Abstract
Spinocerebellar ataxia type 5 (SCA5) is an autosomal dominant neurodegenerative disorder caused by mutations in β-III spectrin. A mouse lacking full-length β-III spectrin has a phenotype closely mirroring symptoms of SCA5 patients. Here we report the analysis of heterozygous animals, which show no signs of ataxia or cerebellar degeneration up to 2 years of age. This argues against haploinsufficiency as a disease mechanism and points towards human mutations having a dominant-negative effect on wild-type (WT) β-III spectrin function. Cell culture studies using β-III spectrin with a mutation associated with SCA5 (L253P) reveal that mutant protein, instead of being found at the cell membrane, appears trapped in the cytoplasm associated with the Golgi apparatus. Furthermore, L253P β-III spectrin prevents correct localization of WT β-III spectrin and prevents EAAT4, a protein known to interact with β-III spectrin, from reaching the plasma membrane. Interaction of β-III spectrin with Arp1, a subunit of the dynactin–dynein complex, is also lost with the L253P substitution. Despite intracellular accumulation of proteins, this cellular stress does not induce the unfolded protein response, implying the importance of membrane protein loss in disease pathogenesis. Incubation at lower temperature (25°C) rescues L253P β-III spectrin interaction with Arp1 and normal protein trafficking to the membrane. These data provide evidence for a dominant-negative effect of an SCA5 mutation and show for the first time that trafficking of both β-III spectrin and EAAT4 from the Golgi is disrupted through failure of the L253P mutation to interact with Arp1.
INTRODUCTION
Spinocerebellar ataxia type 5 (SCA5) is an autosomal dominant neurodegenerative disease. It is characterized by gait and limb ataxia, dysarthria and uncoordinated eye movements, and arises from dysfunction and degeneration of the cerebellum (1–3). Different mutations in the gene encoding β-III spectrin (SPTBN2) were identified as the genetic cause of SCA5 in three independent families (3).
Spectrins are heterotetramers comprising two α- and two β-subunits. The α- and β-subunits associate laterally, forming anti-parallel heterodimers which interact head-to-head to form the functional heterotetramer (4,5). Short actin filaments then link the spectrin tetramers together forming a flexible spectrin network attached to the inner leaf of the membrane bilayer. They were originally discovered in erythrocytes and shown to be critical for mechanical support and maintenance of structural membrane integrity, with defects resulting in hereditary elliptocytosis and spherocytosis (6–9). Spectrins also play important roles in stabilizing cell–cell contacts and localizing ion channels and cell adhesion molecules within specific subdomains of the plasma membrane (10–12).
Vertebrates have two α-subunits (αI/αII), four β-subunits (βI–βIV) and a β-H subunit creating diversity and specialization of function (13). The mammalian erythrocyte spectrin (αI/βI) is found in striated muscle and a subset of neurons, whereas αII/βII, αII/βIII and αII/βIV are the major forms in non-erythroid vertebrate tissues. β-III spectrin is primarily expressed in the nervous system, with the highest levels of expression in the cerebellum, where it is found in Purkinje cell soma and dendrites (14,15). Originally, it was shown to associate with the Golgi apparatus (16), but this staining is now thought to have arisen from antibody cross-reactivity (17). We have previously shown β-III spectrin to stabilize EAAT4, the glutamate transporter predominantly expressed in Purkinje cells, at the cell surface (15). Other investigators have found the N-terminal actin-binding region of β-III spectrin interacts with Arp1, a subunit of the dynactin–dynein complex (18). A role for β-III spectrin in dynein-mediated vesicular transport is implied by observation of axonal transport defects in Drosophila expressing mutant forms of β spectrin, which are exacerbated by the expression of mutant dynein and dynactin (19).
We recently generated a functional β-III spectrin knockout mouse (β-III−/−) and found that from a young age it showed characteristic features of cerebellar ataxia, suggesting a loss of β-III spectrin function underlies SCA5 pathogenesis (20). Since the human disease is autosomal dominant, this finding indicates that either the mutant forms of β-III spectrin are simply inactive and the disease arises from haploinsufficiency or, in addition to being non-functional, the mutant subunits also have a dominant-negative effect and suppress the function of wild-type (WT) spectrin. In order to investigate these two possibilities, we analysed heterozygous animals for signs of motor deficits and cerebellar degeneration. We show here that even at 2 years of age, heterozygous animals show no signs of ataxia or cerebellar pathology. Instead, using cell culture studies, we provide evidence that β-III spectrin containing a mutation associated with SCA5 (L253P) has a dominant-negative effect on WT protein function and interferes with membrane protein trafficking.
RESULTS
Heterozgous β-III+/− spectrin mice show no signs of ataxia or cerebellar degeneration
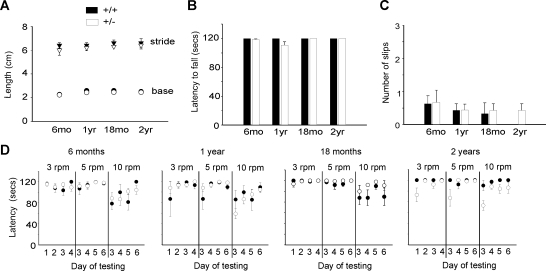
We previously reported that homozygous β-III spectrin-deficient mice (β-III−/−) develop characteristics of ataxia including a wider hind-limb gait, progressive motor incoordination, cerebellar atrophy and Purkinje cell loss (20). To determine whether heterozygous (β-III+/−) mice eventually show signs of ataxia, we carried out behavioural tests and histological analysis on mice aged 6 months to 2 years of age. Analysis of footprint patterns showed no significant difference in base width or stride length between β-III+/− and WT littermates (Fig. 1A). There was also no significant difference in motor performance between the genotypes, heterozygous animals performing as well as WT controls on a stationary rod (Fig. 1B), an elevated beam (Fig. 1C) and a rotating rod task (Fig. 1D).
Figure 1.
Progressive motor deficits not seen in heterozygous β-III+/− mice. (A) Footprint analysis, base width and stride length of 6-month to 2-year old mice. (B) Ability to remain on stationary rod. Mice were given four consecutive trials, with maximum time of 60 s. (C) Number of hind-limb slips age-matched WT and β-III+/− mice made when crossing narrow, elevated beam. (D) Latency of WT and β-III+/− animals to fall from rotarod at 3, 5 and 10 rpm. Mice were given four trials per day and allowed a maximum retention time of 120 s per trial. All data are means ± SEM (WT n = 3–7, β-III+/− n = 7–9).
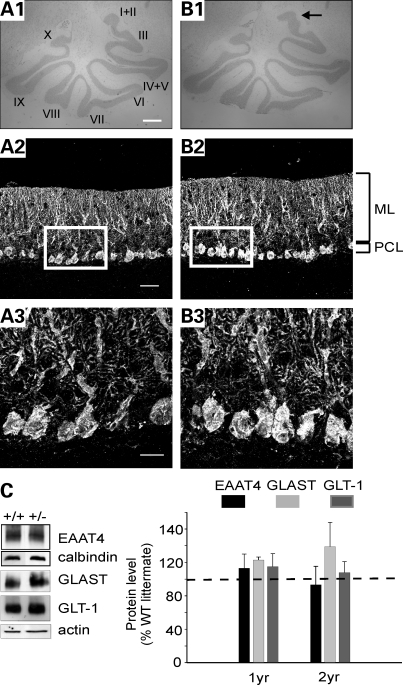
Cerebellar sections stained for Nissl demonstrated that the size and morphology of the cerebellum appeared normal in 2-year old β-III+/− mice, apart from slight differences in folia I and II (Fig. 2A1 and B1). Immunostaining for calbindin showed no changes to Purkinje cell morphology in β-III+/− mice (Fig. 2A2 and A3, and B2 and B3), whereas quantification of Purkinje cell density and molecular layer thickness revealed no cell loss or cerebellar atrophy (WT, 39.6 ± 3.4; β-III+/−, 37 ± 8.6 cell/mm; P = 0.73 and WT, 181.7 ± 7.1; β-III+/−, 197.7 ± 11.4 µm; P = 0.37; n = 3 of each genotype). We also saw no significant reduction in glutamate transporter levels in β-III+/− mice (Fig. 2C), providing additional evidence that the loss of EAAT4 and GLAST in β-III−/− mice (20) may be important aspects of disease pathogenesis. Therefore, β-III+/− mice display none of the characteristics of cerebellar ataxia, arguing against haploinsufficiency as a disease mechanism in the mouse, and hence arguing for a dominant-negative effect of mutant β-III spectrin on WT β-III spectrin function associated with SCA5.
Figure 2.
No cerebellar pathology in β-III+/− mice. Histological analysis of cerebellum from 2-year old WT (A) and β-III+/− (B) mice. (A1, B1) Cresyl violet stain shows whole cerebellar morphology. (A2–B3) Calbindin immunostaining reveals Purkinje cell morphology (ML, molecular layer; PCL, Purkinje cell layer; scale bars: A1 and B1, 500 µm; A2 and B2, 50 µm; A3 and B3, 20 µm). (C) Representative western blot and densitometry data quantifying levels of plasma membrane glutamate transporters in 2-year-old β-III+/− and WT animals. EAAT4 levels normalized with calbindin, a Purkinje cell specific marker. GLAST and GLT1 normalized with actin. All data are means ± SEM (n = 3 of each genotype).
β-III spectrin associates with the Golgi apparatus when Leu253 is substituted by proline
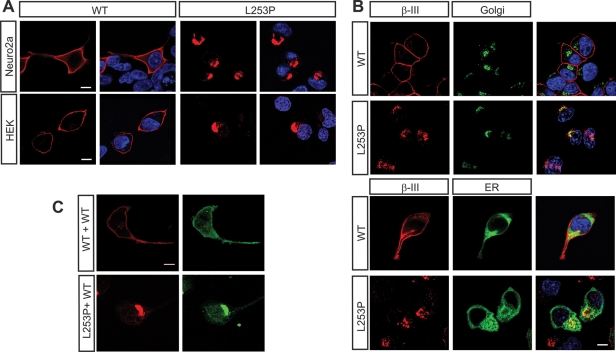
To investigate potential dominant-negative effects, we transfected Neuro2a and human embryonic kidney (HEK) 293T cells with constructs encoding either myc-tagged WT β-III spectrin or β-III spectrin containing a mutation associated with SCA5. The missense mutation (L253P) found in one family with SCA5 was introduced by site-directed mutagenesis into rat β-III spectrin cDNA. The leucine 253 residue and the N-terminus of β-III spectrin are highly conserved from fly to human (3). Immunostaining with an anti-c-myc antibody revealed that, unlike WT, L253P β-III spectrin appears to accumulate in a discrete intracellular location and is no longer found at the plasma membrane (Fig. 3A). No difference in the cellular distribution was seen between the two cell lines examined. We therefore used Neuro2a cells, unless otherwise stated, for all subsequent experiments since β-III spectrin is predominantly a neuronal protein (14–16). To elucidate the intracellular distribution, we co-expressed β-III spectrin constructs with either a Golgi or an endoplasmic reticulum (ER) marker. This revealed that L253P β-III spectrin appears to associate with the Golgi apparatus (Fig. 3B).
Figure 3.
Cellular localization of full-length L253P β-III spectrin overlaps with Golgi marker. (A) Neuro2a and HEK293 cells transfected with either myc-tagged WT or L253P β-III spectrin, fixed and stained using anti-c-myc antibody. Nucleus stained with DAPI (blue). (B) Neuro2a cells cotransfected with either a Golgi (green) or ER (green) marker and myc-tagged WT or L253P β-III spectrin (red). Nucleus stained with DAPI (blue). (C) Neuro2a cells cotransfected with YFP-tagged WT β-III spectrin and either myc-tagged WT or L253P β-III spectrin. Cells immunostained using anti-c-myc antibody (red) and anti-GFP antibody (green). All images are representative of three independent experiments (scale bar, 10 µm).
To determine the effect of L253P β-III spectrin on WT protein, we co-expressed yellow fluorescent protein (YFP)-tagged WT β-III spectrin with myc-tagged L253P β-III spectrin. We found that the presence of L253P β-III spectrin resulted in WT β-III spectrin–YFP being trapped in the same intracellular location as L253P (Fig. 3C). In contrast, WT β-III spectrin–YFP was found at the plasma membrane when myc-tagged WT β-III spectrin was co-expressed. This finding suggests that the presence of the L253P missense mutation confers a dominant-negative effect on WT β-III spectrin protein.
L253P β-III spectrin interferes with protein trafficking and fails to interact with Arp1
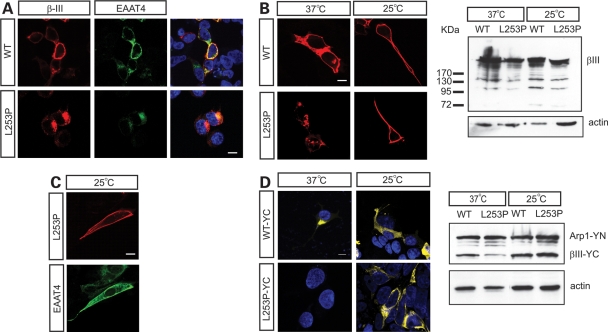
EAAT4 is known to interact directly with and be stabilized at the cell membrane by β-III spectrin (15). Moreover, a decrease in EAAT4 protein is seen in young β-III−/− mice (20) and dramatic changes in EAAT4 distribution are seen in SCA5 autopsy tissue (3). Taken together, these findings suggest that EAAT4 may play an important role in disease pathogenesis. We therefore examined what effect the expression of L253P β-III spectrin had on EAAT4 cellular distribution. Immunofluorescence microscopy revealed accumulation of EAAT4 at the Golgi apparatus when L253P was co-expressed compared with WT β-III spectrin, suggesting a disruption to protein trafficking (Fig. 4A). To test whether a lower temperature could rescue the defect, we incubated transfected cells at 25°C for an additional 12 h before immunostaining. We found that the permissive temperature resulted in L253P β-III spectrin reaching the plasma membrane (Fig. 4B). Western blot analysis confirmed protein levels were not altered by the temperature shift but did show that the expression of L253P β-III spectrin was less than WT protein (Fig. 4B). In addition EAAT4 was no longer trapped intra-cellularly in cells expressing L253P β-III spectrin when incubated at the lower temperature (Fig. 4C).
Figure 4.
L253P β-III spectrin does not interact with Arp1 and interferes with protein trafficking. (A) Neuro2a cells cotransfected with EAAT4 and either myc-tagged WT or L253P β-III spectrin. Cells immunostained using anti-c-myc antibody (red), anti-EAAT4 antibody (green) and nucleus stained with DAPI (blue). (B) Cells transfected with either myc-tagged WT or L253P β-III spectrin and 24 h after transfection incubated at 37 or 25°C for a further 12 h. Cells immunostained using anti-c-myc antibody (red). Western blot analysis of cell homogenates probed with anti-c-myc antibody. (C) Cells expressing myc-tagged L253P β-III spectrin and EAAT4 incubated at 25°C for a further 12 h. Cells immunostained using anti-c-myc antibody (red) and anti-EAAT4 antibody (green). (D) BiFC assay using cells transfected with Arp1 fused to the N-terminal half of YFP (YN-Arp1) and the actin-binding region of β-III spectrin, with or without the L253P substitution, fused to the C-terminal half of YFP (YC-β-III). Twenty-four hours after transfection cells were incubated at 37 or 25°C for a further 12 h. Western blot analysis of cell homogenates probed with anti-GFP antibody. All images are representative of three independent experiments (scale bar, 10 µm).
A role for β-III spectrin in vesicular trafficking has been proposed given its ability to interact with Arp1 and copurify with dynein and dynactin on intracellular vesicles from rat brain (18). We used a biomolecular fluorescence complementation (BiFC) assay to investigate whether the L253P mutation interfered with the ability of the N-terminus of β-III spectrin to interact with Arp1. The BiFC technique is based on the generation of a fluorescent signal when the two halves of enhanced YFP are brought together, mediated by the association of two interacting partners fused to the YFP fragments (21,22). We cloned full-length Arp1 and the N-terminus of β-III spectrin (amino acids 1–294) downstream of the N-terminal (YN) and C-terminal fragments (YC) of YFP, respectively, and transfected HEK 293T cells with the expression vectors. Co-expression of YN–Arp1 and WT YC–β-III spectrin yielded fluorescence, but no fluorescence was observed when L253P YC–β-III spectrin was co-expressed with YN-Arp1 (Fig. 4D), indicating that the mutation does indeed eliminate the interaction between β-III spectrin and Arp1. However, incubation at 25°C for an additional 12 h produced a fluorescence signal (Fig. 4D), suggesting that a conformational change underlies the observed lack of interaction at 37°C. Western blot analysis confirmed that all proteins were expressed at 37°C.
Unfolded protein response not induced by L253P expression
A number of neurodegenerative diseases have been shown to be associated with the accumulation of abnormal protein, impaired ER homeostasis and activation of the unfolded protein/endoplasmic stress response (UPR) (23–26). The UPR can be triggered by a block in trafficking at the ER and Golgi, as well as the accumulation of unfolded or misfolded proteins in the ER. Therefore, since L253P appears to accumulate intra-cellularly and disrupt the trafficking of proteins through the Golgi, we investigated whether the UPR was induced by the expression of L253P.
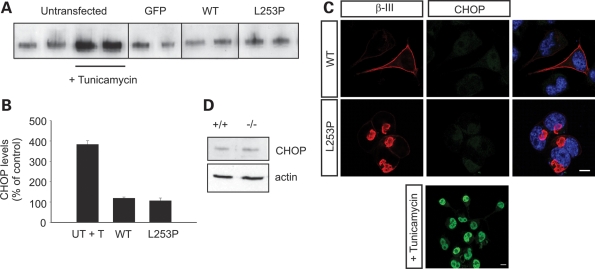
We looked at increased expression levels and nuclear translocation of the transcription factor growth arrest and DNA damage/C/EBP-homologous protein (GADD153/CHOP), a commonly used indicator of ER stress and thought to be a downstream effector of all three UPR pathways (27,28). Western blot analysis revealed no difference in the level of CHOP protein when L253P β-III spectrin was expressed compared with WT β-III spectrin (Fig. 5A and B). CHOP levels were normalized to those obtained when GFP was overexpressed, and the pharmacological induction of ER stress using tunicamycin, a blocker of N-linked glycosylation, confirmed the ability to induce CHOP expression in the cell culture system. Immunofluorescence microscopy confirmed the absence of CHOP activation in cells transfected with L253P as no nuclear staining was observed, in comparison with high levels observed in tunicamycin-treated cells (Fig. 5C). Finally, we only saw a small increase in the expression of CHOP in symptomatic β-III−/− mice compared with litter mate controls (116 ± 7.8% of WT; n = 4 of each genotype; P = 0.11). These data suggest that UPR is not a major consequence of β-III spectrin loss and is unlikely to underlie the Purkinje cell degeneration observed in the β-III−/− mouse model of ataxia. Furthermore, UPR appears not to be a downstream effect of a β-III spectrin mutation associated with SCA5.
Figure 5.
No induction of unfolded protein response by L253P β-III spectrin compared with WT β-III spectrin. (A) Total cellular homogenates of Neuro2a cells transfected with constructs encoding GFP, WT β-III spectrin, L253P β-III spectrin or untransfected cells treated with tunicamycin for 12 h resolved by SDS–PAGE, and immunoblotted using anti-GADD153 antibody. (B) Data quantified by densitometry (means ± SEM). (C) Representative confocal images of cells immunostained for CHOP (green) and nucleus stained with DAPI (blue). Scale bar, 10 µm. (D) Total cerebellar homogenates of symptomatic β-III−/− and age-matched WT mice resolved by SDS–PAGE, immunoblotted using anti-GADD153 antibody.
DISCUSSION
A number of dominantly inherited human diseases have recently been shown to arise from haploinsufficiency (29–31). Often in these cases, the genes involved encode proteins where a correct stoichiometry is vital for function and a single WT gene copy is insufficient. Examples are transcription factors or proteins that form macromolecular complexes. Since spectrin functions as a heterotetramer and assembly appears to be rate limited by the β subunit (32), one possibility is that SCA5 arises from β-III spectrin haploinsufficiency due to the mutant β polypeptides failing to associate with other subunits. Here we provide strong evidence that haploinsufficiency is unlikely to be the disease mechanism, as we see no signs of motor deficits or cerebellar degeneration in heterozygous β-III+/− mice even at 2 years of age. This indicates that the loss of β-III spectrin function, thought to be important in disease pathogenesis (20), must arise from mutations associated with SCA5 having a dominant-negative effect on WT β-III spectrin function. It is possible that the heterozygous animals do not live long enough for a phenotype to be detected or the motor tasks used are not sensitive enough to detect minor motor deficits. However, our cell culture studies do indicate that the proline substitution found in one SCA5 family (L253P) appears to have a dominant-negative effect on WT β-III spectrin, preventing protein trafficking from the Golgi apparatus.
Previously, β-III spectrin was shown to stabilize EAAT4 at the plasma membrane (15) and, using total internal reflection fluorescence microscopy, Ranum and co-authors (3) revealed that the in-frame deletion found in the Lincoln SCA5 pedigree fails to stabilize EAAT4 at the cell surface to the same extent as WT β-III spectrin. Here we now show that β-III spectrin also has a role in the vesicular trafficking of EAAT4 from the Golgi to the plasma membrane and the L253P mutation disrupts this process. The observation that a large number of vesicles are found surrounding Golgi cisternae in β-III−/− mice (20) supports this cellular function. Further we have shown that the L253P substitution prevents the normal interaction between Arp1 and the N-terminus of β-III spectrin. Based on their work in Drosophila, a link between defects in the dynein–dynactin complex and SCA5 pathogenesis is suggested by Lorenzo et al. (19). When flies overexpressing mutant human β-III spectrin (the Lincoln mutation) were crossed with either a hypomorphic dynein heavy chain allele or a dominant mutation in the p150Glued subunit of dynactin, the larvae displayed exacerbated posterior paralysis and slowing of vesicle transport, inferring a synergistic effect between spectrin and dynein–dynactin. Similarly, the rough eye phenotype in flies overexpressing either the Lincoln or the L253P mutation was enhanced by the dynein–dynactin mutants. Here we provide the first direct evidence that a SCA5 mutation interferes with the ability of spectrin to interact with a component of the dynactin complex, highlighting how normal trafficking can be disrupted in SCA5. The fact both the interaction with Arp1 and protein trafficking defects were rescued by incubating at a lower temperature suggests that the L253P substitution results in a protein conformation defect. Ongoing work by Ranum and colleagues supports temperature sensitivity of conformation of the mutant human protein (K.A. Dick Krueger and L.P.W. Ranum personal communication). Importantly, a direct role for dynein in the transport of proteins into dendrites has recently been shown (33), supporting the possibility that interfering with Arp1 binding will result in defects to protein trafficking within the Purkinje cell dendritic tree of SCA5 patients.
Irrespective of the mechanism, the intracellular accumulation of proteins can lead to ER stress, the induction of UPR, and apoptotic cell death. However, we see no major induction of GADD153/CHOP, a downstream effector of UPR in transfected cells or in symptomatic β-III−/− mice. Taken together, these results suggest that unlike other neurodegenerative disorders, the disease mechanism in SCA5 does not involve the induction of UPR. Instead, the actual loss of membrane proteins and their cellular functions appears to be more critical for disease pathogenesis. The expression of mutant forms of β-III spectrin in Purkinje cells and identification of other membrane proteins that are altered will be fundamental to fully understanding the mechanisms of Purkinje cell dysfunction and degeneration. The N-terminus of β spectrin is known to bind actin and 4.1, forming a spectrin/4.1/actin junction. Protein 4.1 has been shown to bind several membrane proteins and be required for their stable cell-surface expression (34–36). Incorporation of a mutant β subunit could alter the conformation of the whole tetramer preventing normal function and interaction with proteins including protein 4.1. Future research should investigate whether different mutations associated with SCA5 alter the ability of β-III spectrin to interact with components of the dynein–dynactin complex or with protein 4.1.
In summary, the present work has shown that mutant β-III spectrin disrupts protein trafficking from the Golgi apparatus through elimination of normal interaction between β-III spectrin and the dynactin component Arp1. This helps explain the mislocalization of membrane proteins seen in SCA5. Furthermore, haploinsufficiency is not supported as a disease mechanism as indicated by a lack of disease phenotype in mice heterozygous for loss of β-III spectrin.
MATERIALS AND METHODS
Analysis of β-III+/− mice
All genotyping, motor tasks, histology and western blotting analysis were carried out as previously described (20). Seven days prior to the behavioural tests, old mice were habituated to the test environment by handling (10 min each day).
Antibodies
Sagittal cerebellar sections were immunostained using mouse anti-calbindin D (1:50) and cyanine 3 (Cy3)-conjugated goat anti-mouse IgG (Jackson Laboratories). Mouse anti-c-myc (Ab-1, Calbiochem) and either Cy3- or Cy2-conjugated goat anti-mouse IgG (Jackson Laboratories) were used to detect pRK5-myc-tagged constructs. YFP-tagged β-III spectrin was detected with rabbit anti-GFP (Molecular Probes, Invitrogen) and fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Cappel). Polyclonal antibodies against EAAT4, GLAST and GLT1 were a kind gift of Jeffrey Rothstein, and mouse anti-actin, -calbindin and rabbit anti-GADD153 obtained from Sigma and Santa Cruz, respectively.
Plasmids
The missense mutation L253P was introduced using the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions using pRK5-myc-tagged β-III spectrin as template and 5′-GGCCTGACGAAGCCCCTGGATCCTGAAG-3′ and 5′- CTTCAGGATCCAGGGGCTTCGTCAGGCC-3′ as primers. Full-length WT β-III spectrin and the first 294 amino acids of WT and L253P β-III spectrin were amplified by PCR using primers that introduced either NotI and ClaI (5′-ATTTGCGGCCGCATGAGCAGCACCCTGTCACCCA-3′ and 5′-CCATCGATTTTGTTCTTCTTAAAGAAGCTGAAT-3′) or BspEI and XbaI (5′-CCTCCGGAATGAGCAGCACCCTGTCACCCA-3′ and 5′-GCTCTAGACTAGCCAATTCTTTTGCCTTCCAC-3′) restriction sites, respectively, and the products cloned into pcDNA3.1(zeo)-YC vector (kind gift of Stephen Michnick). Full-length Arp 1 was amplified using Quickclone cDNA (Clontech) as template and cloned into the BspEI and XbaI sites of pcDNA3.1(zeo)-YN. Other plasmids were pCDNA3.1 rat EAAT4 (15) and Golgi (pECFP-golgi) and ER (pDsRed2-ER) markers from Clontech.
Cell culture and transfections
HEK 293T cells were grown in minimum essential medium (MEM, Sigma) containing 10% fetal bovine serum, 10 mm glutamine, 1× non-essential amino acids and antibiotics (penicillin and streptomycin). Mouse neuronal 2a (N2a) cells were grown in Dulbecco's modified Eagle's medium (DMEM, Gibco) containing the aforementioned components and supplemented with 4.5 g/l glucose and 0.11 g/l sodium pyruvate (Gibco). For cell homogenates, cells were plated onto 35 mm dishes and for microscopic observation cells were plated onto poly-l-lysine-coated coverslips in 35 mm dishes. A total of 2 µg of DNA was used to transfect cells with Fugene HD at a ratio of 3:2 according to the manufacturer's instructions (Roche). Twenty-four hours post-transfection cells were either harvested for western blot analysis, fixed with 4% paraformaldehyde for immunostaining, maintained at 37 or 25°C for an additional 12 h (to test for temperature sensitivity shown by K.A. Dick Krueger et al., manuscript submitted) or treated with 2 µg/ml tunicamycin (Calbiochem) for 12 h. All coverslips were mounted using hard set vectashield containing DAPI unless Cy2-conjugated goat anti-mouse IgG was used. In this instance, vectashield was used as mounting agent (Vector Laboratories, Burlingame, CA, USA).
Microscopy
Images were captured with an Olympus BX51 microscope or using a Zeiss Axiovert confocal laser scanning microscope. All acquisition settings were kept constant between samples, and colours were applied using Image J.
Statistics
Statistical analysis was performed using Student's t-test, two sample assuming unequal variance, apart from densitometry analysis of western blots where one sample t-test was used with a predicted value of 100% for the WT.
FUNDING
This work was supported by grants from The Wellcome Trust (077946), the National Institutes of Health (NS056158) and a MRC PhD studentship (to Y.L.C.).
ACKNOWLEDGEMENTS
We thank Jeffrey Rothstein, Laura Ranum and Paul Skehel for help and useful discussions.
Conflict of Interest statement. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Stevanin G., Herman A., Brice A., Durr A. Clinical and MRI findings in spinocerebellar ataxia type 5. Neurology. 1999;53:1355–1357. doi: 10.1212/wnl.53.6.1355. [DOI] [PubMed] [Google Scholar]
- 2.Burk K., Zuhlke C., Konig I.R., Ziegler A., Schwinger E., Globas C., Dichgans J., Hellenbroich Y. Spinocerebellar ataxia type 5: clinical and molecular genetic features of a German kindred. Neurology. 2004;62:327–329. doi: 10.1212/01.wnl.0000103293.63340.c1. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda Y., Dick K.A., Weatherspoon M.R., Gincel D., Armbrust K.R., Dalton J.C., Stevanin G., Durr A., Zuhlke C., Burk K., et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 2006;38:184–190. doi: 10.1038/ng1728. doi:10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 4.Shotton D.M., Burke B.E., Branton D. The molecular structure of human erythrocyte spectrin: biophysical and electron microscopic studies. J. Mol. Biol. 1979;131:303–329. doi: 10.1016/0022-2836(79)90078-0. doi:10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- 5.Ungewickell E., Gratzer W. Self-association of human spectrin: a thermodynamic and kinetic study. Eur. J. Biochem. 1978;88:379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. doi:10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- 6.Bodine D.M.I.V., Birkenmeier C.S., Barker J.E. Spectrin deficient inherited haemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984;37:721–729. doi: 10.1016/0092-8674(84)90408-2. doi:10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- 7.Tse W.T., Lecomte M.C., Costa F.F., Garbarz M., Feo C., Boivin P., Dhermy D., Forget B.G. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J. Clin. Invest. 1990;86:909–916. doi: 10.1172/JCI114792. doi:10.1172/JCI114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher P.G., Tse W.T., Coetzer T., Lecomte M.C., Garbarz M., Zarkowsky H.S., Baruchel A., Ballas S.K., Dhermy D., Palek J. A common type of the spectrin alpha I 46-50a-kD peptide abnormality in hereditary elliptocytosis and pyropoikilocytosis is associated with a mutation distant from the proteolytic cleavage site. Evidence for the functional importance of the triple helical model of spectrin. J. Clin. Invest. 1992;89:892–898. doi: 10.1172/JCI115669. doi:10.1172/JCI115669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker P.S., Tse W.T., Lux S.E., Forget B.G. β spectrin kissimmee: a spectrin variant associated with autosomal dominant hereditary spherocytosis and defective binding to protein 4.1. J. Clin. Invest. 1993;92:612–616. doi: 10.1172/JCI116628. doi:10.1172/JCI116628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komada M., Soriano P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell. Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. doi:10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura K., Akiyama H., Komada M., Kamiguchi H. betaIV-spectrin forms a diffusion barrier against L1CAM at the axon initial segement. Mol. Cell. Neurosci. 2007;34:422–430. doi: 10.1016/j.mcn.2006.11.017. doi:10.1016/j.mcn.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler A., Teichberg V.I. Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J. 1998;17:3931–3939. doi: 10.1093/emboj/17.14.3931. doi:10.1093/emboj/17.14.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett V., Baines A.J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi G., Orita S., Naito A., Maeda M., Igarashi H., Sasaki T., Takai Y. A novel brain-specific isoform of β spectrin: isolation and its interaction with Munc13. Biochem. Biophys. Res. Commun. 1998;248:846–851. doi: 10.1006/bbrc.1998.9067. doi:10.1006/bbrc.1998.9067. [DOI] [PubMed] [Google Scholar]
- 15.Jackson M., Song W., Liu M.-Y., Jin L., Dykes-Hoberg M., Lin C.-L.G., Bowers W.B., Federoff H.J., Sternweis P.C., Rothstein J.D. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. doi:10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- 16.Stankewich M.C., Tse W.T., Peters L.L., Ch'ng Y., John K.M., Stabach P.R., Devarajan P., Morrow J.S., Lux S.E. A widely expressed βIII spectrin associated with Golgi and cytoplasmic vesicles. Proc. Natl Acad. Sci. USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. doi:10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough L.L., Fan J., Chu S., Winnick S., Beck K.A. Golgi localization of Syne-1. Mol. Biol. Cell. 2003;14:2410–2424. doi: 10.1091/mbc.E02-07-0446. doi:10.1091/mbc.E02-07-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holleran E.A., Ligon L.A., Tokito M., Stakewich M.C., Morrow J.S., Holzbaur L.F. βIII spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. doi:10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo D.N., Li M.-G., Mische S.E., Armbrust K.R., Ranum L.P.W., Hays T.S. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J. Cell. Biol. 2010;189:143–158. doi: 10.1083/jcb.200905158. doi:10.1083/jcb.200905158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins E.M., Clarkson Y.L., Sabatier N., Longhurst D.M., Millward C.P., Jack J., Toraiwa J., Watanabe M., Rothstein J.D., Lyndon A.R., et al. Loss of β-III spectrin leads to Purkinje cell dysfunction recapitulating the behaviour and neuropathology of spinocerebellar ataxia type 5 in humans. J. Neurosci. 2010;30:4857–4867. doi: 10.1523/JNEUROSCI.6065-09.2010. doi:10.1523/JNEUROSCI.6065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C.D., Chinenov Y., Kerppola T.K. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. doi:10.1016/S1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 22.Hu C.D., Kerppola T.K. Simultaneous visualization of multiple protein interactions in living cells using multicolour fluorescence complementation analysis. Nat. Biotechnol. 2003;21:539–545. doi: 10.1038/nbt816. doi:10.1038/nbt816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkin J.D., Farg M.A., Turner B.J., Tomas D., Lysaght J.A., Nunan J., Rembach A., Nagley P., Beart P.M., Cheema S.S., Horne M.K. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. doi:10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- 24.Nagata T., Ilieva H., Murakami T., Shiote M., Narai H., Ohta Y., Hayashi T., Shoji M., Abe K. Increased ER stress during motor neuron degeneration in a transgenic mouse model of amyotrophic lateral sclerosis. Neurol. Res. 2007;29:767–771. doi: 10.1179/016164107X229803. doi:10.1179/016164107X229803. [DOI] [PubMed] [Google Scholar]
- 25.Kim S.J., Zhang Z., Hitomi E., Lee Y.C., Mukherjee A.B. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum. Mol. Genet. 2006;15:1826–1834. doi: 10.1093/hmg/ddl105. doi:10.1093/hmg/ddl105. [DOI] [PubMed] [Google Scholar]
- 26.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J. Neuroinflammation. 2009;6:41–53. doi: 10.1186/1742-2094-6-41. doi:10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. doi:10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 28.Xu C., Bailly-Maitre B., Reed J.C. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. doi:10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veitia R.A., Birchler J.A. Dominance and gene dosage balance in health and disease: why levels matter! J. Pathol. 2010;220:174–185. doi: 10.1002/path.2623. [DOI] [PubMed] [Google Scholar]
- 30.Dang V.T., Kassahn K.S., Marcos A.E., Ragan M.A. Identification of human haploinsufficient genes and their genomic proximity to segmental duplications. Eur. J. Hum. Genet. 2008;16:1350–1357. doi: 10.1038/ejhg.2008.111. doi:10.1038/ejhg.2008.111. [DOI] [PubMed] [Google Scholar]
- 31.Iwaki A., Kawano Y., Miura S., Shibata H., Matsuse D., Li W., Furuya H., Ohyagi Y., Taniwaki T., Kira J., Fukumaki Y. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J. Med. Genet. 2008;45:32–35. doi: 10.1136/jmg.2007.053942. doi:10.1136/jmg.2007.053942. [DOI] [PubMed] [Google Scholar]
- 32.Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J. Cell. Biol. 1987;105:1417–1424. doi: 10.1083/jcb.105.3.1417. doi:10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapitein L.C., Schlager M.A., Kuijpers M., Wulf P.S., van Spronsen M., MacKintosh F.C., Hoogenraad C.C. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. doi:10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 34.Anderson R.A., Lovrien R.E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307:655–658. doi: 10.1038/307655a0. doi:10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- 35.Coleman S.K., Cai C., Mottershead D.G., Haapalahti J.-P., Keinanen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J. Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D.-T., Makino Y., Sharma K., Hayashi T., Neve R., Takamiya K., Huganir R.L. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. doi:10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]