytokeratin 5 (1 : 100) (rabbit polyclonal, Abcam), and cytokeratin 1 (1 : 250) (rabbit polyclonal, Abcam). Antibodies chosen of murine origin. MOM kit (Vector) was used to limit background staining from the secondary antibody.
Immunofluorescence stains were imaged using the Olympus fluoview FV1000 confocal microscope or an IX70 microscope equipped with a Spot RTKE digital camera. Intensity was assessed using MetaMorph analysis (Universal Imaging Corp.). Integrated intensity was based on total area and staining intensity at individual pixels. All wound biopsies were stained at the same time to eliminate staining variations.
Statistical analysis
Results are expressed as mean ± SD. Statistical differences between groups were determined by the Student’s t test. Paired analysis was performed between all groups. Comparisons over time were performed by ANOVA. Significance was deemed at p < 0.05.
RESULTS
Delayed reepithelialization in the absence of CXCR3 signaling
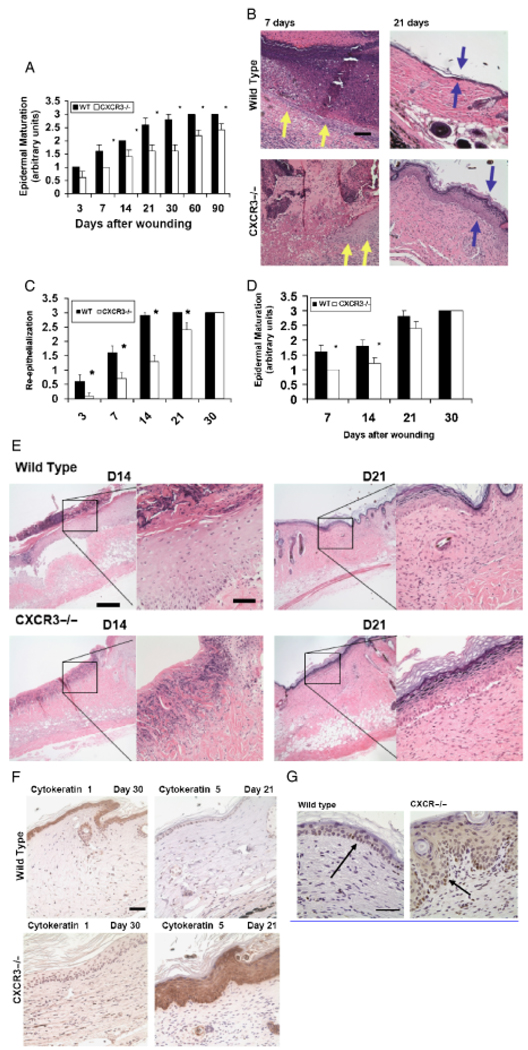
Previous reports have shown that CXCR3 signaling enhances basal motility in cultured keratinocytes, but at only half the rate of classic growth factor receptors such as EGFR and PDGFR, which are activated during wound healing.7,14,15 As such it is not obvious whether the CXCR3 ligands play a significant role in epidermal maturation including reepithelialization. To test this, full-thickness and partial-thickness excisional wounds were made on the dorsal midsection of CXCR3−/− and wild-type (WT, control) mice. Although CXCR3 signaling appears additive with classic growth factors in culture,7 it was surprising to find a significant delay in wound reepithelialization and epidermal maturation in the CXCR3−/− mice even after 30 days (Figure 1A and B). At days 21 and 30 the CXCR3−/− mice showed a 40% reduction in epidermal maturation. This persisted even at 90 days postwounding, when there was a 20% difference in the CXCR3−/− mice in comparison with WT (Figure 1A). This impediment of epidermal maturation was seen histologically by the delay in reepithelialization and epidermal maturation in the CXCR3−/− wounds while the WT tongue had migrated over the provisional matrix to separate the wound crust at day 7 (Figure 1C, as noted by yellow arrows). Additionally at day 21 the CXCR3−/− epidermal layer was hypertrophic (Figure 1C, blue arrows).
Figure 1.
Delayed reepithelialization and maturation in the absence of CXCR3. (A) Histological analyses of epidermal maturation of 2 cm full-thickness wounds demonstrated a lag in wounds in the CXCR3−/− mice in comparison to those in wild-type mice throughout the first 90 days. (B) Representative histological sections of 7- and 21-day-old wounds stained with hematoxylin and eosin (H&E) reveals a hindrance in maturation in the CXCR3−/− wounds, with a delay in the epithelial tongue (yellow arrows) and multilayered epidermis (blue arrows). (C) In partial-thickness wounds, reepithelialization, determine by histological examination, also revealed a delay in the wounds in CXCR3−/− mice as late as day 21. (D) Partial-thickness wounds approximately 7 × 10 and 0.02–0.03 mm deep were made on the skin with a specially modified dermatotome. Epidermal maturation showed a delay for the first 14 days postwounding. (E) Representative photomicrographs show the migrating epithelium. (F) Lack of maturity marker cytokeratin 1 and positive staining of immaturity marker cytokeratin 5 suggesting a continued regenerating epidermis as late 30-day postwounding in CXCR3−/− wound. (G) Proliferation marker (Ki67) staining indicated the wounds in CXCR3−/− mice contained proliferating keratinocytes (arrows) even 30 days postwounding. Shown in A and C are mean ±SD (n=6; *p < 0.05); others are representative of six mice. The photomicrographs measure 300 µm on each side; original magnification ×400. The photomicrographs in (D) measure 300 µm on each side; original magnification, ×100 and ×400, and in (G) original magnification was ×600. The black bar in the photomicrographs measures 50 µm. CXCR3, cysteine-X amino acid-cysteine receptor 3.
While much of wound closure in full-thickness wounds is achieved by matrix contraction, this is deficient in the absence of CXCR3 signaling1 and the perceived limited reepithelialization may simply have resulted from a deficit in contraction. As such, we deemed it important to assess the effects of CXCR3 signaling without contraction playing a role. Partial-thickness wounds were created using a modified dermatotome for mouse skin.12,13 We found that during the initial phases of healing epidermal maturation was impaired, resulting in a lag in reepithelialization throughout the first 14 days (Figure 1D). Photomicrographs of the histopathological analysis revealed that CXCR3−/− wounds lack the complete and well-connected epithelium observed in the healed wounds in WT animals. This was noted as the epidermis lacking a cleanly defined layer of basal keratinocytes and the predilection for the epidermis to separate from the dermis during fixing and staining (Figure 1E); it must be noted that we did not process the tissue appropriately to detect such basal lamina integrity issues by electron microscopy (but rather analyzed by immunostaining). The regenerating epidermis and epidermal tongue in the CXCR3−/− wounds stained negative or only weakly positive for cytokeratin 1, a marker of maturity or keratinocyte differentiation, compared with the positive staining seen in the WT. In contrast the epithelium in the CXCR3−/− wounds stained strongly for cytokeratin 5, a marker for epithelial immaturity or keratinocyte dedifferentiation, whereas the WT wounds resembles the low levels seen in normal epidermis (Figure 1F). Immaturity is also noted by the levels of Ki67 staining 30 days postwounding, confirming the excessive proliferative state of the CXCR3−/− wounds (Figure 1G), persisted well into the resolving phase. These data provide evidence that CXCR3 signaling is necessary for the timely migration of keratinocytes over the wound and maturation of the epidermal layers.
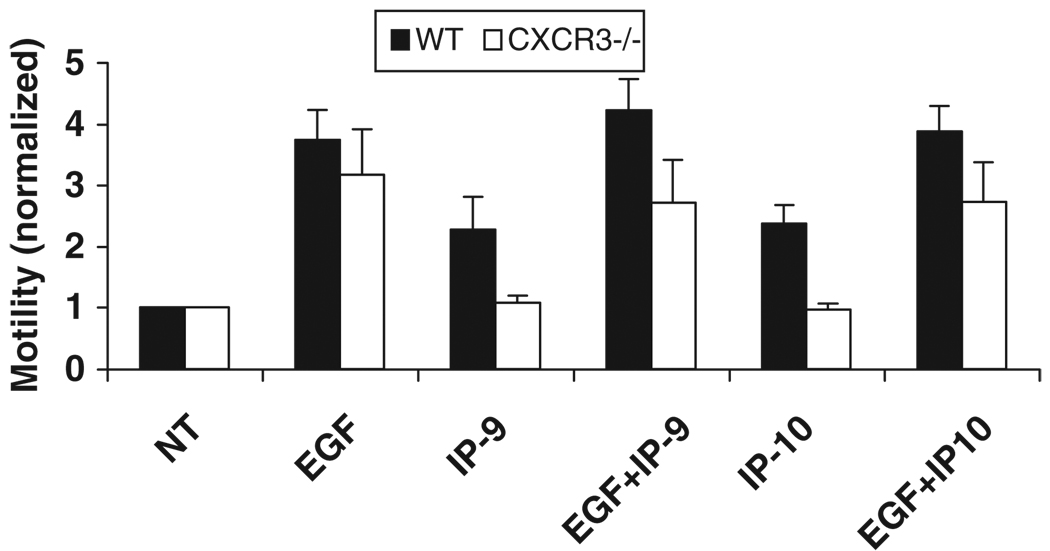
The above results show in vivo that CXCR3 plays a role in keratinocyte function. To further verify that the inhibition of wound closure is due to the lack of CXCR3 and not other endogenous factors we analyzed the migration of keratinocytes from CXCR3−/− mice in the presence of IP-9 and IP-10. Keratinocytes isolated from CXCR3−/− mice (primary cells in passages 2–3) were challenged with EGF and/or CXCR3 ligands (IP-9 and IP-10) and tested for their ability to migrate into the denuded area in a wound scratch assay. Neither IP-9 nor IP-10 induced motility in the keratinocytes from the CXCR3−/− mice, though the positive controls with EGF show that the pathways for migration are functional (Figure 2).
Figure 2.
Cell motility in response to soluble wound factors. Primary keratinocytes were isolated from wild-type (WT) and CXCR3−/− mice and analyzed for response to a wound growth factor (EGF for the EGF receptor network) and the CXCR3 ligands (IP-9 and IP-10). In a two-dimensional in vitro “wound healing” assay, keratinocytes from both mice were equally responsive to EGF, but only the WT keratinocytes responded to IP-9 and IP-10 for induction of migration (mean ± SD; n=3; *p < 0.05). CXCR3, cysteine-X amino acid-cysteine receptor 3; EGF, epidermal growth factor.
Immaturity of the epidermis in the absence of CXCR3
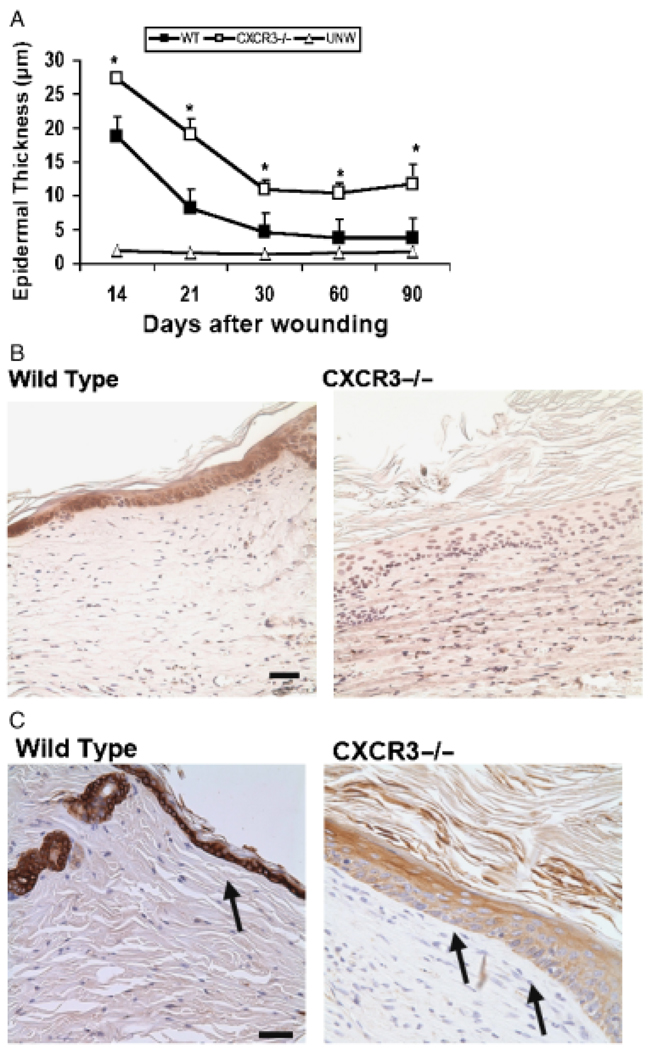
Our initial examination of wound healing (Figure 1B and D) revealed not only delayed epidermal coverage (Figure 1B) but also immaturity of the keratinocytes (Figure 1D) even when the wound was covered. During the initial reepithelialization, there was a hypercellular, thickened epidermis less well connected to the underlying structures than mature epidermis (Figure 1D). When the epithelial layer reaches a state of contact inhibition, the keratinocytes redifferentiate with up-regulation of E-cadherin and cytokeratins indicative of mature keratinocytes, with E-cadherin expression being higher in a mature epidermis as the adhesion between differentiated cells are stronger and more stable.16 Wounds in the CXCR3−/− mice presented an immature epidermis which remained several cell layers thicker than comparably aged wounds in the WT mice (Figure 3A). Consistent with this, these wounds expressed lower levels of keratinocyte E-cadherin (Figure 3B) and cytokeratin 18 (Figure 3C). Because cytokeratins interact with desmosomes and hemidesmosomes, thus contributing to cell–cell adhesion, this finding suggests that CXCR3 not only affects the rate of keratinocyte reepithelialization but the adhesiveness between the keratinocytes of this layer as well.
Figure 3.
Epidermal hypercellularity persists in wounds in mice lacking CXCR3. (A) Quantitative measurements of epidermal cell layers show CXCR3−/− mice wounds to be significantly thicker as late as 90-day postwounding in comparison with wild-type (WT) mice (mean ± SD; n=6; *p < 0.05). (B) Immunostaining localization of E-cadherin staining at 30 days postwounding shows CXCR3−/− wounds as presenting a still dedifferentiated epidermis with no or very little detectable staining. (C) Histological sections taken at day 90 postwounding were stained with cytokeratin 18 (arrows). The CXCR3−/− wounds exhibit a thicker hypercellular epithelial layer with less cytokeratin in the basal layer but excessive superficial keratinization. The images shown in B and C are representative of six mice. The photomicrographs measure 300 µm on each side; original magnification, ×400. The black bar in the photomicrographs measures 50 microns. CXCR3, cysteine-X amino acid-cysteine receptor 3.
Basement membrane remodeling is delayed in the absence of CXCR3
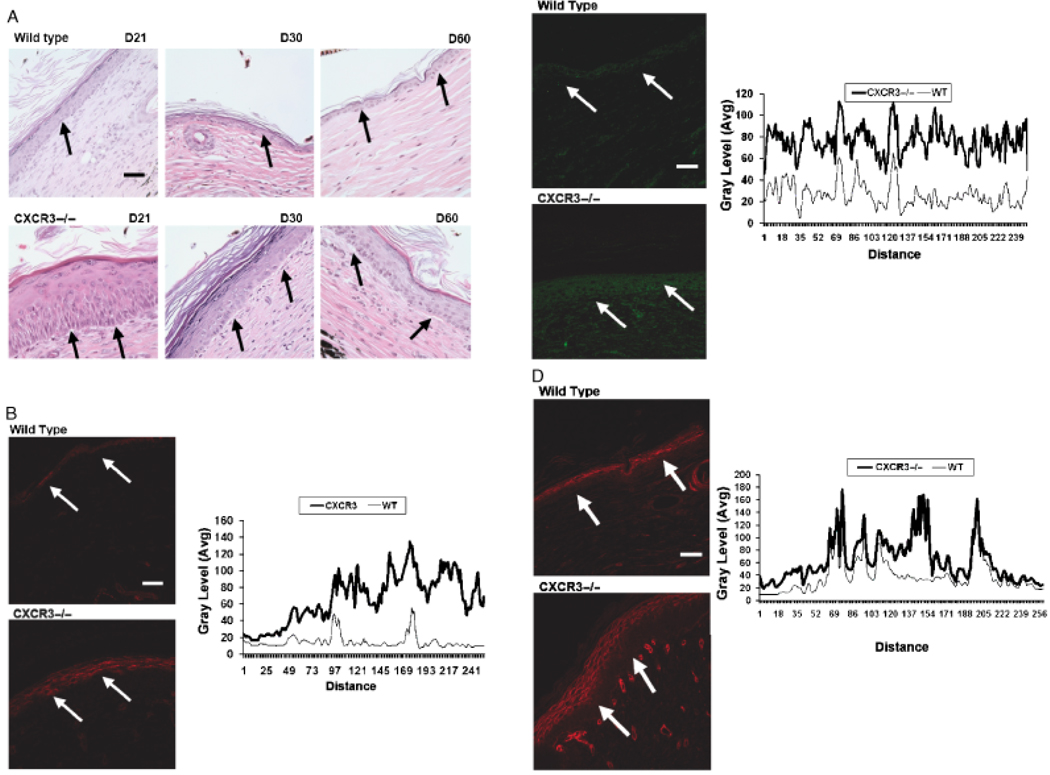
An intact basement membrane is necessary for the integrity and functioning of the skin. Early on a provisional wound matrix and the remodeling epidermis generated present ontological components including fibronectin, tenascin-C, collagen IV, and laminin 5.10,15 As the wound resolves, these are replaced by collagen I and mature laminins. Because the basal keratinocytes appeared less differentiated in the wounds of the CXCR3−/− mice, we examined the basement membrane area. Again, we noted that the epidermis and dermis could separate during the fixing and staining of the tissue in the CXCR3−/− wound throughout all healing phases (Figure 4A) as reflected in the appearance of areas of separation between the basement membrane and the epidermis, though this was not seen at day 60. During the early stages of epidermal wound healing there were high and discontinuous levels of basement membrane proteins, as the wound matured levels of expression decreased and the protein appearance became linear. Wounds in the CXCR3−/− mice continued to express high amounts of collagen IV and laminin 5 at 60 days for collagen IV (Figure 4B) and 90 days for laminin 5 (Figure 4C), well after they were no longer readily detectable in the wounds of the WT mice. Additionally, the basement membrane is a polarized structure in which the attachment to the dermis is anchored by fibrils composed mainly of collagen VII. Collagen VII expression in CXCR3−/− wounds was noted at 60 days to resemble that of a immature remodeling wound with seemingly ectopic expression of this basement membrane component, 17,18 whereas the WT wound expression appeared linear typical of normal mature skin (Figure 4D).
Figure 4.
Delayed in basement membrane remodeling in wounds in mice lacking CXCR3. (A) Representative histological sections of wounds stained with hematoxylin and eosin (H&E) show the immature basement membrane of wounds in CXCR3−/− mice with appearance of gaps between the dermis and epidermis that occur during the fixing and staining process (arrows). (B–D) Immunofluorescence localization of (B) Collagen IV (90 days postwounding), (C) Laminin 5 (60 days postwounding), and (D) Collagen VII (60 days postwounding) shows increased expression in the wounds in CXCR3−/− mice in comparison to those in wild-type (WT) mice. The basement membrane is late in the healing phase of WT mice and resembles a quiescent epidermis basement membrane junction of unwounded skin (not shown). B–D Quantitative measurements of the fluorescence staining in the epidermis portion of the tissues were measured by scanning fluorescence levels across the wounds (demarcated by arrows). All images are representative of six mice. The photomicrographs measure 300 µm on each side; original magnification, ×400. The white bar in the photomicrographs measures 50 microns. CXCR3, cysteine-X amino acid-cysteine receptor 3.
DISCUSSION
Skin is the largest organ of the body, and is formed during development through a highly orchestrated process involving two distinct cell compartments each of which must transition to a mesenchymal-like proliferative and migratory phase and then revert towards terminal differentiation. Soluble factors have been proposed as communicators to synchronize the wound response that coordinates the maturation of the epithelial ectodermal (epidermal) and mesenchymal mesodermal (dermal) layers. Previously published results show that signaling through the CXCR3 receptor plays a major role in wound resolution.1 This receptor and its four genetically and spatio-temporally distinct ligands CXCL4 (PF4), CXCL9 (MIG), CXCL10 (IP-10), and CXC11 (IP-9) contribute to all phases of wound healing. However, the overall correlative data have presented CXCR3 signaling as an important organizer in wound repair.1,6,7 Wounds in mice lacking this receptor present impaired maturation during the later resolving phase with a hypercellular, disorganized, vascular, and immature dermis.1 Germane to these studies is how the progression of dermal maturation is connected to the remodeling of the epidermis and formation of the delineating basement membrane that is integral to skin functioning. As such, we postulated that in addition to dermal maturation, CXCR3 signaling contributes to the timely reepithelialization and expression of the basement membrane components and in turn serves as one of the key communicators that determine the status of epidermal maturation.
Wounds in CXCR3-devoid mice lacked timely and proper reepithelialization, shown herein, along with previously described retarded dermal maturation1 that the wounds did eventually close is not surprising given the redundant nature of wound repair. What was unexpected is the extensiveness of the maturation defects as elimination of most individual factors/receptors implicated in repair lead to more focal impairments if any.19 Earlier we had reported a relative delay in appearance and deficit of fibronectin in the provisional wound matrix.1 As this molecule is stimulatory for dedifferentiated keratinocytes, the lack of key molecules may be responsible. However, this possible explanation of relative keratinocyte quiescence is contrary to the hyperproliferative and thus cellularly thicker epidermis noted even months after wounding in these mice. The keratinocytes isolated from these mice do not migrate in response to CXCR3 signaling, though the EGFR response is intact. Rather, this delayed reepithelialization in the face of a hyperproliferative epidermis, suggests a dysfunctional signaling system in addition to the lack of direct effects of CXCR3.
As both ontological layers of the wounds were affected in mice lacking CXCR3 signaling, we examined the transition from a pan–wound-depth provisional matrix to a mature dermis with a specialized junctional basement membrane. This latter structure is critical for the various barrier functions of the skin as it helps to direct keratinocyte biology.15 In concord with the hyperproliferative and immature epidermis, the development of this barrier was deficient even after 60 days. This deficiency is not global at this extended time with some of the proteins and the interactions between dermis and epidermis appearing to be resolved whereas collagen IV still presented as diffuse. At 60 days, the healed wounds in WT mice presented a basement membrane practically indistinguishable from that of unwounded skin. This failure to establish a mature basement membrane would allow prostimulatory factors that are present in the dermis to directly access the keratinocytes, possibly driving the epidermal hypercellularity. Interestingly, it is not just that a provisional immature matrix persisted but that there also appeared to be a lag in generating the initial provisional matrix components. 9 This suggests that the regenerative phase is both slightly delayed and significantly prolonged in the absence of CXCR3 signals. The early delay of the activation of expression of basement membrane components and the later noted poor attachment may explain the continued remodeling state of the epidermis in the CXCR3−/− wounds. These data would imply that CXCR3 signaling is needed for not only timely but proper maturation of the epidermis, suggesting that deficiencies of the receptor could lead to prolonged healing and quite possibly excessive scarring.
An obvious question arises as to the molecular underpinnings by which CXCR3 signaling would lead to wound maturation. This is the subject of a larger study or studies beyond the scope of the present communication. However, it appears to be part of the generally differentiation-inductive program initiated upon CXCR3 signaling. One aspect that affects fibroblasts involves the prevention of rear deadhesion secondary to inhibition of μ-calpain (calpain2) that channels the fibroblasts to a contractile state leading to differentiation.8,20 The increased keratinocyte motility, via μ–calpain (calpain1) activation-related lessened adhesiveness, 7 drives the advancing epidermal tongue toward ultimate contact inhibition and redifferentiation. As the delineating basement membrane derives from the mature cells in both the dermis and epidermis, this follows from the maturation of the wound. However, the above are just intermediary mechanisms and not the ultimate differentiation control signals. Thus, it is not evident whether the CXCR3 drives maturation and basement membrane synthesis directly or indirectly.
In summary, we demonstrated in the present study that expression of CXCR3 promotes wound healing by acting in a biphasic manner to accelerate reepithelialization while at the same time promoting matrix maturation with the establishment of a basement membrane that acts as a stop signal to epidermal repopulation. Our model thus places CXCR3 signaling as an important system needed to synchronize reepithelialization with the maturation of the superficial dermis to promote proper and functional healing.
ACKNOWLEDGMENTS
We thank Diane George and Dr. Joseph Newsome for providing suggestions and discussions. These studies were supported by grants from the National Institute of General Medical Science of the National Institutes of Health (USA). Services in kind were provided by the Pittsburgh VA Medical Center.
REFERENCES
- 1.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, Newsome J, Hebda PA, Wells A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tensen CP, Flier J, vanderRaaij-Helmer EM, Sampat-Sardjoepersad S, vanderSchors RC, Leurs R, Scheper RJ, Boorsma DM, Willemze R. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Investig Dermatol. 1999;112:716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani P, Annunziato F, Lasagni I, Lazzeri E, Beltrame C, Francalanci M, Ugoccioni M, Galli G, Cosmi L, Maurenzig L, Baggliolini M, Maggi E, Romagnani S, Serio M. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Investig. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oynebraten I, Bakke O, Brandzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- 5.Satish L, Yager D, Wells A. ELR-negative CXC chemokine IP-9 as a mediator of epidermal-dermal communication during wound repair. J Investig Dermatol. 2003;120:1110–1117. doi: 10.1046/j.1523-1747.2003.12230.x. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar R, Yates C, Wells A. IP-10 blocks VEGF-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satish L, Blair HC, Glading A, Wells A. IP-9 (CXCL11) induced cell motility in keratinocytes requires calcium flux-dependent activation of μ-calpain. Mol Cell Biol. 2005;25:1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen FD, Asnes CF, Chang P, Elson EL, Lauffenburger DA, Wells A. EGF-induced matrix contraction is modulated by calpain. Wound Repair Regen. 2002;10:67–76. doi: 10.1046/j.1524-475x.2002.10701.x. [DOI] [PubMed] [Google Scholar]
- 9.Yates C, Whaley D, Yen A, Kulesekaran P, Hebda PA, Wells A. ELR-negative CXC chemokine CXCL11 (IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol. 2008;173:643–652. doi: 10.2353/ajpath.2008.070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElGhalbzouri A, Ponec M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004;12:359–367. doi: 10.1111/j.1067-1927.2004.012306.x. [DOI] [PubMed] [Google Scholar]
- 11.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebda PA, Klingbeil CK, Abraham JA, Fiddes JC. Basic fibroblast growth factor stimulation of epidermal wound healing in pigs. J Investig Dermatol. 1990;95:626–631. doi: 10.1111/1523-1747.ep12513528. [DOI] [PubMed] [Google Scholar]
- 13.Hebda PA, Whaley DL, Kim H-G, Wells A. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Repair Regen. 2003;11:373–379. doi: 10.1046/j.1524-475x.2003.11510.x. [DOI] [PubMed] [Google Scholar]
- 14.Babu M, Wells A. Dermal–epidermal communication in wound healing. Wounds. 2001;13:183–189. [Google Scholar]
- 15.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi M, Matsuzaki T, Ihara S. Expression of P-cadherin distinct from that of E-cadherin in re-epithelialization in neonatal rat skin. Dev, Growth Differentiation. 2005;47:75–85. doi: 10.1111/j.1440-169x.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 17.Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104:611–621. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassel S, Unsold C, Schacke H, Bruckner-Tuderman L, Bruckner P. Collagen XVI is expressed by human dermal fibroblasts and keratinocytes and is associated with the microfibrillar apparatus in the upper papillary dermis. Matrix Biol. 1999;18:309–317. doi: 10.1016/s0945-053x(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 19.Scheid A, Meuli M, Gassmann M, Wenger RH. Genetically modified mouse models in studies on cutaneous wound healing. Exp Physiol. 2000;85:687–704. [PubMed] [Google Scholar]
- 20.Shiraha H, Gupta K, Glading A, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–253. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]