Abstract
Generation of early T cells by coculturing stem cells on notch-ligand-expressing OP9 stromal cells (OP9-DL1) has been widely reported. However, further differentiation of these cells into mature, antigen-specific, functional T cells, without retroviral transduction of T cell receptors (TcRs), is yet to be achieved. In the thymic niche this differentiation is controlled by the interaction of developing TcRs with major histocompatibility (MHC) molecules on stromal cells. We hypothesized that by providing exogenous antigen-specific MHC/TcR signals, stem and progenitor cells could be engineered into functional, effector T cells specific for the same antigen. Here we demonstrate that both thymus-derived immature T cells (double positive [DP]: CD4+CD8+) and mouse embryonic stem cells can be efficiently differentiated into antigen-specific CD8+ T cells using either MHC tetramers or peptide-loaded stromal cells. DP cells, following MHC/TcR signaling, retained elevated recombination activating gene-1 levels, suggesting continuing TcR gene rearrangement. Both DP and embryonic stem-cell-derived CD8+ T cells showed significant cytotoxic T lymphocytes activity against antigen-loaded target cells, indicating that these cells are functional. Such directed differentiation strategy could provide an efficient method for generating functional, antigen-specific T cells from stem cells for potential use in adoptive T cell therapy.
Introduction
T cells or T lymphocytes are a group of white blood cells essential for generating long-term immunity through cell-mediated immune response. The presence of T cell receptors (TcRs) on their surface functionally distinguishes them from other lymphocyte types, such as B cells and natural killer cells. T cells are developmentally unique from other blood lineage cells since their development and maturation takes place exclusively in the thymus, and not in the bone marrow. Hematopoietic stem cells (HSCs) migrate from the bone marrow to the thymus, and through a series of highly specific and regulated intercellular signals, they differentiate into functional T cells. It is well established that notch/delta-like ligands (DLL) signaling, presented through thymic stromal cells, is necessary for T lineage commitment of HSCs and generates immature T cells that are CD4+CD8+ double positive (DP).1 These DP cells further mature into CD4+ or CD8+ single-positive (SP) T cells through the engagement of TcRs with specific major histocompatibility (MHC) complexes present on thymic stromal and epithelial cells. Specifically, interaction of the developing TcRs with class I MHCs produces mature CD8+ SP T cells, most of which are cytotoxic T lymphocytes (CTLs) or killer T cells.2 These cells are responsible for destroying pathogen-infected cells as well as tumor cells and play a crucial role in the immune system.
Ex vivo manipulated autologous immune cells (T cells or dendritic cells) have been explored for cell therapy against cancers and infectious diseases. This approach, termed adoptive transfer, has shown considerable promise in human malignant melanoma, leukemia, renal cell cancer, non-Hodgkin lymphoma, multiple myeloma, and prostate cancer.3–9 Although such ex vivo training and expansion of mature antigen-specific T cells has been reported,9–12 the concept is severely constrained by the limited availability of donor cells suitable for collection, expansion, and transfer,13 as well as the time required to expand and train autologous T cells in vitro. These limitations can be addressed through robust and reproducible in vitro generation of functional, transplantable T cells from embryonic stem (ES) or adult stem cells, which has the capability to self-renew indefinitely.14
With the advent of modern tissue engineering concepts and emerging cellular transplantation therapies, stem-cell-derived therapeutics are increasingly becoming a clinical reality. For example, transplantation of marrow-derived hematopoietic progenitors has shown excellent success in treating several cancers.15–18 In recent years, considerable progress has been made in directing stem cells into T cells in vitro. Specifically, the use of various notch-signaling DLL ligands, presented either by retrovirally transfected stromal cells19,20 or by coating and immobilizing on to tissue culture plate21,22 and microbeads,23 has enabled efficient generation of early T cells from both ES and adult stem cells. However, engineering matured, functional, antigen-specific T cells in vitro from these early stem-cell-derived T cells has not been possible without first retrovirally transfecting antigen-specific TcRs to the stem cells.20 Such retroviral transfection introduces significant complexity and regulatory concerns that would hinder eventual clinical application of these cells. The development of new tissue engineering strategies for efficient generation of functional T cells from stem or progenitor cells without the use of retroviral transfection is therefore critical for the ultimate clinical applicability of adoptive T cell therapy.
The OP9-DL1 system has been the most well established and most extensively used approach for in vitro differentiation of stem cells toward the T cell lineage.19,24,25 This murine bone-marrow-derived stromal cell line, genetically modified to stably express the DLL1 notch ligand, can support CD8+ lineage differentiation from murine ES cells19,24,26 or from adult progenitors of both human24 and mouse origin.25,27,28 T cell progenitors generated from the OP9-DL1 supportive system were shown to be fully functional after transplantation into immunodeficient mice.19 Not only were recipient T cell compartments reconstituted, but responses to lymphocytic choriomeningitis virus (LCMV) infection also were achieved.19 In addition, Zhao et al.20 used the OP9-DL1 system for extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic progenitors. Despite these accomplishments, none of these methods have provided an efficient way to generate therapeutic antigen-specific T cells suitable for transplantation, directly from native stem cells.
Over the past decade, various studies have shown that in the thymus, interactions of immature (DP) T cells with MHC molecules is an essential step for the generation of mature, functional SP (CD4+ or CD8+) T cells.1 On the basis of our current understanding of such MHC-directed TcR maturation in the thymus and the demonstrated efficiency of antigen presentation by peptide-loaded MHC (pMHC) tetramers in vitro,10,11,29 it is reasonable to propose that antigen-specific T cells could be directly generated in vitro by efficiently presenting MHC-antigen complexes to stem cells that are already committed to the T cell lineage (i.e., early, immature T cells). Indeed, both the instructive and stochastic models of T cell generation1 specify a role for pMHC in the selection and development of CD4+ (MHC II) and CD8+ (MHC I) T cells.
Antigenic pMHC tetramers conjugated with fluorescently labeled streptavidin molecules are typically employed to enumerate antigen-specific T cells.30 Maus et al.10,29 developed bead-based artificial antigen-presenting cells displaying pMHC tetramers along with various costimulatory molecules (i.e., anti-CD3, anti-CD28, and 4-1BB) for ex vivo activation and expansion of antigen-specific T cells isolated from peripheral blood. Further, Savage et al.11 utilized soluble class I pMHC tetramers to expand antigen-specific cytotoxic CD8+ T cells in vitro and in vivo. Despite the limitations of these methods, in vitro engagement of pMHC tetramers with TcR, along with subsequent TcR signaling, achieved significant clonal expansion of antigen-specific T cells.10,29 In addition, it was demonstrated that soluble pMHC tetramers are more efficient than soluble pMHC monomers in activation and expansion of antigen-specific T cells. This multidirectional mode of antigen presentation may enhance TcR recognition, leading to the probable engagement of multiple TcRs by a single tetramer so as to benefit the activation and expansion of T cells. Therefore, we hypothesized that by coculturing with antigenic pMHC class I tetramers, which can induce pMHC I/TcR interactions in stem-cell-derived immature T cells, we can create an optimal environment for the direct production of antigen-specific, cytotoxic CD8+ T cells.
Here we show that using the MHC/TcR signaling approach, both thymic T cell progenitors and mouse ES cells can be efficiently engineered into CD8+ mature T cells specific for an antigenic peptide. The resulting CD8+ T cells showed robust CTL activity against peptide-loaded EL4 syngeneic target cells. This was accompanied by the maintenance of recombination activating gene-1 (RAG1) expression, indicating that TcR gene rearrangement was maintained as a result of pMHC–TcR interaction. This relatively simple culture system could ultimately provide a means for high-throughput production of therapeutic T cells and for the development of scalable methods for generation of T cells from ES cells and adult stem cells in vitro.
Materials and Methods
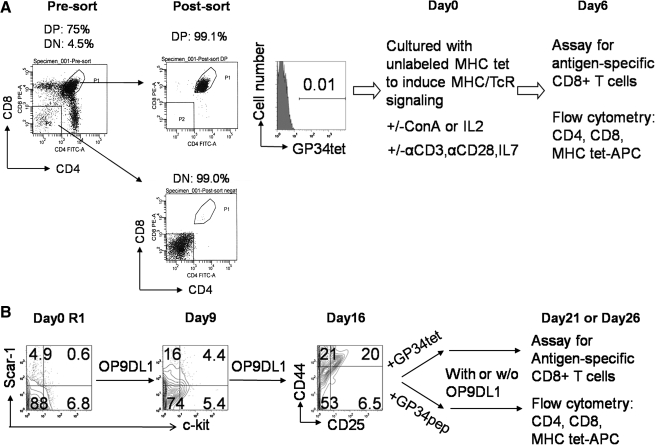
The detailed experimental schemes and starting cell population characterizations for both DP thymocyte differentiation and ES cell differentiation are shown in Figure 1.
FIG. 1.
Schematic of double-positive (DP) thymocyte and embryonic stem (ES) cell differentiation into antigen-specific, functional CD8+ T cells. (A) DP cell differentiation protocol: presort thymocytes show 75% CD4+CD8+ DP thymocytes and 4.5% of CD4−CD8− double-negative (DN) thymocytes; postsort analysis of purified thymocytes shows 99.1% DP thymocytes with negligible percentage (0.01%) staining positive with antigen-presenting cell (APC)–labeled major histocompatibility (MHC) class I GP34 tetramer (GP34tet) (from all gated live cells). The purified DP thymocytes were placed into differentiation cultures containing unlabeled GP34tet (or other unlabeled tetramers) with various supplemental factors (as shown) at day 0. Cells were harvested at day 6 for flow cytometric analysis of CD4, CD8, and MHC tetramer specificity, using APC-labeled GP34tet (or other unlabeled tetramers). (B) ES cell differentiation protocol: at day 0, 0.6% R1 ES cells were c-kit+/sca-1+. After 9 days coculture on OP9-DL1 monolayer, nonadherent single cells were isolated. These cells showed 4.4% of c-kit+/sca-1+ (hematopoietic progenitors). To generate antigen-specific T cells, these nonadherent cells were further cultured and transferred at day 16 into cultures supplemented with either GP34tet or GP34 peptide (GP34pep). Cells were harvested at day 21 or 26 for flow cytometry analysis of antigen-specific CD8+ T cells.
Cell lines and biological reagents
Mouse R1 ES cells31 with 129/Sv × 129/Sv-CP background were obtained from Dr. A. Nagy (Mount Sinai Hospital, Ontario, Canada). This cell line can also be purchased from American Type Culture Collection (ATCC). Leukemia inhibitory factor (LIF)–producing embryonic fibroblast cells (STO cells) were obtained from Ms. S. Maika (University of Texas at Austin). Murine embryonic fibroblasts were purchased from ATCC. Notch DL1 ligand functionalized mouse bone marrow stromal cells (OP9)19 were a gift from Dr. J.C. Zuniga-Pflucker (Toronto, Canada). EL4 cells were a gift from Dr. M. Poenie (University of Texas at Austin). All tetramers and peptides were purchased from the MHC Tetramer Core Laboratory at Baylor College of Medicine or Beckman Coulter Inc. The tetramers were formed by first refolding MHCs in the presence of desired antigenic peptide. The achieved pMHC monomers were then biotinylated and conjugated to the four biotin-binding sites on streptavidin.30 Tetramers without fluorescent labels were used for differentiation cultures, whereas tetramers with fluorescent-labeled streptavidin core were used to enumerate antigen-specific T cells by flow cytometry (fluorescence-activated cell sorting [FACS]). All antibodies for cell surface staining were purchased from BD Pharmingen. Functional-grade anti-CD3 (clone 245-2C11) and anti-CD28 (clone 37.51) antibodies were purchased from eBioscience. Interleukin-2 (IL-2), IL-7, and Flt3 ligands were purchased from Peprotech. Supernatant from Concanavalin A–stimulated rat spleen cells (henceforth referred as ConA) was produced as previously described.32
Thymocyte isolation, culture, and differentiation
Briefly, thymocytes were collected from 4–5-week-old female C57BL/6 mice (Jackson Laboratory) and stained with anti-CD4 and anti-CD8 antibodies. FACS-purified (BD FACSAria) CD4+CD8+ DP thymocytes were stimulated with unlabeled LCMV pMHC class I tetramers. All cultures were maintained in RPMI 1640 supplemented with 10% heat-inactivated and charcoal-striped fetal calf serum, 10 μg/mL anti-CD3, 5 μg/mL anti-CD28, 10 ng/mL IL-7, 15% ConA supernatant, or 10 ng/mL IL-2. IL-7 was replenished every 3 days. At day 6 or after (times specified in Results section), all cultured cells were collected to analyze expression of cell surface markers (CD4, CD8, etc.), and the antigen specificity of the TcRs were assessed by flow cytometry using fluorescently labeled tetramers.
ES cell culture and differentiation
As previously described,33 undifferentiated mouse R1 ES cells were maintained and expanded on irradiation-inactivated STO cells in Dulbecco's modified Eagle's medium (Invitrogen) containing 20% defined fetal bovine serum (FBS) (Hyclone), 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin. The LIF-producing STO cells can be replaced by mitomycin-inactivated murine embryonic fibroblasts with supplementation of LIF (Chemicon). R1 ESC/OP9-DL1 coculture was modified from a previously described method.19 Briefly, 5 × 104 ES cells were seeded onto 50%–60% confluent OP9-DL1 monolayers in T75 flasks in OP9 medium (Dulbecco's modified Eagle's medium supplemented with 20% FBS [Stem Cell Technologies], 2.2 g/L sodium bicarbonate, 100 U/mL penicillin, and 100 μg/mL streptomycin), in the absence of LIF or any other additional cytokines. On day 5 of culture, cells were disrupted by treatment with 0.25% trypsin–ethylenediaminetetraacetic acid (Invitrogen). The resulting single-cell suspension was preplated for 30 min, and then nonadherent cells were re-plated onto a fresh OP9-DL1 monolayer with fresh OP9 medium containing 5 ng/mL Flt3 ligand,34 at a density of 6 × 105 cells per T75 flask. On day 8 of coculture and every 4 days thereafter, nonadherent ES-cell-derived hematopoietic cells were collected by vigorous pipetting, filtered through a 40 μm nylon mesh, and then were transferred onto fresh OP9 monolayers in fresh OP9 medium. On day 8 of culture, another 5 ng/mL of Flt3 ligand was added together with 5 ng/mL IL-7.34 Both cytokines were supplemented to all subsequent passages. To generate antigen-specific T cells, 1 μg/mL of LCMV GP34 peptide (GP34pep) was added into cultures at day 16 and replenished every 4–5 days after cell passage. At days 8, 12, 16, 21, and 26, all nonadherent cells were collected and FACS analysis was performed to determine expression of hematopoietic progenitor markers (c-kit [clone ack45], sca-1 [clone D7])19; B cell marker (CD19 [clone 1D3])19; and T cell markers (Thy1.2 [clone 53–2.1], CD44 [clone IM7], CD25 [clone 7D4], CD4 [clone RM4-5], and CD8 [clone 53–6.7]),1 and to assess the antigen specificity of TcR.
CTL killing assay
The functionality of antigen-specific CD8+ T cells derived from DP thymocytes or ES cells was assessed in CTL killing assays performed as previously described (CyToxiLux Plus; OncoImmunin).35,36 All viable cells obtained from the differentiation cultures were counted as effector cells. EL4 target cells were maintained in RPMI-1640, supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 50 mM HEPES (pH 7.4), 100 U/mL penicillin, and 100 μg/mL streptomycin. EL4 target cells were labeled fluorescently as per the CyToxiLux Plus kit instructions. About 1 μg/mL LCMV peptides were loaded onto EL4-expressing MHC class I by incubation for 1 h at 37°C. Coincubation of effector and target cells was carried out for 3 h at 37°C, followed by 1 h incubation with the substrate of activated caspase-3. The percentage of activated caspase-3-positive EL4 cells was determined by flow cytometry.
Reverse transcription-polymerase chainreaction analysis of RAG1 expression in thymocytes
After 2 or 6 days of DP thymocyte cultures, RNA was prepared by Trizol (Invitrogen) extraction of all cultured DP thymocytes. RNA was also extracted from untreated DP and double-negative (DN) thymocytes after FACSAria sorting. The DN thymocytes used in the reverse transcription-polymerase chain reaction (RT-PCR) analysis were unfractionated from stromal or other CD4−CD8− non-T cells. The SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) was used to synthesize cDNA. PCR supermix (Invitrogen) was used to amplify RAG1 cDNA with forward 5′-CCAAGCTGCAGACATTCTAGCACTC-3′ and 5′-CTGGATCCGGAAAATCCTGGCAATG-3′ reverse primers. Forward primer 5′-CCTTCATTGACCTCAACTAC-3′ and reverse primer 5′-GGAAGGCCATGCCAGTGAGC-3′ were used for glyceraldehyde 3-phosphate dehydrogenase.
Flow cytometry and statistical analysis
After fluorescent antibody staining, cells were resuspended in 300 μL of phosphate-buffered saline and samples were analyzed using FACSCalibur (BD Biosciences). Live cells were distinguished from dead cells by gating using the forward and side scatter signals as well as by exclusion of 7-amino actinomycin D (eBioscience) or propidium iodide (Invitrogen)–stained dead cells. Nonstained cells or appropriate isotype-control-stained cells were used to evaluate background fluorescence. All FACS data were analyzed with FlowJo (version 7.2.5; Tree Star). Significant differences between experimental and control groups were evaluated using Student's t-test and a p-value of <0.05 was considered to be significant.
Results
Antigen-specific CD8+ SP T cells can be efficiently generated from immature DP thymocytes
To test our hypothesis and to evaluate the efficiency of pMHC I tetramers in inducing pMHC I/TCR signaling, we first evaluated whether DP cells, already committed to the immature T cell lineage, can be differentiated to mature CD8+ SP T cells that are antigen-specific and functional. DP cells were purified to about 99% homogeneity by FACSAria sorting (Fig. 1A). Purified DP cells were then stimulated by MHC tetramers and were maintained in RPMI medium supplemented with factors that stimulate growth, survival (IL-7,37–39 IL-2, or ConA supernatant), expansion, and differentiation (IL-2, anti-CD3, anti-CD28)20 of antigen-specific CD8+ T cells10,29 (Fig. 1A).
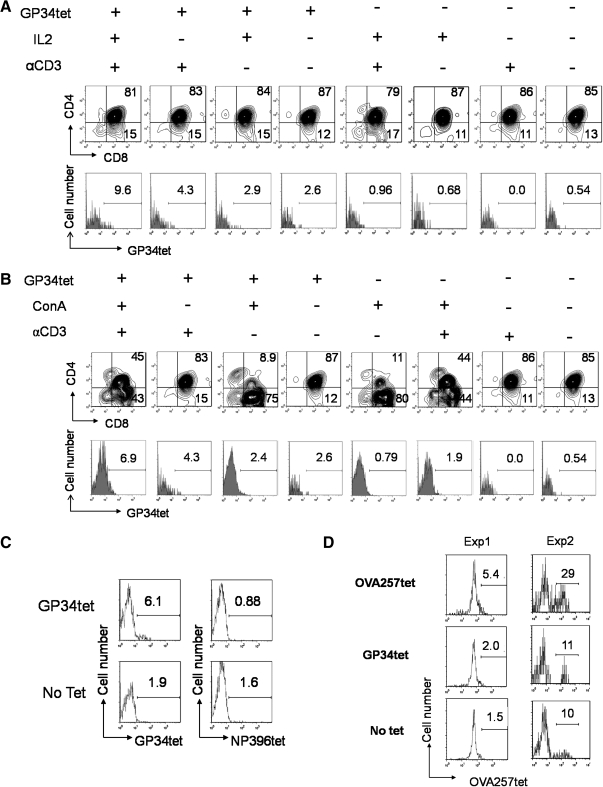
The effect of LCMV GP34 (GP34-41: AVYNFATC) pMHC I (H-2Kb) tetramers (GP34tet) on generation of GP34-specific CD8+ T cells is shown in Figure 2. After 6 days of culture, higher percentages of GP34 antigen-specific CD8+ T cells were observed in GP34tet-treated samples than in untreated controls (Fig. 2A: 9.6% vs. 0.96%; 4.3% vs. 0.0%; 2.9% vs. 0.68%; 2.6% vs. 0.54%, and Fig. 2B: 6.9% vs. 1.9%; 2.4% vs. 0.79%).
FIG. 2.
DP thymocytes can be converted into antigen-specific CD8+ T cells in vitro using MHC class I tetramers. Purified DP cells (Fig. 1A) were placed into differentiation cultures containing 10 ng/mL interleukin-7 (IL-7) and 5 μg/mL anti-CD28 (αCD28) in the presence (+) or absence (−) of other supplemental factors (as shown). MHC class I tetramers loaded with lymphocytic choriomeningitis virus (GP34tet or NP396tet) or ovalbumin OVA257tet peptides were used to induce differentiation into antigen-specific CD8+ single-positive (SP) T cells. Cells were harvested at day 6 and analyzed as described in Figure 1. (A) Effect of IL-2 and αCD3 supplementation on GP34tet-induced differentiation. (B) Effect of replacing IL-2 with Concanavalin A (ConA) supernatant on GP34tet-induced differentiation. (C) Specificity of tetramer staining: coculture of DP cells with GP34tet-induced GP34-specific CD8+ SP T cells but not NP396-specific cells. (D) Generation of OVA-specific CD8+ SP T cells from two representative experiments (Exp): coculture of DP cells with OVA257tet induced significantly enhanced staining for OVA257tet-APC compared controls. Data in (A–C) are representative from at least three independent experiments.
Supplementing tetramer-treated DP cells with IL-2 alone had minimal effect (Fig. 2A: from 2.6% to 2.9%), whereas anti-CD3 enhanced GP34-specific CD8+ cells (Fig. 2A: 2.6%–4.3%). However, addition of both anti-CD3 and IL-2 led to a synergistic increase (Fig. 2A, up to 9.6%). Similar results were observed by substituting IL-2 with ConA supernatant32 (Fig. 2B). The effect of ConA supernatant was examined as IL-2 is the best characterized lymphokine in it.40 Addition of GP34tet-induced antigen-specific CD8+ T cells both in the absence (Fig. 2B: 2.6% vs. 0.54%) or in the presence (Fig. 2B: 6.9% vs. 1.9%; 4.3% vs. 0.0%) of anti-CD3. Further, addition of both anti-CD3 and ConA supernatant led to synergistic increase. These GP34tet-induced CD8+ T cells were only minimally reactive with an irrelevant LCMV-derived (NP396-404: FQPQNGQFI) pMHC I (H-2Db) tetramer (NP396tet; Fig. 2C) or with an ovalbumin (OVA257-264: SIINFEKL) pMHC I (H-2Kb) tetramer (OVA257tet; Fig. 2D), confirming the specificity of our tetramer staining.
To confirm the versatility of this system, the same protocol was repeated with OVA257tet treatment of DP cells. As shown in Figure 2D (two independent experiments), CD8+ T cells specific for OVA257tet were efficiently generated, indicating that antigen-specific T cell generation is not limited to GP34tet.
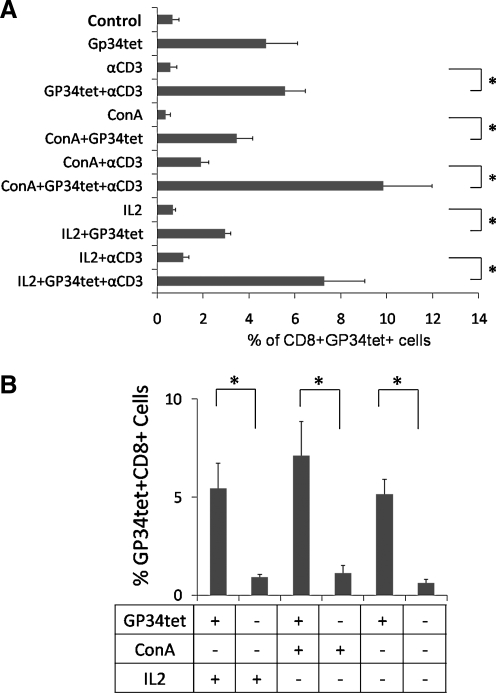
The GP34tet-induced T cell generation experiments were repeated more than three times with similar results. As shown in Figure 3A and B, statistically significant higher percentages (p < 0.05) of GP34-specific CD8+ T cells were generated when DP cells were cultured with antigen-loaded GP34tet in the presence of IL-2 or Con A.
FIG. 3.
Efficiency of converting DP thymocytes into GP34-specific CD8+ T cells using tetramer signaling. (A) The effect of GP34tet, ConA supernatant (ConA), and anti-CD3 (αCD3) in inducing GP34-specific CD8+ (CD8+GP34tet+) T cells. Bar graph shown is the percentage of CD8+GP34tet+ cells generated under 12 different culture conditions; 3 or more independent experiments were performed for each condition. *p-value < <0.05. (B) The effect of GP34tet in inducing GP34-specific, CD8+ T cells. Percent CD8+GP34tet+ cells generated in GP34tet-treated (+) conditions was compared to that in GP34tet untreated (−) conditions, with (+) or without (−) IL-2 or ConA. *p-Value < 0.05. All cultures were supplemented with IL-7 and anti-CD28. Control group indicates culture with IL-7 and anti-CD28 alone. Error bars shown are standard errors of mean.
In vitro differentiated GP34-specific CD8+ T cells from DP thymocytes are cytolytic to GP34pep-loaded target cells
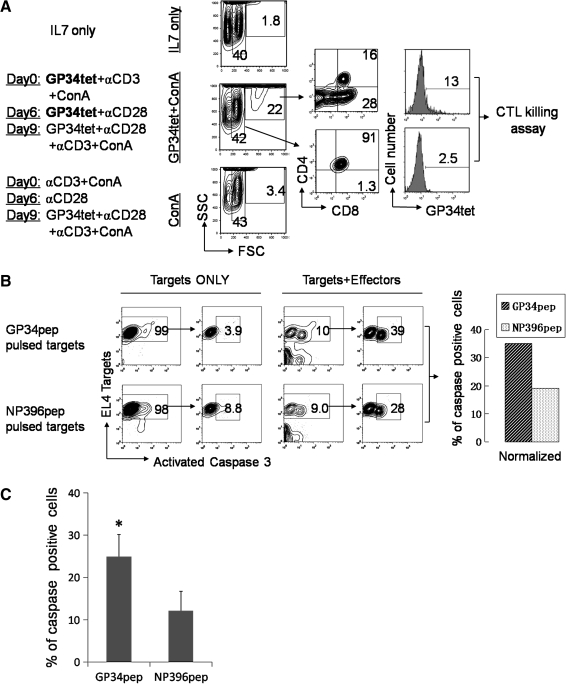
An activated caspase-3-based CTL killing assay36,41 was performed to determine the functionality of those in vitro differentiated CD8+ T cells. Since a large number of antigen-specific CD8+ T cells are required to perform this assay, cultured thymocytes were collected at day 12 instead of day 6 (Fig. 4A). At day 9, GP34tet, together with anti-CD28, anti-CD3, ConA supernatant, and IL-7, was added into the culture to stimulate the proliferation and activation of antigen-specific CD8+ T cells. As shown in Figure 4A, a distinct population of cells with high forward scatter (22% of total cells) were observed in GP34tet + ConA supernatant cultures compared to cultures with ConA supernatant but lacking GP34tet (3.4%) or cultures with only IL-7 (1.8%). As indicated in the GP34 + ConA condition, ∼28% of the high forward scatter population was CD8+ T cells. By contrast, only 1.3% cells with low forward scatter were CD8+ T cells, whereas 91% were DP cells. Further analysis of this high forward scatter fraction showed that 13% of these cells were GP34tet+, confirming that these cells are the cells of interest. In the CTL killing assay (Fig. 4B), GP34tet-specific T cells derived from DP thymocytes showed higher CTL activity against GP34peptide-loaded EL4 target cells (35.1%) than the control NP396 peptide-loaded EL4 target cells (19.2%). A summary with statistical analysis of the percent caspase-positive EL4 target cells from three representative killing assays is provided in Figure 4C. As shown, statistically higher percentage (p < 0.05, paired t-test) of antigen-specific cell killing was observed in GP34tet-generated CD8+ T cells.
FIG. 4.
In vitro derived CD8+ T cells from DP thymocytes are cytolytic to peptide-pulsed EL4 target cells. (A) Generation of GP34-specific CD8+ T cells for cytotoxic T lymphocyte (CTL) killing assay. Purified DP thymocytes were cultured as indicated on the left panel. IL-7 was replenished every 3 days. GP34tet, αCD3, αCD28, and ConA supernatant were re-added at day 9 to increase the number of antigen-specific CD8+ T cells. Center panel: all cells were harvested at day 12 for fluorescence-activated cell sorting (FACS) analysis using forward (FSC) and side (SSC) scatter. Specific cell populations (as shown) were evaluated for CD4, CD8, and GP34tet specificity (right panels). The 13% GP34tet-positive cells is related to the 28% of the large forward scattering CD8+ cells. (B) Analysis of GP34-specific CTL killing. All live cells from the GP34tet + ConA condition (A) were counted as Effectors. GP34pep or NP396 control peptide-pulsed EL4 cells were used as Targets at Effector:Target ratios of 25:1. The left panel of “Targets ONLY” and “Targets + Effectors” shows all gated live cells. Since apoptosis is examined in EL4 Targets, EL4 cells that stain positive (y axis) were gated and the percentage of caspase-3-positive cells (x axis) in this population was determined (right panel). Antigen-specific killing was calculated by subtracting the percentage of caspase-3-positive cells in the absence of Effectors (Targets ONLY) from values obtained in the presence of both Targets and Effectors. GP34tet-specific T cells derived from DP thymocytes showed significantly higher CTL activity against GP34pep-pulsed EL4 target cells (39% − 3.9% = 35.1%) than the control NP396 peptide-pulsed EL4 target cells (28% − 8.8% = 19.2%). (C) Summary of the percent caspase-positive EL4 target cells from three representative killing assays. *p-Value < 0.05, paired t-test. Error bars shown are standard errors of mean.
Addition of tetramer sustains RAG1 activity in differentiating thymocytes
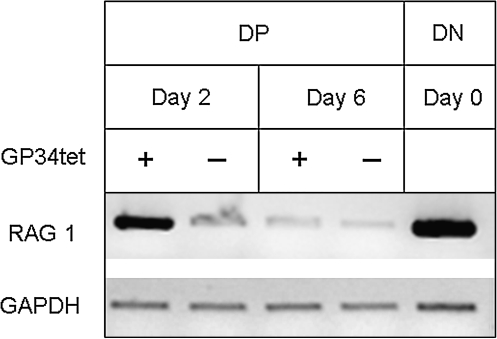
RAG1 catalysis is indispensable for TcR gene rearrangement. RT-PCR analysis was conducted to determine the mRNA levels of RAG1 in GP34tet-induced thymocytes. After 2 days of culture, we observed higher levels of RAG1 mRNA in GP34tet-treated DP thymocytes, whereas untreated controls had low levels of RAG1 mRNA (Fig. 5). No RAG1 was detected in “no RT” controls. These data indicate continued and/or newly initiated TcR gene rearrangement in the GP34tet-treated cells. RAG1 levels were diminished at day 6 in both GP34tet-treated cells and untreated controls.
FIG. 5.
Semiquantitative RT-polymerase chain reaction (PCR) analysis of RAG1 mRNA level in DP thymocytes cultured in vitro. DP thymocytes were treated with αCD3, αCD28, IL-2, and IL-7 with (+) or without (−) GP34tet in culture as described in Figure 2A. Cultured DP thymocytes were harvested on days 2 and 6 for RT-PCR analysis. FACSAria-purified, untreated (day 0) DN thymocytes (see Fig. 1A) served as a positive control for RAG1 (550 bp) activity. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (593 bp) was used as internal loading control. The possibility of false-positive RAG1 expression due to genomic DNA contamination in RNA preparation was excluded by employing a No RT condition in cDNA preparation, in which all components for cDNA synthesis were added except for reverse transcriptase. Data shown are negative images of the representative ethidium-bromide-stained gels for the PCR-amplified products. At day 2, GP34tet-treated DP cells showed RAG1 activity compared to untreated DP cells.
Antigen-specific CD8+ T cells can be efficiently generated from ES cells
Next, the validity of this approach in generating functional, GP34-specific T cells directly from ES cells was evaluated. We reasoned that this can be achieved by either (1) loading an antigenic peptide onto the MHC I molecules expressed on the OP9-DL1 cells or (2) using a pMHC I tetramer during coculture with OP9-DL1 cells. ES cells were first directed into early T cells by culturing on OP9-DL1 monolayer for 16 days (Fig. 1B). The T lineage commitment of ES cells was monitored by checking hematopoietic progenitor markers (c-kit+/sca-1+) and DN T cell markers (CD44/CD25) (Fig. 1B). Day 16 differentiated ES cells were harvested and transferred onto either GP34pep-loaded OP9-DL1 monolayers (GP34pep/OP9-DL1), onto OP9-DL1 monolayers in which GP34tet was added into the medium (GP34tet/OP9-DL1), into GP34tet in medium lacking OP9-DL1 monolayers (GP34tet), or onto OP9-DL1 monolayers alone (OP9-DL1) (Fig. 6A). At day 26, the percentage of CD8+ T cells was assessed using flow cytometry as described above. As shown in Figure 6A, only conditions and all conditions that employed pMHC I/TcR signaling generated high levels (ranging from 23% to 41%) of GP34-specific CD8+ T cells. The GP34pep/OP9-DL1 culture condition produced the highest percentage of total CD8+ T cells (54%), whereas the percentage of CD8+ T cells that were GP34 specific was higher in GP34tet/OP9-DL1-treated cells (41%).
FIG. 6.
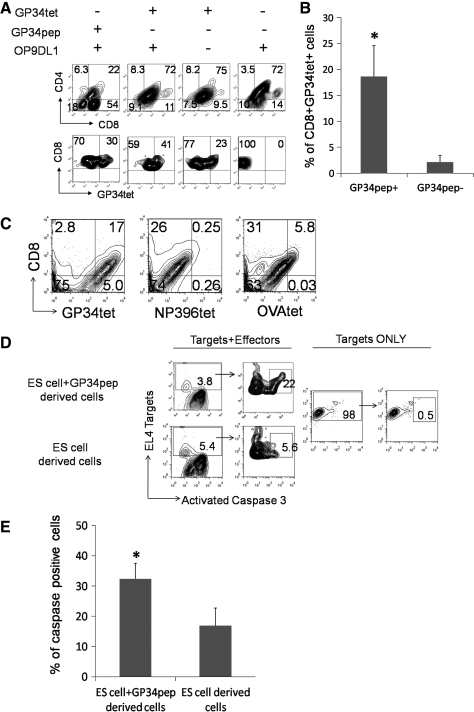
Antigen-specific CD8+ SP T cells can be efficiently generated in vitro from ES cells and are cytolytic to peptide-pulsed EL4 target cells. (A) Upper panel: FACS analysis of CD4 and CD8 expression of cells differentiated under various conditions. Lower panel: percentage of ES-derived CD8+GP34tet+ cells after 26 days in culture on OP9-DL1 monolayers (ESC/OP9-DL1 cocultures) in the presence (+) or absence (−) of GP34pep, GP34pep-loaded tetramers (GP34tet), or OP9-DL1 cells. (B) A summary of the percent CD8+GP34tet+ cells after 21 or 26 days in culture on OP9-DL1 monolayers (ESC/OP9-DL1 cocultures) in the presence (+) or absence (−) of GP34pep. *p-Value < 0.05. Error bars shown are standard errors of mean. (C) Specificity of GP34tet staining on ES-cell-derived CD8+ T cells: GP34-specific CD8+ T cells were generated by ESC/OP9-DL1 cocultures supplemented at day 16 with GP34pep, ConA supernatant, αCD3, αCD28, and IL-7. Cells were harvested at day 21 and stained with GP34tet, NP396tet, or OVA257tet. (D) Measurement of CTL activity of CD8+ T cells generated from ES cells in vitro: cells generated using the GP34pep (ES cells + GP34pep-derived cells) or cells with random antigen specificity (ES-cell-derived cells) were collected on day 26 from cocultures as indicated in (A). All live nonadherent cells (excluding OP9-DL1 stromal cells) were collected and counted as Effectors. GP34pep-pulsed EL4 cells were used as Targets at an Effector:Target ratio of 25:1. Target ONLY shows the background caspase activity of EL4 cells in the absence of effectors. Since apoptosis is examined in EL4 Targets, EL4 cells that stain positive (y axis) were gated and the percentage of caspase-3-positive cells (x axis) in this population was determined (right panel). (E) Percent caspase-positive EL4 target cells from three representative killing assays. *p-Value < 0.05, paired t-test. Error bars shown are standard errors of mean.
These experiments were repeated thrice with similar results. A summary with statistical analysis of the percent GP34-specific CD8+ T cells generated in all three experiments is provided in Figure 6B. As shown, significantly higher (p < 0.05) antigen-specific T cells were generated in GP34pep-treated cultures.
The effect of using ConA supernatant with anti-CD3, anti-CD28, and IL-7 was also evaluated in ES cells. At day 16 of ESC/OP9-DL1 coculture, GP34pep was loaded onto OP9-DL1 monolayers, supplemented with anti-CD3, anti-CD28, IL-7, and ConA supernatant in culture media. Cells were harvested at day 21 and stained with GP34tet, NP396tet, or OVA257tet for FACS analysis. As shown in Figure 6C, GP34pep induction with ConA supernatant produced a higher percentage of ES-cell-derived CD8+GP34tet+ cells (17%) than CD8+OVA257tet+ cells (5.8%) and CD8+NP396tet+ (0.25%). These results also confirmed the specificity of tetramer staining on ES-cell-derived cells as GP34pep-induced ES cells showed minimal staining for OVA257tet and NP396tet (Fig. 6C).
GP34-specific CD8+ T cells generated from ES cells demonstrate efficient CTL activity
To evaluate whether ES-cell-derived antigen-specific CD8+ T cells were functional, day 16 cells from ESC/OP9-DL1 coculture were transferred onto GP34pep-loaded or no exogenous peptide-loaded (i.e., carrying endogenous peptides only) OP9-DL1 monolayers and cultured for another 10 days. At day 26, all live cells, excluding OP9-DL1 stromal cells, were collected for CTL killing assays. As shown in Figure 6D, CD8+ T cells generated by GP34pep treatment (ES cells + GP34pep) showed higher levels (22%) of CTL activity against GP34pep-loaded EL4 target cells compared to cells from control samples (5.6%). The EL4 target only group (assay control) showed minimal activity. A summary of the percent caspase-positive EL4 target cells from three independent killing assays is provided in Figure 6E. As shown, significantly higher (p < 0.05; paired t-test) CTL activity was seen in GP34pep-treated cultures than in no peptide-treated cultures.
Discussion
We have developed a simple system for generating antigen-specific, cytotoxic CD8+ T cells in vitro from immature DP thymocytes or from mouse ES cells by artificially inducing MHC-TcR signaling. In both the thymocyte cultures and the ES cell cultures, MHC I/TcR signaling that was mediated through antigen-loaded tetramers or antigenic peptide-loaded stromal cells led to successful generation of antigen-specific functional CD8+ T cells. Our data also showed that addition of IL-2/ConA or anti-CD3 into thymic progenitor (DP cells) cultures increased the percentage of antigen-specific T cells.
A central question raised by our results is the following: What is the underlying molecular mechanism for driving generation of antigen-specific SP T cells in vitro? Although this needs to be established in future studies, there are two possible pathways: (1) clonal expansion of the randomly generated, antigen-specific T cells (either among the DP-derived CD8+ T cells or among the ES-derived CD8+ T cells) and/or (2) active TcR gene rearrangement in the presence of continuous antigen-specific, MHC/TcR signaling as provided by the tetramer or MHC molecules on OP9 cells. We believe that both these mechanisms could be in play. We presented preliminary evidence that supports the possibility of tetramer-induced gene rearrangement. In individual developing thymocytes, successive TcR gene rearrangement at the same TcRα locus has been observed.42,43 This event, termed receptor editing, can occur repeatedly until the appropriate rearrangement signals for a thymocyte to be positively selected are achieved. This potential for successive rearrangements eventually leads to altered antigen receptor specificity and plays an important role in determining the preimmune repertoire of Ag-specific TcRs.44,45 Expression of the TcR is not in itself sufficient to shut off gene rearrangement. Thus, both self- and non-self-antigens are able to induce TcR editing by engaging with existing TcR.42,46 In light of these observations, we suggest that interactions with non-self-peptide–MHC complexes might induce the development of T cells with TcRs that are specific for that peptide. Indeed, our data indicate that non-self-peptides presented by both OP9-DL1 stromal cells and tetramers skew the differentiation of functional CD8+ T cell from both ES cells and immature DP thymocytes. Future studies are needed to study the polyclonality of the antigen-specific T cells to further determine whether they are expanded from a single clone or are actually induced to differentiate through MHC/TcR signaling.
One argument in favor of the clonal expansion mechanism is that IL-2 (together with anti-CD3 and anti-CD28) has been shown to have similar effects on in vitro expansion of T cells.10,29 However, it is also still possible that maturation of DP T cells to CD8+ T cells took place in the thymocyte cultures. It has been demonstrated that IL-2 and anti-CD3 augment differentiation of HSC-derived DP T cells into SP T cells.20 In addition, IL-7 and anti-CD28 were employed in all our thymocyte culture systems. IL-7 was used as it is crucial for the survival and homeostasis of T cells, and it induces chromatin remodeling at the antigen receptor loci in lymphoid progenitors.37–39 We employed anti-CD28 because it has been reported to function as an important costimulatory factor in the clonal expansion of antigen-specific T cells.10,29 We observed no significant effect of IL-7 and anti-CD28 on generation of antigen-specific T cells in our DP thymocyte cultures (data not shown). The lack of this effect suggests that the primary mechanism may not be clonal expansion.
Although GP34-specific CTL killing was significantly higher than background controls, we observed, on average, somewhat high nonspecific background in our CTL killing assays. This high background could derive from the fact that all cells after culture were collected and were used as effector cells (due to cell number limitations). In addition, in the thymocyte-derived T cell killing assay, since the NP396 peptide does not bind to the same MHC molecule as the GP34pep, the background killing could be caused by the recognition of endogenous H2-Kb MHC I presented peptides on the EL4 target cells. This could be confirmed in future experiments by mixing unpulsed target cells with GP34-specific T cells.
One argument against using such ES-cell-derived, in vitro differentiated, functional T cells for therapy is based on safety concerns arising from the fact that these cells do not undergo negative selection against self-antigens. However, the observations that not all self-antigens are likely to be expressed in the thymus and that the thymus regresses in puberty indicate an absolute need for peripheral tolerance to complement the central tolerance mechanisms and thus T cells escaping central tolerance, such as these in vitro differentiated cells from ES cells in this study, may circumvent autoimmune reaction by these tolerance mechanisms or result in autoreaction without a precipitating autoimmune disease.47 In addition, our results have shown that T cells produced in vitro display significant peptide-specific binding and peptide-specific cell lysis. It is less likely that a T cell bears two dominant specificities of the TcR repertoire at the same time; that is, one for non-self-peptide, the other for self. However, further in vivo investigation is needed to address this issue. One possible solution could be coculture of these antigen-specific T cells with a patient's peripheral blood-antigen-presenting cells to induce negative selection and separate only the nonreactive (i.e., no apoptotic) T cells.
Although MHC-matched ES cells are difficult to avail, somatic cell nuclear transfer48 could circumvent this limitation. In addition, induced pluripotent stem (iPS) technology49,50 could greatly facilitate the application of this system in the clinic since one can start with MHC-matched, patient-specific iPS cells. We are currently working on evaluating if such antigen-specific T cells can also be generated from iPS cells.
In summary, we have developed an in vitro system to generate therapeutic T cells from pluripotent ES cells and progenitors. This relatively simple culture system could ultimately provide a means for high-throughput production of therapeutic T cells and for the development of scalable methods for generation of T cells from ES cells and adult stem cells in vitro.
Acknowledgments
We thank Cassandra Horne, Richard Salinas, and the Institute of Cell and Molecular Biology at The University of Texas at Austin for help with cell sorting and Dr. Irina Fernandez for comments and discussion on the article. This research was partially supported by the National Science Foundation CAREER award and National Institutes of Health (Grant R21HL089843).
Author Contributions
J.L., H.N., P.W.T., and K.R. planned and designed experiments; J.L. and H.N. conducted experiments, and acquired and analyzed data; J.L., H.N., P.W.T., and K.R. interpreted the data and wrote the article.
Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Germain R.N. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 2.Singer A. Adoro S. Park J.H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontaine P. Roy-Proulx G. Knafo L. Baron C. Roy D.C. Perreault C. Adoptive transfer of minor histocompatibility antigen-specific T lymphocytes eradicates leukemia cells without causing graft-versus-host disease. Nat Med. 2001;7:789. doi: 10.1038/89907. [DOI] [PubMed] [Google Scholar]
- 4.Schultze J.L. Anderson K.C. Gilleece M.H. Gribben J.G. Nadler L.M. A pilot study of combined immunotherapy with autologous adoptive tumour-specific T-cell transfer, vaccination with CD40-activated malignant B cells and interleukin 2. Br J Haematol. 2001;113:455. doi: 10.1046/j.1365-2141.2001.02760.x. [DOI] [PubMed] [Google Scholar]
- 5.Galea-Lauri J. Darling D. Mufti G. Harrison P. Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rhee F. Barrett J. Adoptive transfer of Ag-specific T cells to prevent CMV disease after allogeneic stem-cell transplantation. Cytotherapy. 2002;4:3. doi: 10.1080/146532402317251473. [DOI] [PubMed] [Google Scholar]
- 7.Engleman E.G. Dendritic cell-based cancer immunotherapy. Semin Oncol. 2003;30:23. doi: 10.1016/s0093-7754(03)00229-x. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong A.C. Dermime S. Mulryan K. Stern P.L. Bhattacharyya T. Hawkins R.E. Adoptive transfer of anti-idiotypic T cells cure mice of disseminated B cell lymphoma. J Immunother. 2004;27:227. doi: 10.1097/00002371-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Morgan R.A. Dudley M.E. Wunderlich J.R. Hughes M.S. Yang J.C. Sherry R.M. Royal R.E. Topalian S.L. Kammula U.S. Restifo N.P. Zheng Z. Nahvi A. de Vries C.R. Rogers-Freezer L.J. Mavroukakis S.A. Rosenberg S.A. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maus M.V. Thomas A.K. Leonard D.G. Allman D. Addya K. Schlienger K. Riley J.L. June C.H. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 11.Savage P. Millrain M. Dimakou S. Stebbing J. Dyson J. Expansion of CD8+ cytotoxic T cells in vitro and in vivo using MHC class I tetramers. Tumour Biol. 2007;28:70. doi: 10.1159/000099152. [DOI] [PubMed] [Google Scholar]
- 12.Oelke M. Maus M.V. Didiano D. June C.H. Mackensen A. Schneck J.P. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 13.Daley G.Q. From embryos to embryoid bodies: generating blood from embryonic stem cells. Ann N Y Acad Sci. 2003;996:122. doi: 10.1111/j.1749-6632.2003.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 14.Ying Q.L. Nichols J. Chambers I. Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 15.Storb R. Allogeneic hematopoietic stem cell transplantation—yesterday, today, and tomorrow. Exp Hematol. 2003;31:1. doi: 10.1016/s0301-472x(02)01020-2. [DOI] [PubMed] [Google Scholar]
- 16.Little M.T. Storb R. The future of allogeneic hematopoietic stem cell transplantation: minimizing pain, maximizing gain. J Clin Invest. 2000;105:1679. doi: 10.1172/JCI10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maris M. Storb R. The transplantation of hematopoietic stem cells after non-myeloablative conditioning: a cellular therapeutic approach to hematologic and genetic diseases. Immunol Res. 2003;28:13. doi: 10.1385/IR:28:1:13. [DOI] [PubMed] [Google Scholar]
- 18.Storb R.F. Lucarelli G. McSweeney P.A. Childs R.W. Hematopoietic cell transplantation for benign hematological disorders and solid tumors. Hematol Am Soc Hematol Educ Program. 2003;1:372. doi: 10.1182/asheducation-2003.1.372. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt T.M. de Pooter R.F. Gronski M.A. Cho S.K. Ohashi P.S. Zuniga-Pflucker J.C. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y. Parkhurst M.R. Zheng Z. Cohen C.J. Riley J.P. Gattinoni L. Restifo N.P. Rosenberg S.A. Morgan R.A. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varnum-Finney B. Dallas M.H. Kato K. Bernstein I.D. Notch target Hes5 ensures appropriate Notch induced T- versus B-cell choices in the thymus. Blood. 2008;111:2615. doi: 10.1182/blood-2007-03-079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varnum-Finney B. Brashem-Stein C. Bernstein I.D. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 23.Taqvi S. Dixit L. Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J Biomed Mater Res A. 2006;79:689. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- 24.La Motte-Mohs R.N. Herer E. Zuniga-Pflucker J.C. Induction of T-cell development from human cord blood hematopoietic stem cells by delta-like 1 in vitro. Blood. 2005;105:1431. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt T.M. Zuniga-Pflucker J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 26.Besseyrias V. Fiorini E. Strobl L.J. Zimber-Strobl U. Dumortier A. Koch U. Arcangeli M.L. Ezine S. Macdonald H.R. Radtke F. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. 2007;204:331. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal M. Borowski C. Palomero T. Ferrando A.A. Oberdoerffer P. Meng F. Ruiz-Vela A. Ciofani M. Zuniga-Pflucker J.C. Screpanti I. Look A.T. Korsmeyer S.J. Rajewsky K. von Boehmer H. Aifantis I. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terra R. Louis I. Le Blanc R. Ouellet S. Zuniga-Pflucker J.C. Perreault C. T-cell generation by lymph node resident progenitor cells. Blood. 2005;106:193. doi: 10.1182/blood-2004-12-4886. [DOI] [PubMed] [Google Scholar]
- 29.Maus M.V. Riley J.L. Kwok W.W. Nepom G.T. June C.H. HLA tetramer-based artificial antigen-presenting cells for stimulation of CD4+ T cells. Clin Immunol. 2003;106:16. doi: 10.1016/s1521-6616(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 30.Bodinier M. Peyrat M.A. Tournay C. Davodeau F. Romagne F. Bonneville M. Lang F. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat Med. 2000;6:707. doi: 10.1038/76292. [DOI] [PubMed] [Google Scholar]
- 31.Nagy A. Rossant J. Nagy R. Abramow-Newerly W. Roder J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaattari S.L. Rittenberg M.B. Concanavalin A supernatant recruits antigen-insensitive IgG memory B lymphocyte precursors into an antigen-sensitive precursor pool. J Immunol. 1982;128:720. [PubMed] [Google Scholar]
- 33.Taqvi S. Roy K. Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6024. doi: 10.1016/j.biomaterials.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Baird A.M. Gerstein R.M. Berg L.J. The role of cytokine receptor signaling in lymphocyte development. Curr Opin Immunol. 1999;11:157. doi: 10.1016/s0952-7915(99)80027-2. [DOI] [PubMed] [Google Scholar]
- 35.Barber D.L. Wherry E.J. Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 36.Liu L. Chahroudi A. Silvestri G. Wernett M.E. Kaiser W.J. Safrit J.T. Komoriya A. Altman J.D. Packard B.Z. Feinberg M.B. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8:185. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- 37.Fry T.J. Mackall C.L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 38.Hofmeister R. Khaled A.R. Benbernou N. Rajnavolgyi E. Muegge K. Durum S.K. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 39.Muegge K. Vila M.P. Durum S.K. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993;261:93. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 40.Raulet D.H. Hunig T. Parker D.C. T cells produce TRF in response to Con A and factors in T cell hybridoma supernatants. J Immunol. 1982;128:908. [PubMed] [Google Scholar]
- 41.Jerome K.R. Sloan D.D. Aubert M. Measurement of CTL-induced cytotoxicity: the caspase 3 assay. Apoptosis. 2003;8:563. doi: 10.1023/A:1026123223387. [DOI] [PubMed] [Google Scholar]
- 42.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 43.Petrie H.T. Livak F. Burtrum D. Mazel S. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J Exp Med. 1995;182:121. doi: 10.1084/jem.182.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahan C.J. Fink P.J. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- 45.Yokosuka T. Takase K. Suzuki M. Nakagawa Y. Taki S. Takahashi H. Fujisawa T. Arase H. Saito T. Predominant role of T cell receptor (TCR)-alpha chain in forming preimmune TCR repertoire revealed by clonal TCR reconstitution system. J Exp Med. 2002;195:991. doi: 10.1084/jem.20010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bas A. Hammarstrom S.G. Hammarstrom M.L. Extrathymic TCR gene rearrangement in human small intestine: identification of new splice forms of recombination activating gene-1 mRNA with selective tissue expression. J Immunol. 2003;171:3359. doi: 10.4049/jimmunol.171.7.3359. [DOI] [PubMed] [Google Scholar]
- 47.Zakrzewski J.L. Kochman A.A. Lu S.X. Terwey T.H. Kim T.D. Hubbard V.M. Muriglan S.J. Suh D. Smith O.M. Grubin J. Patel N. Chow A. Cabrera-Perez J. Radhakrishnan R. Diab A. Perales M.A. Rizzuto G. Menet E. Pamer E.G. Heller G. Zuniga-Pflucker J.C. Alpdogan O. van den Brink M.R. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 48.Gurdon J.B. Byrne J.A. Simonsson S. Nuclear reprogramming and stem cell creation. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11819. doi: 10.1073/pnas.1834207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okita K. Ichisaka T. Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 50.Wernig M. Meissner A. Foreman R. Brambrink T. Ku M. Hochedlinger K. Bernstein B.E. Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]