Abstract
Background
Interpersonal communication problems are common among persons with schizophrenia and may be tied, in part, to deficits in theory of mind – the ability to accurately perceive the attitudes, beliefs, and intentions of others. Particular difficulties might be expected in the processing of counterfactual information such as sarcasm or lies.
Method
The present study included 50 schizophrenia or schizoaffective outpatients and 44 demographically comparable healthy adults who were administered Part III of The Awareness of Social Inferences Test (TASIT; a measure assessing comprehension of sarcasm vs. lies) as well as measures of positive and negative symptoms and community functioning.
Results
The TASIT data were analyzed using a 2 (group: patients vs. healthy adults) x 2 (condition: sarcasm vs. lie) repeated measures ANOVA. The results showed significant effects for group, condition, and the group x condition interaction. Compared to controls, patients performed significantly worse on sarcasm but not lie scenes. Within-group contrasts showed patients to perform significantly worse on sarcasm vs. lie scenes; controls performed comparably on both. In patients, performance on the TASIT showed a significant correlation with positive, but not negative symptoms. The group and interaction effects remained significant when rerun with a subset of patients with low level positive symptoms. The findings for a relationship between TASIT performance and community functioning were essentially negative.
Conclusions
The findings replicate a prior demonstration of difficulty in the comprehension of sarcasm using a different test, but are not consistent with previous studies showing global ToM deficits in schizophrenia.
INTRODUCTION
Schizophrenia is characterized by impaired social functioning, commonly manifested in disturbances in communication (Sperber & Wilson, 2002). These disturbances may be attributed, in part, to impairments in theory of mind (ToM) -- the ability to represent one’s own and other persons’ mental states in terms of beliefs, attitudes, thoughts, and emotions (Frith, 1992; Leslie et al. 2004; Premack & Woodruff,1978). Developmentally, it is present in simple forms as early as 18 months of age (Leslie, 1987) and continues to develop through childhood and early adolescence (Baron-Cohen, 1995).
ToM deficits in schizophrenia are well-documented (see Brune, 2005; Corcoran, 2001; Harrington et al, 2005 for reviews; see Sprong et al. 2007 for meta-analysis). Harrington et al. found evidence of ToM impairment in 27 of the 30 studies reviewed and the findings appeared largely independent of impairments in neurocognition (e.g., memory, attention). ToM deficits have been noted on tasks assessing first- and second-order false beliefs (Frith & Corcoran, 1996; Roncone et al. 2002), as well as higher-order tasks involving comprehension of subtle suggestions (e.g., hinting), humor, metaphor, and irony (Brune, 2005; Corcoran et al. 1995; Corcoran et al. 1997; Marjoram et al. 2005a; Mazza et al. 2001; Mitchley et al. 1998; Mo et al. 2008). These deficits do not appear directly linked to psychiatric symptoms. Some studies have shown relationships with paranoid symptoms, negative symptoms, and formal thought disorder, but others have not (Marjoram et al. 2005b; Roncone et al. 2002; Sprong et al. 2007; see Harrington et al. 2005 for review). More consistent evidence supports a stable ToM deficit across acute and remitted clinical states (Herold et al. 2002; Inoue et al. 2006; Janssen et al. 2003; Randall et al. 2003; Sprong et al. 2007).
Persons with schizophrenia may experience particular difficulty in social situations that involve the processing of counterfactual information such as when someone makes a sarcastic remark or tells a white lie that requires consideration of social cues beyond the literal meaning of the message. According to McDonald et al. (2003), counterfactual verbal exchanges occur when the literal meaning of a message is contradicted by situational context. The true meaning is accessible only by incorporating social cues and other sources of information that place verbal statements into context. Difficulties understanding conversational exchanges involving counterfactual information are often noted in persons with traumatic brain-injury (TBI) and have been linked to impairments in social functioning (Dennis et al., 2001; McDonald, 2000; McDonald et al., 2004; McDonald & Flanagan, 2004).
We are aware of only one previous study that assessed the ability to detect sarcasm in a schizophrenia sample (Leitman et al. 2006). Relative to healthy controls, the schizophrenia group showed a decreased sensitivity to detect sarcasm plus an increased bias towards sincerity. Sarcasm perception scores did not significantly correlate with overall positive or negative symptoms. The relationship between the ability to detect sarcasm and community functioning was not examined. Regarding white lies, a few studies have examined the ability of children and adults with schizophrenia to infer the mental states of others using second-order ToM stories involving deception or lying (Happe & Frith, 1994), and the findings have been mixed (Brune, 2003; Mazza et al., 2001; Pilowsky et al., 2000) or difficult to interpret because the detection task scores were part of a composite including second-order ToM stories not involving deception (Randall et al., 2003).
ToM studies in schizophrenia have relied heavily on paper-and-pencil measures such as short stories or sequential picture sets of line drawings (Happe, 1993; Langdon et al. 2002; Mo et al. 2008), a number of which were passed down from the developmental literature examining the social development of healthy children (Doody, 1998) and those with autism spectrum disorders (Baron-Cohen et al. 1985; Happe, 1993). These measures are not ideally suited for adults with schizophrenia because they are tailored for children and consequently relatively easy, and also are potentially confounded with reading, comprehension, and working memory abilities. Further, paper-and-pencil measures do not capture dynamic characteristics such as facial expressions, voice tones, and gestures that are central to social communication and convey meaning apart from the content of speech. Compared to written texts, video clips of social interactions may be more ecologically valid, reduce potential confounds, and be more engaging for participants.
For the present study we used The Awareness of Social Inference Test (TASIT Part III: Social Inference - Enriched; McDonald et al. 2003) that was originally developed for use with persons with traumatic brain injury (TBI). The test includes videoed vignettes of everyday situations enacted by professional method actors. Part III assesses the ability to draw inferences about the thoughts, intentions, beliefs, and feelings of others engaged in conversational exchanges involving lies or sarcasm. Test-retest reliability of Part III (Form A) was .83 in a small sample of TBI patients (n = 18; McDonald et al. 2006). Discriminant validity of this TASIT component was demonstrated in a study comparing 12 TBI patients with 12 age-, education-, and occupation-matched healthy controls (McDonald et al., 2003). TBI patients performed significantly worse than controls on the sarcasm subscale and overall composite, but not the lie subscale. In the TBI group (McDonald et al., 2006), performance on Part III significantly (positively) correlated with performance on an emotion perception task (Ekman photos; Ekman & Friesen, 1976), but not social problem-solving ability (Assessment of Interpersonal Problem-Solving Skills; AIPSS; Donahoe et al., 1990) or first- and second-order false belief ToM tasks (Bibby & McDonald, 2004). There are no published reports of studies using the TASIT in schizophrenia; one co-author used it in a study of psychometrically-defined schizotypy (Jahshan & Sergi, 2007).
The present study’s aims were to: a) compare ToM abilities in conversational exchanges involving counterfactual information (lies, sarcasm) in a sample of stable, chronic schizophrenia outpatients vs. demographically comparable healthy adults, and b) examine the relationship of these abilities with community functioning in the patient sample. Based on the ToM literature in schizophrenia, we hypothesized schizophrenia patients to be impaired relative to healthy adults on perception of both sarcasm and lies. There is very little data on the relationship between ToM and community functioning in schizophrenia. Our examination of this relationship was exploratory.
METHOD
Subjects
The sample included 50 persons who met DSM-IV criteria for schizophrenia or schizoaffective disorder and 43 demographically comparable healthy adults. This project was part of the UCLA Translational Research Center for Neurocognition and Emotion (K.H. Nuechterlein, PI). All participants were considered to have chronic illness (i.e., onset of psychotic symptoms at least 5 years prior to participation), were clinically stable, and were previously enrolled in the UCLA Aftercare Program. None of the schizoaffective disorder study participants met criteria for current mood episode at the time of enrollment. Psychiatric diagnosis was re-confirmed for this study with a follow-up Structured Clinical Interview for DSM-IV (SCID-I/P; First et al. 1996). Training of diagnostic interviewers involved viewing videotapes and conducting live interviews to establish adequate inter-rater reliability. A minimum kappa of .75 was required of raters on symptom presence. Final diagnosis was determined during case conferences following presentation and review of interview data, information from previous SCID-I/P, and collateral information (e.g., medical records, informants). Inclusion criteria for schizophrenia participants included: (a) DSM-IV diagnosis of schizophrenia or schizoaffective disorder, depressed subtype, (b) age between 18 and 60 years, (c) estimated IQ > 70, (d) able to understand spoken English sufficiently to comprehend testing procedures, and (e) at least five years since the onset of their first psychotic episode. Exclusion criteria included: (a) history of clinically significant neurological disorder (e.g., epilepsy), (b) history of head trauma with loss of consciousness greater than one hour, and (c) physical, cognitive, or language impairment of such severity to adversely affect the validity of the data, (d) alcohol or substance use disorder within the past six months, and (e) current treatment of psychotic symptoms with only conventional antipsychotic medication.
Healthy adults were recruited through newspaper ads, community colleges, trade schools, and the internet. Healthy adults were selected to be comparable as a group to the schizophrenia group on age, gender, race/ethnicity, handedness, parental educational level, and community of residence. This goal was accomplished by regular review of group means on these demographic variables and making recruitment adjustments accordingly. Exclusion criteria for the healthy adults included history of a schizophrenia spectrum disorder (avoidant, paranoid, schizotypal, or schizoid personality disorders), bipolar disorder, recurrent major depression or more than one year of depressive symptoms, post traumatic stress disorder, neurological disorder, significant head injury, alcohol/substance use disorder, or presence of a family history of psychotic disorder among first-degree relatives. All potential control subjects received a SCID-I and SCID-II (Cluster A and C sections) to assess selection criteria. Table 1 presents the demographic, illness chronicity, and symptom characteristics of schizophrenia participants and the demographic characteristics of the healthy adults. Written informed consent was obtained from all study participants based on a complete description of the study.
Table 1.
Demographic and clinical characteristics.
| Variable | Schizophrenia Participants (n = 49) | Healthy Adults (n = 43) |
|---|---|---|
| Age (yrs.) | 34.5 (7.8) | 32.7 (5.4) |
| Education (yrs.) | 13.9 (1.6) | 14.4 (1.7) |
| Parental education (yrs.) | 15.1 (3.0) | 14.4 (2.9) |
| Gender (% male) | 63.3 | 72.1 |
| Ethnicity (% white) | 83.7 | 90.7 |
| WTARa | 38.2 (9.3) | 42.2 (6.7) |
| SAPS totalb | 3.6 (2.7) | |
| SANS totalb | 7.2 (4.3) |
WTAR = Wechsler Test of Adult Reading; significant group difference; t(83) = 2.32, p < .02; 7 subjects were missing WTAR scores (3 in schizophrenia group; 4 in healthy adult group);
SAPS = Scale for Assessment of Positive Symptoms; SANS = Scale for Assessment of Negative Symptoms; the SAPS and SANS total scores were calculated by summing the global scores from the respective subscales. The group means and standard deviations are presented in the table.
Procedure
All study participants were administered the TASIT (Part III: Social Inference – Enriched; Form A) as part of their participation in a project on social cognition within the UCLA Translational Research Center. Parts I and II were not administered due to consideration of participant burden resulting from multiple Center projects administering other assessments to the same persons. Part III consists of 16 videoed scenes, each lasting 15 to 60 seconds, depicting lies or sarcasm (8 of each presented in a fixed random order; see Appendix for examples). The lie scenes involved either white lies or sympathetic lies. Each scene was administered only once. A prologue/epilogue or camera edit provided information to the viewer about the nature of the conversational exchange. Participants were provided a record form and asked to answer four types of forced-choice (yes/no) questions: The first question asks the participant to think about what one character in the scene is doing to the other, that is what he/she is trying to make the other person think or feel. The second question asks what the character is trying to say to the other person, that is what is the message he/she is trying to get across. The third question asks what the character is thinking, that is what is his/her underlying belief. The fourth question asks what the character is feeling, that is what emotion he/she is feeling or how he/she feels towards the other person or the situation. A practice scene is provided at the beginning to familiarize participants with the questions. During administration, the videotape is paused between each scene to allow the participant time to answer the four questions respective to that scene. The test is not timed, but required approximately 15 minutes for administration with schizophrenia participants in the current study. The test provides an overall total score (maximum = 64), plus subtotals for each type of question (Do, Say, Think, Feel), scene condition (lie vs. sarcasm), and type of cue provided (visual vs. text).
In addition to the TASIT, all schizophrenia participants received measures of positive symptoms, negative symptoms, pre-morbid intelligence, and community functioning (Role Functioning Scale, Global Functioning Scale) described briefly below. The two community functioning measures were administered to provide continuity in the assessment of this area across Translational Research Center samples. Ratings for the community functioning measures were based in part on collateral information from the Community Adjustment Form (Stein & Test, 1980), a comprehensive, multi-factorial, semi-structured interview.
Scale for Assessment of Positive Symptoms (SAPS; Andreasen, 1984a) - This interview-based measure of positive symptoms of schizophrenia assesses four domains (hallucinations, delusions, bizarre behavior, positive formal thought disorder). The scale yields a global score for each domain (score range = 0 – 5 with 0 indicating no symptom presence and 5 indicating severe presence). An overall composite was calculated by summing the global scores (score range = 0 – 20).
Scale for Assessment of Negative Symptoms (SANS; Andreasen, 1984b) – This interview-based measure of negative symptoms associated with schizophrenia assesses five domains (affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention). The attention subscale was dropped because it is not believed to represent a core component of negative symptoms (Blanchard & Cohen, 2006). The scale yields a global score for each domain (score range = 0 – 5 with 0 indicating no impairment and 5 severe impairment). An overall composite was calculated by summing the global scores (score range = 0 – 20).
Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) – This test of general word knowledge is frequently used as an estimate of pre-morbid intelligence and requires the individual to read aloud 50 irregularly pronounced words. The dependent score was the total number of words correctly pronounced (score range = 0 – 50).
Role Functioning Scale (McPheeters, 1984) – This measure assesses level of role functioning in four areas of everyday life: (a) Working productivity, (b) Independent living and self care, (c) Family relationships, and (d) Social network relationships. The time frame is the individual’s functioning within the past month. Scores on each subscale range from 1 (very low) to 7 (optimal).
Global Social and Role Functioning Scale (GSRFS; Auther et al. 2006; Cornblatt et al. 2007; Niendam et al. 2006) – This global measure assesses two areas of functioning (role and social) at three time points (highest, lowest, current). The current study focused on current level of functioning. Scores on the role and social functioning subscales range from 1 (very low) to 10 (superior).
Statistical analyses
All analyses were conducted using SAS (2002). Initially, we examined the TASIT data for distribution normality and outliers. The lie scene data were negatively skewed and consequently transformed along with all other TASIT scores using a square root transformation. One study participant’s data were dropped due to questionable validity yielding n=49 in the schizophrenia group for the data analyses. Demographic characteristics of schizophrenia participants vs. healthy adults were compared using independent t-tests for continuous variables and chi-square tests for categorical data. Using the SAS PROC MIXED procedure, a 2 (group: schizophrenia participants vs. healthy adults) x 2 (condition: sarcasm vs. lie) repeated-measures ANOVA was performed and followed up with tests for gender effects. We collapsed across the four question types because preliminary analyses with specific variables (i.e., do, say, think, feel) did not reveal any significant three-way interactions and indicated a similar overall pattern of results. Hence, we opted to use only the subscale scores for lies and sarcasm and the overall total score in the current analyses for clarity and ease of presentation.
To examine the potential role of symptom severity on observed group differences in TASIT performance, we examined the correlations of the TASIT with SAPS and SANS total scores in the schizophrenia group. When significant relationships were found, we reran the 2 × 2 repeated measures ANOVA using a subset of schizophrenia participants with low level symptoms to see if previously observed findings were not tied to symptom severity. Analysis of covariance was not feasible due to the absence of symptom data in the healthy adult group. Lastly, we performed correlational analyses to examine the relationship between TASIT performance and community functioning in the schizophrenia group. To control for experiment-wise error due to multiple comparisons, we used a conservative p value of .01 based on the number of outcome measures (n = 6) for these tests of significance. For significant or trend (p < .05) relationships, we conducted follow-up regression analyses controlling for symptoms.
RESULTS
Demographic comparisons
Schizophrenia participants did not significantly differ from healthy adults in age, gender composition, education, parental education, or ethnicity, but did on the WTAR (t(84) = 2.36, p < .02; see Table 1). Based on findings that impairments in intellectual functioning and academic performance are noted in persons with schizophrenia prior to onset of illness (Reichenberg et al. 2002; Weiser et al. 2007), we opted not to treat this putative measure of “pre-morbid” intelligence as a covariate in the analyses.
Group comparisons on the TASIT
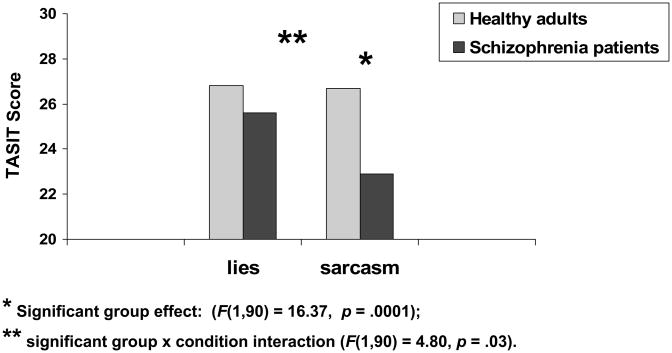
The results from the 2 × 2 ANOVA revealed significant effects of group (F(1,90) = 16.37, p = .0001), condition (F(1,90) = 4.40, p = .04), and group x condition interaction (F(1,90) = 4.80, p = .03; see Figure 1). Follow-up between-group contrasts revealed that schizophrenia participants differed from controls on sarcasm (F(1,90) = 18.10, p = .0001; Cohen’s d = .95), but not lie scenes (F(1,90) = 2.55, p = .11; Cohen’s d = .33). Follow-up within-group contrasts showed that healthy adults performed comparably on both sarcasm and lie scenes (F(1,90) = 0.00, p = .95; lie mean = 26.8 (3.7); sarcasm mean = 26.7 (3.5)); schizophrenia participants performed significantly worse on sarcasm relative to lie scenes (F(1,90) = 9.84, p = .002; lie mean = 25.6 (3.8); sarcasm mean = 22.9 (4.4)). The follow-up analyses adding gender to the repeated measures ANOVA did not reveal any significant effects for gender or the gender x condition interaction. In sum, schizophrenia participants showed greater difficulty with sarcasm than lie scenes relative to healthy adults.
Figure 1.
* Significant group effect (F(1, 90) = 16.37, p = .0001);
** Significant group × condition interaction (F(1, 90) = 4.80, p = .03).
Group comparisons on the TASIT (controlling for symptoms)
Examination of results from the bivariate correlational analyses involving the TASIT and patient symptom measures revealed significant relationships between the TASIT total score and selected indices from the SAPS, but not the SANS (see Table 2). On the SAPS, significant relationships were found with delusions (r = −.31, p = .04), positive formal thought disorder (r = −.52, p = .001), and SAPS total score (r = −.49, p = .001), but not hallucinations or bizarre behavior. To see if the schizophrenia vs. healthy adult group differences on the TASIT remained in a subset of schizophrenia participants who were in a clinically remitted state, we re-ran the between-group analyses using only participants who did not show clinically significant elevations on any of the SAPS subscales (i.e., ratings of 2 or less; n = 30). These analyses revealed significant effects of group (F(1,71) = 5.95, p = .02), condition (F(1,71) = 4.27, p = .04), and group x condition interaction (F(1,71) = 4.62, p = .035). Follow-up between-group contrasts revealed that schizophrenia participants performed significantly worse than controls on sarcasm (F(1,71) = 9.22, p = .003; Cohen’s d = .71), but not lie scenes (F(1,71) = 0.16, p = .69; Cohen’s d = .00). Follow-up within-group contrasts showed that healthy adults performed comparably on both sarcasm and lie scenes (F(1,71) = 0.00, p = .95); schizophrenia participants performed significantly worse on sarcasm relative to lie scenes (F(1,71) = 7.75, p = .007). These findings were essentially the same as those noted with the full schizophrenia sample.
Table 2.
Correlations between TASIT total score and positive and negative symptoms.
| Symptom scale | r |
|---|---|
| SAPS | |
| Hallucinations | −.21 |
| Delusions | −.31* |
| Bizarre behavior | −.01 |
| Positive formal thought disorder | −.52** |
| SAPS total | −.49** |
| SANS | |
| Affective flattening | −.16 |
| Alogia | −.07 |
| Avolition - Apathy | −.15 |
| Anhedonia - Asociality | −.19 |
| SANS total | −.17 |
p < .05;
p < .001
TASIT and community functioning
Table 3 presents the data examining the relationship between the TASIT sarcasm and lie subscales and total score with community functioning. Using a threshold of alpha = .01 to adjust for multiple comparisons, there was one trend finding between the TASIT total score and current social functioning from the GSRFS (r = .30, p = .04). No other noteworthy relationships were observed (all p’s > .07). To see if the lone trend relationship remained present after controlling for positive symptoms, we ran a regression analysis with two independent variables (SAPS total and TASIT total score) entered simultaneously. The results revealed that only the SAPS total was significant (p = .04) accounting for 16.35% of the variance in current social functioning. The amount of additional variance accounted for by the TASIT over and above the SAPS was negligible (R2 change = 1.35%).
Table 3.
Correlations between TASIT scores and community functioning measures.
| Sarcasm subscale | Lie subscale | TASIT total score | |
|---|---|---|---|
| Community functioning measure | |||
| Role Functioning Scale | |||
| Work productivity | .01 | .15 | .10 |
| Independent living | .15 | .23 | .27 |
| Family network relationships | .10 | .20 | .20 |
| Immediate social relationships | −.01 | .08 | .04 |
| Global Social and Role Functioning Scale | |||
| Current role functioning | −.10 | .21 | .06 |
| Current social functioning | .23 | .19 | .30* |
p = .04
DISCUSSION
This study is the first to report findings of schizophrenia patients on the TASIT, a videotape measure of ToM that assesses the ability to draw inferences about the intentions, beliefs, and feelings of others in social interactions involving counterfactual statements (i.e., lies vs. sarcasm). The primary finding was that schizophrenia vs. control group differences were largely accounted for by differences in the ability to comprehend sarcasm. Compared with demographically comparable healthy adults, schizophrenia participants showed greater difficulty comprehending sarcasm than lies. This finding was present with the full schizophrenia sample as well as a subset with low level positive symptoms. Hence, the relative discrepancy in the ability to comprehend sarcasm does not appear to be an artifact of psychotic symptoms.
Our findings are consistent with the lone previous report on sarcasm involving a schizophrenia sample (Leitman et al. 2006). In that study, inpatients and outpatients with schizophrenia or schizoaffective disorder were compared with a healthy control group. The ability to detect sarcasm was assessed using the attitudinal subtest from the Aprosodia Battery (Orbelo et al. 2005) that included an audiotape presentation of 10 semantically neutral sentences (e.g., “This looks like a safe boat.”) recorded by a female speaker in both a sincere and a sarcastic tone of voice. Participants’ task was to ascertain whether the speaker was being sarcastic or sincere. Though the methods used in the Leitman et al. study were considerably different than those used in the present one, the results provide convergent evidence for a deficit in this area of processing.
We can only speculate about the reasons for impairment in the perception of sarcasm but not lies in persons with schizophrenia. Leitman et al. (2006) proposed that the observed deficits in sarcasm might be due to difficulties in the processing of physical features that give rise to the percept of sarcasm (i.e., alterations in stress and prosody) rather than a more general ToM deficit. In line with this possible explanation, it is noteworthy that the physical cues that “tip off” the observer about whether someone is telling a white lie or being sarcastic are present only for the latter. For example, persons typically show little change in expression when telling a white lie in an attempt to conceal the deception. In contrast, persons making a sarcastic remark often present with exaggerated facial expression, voice tone, and gestures for effect. In the TASIT Part III scenes, the determination of white lies places fewer demands on information processing. Social cues are less relevant and the determination can be made by viewing the camera shot or prologue/epilogue that reveals the true state of affairs to one of the characters but not the other. For the sarcasm scenes, this supplemental information is helpful but not sufficient to make an accurate determination. The viewer must attend to and accurately process subtle changes in paralinguistic and other social cues from the characters’ conversational exchange in addition to information from the camera shot or prologue/epilogue. The findings from the present study suggest that persons with schizophrenia may not be impaired in ToM globally, but only certain areas with greater information processing load or perhaps, those ToM areas more intimately tied to the ability to perceive facial expression and paralinguistic cues. Interestingly, impaired perception of sarcasm but not lies was also found in the lone study with TBI patients using the TASIT (McDonald et al., 2003).
Despite the observed differences in comprehension of sarcasm vs. lies in the present study, care should be taken in interpreting these findings as definitive of a differential deficit. Testing for a differential deficit requires measures to be equated on true score reliability, variance, and level of difficulty (Chapman & Chapman, 1978). For the present study, no a priori efforts were made to ensure that the two conditions were equated on these psychometric properties. However, interpretation of findings should also consider two other sources of information, the TASIT’s administration methods and healthy adult data from the present study. It would be difficult to argue that schizophrenia participant difficulties on sarcasm vs. lies were due to differences in the methods used to assess these conditions. For both sarcasm and lies, the structure of the scenes was highly similar (e.g., characters, settings, length of video clip), and the type of questions and response format were the same. Post hoc examination of subscale scores for sarcasm vs. lies in the healthy adult group revealed highly comparable levels of performance accuracy and variance for both types of scenes, levels comparable with published norms for this test (McDonald et al. 2006). Hence, even though the measures were not deliberately manipulated for the purpose of testing a differential deficit, they were fairly well matched.
Examination of the relationship between TASIT performance and community functioning yielded negative findings. Of 18 comparisons, one trend finding emerged and the significance of that relationship disappeared after consideration of positive symptoms. In hindsight, it is not clear that we should expect one. Despite the substantial number of studies documenting the presence of ToM deficits in schizophrenia, few have examined the relationship between ToM and community functioning. A recent review (Couture et al. 2006) revealed four published reports of ToM and functional outcome in schizophrenia; only one examined community functioning and the findings were mixed (Roncone et al., 2002). Perhaps, ToM is less directly tied to broader community functioning that can be influenced by a number of variables (e.g., family support, employment opportunities, social environment) and instead, is more closely related to narrower areas within interpersonal functioning such as social communication. For example, it might be expected that the ability to “read” other persons’ mental states would be necessary to communicate effectively, and this ability may in turn facilitate the formation and maintenance of social relationships, though the latter could be influenced by other variables as well. There is some, albeit limited, evidence to support this notion (McCabe et al., 2004).
Our findings indicated a significant relationship between ToM ability and positive, but not negative symptoms. Specifically, TASIT performance was related to SAPS subscales measuring severity of delusions and positive formal thought disorder, as well as overall positive symptom severity. In contrast, TASIT performance did not show a significant relationship with overall severity of negative symptoms or any of the negative symptom subscales. The findings for negative symptoms are generally consistent with those reported by Leitman et al. (2006) that also used the SANS. However, that study did not find a significant relationship between sarcasm and positive symptoms, perhaps because they used a different measure (BPRS vs. SAPS). In general, reviews of the literature have yielded mixed findings for a relationship between ToM and positive and negative symptoms. The inconsistencies across studies may be due to differences in the samples, the measures used to assess symptom severity, the area of ToM assessed (e.g., first-and second-order false beliefs, sarcasm, irony), and the ToM measures used.
The study is limited by the lack of inclusion of other measures of ToM to evaluate how comprehension of sarcasm and lies compares with other ToM abilities such as understanding first- and second-order false beliefs, metaphors, jokes, empathy, or adult faux pas. Also, a broad-based neurocognitive battery was not administered, so we cannot make claims about the independence of observed group effects on this measure relative to differences in general cognitive functioning. The schizophrenia sample was comprised of stable chronic outpatients and we do not know if these findings may extend to more acute patients or those early in the course of their illness.
In sum, this report is the first to our knowledge to use videotape recordings of social interactions to assess ToM in schizophrenia. In terms of ecological validity, the TASIT has advantages over paper-and-pencil measures and more closely captures the kinds of ToM processes involved in daily interactions with others in everyday conversations. This study showed a differential impairment in the ability to perceive sarcasm vs. lies for persons with schizophrenia. Though intriguing, this finding requires replication in other independent studies using the TASIT or similar measures.
Acknowledgments
We thank the patients for their participation in the project, and Mike DeGroot, Robin Kite, Jeff Nishii, and Samantha Swain for their help in the data collection. The project was supported by a NIMH Center grant to Keith Nuechterlein (P50 MH 66586).
APPENDIX
Scene 2 (sarcasm)
Ruth: Did Rosie write on your book? She sometimes does that.
Gary: No. No. She didn’t write on it.
Ruth: Well, I had to punish her for it the other day.
Camera Shot: Gary holds up the book for Ruth to see. The page is covered in red crayon scribbles.
Ruth: I thought she learned her lesson.
Gary: Well, you certainly taught her a good lesson.
Scene 3 (lie)
Jane: Hi.
Rowan: Hey.
Jane: How’s it going?
Rowan: Good. Billy brought back your CDs. You better check if they’re okay.
Camera Shot: Jane opens the CD case and finds it empty, but she doesn’t show it to Rowan.
Jane: Yeah, they’re okay.
Rowan: He can be so careless sometimes. I’d never lend him anything.
Jane: Oh, I wouldn’t call him careless. He can borrow my CDs any time.
References
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Auther AM, Smith CW, Cornblatt BA. Global Functioning Scale: Social (GFS: Social) Zucker Hillside Hospital; Glen Oaks, NY: 2006. [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Bradford/MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a ‘theory of mind’? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bibby H, McDonald S. Theory of mind after traumatic brain injury. Neuropsychologia. 2004;43:99–104. doi: 10.1016/j.neuropsychologia.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bulletin. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M. Theory of mind and the role of IQ in chronic disorganized schizophrenia. Schizophrenia Research. 2003;60:57–64. doi: 10.1016/s0920-9964(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Brune M. ‘Theory of mind’ in schizophrenia: a review of the literature. Schizophrenia Bulletin. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Corcoran R. Theory of mind and schizophrenia. In: Corrigan PW, Penn DL, editors. Social Cognition and Schizophrenia. American Psychological Association; Washington, DC: 2001. pp. 149–174. [Google Scholar]
- Corcoran R, Cahill C, Frith CD. The appreciation of visual jokes in people with schizophrenia: a study of ‘mentalizing’ ability. Schizophrenia Research. 1997;24:319–327. doi: 10.1016/s0920-9964(96)00117-x. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating ‘theory of mind’ in people with schizophrenia. Schizophrenia Research. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zingerg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Roberts DL, Penn DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;32 (Suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe CP, Carter MJ, Bloem WD, Hirsch GL, Laasi N, Wallace CJ. Assessment of interpersonal problem-solving skills. Psychiatry. 1990;53:329–339. doi: 10.1080/00332747.1990.11024517. [DOI] [PubMed] [Google Scholar]
- Doody GA, Gotz M, Johnstone EC, Frith CD, Owens DG. Theory of mind and psychoses. Psychological Medicine. 1998;28:397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychological Press; Palo Alto, CA: 1976. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P, Version 2.0) American Psychiatric Press Inc; Washington, DC: 1996. [Google Scholar]
- Frith CD. The Cognitive Neuropsychology of Schizophrenia. Psychology Press; Hove, UK: 1992. [Google Scholar]
- Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychological Medicine. 1996;26:521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Happe FGE. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition. 1993;48:101–119. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cognitive Neuropsychiatry. 2005;10:249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- Herold R, Tenyi T, Lenard K, Trixler M. Theory of mind deficit in people with schizophrenia during remission. Psychological Medicine. 2002;32:1125–1129. doi: 10.1017/s0033291702005433. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yamada K, Hirano M, Shinohara M, Tamaoki T, Iguchi H, Tonooka Y, Kanba S. Impairment of theory of mind in patients inremission following first episode of schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:326–328. doi: 10.1007/s00406-006-0608-z. [DOI] [PubMed] [Google Scholar]
- Jahshan CS, Sergi MJ. Theory of mind, neurocognition, and functional status in schizotypy. Schizophrenia Research. 2007;89:278–286. doi: 10.1016/j.schres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Jolles J, van Os J. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatrica Scandinaviaca. 2003;108:110–117. doi: 10.1034/j.1600-0447.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: the role of poor pragmatics and poor mind-reading. Psychological Medicine. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- Leitman D, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychological Medicine. 2006 doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- Leslie A. Pretence and representation: the origins of ‘theory of mind’. Psychological Review. 1987;94:412–426. [Google Scholar]
- Leslie AM, Friedman O, German TP. Core mechanisms in “theory of mind”. Trends in Cognitive Science. 2004;8:528–533. doi: 10.1016/j.tics.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Tansley H, Miller P, MacIntyre D, Cunningham Owens DG, Johnstone EC, Lawrie S. A theory of mind investigation into the appreciation of visual jokes in schizophrenia. BMC Psychiatry. 2005a;5:12. doi: 10.1186/1471-244X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram D, Gardner C, Burns J, Miller P, Lawrie SM, Johnstone EC. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cognitive Neuropsychiatry. 2005b;10:347–359. doi: 10.1080/13546800444000092. [DOI] [PubMed] [Google Scholar]
- Mazza M, De Risio A, Surian L, Roncone R, Casacchia M. Selective impairments of theory of mind in people with schizophrenia. Schizophrenia Research. 2001;47:299–308. doi: 10.1016/s0920-9964(00)00157-2. [DOI] [PubMed] [Google Scholar]
- McCabe R, Leudar I, Antaki C. Do people with schizophrenia display theory of mind deficits in clinical interactions? Psychological Medicine. 2004;34:401–412. doi: 10.1017/s0033291703001338. [DOI] [PubMed] [Google Scholar]
- McDonald D. Exploring the process of inference generation in sarcasm: a review of normal and clinical studies. Brain and Language. 1999;68:486–506. doi: 10.1006/brln.1999.2124. [DOI] [PubMed] [Google Scholar]
- McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disability and Rehabilitation. 2006;28:1529–1542. doi: 10.1080/09638280600646185. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: a new clinical tool for assessing social perception after traumatic brain injury. Journal of Head Trauma and Rehabilitation. 2003;18:219–238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Mental Health Journal. 1984;20:44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- Mitchley NJ, Barber J, Gray JM, Brooks DN, Livingston MG. Comprehension of irony in schizophrenia. Cognitive Neuropsychiatry. 1998;3:127–138. [Google Scholar]
- Mo S, Su Y, Chan RCK, Liu J. Comprehension of metaphor and irony in schizophrenia during remission: the role of theory of mind and IQ. Psychiatry Research. 2008;157:21–29. doi: 10.1016/j.psychres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, Cannon TD. Global Functioning Scale: Role (GFS: Role) University of California; Los Angeles, CA: 2006. [Google Scholar]
- Orbelo DM, Grim MA, Talbott RE, Ross ED. Impaired comprehension of affective prosody in elderly subjects is not predicted by age-related hearing loss or age-related cognitive decline. Journal of Geriatric Psychiatry and Neurology. 2005;18:25–32. doi: 10.1177/0891988704272214. [DOI] [PubMed] [Google Scholar]
- Roncone R, Falloon IR, Mazza M, De Risio A, Pollice R, Necozione S, Morosini P, Casacchia M. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35:280–288. doi: 10.1159/000067062. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a ‘theory of mind’? Behavioral and Brain Sciences. 1978;4:515–526. [Google Scholar]
- Randall F, Corcoran R, Day JC, Bentall RP. Attention, theory of mind, and causal attributions in people with persecutory delusions: a preliminary investigation. Cognitive Neuropsychiatry. 2003;8:287–294. doi: 10.1080/135468000057. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, Kaplan Z, Davidson M. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. American Journal of Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Roncone R, Falloon IR, Mazza M, De Risio A, Pollice R, Necozione S, Morosini P, Casacchia M. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35:280–288. doi: 10.1159/000067062. [DOI] [PubMed] [Google Scholar]
- Sperber D, Wilson D. Pragmatics, modularity and mind-reading. Mind and Language. 2002;17:3–23. [Google Scholar]
- Stein LI, Test MA. Alternatives to mental hospital treatment: I. Conceptual model treatment program and clinical evaluation. Archives of General Psychiatry. 1980;37:392–397. doi: 10.1001/archpsyc.1980.01780170034003. [DOI] [PubMed] [Google Scholar]
- SAS Version 9.1. SAS Institute; Cary, NC: 2002. [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Aharon-Peretz J. The neuroanatomical basis of understanding sarcasm and its relationship to social cognition. Neuropsychology. 2005;19:288–300. doi: 10.1037/0894-4105.19.3.288. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. British Journal of Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading (WTAR) The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Weiser M, Reichenberg A, Rabinowitz J, Nahon D, Kravitz E, Lubin G, Knobler HY, Davidson M, Noy S. Impaired reading and mathematical abilities in male adolescents with average or above general intellectual abilities are associated with comorbid and future psychopathology. Journal of Nervous and Mental Disease. 2007;195:883–890. doi: 10.1097/NMD.0b013e31815928b0. [DOI] [PubMed] [Google Scholar]