Abstract
Purpose
To compare optical coherence tomography (OCT) and scanning laser polarimetry (GDx) measurements of the retinal nerve fiber layer (RNFL) in multiple sclerosis (MS) patients with and without optic neuritis (ON).
Methods
OCT and GDx were performed on 68 MS patients. Qualifying eyes were divided into two groups: 51 eyes with an ON history ≥ 6 months prior (ON eyes), and 65 eyes with no history of ON (non-ON eyes). Several GDx and OCT parameters and criteria were used to define an eye as abnormal, for example, GDx nerve fiber indicator (NFI) above 20 or 30, OCT average RNFL thickness and GDx temporal-superior-nasal-inferior-temporal average (TSNIT) below 5% or 1% of the instruments’ normative database. Agreement between OCT and GDx parameters was reported as percent of observed agreement, along with the AC1 statistic. Linear regression analyses were used to examine the relationship between OCT average RNFL thickness and GDx NFI and TSNIT.
Results
All OCT and GDx measurements showed significantly more RNFL damage in ON than in non-ON eyes. Agreement between OCT and GDx parameters ranged from 69–90% (AC1 0.37–0.81) in ON eyes, and 52–91% (AC1 = 0.21–0.90) in non-ON eyes. Best agreement was observed between OCT average RNFL thickness (P < 0.01) and NFI (>30) in ON eyes (90%, AC1 = 0.81), and between OCT average RNFL thickness (P < 0.01) and GDx TSNIT average (P < 0.01) in non-ON eyes (91%, AC1 = 0.90). In ON eyes, the OCT average RNFL thickness showed good linear correlation with NFI (R2 = 0.69, P < 0.0001) and TSNIT (R2 = 0.55, P < 0.0001).
Conclusions
OCT and GDx show good agreement and can be useful in detecting RNFL loss in MS/ON eyes.
Keywords: multiple sclerosis, optic neuritis, axonal loss, retinal nerve fiber layer, GDx, OCT
Multiple Sclerosis (MS) is a demyelinating disease of the central nervous system (CNS), in which the optic nerve is particularly vulnerable. Demyelination of the optic nerve can lead to retinal ganglion cell loss and nerve atrophy. Optic neuritis (ON) is the presenting manifestation in 15%–20% of MS patients, with an additional 40% suffering an attack at some point during the course of the disease.1,2 Studies using funduscopic examination,3,4 direct axonal counting in postmortem tissue 5 and optical imaging devices 6–11 have found that after experiencing an episode of ON, the retinal nerve fiber layer (RNFL) decreases in thickness due to axonal loss. Eyes of MS patients without a history of ON can also have reduced RNFL thickness.6,8,9,12 Because the RNFL consists of axons of the optic nerve which is a tract of the CNS, studying the thickness of the RNFL gives insights into the status of the CNS more generally.
Optical coherence tomography (OCT) and scanning laser polarimetry with variable corneal compensation (GDx) are noninvasive, quantitative techniques that measure the thickness of the RNFL with good reproducibility.13 Both techniques have been widely used in the clinic and research for detecting RNFL loss in glaucoma,14 and recently in ON/MS.15–17 OCT and GDx use different optical principles to image the RNFL.14 OCT uses the principles of low coherence interferometry to determine the RNFL thickness, in micrometers, based on the structure’ reflectivity.18 GDx measures the phase retardation induced by RNFL birefringence, a property that depends on the integrity of axons’ internal structures such as microtubules and neurofilaments, and the peripapillary RNFL thickness is then estimated from the retardation values.19 The OCT assesses the entire optic disc circumference and has the ability to differentiate between layers of the retina, giving a cross-sectional measurement of the RNFL thickness similar to a histological resolution, which enables detection of precise RNFL thickness changes.14,15 However, a recent experiment in monkey eyes 20 suggests that GDx may detect axonal degeneration before RNFL thinning occurs because of its ability to evaluate microtubule density changes. The relative strengths/limitations of OCT and GDx, along with the possibility that the two techniques may identify different stages and/or characteristics of retinal ganglion axonal damage, underscore the importance of directly comparing the two instruments’ ability detecting various optic nerve diseases.
In glaucoma, some studies found both instruments to be useful, with moderate sensitivity and no statistically significant difference;21–23 while others found either OCT 24,25 or GDx 26 to be more sensitive in detecting RNFL loss. In band optic atrophy due to chiasmal compression, both instruments performed similarly in detecting average, superior, inferior, and nasal RNFL loss. It was only in temporal quadrant that GDx VCC is less sensitive than OCT.27 In MS, Zaveri et al 11 determined OCT and GDx had similar abilities distinguishing MS eyes from normal controls, and a “somewhat better” (not statistically significant) ability for OCT in distinguishing MS eyes with a history of ON from those without. In a pilot study involving 12 MS patients, Frohman et al (2009)28 found a stronger correlation between OCT-measured RNFL thickness and optic nerve/brain MRI measures than GDx-measured RNFL thickness.
It is relevant, not only for detecting changes in nerve fiber layer, but also for monitoring disease progression and effectiveness of therapies, to determine whether OCT and GDx provide similar or complementary information with regard to retinal ganglion axonal damage and degeneration in MS patients with (and without) ON. The purpose of this study was to directly compare OCT and GDx measurements of RNFL thickness in a relatively large population of MS patients, and to determine whether one technique is more advantageous than the other for detecting axonal loss in MS eyes with or without a history of ON.
METHODS
Subjects
Sixty-eight patients (1:4.2, M:F) from the MS Eye CARE clinic at the University Eye Institute, with a previous diagnosis of clinical definite multiple sclerosis were enrolled in our study. Sixty-six of the patients had relapsing-remitting (RR) MS and two had secondary progressive (SP) MS. Age range was 21 to 57 years with a mean of 40 ± 9.9 years. Duration of the disease ranged from newly diagnosed to 21 years with a mean of 6.2 ± 5.6 years.
All subjects underwent both OCT and GDx as part of their comprehensive eye examination conducted by one of the experienced neuro-ophthalmologists (R. T. or J. S.), which typically included the following: a detailed history regarding visual symptoms (e.g., the time course of onset and recovery, the presence of eye pain), refraction, visual acuity, extraocular muscle, pupil, color vision, intraocular pressure, threshold visual fields, dilated fundus examination, visual evoked potentials or multifocal visual evoked potential (mfVEP), optic nerve head and RNFL analysis. Related medical records were carefully reviewed. All subjects had one or more magnetic resonance imaging scans for diagnosis and management of MS. Patients with any ocular or systemic conditions other than MS/ON that could affect the visual system were not included in the study.
A history of ON was determined based on clinical signs and symptoms obtained by one or more eye exams as mentioned above.29,30 Other sources of optic atrophy such as glaucoma (or suspect), ischemic optic neuropathy, or compressive optic neuropathy were clearly ruled out. Eyes were divided into two groups: 51 eyes with a history of optic neuritis (ON eyes) and 65 eyes with no history of optic neuritis (non-ON eyes). The last ON episode was ≥ 6 months prior to the measurements allowing time for retrograde degeneration of the RNFL.31–33 Of the 20 eyes eliminated from the study, 16 had ON within 6 months prior to the scans, 1 had an unconfirmed diagnoses of ON, 1 had complete vision loss, 1 had a poor quality GDx, and one had no GDx on record. Patients’ demographic and clinical information is summarized in Table 1.
Table 1.
Subjects’ clinical information.
| ON Eyes (n = 51) | Non-ON Eyes (n = 65) | ||
|---|---|---|---|
| Age | 41.0 ± 9.2 | 40.5 ± 9.9 | |
| Number of ON Attacks | |||
| 1 attack (%) | 37 (73%) | NA | |
| >1 attack (%) | 14 (27%) | NA | |
| Months since last ON attack | 78.9 ± 80.5 | NA | |
| Visual Acuity | |||
| 20/20 or better (%) | 32 (63%) | 60 (92%) | |
| 20/20 to 20/50 (%) | 19 (37%) | 5 (8%) | |
| Years since MS diagnoses | 8.6 ± 6.6 | 5.0 ± 4.4 | |
| HVFa mean deviation (dB) | −5.15 ± 6.36 (n = 47) | −2.26 ± 3.63 (n = 57) | |
HVF refers to Humphery visual field 30-2 or 24-2 threshold tests. Four ON eyes and 8 non-ON eyes had unreliable HVF (fixation loss, false positives or false negatives > 33%) and were excluded for calculating HVF mean deviation.
Our procedures adhered to the tenets of Declaration of Helsinki, and the protocol was approved by the University of Houston Committee for the Protection of Human Subjects. All patients gave informed consent to participate in the study.
The OCT data from sixty-five of the patients were used in a previous study which compared the performance of multifocal visual evoked potential, standard automated perimetry and OCT in detecting visual abnormalities in MS.34
Optical Coherence Tomography
The Stratus OCT 3000 system (Carl Zeiss Meditec, Inc., Dublin, CA) was used by a trained ophthalmic technician to acquire three 3.4 mm diameter circular scans centered on the optic disc (Fast RNFL protocol). Only good-quality scans were accepted. Quality criteria followed recommendations from previous studies,35,36 which included signal strength 7 or better, a well-focused fundus image with the presence of a centered ring around the optic disc, visible retinal pigment epithelium and RNFL with no missing or blank areas within the scan pattern. Overall RNFL thickness along the circumference and sector thickness in every quadrant were automatically calculated by the OCT software and compared with a built-in normative database of age-matched control subjects. Several criteria were used to classify an eye as abnormal, which included OCT average thickness with P < 0.05 or P < 0.01, and “OCT ≥ 1 Quadrant” for which an eye was considered abnormal when any quadrant was classified by the instrument as abnormal P < 0.01 or P < 0.05.
Scanning Laser Polarimetry
Scanning laser polarimetry with variable corneal compensation (GDx VCC; software version 5.5.1; Carl Zeiss Meditec Inc., Dublin, CA) was performed by a trained ophthalmic technician. The principles of the GDx VCC technology have been described in detail elsewhere.37 In essence, polarized light (780 nm) is used to scan a peripapillary region in the posterior pole and the amount of retardation measured in nanometers is converted to the RNFL thickness in micrometers (0.67 nm/µm). Only images with a scan quality score ≥ 8 were analyzed. The RNFL thickness (based on an average of three scans) along a 0.4 mm wide measuring circle (outer diameter of 3.2 mm and inner diameter of 2.4 mm) centered on the optic disc was used. The temporal-superior-nasal-inferior-temporal average (TSNIT), superior average (Sup Avg), and inferior average (Inf Avg) indicates the average RNFL thickness along the entire measuring circle, the superior 120° region, or the inferior 120° region of the measuring circle, respectively. Each parameter was compared with a normative database of age-matched controls built into the instrument’ software. GDx TSNIT was considered abnormal at P < 0.05 or P < 0.01. “GDx Sup/Inf Avg” was considered abnormal if either the superior or inferior average was classified as abnormal by P < 0.05 or P < 0.01.
The GDx VCC also provides a global index called the nerve fiber indicator (NFI). The NFI is calculated using information from the entire RNFL thickness map to indicate the overall integrity of the RNFL, and reports data using an absolute scale from 1 to 100 (1–30 normal, 31–50 borderline, ≥ 51 abnormal,37 rather than probability, to optimize discrimination between healthy and glaucomatous eyes. An advanced neural network discriminating algorithm trained on a large sample of healthy and glaucomatous eyes was used to calculate the NFI.37 It is somewhat unclear how this parameter relates to MS/ON eyes. By examining other studies’ data, it is reasonable to conclude that average NFI increases with age in the healthy population. Specifically, previous studies’ healthy control groups with a mean age of 38.7 ± 13.6,26 40.25 ± 13.49,12 49.9 ± 13.0,25 65 ± 8,22 and 66 ± 9.921 found the mean NFI to be 10 ± 5.8, 17.08, 15.2 ± 7.6, 19.8 ± 6.7, and 19.0 ± 8.2, respectively. In addition to a manufacture recommended criterion of NFI > 30, we evaluated our data using NFI > 20 because of our relatively young study population (mean age of 40 ± 9.9).
Statistical Analysis
Data for ON and non-ON eyes are reported as the mean ± SD. Differences between the two groups were evaluated with two-sample t-tests.
Agreement between OCT and GDx classification of eyes was displayed as an observed percentage of agreement. Any two tests may classify some subjects into the same category by chance. We used the AC1 statistic (see details of calculation in Cheng et al7) to correct for chance agreement. Compared to a commonly used Kappa statistic, AC1 is less dependent on the prevalence of a trait.38
The relationship between OCT average thickness and GDx parameters was examined using linear regression analysis. The coefficient of determination (R2) represents the amount of variation accounted for by the linear relationship.
RESULTS
Means for the GDx parameters and OCT RNFL thickness (both average and quadrant) for ON and non-ON eyes, are presented in Table 2. Each GDx and OCT parameter of ON versus non-ON eyes, was significantly different. More specifically, the three overall parameters, GDx NFI, GDx TSNIT, and OCT average thickness, all had P < 0.0001. This is consistent with studies showing significant decrease in RNFL thickness in ON eyes relative to non-ON eyes.6,7,9
Table 2.
Mean (± SD) of OCT RNFL thickness and GDx parameters for the ON and non-ON groups.
| ON Eyes (n = 51) | Non-ON Eyes (n = 65) | P valuea | |
|---|---|---|---|
| GDx NFI | 34.39 ± 17.72 | 21.37 ± 10.17 | < 0.0001 |
| GDx TSNIT | 48.23 ± 7.43 | 53.00 ± 6.26 | < 0.0001 |
| GDx Sup Avg | 56.33 ± 10.52 | 63.46 ± 8.18 | < 0.001 |
| GDx Inf Avg | 56.80 ± 10.38 | 61.48 ± 7.41 | < 0.01 |
| OCT-Avg (µm) | 78.01 ± 17.43 | 95.24 ± 11.64 | < 0.0001 |
| OCT-T (µm) | 48.08 ± 14.12 | 67.57 ± 15.41 | < 0.0001 |
| OCT-S (µm) | 96.27 ± 23.80 | 115.49 ± 15.48 | < 0.0001 |
| OCT-N (µm) | 63.65 ± 20.97 | 74.75 ± 15.50 | < 0.01 |
| OCT-I (µm) | 103.71 ± 23.95 | 124.00 ± 17.37 | < 0.0001 |
OCT – Avg, T, S, N, I represents the overall average, temporal, superior, nasal and inferior quadrant RNFL thickness, respectively.
P value is based on two-sample t-tests comparing ON and non-ON eyes.
Qualitative Comparison of OCT and GDx Parameters
For each eye, OCT RNFL thickness classification (as normal or abnormal) was compared to that of each GDx parameter to assess agreement using different criteria. For example, OCT average thickness and GDx TSNIT were considered abnormal when below 1% of the instruments’ normative databases. In accordance with the manufacturer’ suggested cutoff value, GDx NFI was classified as abnormal when it was > 30. The number (and percent) of eyes classified as normal and abnormal, using these parameters, is shown in Table 3.
Table 3.
The number (and percent) of eyes in the ON group (A) and non-ON group (B) that are classified as normal or abnormal by OCT average RNFL thickness (P < 0.01), GDx NFI > 30, or GDx TSNIT (P < 0.01).
| Normal GDx NFI |
Abnormal GDx NFI |
Normal GDx TSNIT |
Abnormal GDx TSNIT |
|
|---|---|---|---|---|
| ON eyes (n = 51) | ||||
| Normal OCT | 25 (49%) | 5 (10%) | 28 (55%) | 2 (4%) |
| Abnormal OCT | 0 | 21 (41%) | 6 (12%) | 15 (29%) |
| Non-ON eyes (n = 65) | ||||
| Normal OCT | 56 (86%) | 6 (9%) | 58 (89%) | 4 (6%) |
| Abnormal OCT | 1 (2%) | 2 (3%) | 2 (3%) | 1 (2%) |
Of the 51 ON eyes (Table 3A), OCT average thickness (P < 0.01) classified 21 (41%) as abnormal, while NFI (>30) and TSNIT (P < 0.01) classified 26 (51%) and 17 (33%), respectively, as abnormal. Percent agreement between OCT average thickness (P < 0.01) and GDx NFI (>30) was 90% (AC1 = 0.81), and 84% (AC1 = 0.71) between OCT average thickness (P < 0.01) and GDx TSNIT (P < 0.01).
Of the 65 non-ON eyes (Table 3B), OCT average thickness (P < 0.01), NFI (>30), and TSNIT (P < 0.01) classified 3 (5%), 8 (12%), and 5 (8%) eyes as abnormal, respectively. Percent agreement between OCT average thickness (P < 0.01) and GDx NFI (>30) was 89% (AC1 = 0.87), and 91% (AC1 = 0.90) between OCT average thickness (P < 0.01) and GDx TSNIT (P < 0.01).
We examined the percentage of agreement (and AC1 statistic) between OCT and GDx when different parameters/criteria were used to classify an eye as abnormal (Table 4). Criteria used include global parameters: OCT average thickness or GDx TSNIT below 1% or 5% of the instruments’ normative databases, and GDx NFI > 30 or NFI > 20 (see Methods). We also examined the criterion “OCT ≥ 1 Quadrant” for which an eye was classified as abnormal if any quadrant of OCT measurement was below 5% or 1% of the built in normative database; and the “GDx Sup/Inf Avg” criterion for which abnormality was defined when either the superior or inferior average was below P < 0.05 or P < 0.01. The highest percent agreement for ON eyes (Table 4A) was 90% (AC1 = 0.81), using GDx NFI > 30 and OCT average thickness (P < 0.01) as the criteria. In non-ON eyes (Table 4B), the highest percent agreement (91%, AC1 = 0.90) was between OCT average thickness (P < 0.01) and GDx TSNIT (P < 0.01).
Table 4.
Percent of agreement (and AC1 statistic) between OCT and GDx parameters for the ON (A) and non-ON (B) group.
| GDx Parameter | OCT Avg Thickness (P < 0.01) |
OCT Avg Thickness (P < 0.05) |
OCT ≥ 1 Quadrant (P < 0.01) |
OCT ≥ 1 Quadrant (P < 0.05) |
|---|---|---|---|---|
| The ON group (n = 51) | ||||
| GDx NFI > 30 | 90% (.81) | 82% (.66) | 78% (.57) | 71% (.46) |
| GDx NFI > 20 | 71% (.43) | 84% (.72) | 73% (.48) | 88% (.82) |
| GDx TSNIT (P < 0.01) | 84% (.71) | 69% (.37) | 73% (.46) | 53% (.08) |
| GDx TSNIT (P < 0.05) | 80% (.61) | 76% (.54) | 80% (.61) | 69% (.42) |
| GDx Sup/Inf Avg (P < 0.01) | 88% (.77) | 73% (.48) | 80% (.61) | 61% (.25) |
| GDX Sup/Inf Avg (P < 0.05) | 86% (.73) | 78% (.58) | 82% (.65) | 71% (.46) |
| The non-ON group (n=65) | ||||
| GDx NFI > 30 | 89% (0.87) | 85% (0.80) | 86% (0.83) | 71% (0.56) |
| GDx NFI > 20 | 52% (0.21) | 60% (0.30) | 55% (0.25) | 62% (0.26) |
| GDx TSNIT (P < 0.01) | 91% (0.90) | 89% (0.87) | 88% (0.86) | 72% (0.60) |
| GDx TSNIT (P < 0.05) | 82% (0.77) | 83% (0.77) | 82% (0.76) | 69% (0.52) |
| GDx Sup/Inf Avg (P < 0.01) | 89% (0.88) | 88% (0.85) | 89% (0.87) | 74% (0.62) |
| GDX Sup/Inf Avg (P < 0.05) | 78% (0.73) | 83% (0.77) | 82% (0.76) | 72% (0.56) |
Quantitative Relationship between OCT and GDx Parameters
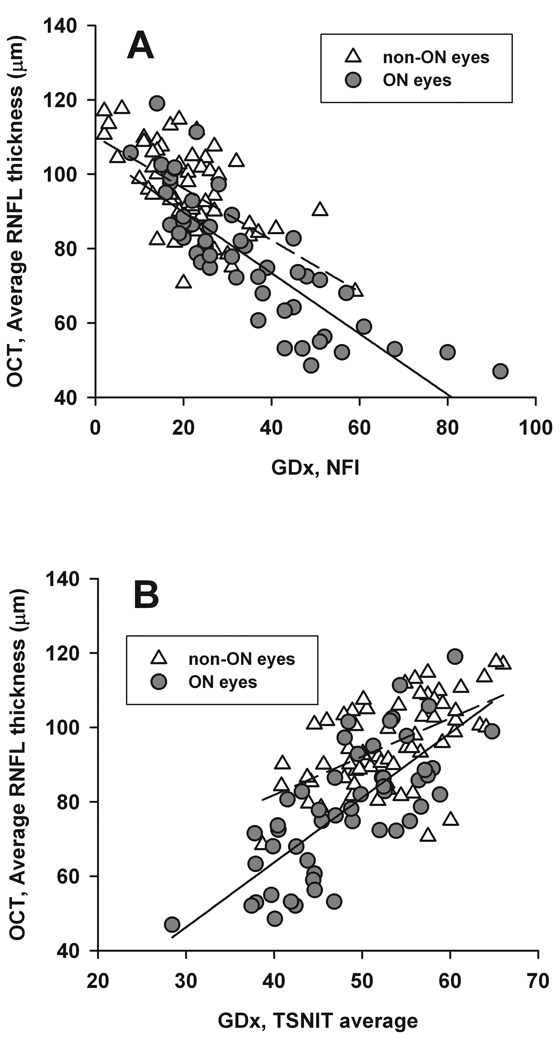
The relationship between OCT average thickness and GDx NFI or TSNIT was studied using linear regression analysis (Figure 1). In ON eyes, the OCT average thickness correlated well with GDx NFI (R2 = 0.69, P < 0.0001) and TSNIT (R2 = 0.55; P < 0.0001). In non-ON eyes, the results showed R2 = 0.37 (P < 0.0001) for OCT versus NFI, and R2 = 0.31 (P < 0.0001) for OCT versus TSNIT.
Figure 1.
Scatter plots and linear regression between OCT average RNFL thickness (µm) and GDx NFI (top), GDx TSNIT average (bottom). Solid line delineates regression for ON eyes, and dashed line for non-ON eyes. In the top figure, R2 is 0.69 (P < 0.0001) for ON eyes, and 0.37 (P < 0.0001) for non-ON eyes. In the bottom figure, R2 is 0.55 (P < 0.0001) for ON eyes, and 0.31 (P < 0.0001) for non-ON eyes.
To eliminate any contamination from intrasubject correlation, we performed regression analysis by including only one eye from each patient (left eye included in bilateral ON patients) and found almost identical results. In ON eyes, the results showed R2 = 0.70 (P < 0.0001) for OCT versus NFI and R2 = 0.57 (P < 0.0001) for OCT versus TSNIT. The results for non-ON eyes showed R2 = 0.39 (P < 0.0001) for OCT versus NFI and R2 = 0.31 (P < 0.0001) for OCT versus TSNIT.
DISCUSSION
It is evident that anterior visual pathway axonal degeneration in MS patients causes RNFL thinning, which is detectable by both OCT and GDx. In agreement with other studies, both OCT 6,7,9 and GDx 39 detected significant RNFL differences in MS patients with a history of ON versus those without a history of ON (Table 2). Agreement between OCT and GDx parameters varied with the criteria selected for each instrument (Table 4). For the ON group, the criteria that achieved the best percent agreement (90%, AC1 = 0.81) were OCT average thickness (P < 0.01) and GDx NFI > 30; and the agreement between “OCT ≥ 1 Quadrant (P < 0.05)” and GDx NFI > 20 was also very good (88%, AC1 = 0.82). Linear regression analysis also revealed good correlation between the average RNFL thickness measured by OCT and GDx NFI (R2 = 0.69) and TSNIT (R2 = 0.55) among the ON eyes (Figure 1). As previously reported,7,34 global parameters in general show better agreements among tests because they are less affected by variability in local measurements. Unlike glaucoma, in which arcuate nerve bundles are more susceptible to damage, axonal loss in ON appear to be relatively “diffuse”, meaning that the defects tend to cross the boundaries set by an OCT quadrant.7 Despite that, careful analysis of local defects on OCT and/or GDx is of great clinical value in detecting mild RNFL loss.
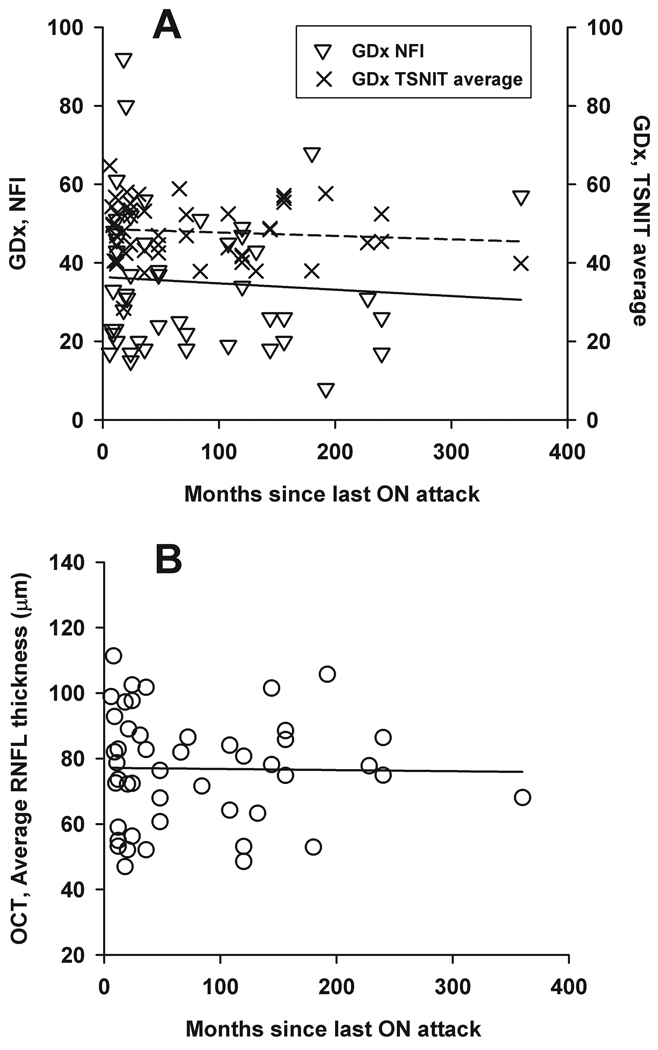
To allow adequate time for retrograde degeneration of the RNFL and minimize the effect of optic disc swelling from acute inflammation, OCT and GDx measurements used in this study were taken at least six months after the last ON attack.31–33 It is controversial whether there is further RNFL thinning beyond six months.32,40 To exam this issue, we used linear regression analysis to investigate the relationship between recovery time from the ON episode and the most significant parameters (OCT average thickness, GDx NFI, and GDx TSNIT) (Figure 2). No relationship (P = 0.52–0.91) was found between recovery time and any of the parameters. Some studies found that patients with a longer history of MS had a more severe reduction in RNFL thickness,6,8 while others did not.41 In our subject population, RNFL parameters (OCT average thickness, GDx NFI, and GDx TNSIT) did not correlate with the duration of MS diagnoses (P = 0.39–0.53, data not shown). The relatively large inter-subject variability in measurements could be an obscuring factor for not observing any relationship in the above regression analysis (see Figure 2). Future longitudinal studies rather than cross-sectional analysis should be used to study RNFL thickness changes over time.
Figure 2.
Scatter plots and linear regression between the time since last ON attack and three parameters: GDx NFI (open triangles and solid line in top figure), GDx TSNIT average (crosses and dashed line in top figure), and OCT average RNFL thickness (bottom). The slopes and the P values for the regression lines are − 0.02 (P = 0.62), 0.00 (P = 0.52), and 0.00 (P = 0.91) for GDx NFI, GDx TSNIT average, and OCT average RNFL thickness, respectively.
It is of clinical interest to compare test sensitivity between the OCT and GDx. The amount of abnormality detected clearly depended on the criteria used (Table 5). For instance, the percent of ON eyes classified as abnormal using “OCT Avg Thickness (P < 0.05)” or “NFI > 30” was 65% and 51%, respectively; less than 80% and 73% using “OCT ≥ 1 Quadrant (P < 0.05)” or “NFI > 20”. It is important to point out that when a lower NFI (see Methods) or an “or-logic” such as “OCT ≥ 1 Quadrant (P < 0.05)” is used, the test sensitivity will increase but the specificity will decrease. The specificities (false-positive rates) for NFI and OCT quadrant criteria are unknown due to a lack of normative control group in our study. Budenz and his colleagues have reported the specificity for “OCT ≥ 1 Quadrant (P < 0.05)” as 95% based on 109 normal subjects with a mean age of 42.8 ± 14.6 years (our subjects’ mean age is similar: 40 ± 9.9),42 or 76% based on 50 normal subjects with a mean age 62.9 ± 12.7 years.43
Table 5.
Number (and percent) of eyes classified as abnormal using different criteria.
| Parameter | ON Eyes (n = 51) | Non-ON eyes (n = 65) |
|---|---|---|
| GDx NFI (> 30) | 26 (51%) | 8 (12%) |
| GDx NFI (> 20) | 37 (73%) | 32 (49%) |
| GDx TSNIT (P < 0.01) | 17 (33%) | 5 (8%) |
| GDx TSNIT (P < 0.05) | 25 (49%) | 11 (17%) |
| GDx Sup/Inf Avg (P < 0.01) | 21 (41%) | 6 (9%) |
| GDx Sup/Inf Avg (P < 0.05) | 26 (51%) | 13 (20%) |
| OCT Avg Thickness (P < 0.01) | 21 (41%) | 3 (5%) |
| OCT Avg Thickness (P < 0.05) | 33 (65%) | 9 (14%) |
| OCT ≥ 1 Quadrant (P < 0.01) | 27 (53%) | 5 (8%) |
| OCT ≥ 1 Quadrant (P < 0.05) | 41 (80%) | 19 (29%) |
Studies have measured decreased RNFL thickness in MS non-ON eyes compared to healthy control eyes indicating that these eyes are also abnormal.6,8,9,12 In these cases, the instruments may be detecting subclinical optic nerve damage. The mean (± SD) OCT average RNFL thickness, GDx TSNIT average, and GDx NFI for our non-ON eyes were 95.24 ± 11.64 µm, 53.00 ± 6.26 µm, and 21.37 ± 10.17, respectively; comparable to those (94.2 µm, 52.25 µm, and 22.68, respectively) reported by Pueyo et al 2008, who studied 50 RRMS patients with similar age (41.36 ± 10.53).8 In the Pueyo 2008 study, mean values of non-ON eyes were significantly different from their age-matched normal controls (104.97 µm, 56.50 µm, and 17.08, respectively). In a recent study by Pueyo et al 2009,12 about one third of the MS non-ON eyes, with normal visual acuity and visual fields, showed RNFL defects on OCT (30%) or GDx (32.5%) measurements. Direct comparison between the present study and Pueyo et al’ 2009 study was not possible. First, the majority of our subjects (66 out of 68) had RRMS, whereas MS subtypes were not specified in Pueyo’ population. There is evidence suggesting that RNFL thinning may be more pronounced in SPMS than RRMS.44,45 Most importantly, the authors did not report the criteria they used to define “abnormal RNFL”. Based on the one example shown (see Figure 3 in Pueyo et al 2009)12, it appeared that for OCT, at least a quadrant (P < 0.05) criterion was used. Interestingly, the percent of abnormality revealed by our OCT quadrant (P < 0.05) criterion was actually very similar to theirs (29% versus 30%). As we mentioned earlier, a quadrant criterion would increase the chance of misclassifying a normal eye as abnormal. In fact, Pueyo et al (2009) reported that “defects in the RNFL were detected by both OCT and GDx in three of 20 control eyes (15%)”, suggesting a false positive rate of at least 15%.12 In general, the present study agrees with previous studies that subclinical atrophy occurs in non-ON eyes, and such defects appear to be relatively mild in relapsing-remitting MS.46 It is important to note that functional tests have revealed more abnormalities in non-ON eyes.47–49 In our recent paper which included the OCT data of 65 MS subjects reported here34, the Humphery visual field (HVF), multifocal VEP (mfVEP), and OCT classified 38%, 29% and 8% of non-ON eyes as abnormal, using criteria with 5% false positive rate for each test. 18% of non-ON eyes showed abnormality on both HVF and mfVEP tests. We concluded that “20–40% of MS eyes with no clinical history of ON are likely to have had a subclinical event somewhere along the visual pathway”.
Of 68 non-ON eyes in the present study, Table 5 shows that the GDx classified more non-ON eyes as abnormal than the OCT did when criteria were set at the same level (e.g. P < 0.01 or 0.05). This was true for comparisons of OCT with either GDX measure, GDx TSNIT or NFI. This orts a prior evidence that birefringent properties of the RNFL may change before RNFL thinning is measurable,20 and that GDx may be useful in subclinical cases.
To examine whether a clinical history of ON in the fellow eye contributed to axonal loss in the non-ON eye, we divided non-ON eyes into two subgroups: the non-ON-a with no ON history in either eye (n = 28), and the non-ON-b with an ON history in the fellow eye (n = 37).
Interestingly, GDx NFI and TSNIT showed significant differences between the two subgroups (P = 0.003 for NFI, and 0.05 for TSNIT), whereas OCT average RNFL thickness did not (P = 0.158). Specifically, the mean (± SD) GDx NFI, TSNIT and OCT average RNFL were 17.32 ± 7.58, 54.70 ±5.30 µm, and 97.5 ±10.4 µm, respectively, for the non-ON-a subgroup; and 24.4 ± 10.9, 51.71 ± 6.69, and 93.5 ±12.3, respectively, for the non-ON-b subgroup. Qualitatively, none of non-ON-a eyes (versus 8 eyes in non-ON-b) were identified as abnormal using “GDx NFI > 30” criterion, and only two (versus 7 in non-ON-b) were classified as abnormal using “OCT Avg Thickness (P < 0.05)” criterion. Thus, it appears that some RNFL damage in non-ON eyes might have resulted from ON attacks in fellow eyes, although this issue is confounded by subclinical events in MS subjects.
In summary, the findings indicated that although there was a discrepancy between the two instruments, both the OCT and GDx can be useful in detecting optic nerve atrophy in MS and ON patients. Using the current technology, inter-patient variability of RNFL thickness makes it difficult to determine precise cutoff values for identifying RNFL damage in the general population. With a baseline reading, preferably before an ON event, this type of measurement could be a good quantitative test for monitoring axonal loss in individual MS/ON patient overtime. Future longitudinal studies involving both MS and normal subjects may provide further insight to the comparative utility of these instruments. In addition, several studies including those from our own have shown that functional tests (e.g., visual evoked potentials, multifocal visual evoked potentials, and standard automated perimetry) detect more abnormalities in MS eyes compared to OCT or GDx,34,50–53 therefore, combining structural and functional measurements is probably the best strategy in monitoring the progression and therapeutic effects in MS.
ACKNOWLEDGMENTS
This study was supported by the NIH Short Term Training in Health Professional Schools grant T35 EY007088 (AQ), a pilot grant from the National Multiple Sclerosis Society (HC), and the NIH CORE-Vision Research grant P30 EY07751 (University of Houston, College of Optometry). We wish to thank Dr. Laura Frishman for helpful comments on the manuscript and Dr. Ying Sheng Hu for helpful discussions on statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors indicate no financial conflict of interest.
REFERENCES
- 1.Cantore WA. Optic neuritis. Pa Med. 1996;99 Suppl:96–98. [PubMed] [Google Scholar]
- 2.Sorensen TL, Frederiksen JL, Bronnum-Hansen H, Petersen HC. Optic neuritis as onset manifestation of multiple sclerosis: a nationwide, long-term survey. Neurology. 1999;53:473–478. doi: 10.1212/wnl.53.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Frisen L, Hoyt WF. Insidious atrophy of retinal nerve fibers in multiple sclerosis. Funduscopic identification in patients with and without visual complaints. Arch Ophthalmol. 1974;92:91–97. doi: 10.1001/archopht.1974.01010010097001. [DOI] [PubMed] [Google Scholar]
- 4.Elbol P, Work K. Retinal nerve fiber layer in multiple sclerosis. Acta Ophthalmol (Copenh) 1990;68:481–486. doi: 10.1111/j.1755-3768.1990.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 5.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124:1813–1820. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- 6.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48:5798–5805. doi: 10.1167/iovs.07-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pueyo V, Martin J, Fernandez J, Almarcegui C, Ara J, Egea C, Pablo L, Honrubia F. Axonal loss in the retinal nerve fiber layer in patients with multiple sclerosis. Mult Scler. 2008;14:609–614. doi: 10.1177/1352458507087326. [DOI] [PubMed] [Google Scholar]
- 9.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, Bucci MG, Pierelli F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–2527. [PubMed] [Google Scholar]
- 10.Steel DH, Waldock A. Measurement of the retinal nerve fibre layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J Neurol Neurosurg Psychiatry. 1998;64:505–509. doi: 10.1136/jnnp.64.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaveri MS, Conger A, Salter A, Frohman TC, Galetta SL, Markowitz CE, Jacobs DA, Cutter GR, Ying GS, Maguire MG, Calabresi PA, Balcer LJ, Frohman EM. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch Neurol. 2008;65:924–928. doi: 10.1001/archneur.65.7.924. [DOI] [PubMed] [Google Scholar]
- 12.Pueyo V, Ara JR, Almarcegui C, Martin J, Guerri N, Garcia E, Pablo LE, Honrubia FM, Fernandez FJ. Sub-clinical atrophy of the retinal nerve fibre layer in multiple sclerosis. Acta Ophthalmol. 2009 doi: 10.1111/j.1755-3768.2009.01527.x. [DOI] [PubMed] [Google Scholar]
- 13.Stein DM, Wollstein G, Schuman JS. Imaging in glaucoma. Ophthalmol Clin North Am. 2004;17:33–52. doi: 10.1016/S0896-1549(03)00102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zangwill LM, Bowd C. Retinal nerve fiber layer analysis in the diagnosis of glaucoma. Curr Opin Ophthalmol. 2006;17:120–131. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 15.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–675. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sergott RC, Frohman E, Glanzman R, Al-Sabbagh A. The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci. 2007;263:3–14. doi: 10.1016/j.jns.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Kallenbach K, Frederiksen J. Optical coherence tomography in optic neuritis and multiple sclerosis: a review. Eur J Neurol. 2007;14:841–849. doi: 10.1111/j.1468-1331.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XR, Knighton RW. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–4593. doi: 10.1167/iovs.05-0532. [DOI] [PubMed] [Google Scholar]
- 20.Fortune B, Cull GA, Burgoyne CF. Relative course of retinal nerve fiber layer birefringence and thickness and retinal function changes after optic nerve transection. Invest Ophthalmol Vis Sci. 2008;49:4444–4452. doi: 10.1167/iovs.08-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L, Felletti M. Comparison between GDx VCC scanning laser polarimetry and Stratus OCT optical coherence tomography in the diagnosis of chronic glaucoma. Acta Ophthalmol Scand. 2006;84:650–655. doi: 10.1111/j.1600-0420.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- 23.Leung CK, Chan WM, Chong KK, Yung WH, Tang KT, Woo J, Tse KK. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, I: correlation analysis in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3214–3220. doi: 10.1167/iovs.05-0294. [DOI] [PubMed] [Google Scholar]
- 24.Bowd C, Zangwill LM, Berry CC, Blumenthal EZ, Vasile C, Sanchez-Galeana C, Bosworth CF, Sample PA, Weinreb RN. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 25.Schrems WA, Mardin CY, Horn FK, Juenemann AG, Laemmer R. Comparison of scanning laser polarimetry and optical coherence tomography in quantitative retinal nerve fiber assessment. J Glaucoma. 2010;19:83–94. doi: 10.1097/IJG.0b013e3181a2fc0e. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Ahn H, Ha SJ, Yeom HY, Seong GJ, Hong YJ. Early glaucoma detection using the Humphrey Matrix Perimeter, GDx VCC, Stratus OCT, and retinal nerve fiber layer photography. Ophthalmology. 2007;114:210–215. doi: 10.1016/j.ophtha.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro ML, Moura FC, Medeiros FA. Scanning laser polarimetry with enhanced corneal compensation for detection of axonal loss in band atrophy of the optic nerve. Am J Ophthalmol. 2008;145:747–754. doi: 10.1016/j.ajo.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Frohman EM, Dwyer MG, Frohman T, Cox JL, Salter A, Greenberg BM, Hussein S, Conger A, Calabresi P, Balcer LJ, Zivadinov R. Relationship of optic nerve and brain conventional and non-conventional MRI measures and retinal nerve fiber layer thickness, as assessed by OCT and GDx: a pilot study. J Neurol Sci. 2009;282:96–105. doi: 10.1016/j.jns.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–1962. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- 30.Optic Neuritis Study Group. The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1991;109:1673–1678. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 31.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 32.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14:893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- 33.Noval S, Contreras I, Rebolleda G, Munoz-Negrete FJ. Optical coherence tomography versus automated perimetry for follow-up of optic neuritis. Acta Ophthalmol Scand. 2006;84:790–794. doi: 10.1111/j.1600-0420.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 34.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Comparison of multifocal visual evoked potential, standard automated perimetry and optical coherence tomography in assessing visual pathway in multiple sclerosis patients. Mult Scler. 2010 Mar 5; doi: 10.1177/1352458509359782. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 36.Vizzeri G, Bowd C, Medeiros FA, Weinreb RN, Zangwill LM. Effect of improper scan alignment on retinal nerve fiber layer thickness measurements using Stratus optical coherence tomograph. J Glaucoma. 2008;17:341–349. doi: 10.1097/IJG.0b013e31815c3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laser Diagnostic Technologies, Inc. RNFL A RNFL Analysis with GDx VCC: A Primer and Clinical Guide. San Diego, CA: Laser Diagnostic Technologies, Inc.; 2004. [Google Scholar]
- 38.Gwet K. Statistical Methods for Inter-Rater Reliability Assessment No. 2. Montgomery Village, MD: Stataxis Consulting; 2002. [Accessed May 2002]. Inter-rater reliability: dependency on trait prevalence and marginal homogeneity. Avialable at: www.stataxis.com. [Google Scholar]
- 39.Iester M, Cioli F, Uccelli A, Papadia M, Bandini F, Mancardi GL, Calabria GA. Retinal nerve fibre layer measurements and optic nerve head analysis in multiple sclerosis patients. Eye (Lond) 2009;23:407–412. doi: 10.1038/sj.eye.6703013. [DOI] [PubMed] [Google Scholar]
- 40.Klistorner A, Arvind H, Garrick R, Graham S, Paine M, Yiannikas C. Correlation of optical coherence tomography and multifocal visual-evoked potential after optic neuritis. Invest Ophthalmol Vis Sci. 2009 Dec 30; doi: 10.1167/iovs.09-4577. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277–287. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 42.Budenz DL, Michael A, Chang RT, McSoley J, Katz J. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 43.Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116:2294–2299. doi: 10.1016/j.ophtha.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–2092. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 45.Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281:74–79. doi: 10.1016/j.jns.2009.02.354. [DOI] [PubMed] [Google Scholar]
- 46.Jindahra P, Hedges TR, Mendoza-Santiesteban CE, Plant GT. Optical coherence tomography of the retina: applications in neurology. Curr Opin Neurol. 2010;23:16–23. doi: 10.1097/WCO.0b013e328334e99b. [DOI] [PubMed] [Google Scholar]
- 47.Keltner JL, Johnson CA, Spurr JO, Beck RW Optic Neuritis Study Group. Baseline visual field profile of optic neuritis. The experience of the optic neuritis treatment trial. Arch Ophthalmol. 1993;111:231–234. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 48.Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis. One-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1994;112:946–953. doi: 10.1001/archopht.1994.01090190094027. [DOI] [PubMed] [Google Scholar]
- 49.Keltner JL, Johnson CA, Cello KE, Dontchev M, Gal RL, Beck RW. Visual field profile of optic neuritis: a final follow-up report from the optic neuritis treatment trial from baseline through 15 years. Arch Ophthalmol. 2010;128:330–337. doi: 10.1001/archophthalmol.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Della Mea G, Bacchetti S, Zeppieri M, Brusini P, Cutuli D, Gigli GL. Nerve fibre layer analysis with GDx with a variable corneal compensator in patients with multiple sclerosis. Ophthalmologica. 2007;221:186–189. doi: 10.1159/000099299. [DOI] [PubMed] [Google Scholar]
- 51.Naismith RT, Tutlam NT, Xu J, Shepherd JB, Klawiter EC, Song SK, Cross AH. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009;73:46–52. doi: 10.1212/WNL.0b013e3181aaea32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, O'Day J, Grigg J, Billson F, Yiannikas C. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol. 2008;64:325–331. doi: 10.1002/ana.21474. [DOI] [PubMed] [Google Scholar]
- 53.Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ. Assessing visual pathway function in multiple sclerosis patients with multifocal visual evoked potentials. Mult Scler. 2009;15:1431–1441. doi: 10.1177/1352458509350470. [DOI] [PMC free article] [PubMed] [Google Scholar]